Abstract
With interest waning in the use of cyclooxygenase-2 (COX-2) inhibitors for inflammatory disease, prostaglandin receptors provide alternative targets for the treatment of COX-2–mediated pathological conditions in both the periphery and the central nervous system. Activation of prostaglandin E2 receptor (PGE2) subtype EP2 promotes inflammation and is just beginning to be explored as a therapeutic target. To better understand physiological and pathological functions of the prostaglandin EP2 receptor, we developed a suite of small molecules with a 3-aryl-acrylamide scaffold as selective EP2 antagonists. The 12 most potent compounds displayed competitive antagonism of the human EP2 receptor with KB 2–20 nM in Schild regression analysis and 268- to 4,730-fold selectivity over the prostaglandin EP4 receptor. A brain-permeant compound completely suppressed the up-regulation of COX-2 mRNA in rat cultured microglia by EP2 activation and significantly reduced neuronal injury in hippocampus when administered in mice beginning 1 h after termination of pilocarpine-induced status epilepticus. The salutary actions of this novel group of antagonists raise the possibility that selective block of EP2 signaling via small molecules can be an innovative therapeutic strategy for inflammation-related brain injury.
Keywords: cAMP, brain inflammation, neurodegeneration, neuroprotection, epilepsy
Cyclooxygenase-2 (COX-2), the inducible isoform of COX, is rapidly up-regulated in damaged tissue, for example in the central nervous system (CNS) after a seizure or cerebral ischemia (1–3). COX-2 induction in CNS overall contributes to inflammation and injury mainly by producing prostanoids (4–7). However, the deleterious cardio- and cerebrovascular side effects from sustained inhibition of COX-2 suggest that some COX-2 downstream prostanoid signaling might be beneficial (8), such that modulation of a specific prostanoid receptor or synthase could be a superior therapeutic strategy compared with generic block of the entire COX-2 cascade. Prostaglandin E2 (PGE2), a dominant enzymatic product of COX-2 in the brain, can activate four G-protein–coupled receptors (GPCRs): EP1, EP2, EP3, and EP4. Among these, EP2 and EP4 receptors are positively coupled through Gαs to cAMP production (9). In turn, cAMP can initiate multiple downstream events mediated by protein kinase A (PKA) or exchange protein activated by cAMP (Epac) (9).
The EP2 receptor is widely expressed in both neurons and glia (3, 10). Neuronal EP2 activation appears to mediate some beneficial effects, such as PKA-dependent neuroprotection in acute models of ischemia and excitotoxicity (3, 11, 12), early neuroprotection following seizures (13), and promotion of spatial learning (14). Conversely, on the basis of the phenotype of EP2 knockout mice, EP2 activation is thought to promote inflammation and neurotoxicity in animal models of neurodegenerative diseases including Alzheimer's disease (15), Parkinson's disease (16), and amyotrophic lateral sclerosis (10). Glial, especially microglial EP2, is considered to play a major role in brain inflammation associated with chronic neurologic disorders (10, 16). Genetic ablation of the EP2 receptor reduces oxidative stress and improves cell survival, accompanied by substantial down-regulation of enzymes that produce reactive oxygen or nitrogen species such as inducible nitric oxide synthase (iNOS), NADPH oxidase (NOX), and COX-2 itself (10). EP2 receptor activation by PGE2 has also been reported to elevate iNOS expression in activated astrocytes by potentiating the response to the inflammatory cytokines TNF-α and IFN-γ (17). Moreover, the EP2 receptor is involved in spinal inflammatory hyperalgesia and neuropathic pain (18, 19). In the past 2 decades inflammation has emerged as a common feature in many if not all chronic neurological disorders and is widely believed to play a pivotal role in subsequent neuropathogenesis (20). For these reasons we hypothesized that pharmacologic block of PGE2/EP2 signaling might represent an innovative strategy to mitigate inflammation and protect neuronal tissue in neurological disorders.
All previous conclusions on the roles of PGE2/EP2 signaling were made on the basis of studies using either a selective EP2 agonist (e.g., butaprost) or mice deficient in the EP2 gene. Parallel data from direct pharmacological inhibition of EP2 receptors are missing, because in contrast to all other prostaglandin receptors no selective antagonists for the EP2 receptor have been reported until recently (21). We developed a set of cell-based time-resolved fluorescence resonance energy transfer (TR-FRET) assays of cAMP formation that are suitable for high-throughput screening (HTS) and we used these assays to discover and then chemically modify novel selective competitive antagonists of this key prostaglandin receptor. Here, we report that a series of small molecules sharing a 3-aryl-acrylamide scaffold show high potency and good selectivity for inhibiting PGE2-induced cAMP accumulation in both human and mouse EP2-expressing cells and have sufficient pharmacokinetic properties to be useful in vivo. These small molecules completely suppressed the EP2–up-regulated inflammatory mediator COX-2 in rat primary microglial cultures. Administration of an EP2 antagonist in mice after pilocarpine-induced status epilepticus (SE) significantly reduced neurodegeneration in hippocampus. These results reinforce the value of the prostaglandin EP2 receptor as a potential neuroprotection target in epilepsy and other inflammation-related neurological disorders. This work was reported in preliminary form (22, 23).
Results
Potent and Selective Antagonists for Human EP2 Receptors.
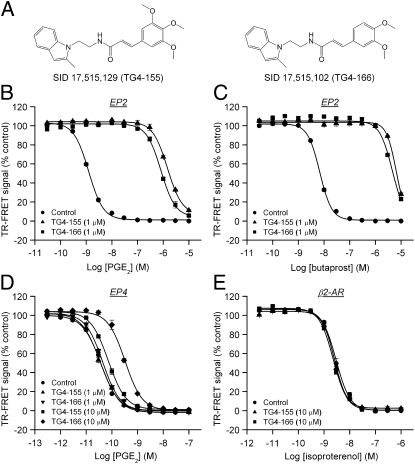
Using a set of cell-based TR-FRET assays of cAMP formation, we screened a library of 262,371 small molecules and identified a series of compounds as antagonists of human prostaglandin EP2 receptor (Figs. S1 and S2). Among those, hits PubChem substance identification number (SID) 17,515,129 (resynthesized as TG4-155) and SID 17,515,102 (TG4-166) are the most potent ones (Fig. 1A) and showed robust inhibition of PGE2 (1 μM)-induced cAMP accumulation (i.e., reduction of the TR-FRET signal) in human EP2-overexpressing C6 glioma (C6G-EP2) cells (11), without affecting prostaglandin EP4 or β2-adrenergic receptors under agonist-saturated conditions (SI Results and Fig. S1). The potency of compounds was further evaluated by their effects on the dose–response curves of PGE2 and butaprost, the EP2 selective agonist, in C6G-EP2 cells. Cells were incubated first with vehicle or a fixed concentration (1 μM) of test compound for 5 min and then with increasing concentrations of PGE2 or butaprost for 40 min to induce cAMP accumulation. Both compounds caused a robust rightward shift in the PGE2 dose–response curve without affecting the maximal response to PGE2. TG4-155 (1 μM) caused 1,120-fold shift and TG4-166 (1 μM) caused a 651-fold shift in the PGE2 EC50 (Fig. 1B). TG4-155 and TG4-166 also caused robust inhibition of the EP2 response to butaprost in C6G-EP2 cells, TG4-155 (1 μM) producing a 962-fold rightward shift and TG4-166 (1 μM) a 678-fold shift in the butaprost EC50 (Fig. 1C). The quantitatively similar extent of inhibition by TG4-155 and TG4-166 against EP2 receptor activation by PGE2 or butaprost demonstrates that EP2 inhibition is not agonist specific.
Fig. 1.
Selective inhibition of EP2 receptor by hit compounds. (A) Chemical structures of TG4-155 and TG4-166. (B) TG4-155 and TG4-166 caused rightward shifts in the PGE2 dose–response curves in C6G-EP2 cells. TG4-155 (1 μM) caused a 1,120-fold shift and TG4-166 (1 μM) caused a 651-fold shift in the PGE2 EC50. (C) TG4-155 (1 μM) caused a 962-fold shift and TG4-166 (1 μM) caused a 678-fold shift in the butaprost EC50 in C6G-EP2 cells. (D) TG4-155 and TG4-166 (1 μM) had no effect on prostaglandin EP4 receptor. At 10 μM, TG4-155 caused only a 1.8-fold shift and TG4-166 caused an 8.6-fold shift in the PGE2 EC50 in HEK-EP4 cells. (E) There was no effect of TG4-155 and TG4-166 (10 μM) on β2-adrenergic receptor as shown by overlapping isoproterenol dose–response curves. Data were normalized as percentage of maximum response; points represent mean ± SEM (n = 4).
We next determined the functional selectivity of these hits for inhibiting EP2 receptors relative to other Gαs-coupled GPCRs. Cells expressing human EP4 or β2-adrenergic receptor were incubated with vehicle, 1 or 10 μM test compound and subsequently stimulated with increasing concentrations of PGE2 or isoproterenol, respectively. TG4-155 and TG4-166 at 1 μM had no effect on prostaglandin EP4 receptors as shown by virtually overlapping PGE2 dose–response curves (Fig. 1D). Even at 10 μM, TG4-155 caused <2-fold shift and TG4-166 caused <10-fold shift in the PGE2 EC50 in HEK-EP4 cells (Fig. 1D). There was also no effect of TG4-155 and TG4-166 (10 μM) on β2-adrenergic receptors as shown by overlapping isoproterenol dose–response curves (Fig. 1E). Both EP4 and β2-adrenergic receptors are Gαs-coupled GPCRs, and EP4 is also activated by PGE2. Therefore, these results suggest that the compounds are selective for the EP2 receptor over EP4 and β2-adrenergic receptors. The selectivity of TG4-155 was further tested in cell-based functional assays against a panel of other GPCRs in leukotriene and prostanoid receptor families. TG4-155 displayed at least 500-fold selectivity for the human EP2 receptor over human BLT1, EP1, EP3, and FP receptors; 345-fold selectivity against human TP receptor; 240-fold selectivity against human IP receptor; and 7-fold selectivity against human DP1 receptor (Fig. S3). In addition, TG4-155 did not show substantial inhibition of COX-1 and COX-2 at 10 μM (Fig. S3). These results indicate that of the nine canonical prostanoid receptors, TG4-155 shows nanomolar antagonist activity against only EP2 and DP1.
EP2 Antagonism Is Competitive.
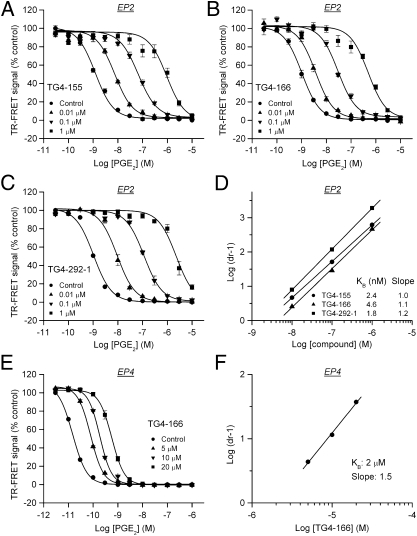
Information on mechanisms of inhibition can be obtained by performing a Schild regression analysis characterized by the equation log(dr − 1) = log XB − log KB, where dose ratio (dr) is the fold shift in EC50, XB is [antagonist], and KB is the equilibrium dissociation constant for the antagonist–receptor complex. A linear regression of log(dr − 1) on log XB with a slope of unity characterizes a competitive antagonism and the KB value indicates the antagonist concentration required for a twofold rightward shift in the dose–response curve. Thus, a lower KB value indicates a higher inhibitory potency. To perform the Schild regression, C6G-EP2 cells were incubated first with vehicle, 0.01, 0.1, or 1 μM of test compound for 5 min and then with increasing concentrations of PGE2 for 40 min to activate EP2 receptors. All compounds induced concentration-dependent, parallel rightward shifts in the PGE2 dose–response curve (Fig. 2 A–C). The Schild regression analyses demonstrated that these compounds have a competitive mechanism of antagonism of EP2 receptor as illustrated by TG4-155 and TG4-166 with KB = 2.4 nM and KB = 4.6 nM, respectively (Fig. 2D and Table 1). TG4-155 displayed a high affinity to human EP2 receptors with Ki = 15 nM in the radioligand binding assay (Fig. S3). The Schild regression analyses also revealed selectivity for EP2 over EP4 because the KB for antagonism of EP4 was 4,730-fold (TG4-155) and 435-fold (TG4-166) higher than for EP2 (Table 1 and Fig. 2 E and F).
Fig. 2.
Competitive antagonism of EP2 receptor. (A–C) Hits TG4-155, TG4-166, and analog TG4-292-1 inhibited PGE2-induced human EP2 receptor activation in a concentration-dependent manner. (D) Schild regression analysis was performed to elucidate the modality of antagonism from these compounds. TG4-155, TG4-166, and TG4-292-1 displayed a competitive antagonism mode of action on EP2 receptor shown by Schild plots. KB = 2.4, 4.6, and 1.8 nM; slopes, 1.0, 1.1, and 1.2 for TG4-155, TG4-166, and TG4-292-1, respectively. (E) EP2 antagonist compounds showed low potency on human EP4 receptor in HEK-EP4 cells, as illustrated by TG4-166. (F) Schild regression analysis was performed to evaluate inhibition of human EP4 receptor by TG4-166. KB = 2 μM; slope, 1.5. Data were normalized as percentage of maximum response; points represent mean ± SEM (n = 4).
Table 1.
Activity, cytotoxicity, and selectivity for EP2 antagonist compounds
| Analog no. | *KB EP2, nM | †CC50, μM | ‡Therapeutic index | §KB EP4, μM | ¶Selectivity index |
| TG4-155 | 2.4 | 172 | 71,700 | 11.4 | 4,730 |
| TG4-166 | 4.6 | 397 | 86,300 | 2.0 | 435 |
| TG4-211-1 | 348 | 360 | 1,030 | 13.7 | 39 |
| TG4-211-2 | 947 | 448 | 473 | 13.3 | 14 |
| TG4-215-2 | 1,490 | 325 | 219 | 15.3 | 10 |
| TG4-161 | 2,520 | 311 | 123 | 13.9 | 6 |
| TG6-109-1 | Inactive | 316 | NA | 18.9 | NA |
| TG4-290-1 | 2.1 | 155 | 73,800 | 5.4 | 2,600 |
| TG4-290-2 | 22.2 | 264 | 11,900 | 1.7 | 77 |
| TG6-10-1 | 21.4 | 81 | 3,790 | 13.4 | 626 |
| TG6-10-2 | 58.8 | 209 | 3,550 | 22.4 | 381 |
| TG4-154 | 1,860 | 182 | 98 | 5.7 | 3 |
| TG6-78 | 185 | 278 | 1,500 | 16.6 | 90 |
| TG4-156 | 214 | 397 | 1,860 | 18.1 | 85 |
| TG6-94-1 | 16.5 | 3,090 | 187,000 | 8.0 | 484 |
| TG6-97-1 | 42.4 | 653 | 15,400 | 11.7 | 277 |
| TG4-215-1 | 18.1 | 146 | 8,070 | 11.4 | 629 |
| TG4-292-2 | 7.2 | 517 | 71,800 | 1.9 | 268 |
| TG6-109-2 | 82.4 | 199 | 2,420 | 30.2 | 367 |
| TG6-94-2 | 3.8 | 89 | 23,400 | 10.6 | 2,800 |
| TG6-97-2 | 7.0 | 684 | 97,700 | 13.9 | 1,990 |
| TG6-94-3 | 18.7 | 46 | 2,460 | 17.2 | 921 |
| TG6-97-3 | 27.8 | 62 | 2,230 | 19.9 | 715 |
| TG4-292-1 | 1.8 | 696 | 387,000 | 2.2 | 1,200 |
| TG4-294-1 | 6.3 | 601 | 95,400 | 3.3 | 525 |
| TG4-294-2 | 1.9 | 481 | 253,000 | 4.7 | 2,470 |
| TG6-110 | 8.0 | 1,240 | 155,000 | 6.9 | 859 |
NA: not applicable.
*KB (nM) value for EP2 receptor from Schild regression analysis.
†Half-maximal cytotoxic concentration (CC50) in C6G cells after 48 h incubation.
‡In vitro therapeutic index determined by CC50/KB for EP2.
§KB (μM) value for EP4 receptor from Schild regression analysis.
¶Selectivity index, determined by KB EP4/KB EP2.
Structure–Activity Relationships.
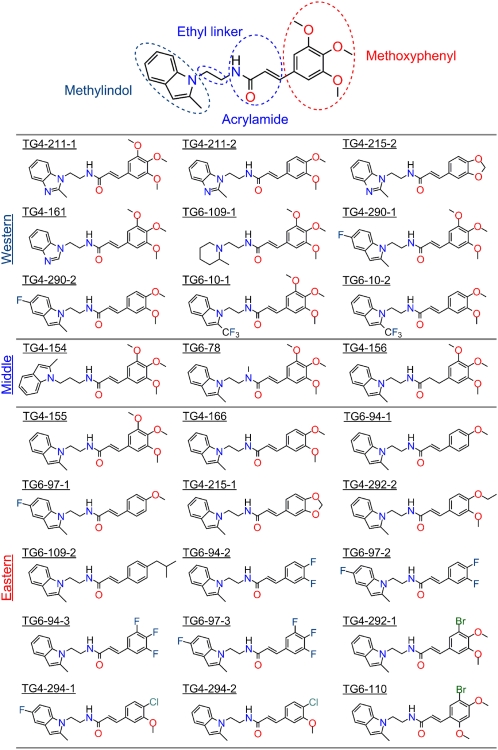
To obtain information on structure–activity relationships (SAR), we synthesized 27 small molecules based on the 3-aryl-acrylamide scaffold in TG4-155, (E)-N-(2-(2-methyl-1H-indol-1-yl)ethyl)-3-(3,4,5-trimethoxyphenyl)acrylamide, and TG4-166, (E)-3-(3,4-dimethoxyphenyl)-N-(2-(2-methyl-1H-indol-1-yl)ethyl)acrylamide (Fig. S4). Four moieties were examined as determinants of compound potency and selectivity: methylindol, ethyl linker, acrylamide, and methoxyphenyl (Fig. 3). All compounds were evaluated for inhibitory potency on EP2 and EP4 receptors by measuring the Schild KB values. Overall, compounds with a methylindol or fluoro-methylindol ring, ethyl linker, acrylamide, and methoxyphenyl or halogenphenyl retain activities in the low nanomolar range (SI Results, Fig. 3, and Table 1). Introduction of fluorine (e.g., TG4-290–1 and TG6-10–1 in the western side and TG6-94–2 in the eastern side) to improve compound pharmacokinetic properties increased the in vitro metabolic stability of compounds, measured in mouse or human liver microsomes (SI Results, Fig. 3, and Fig. S5). All test compounds showed no significant cytotoxicity by measuring the half-maximal cytotoxic concentrations (CC50) in C6G cells (Table 1), as illustrated by TG4-155 and TG4-166 in Fig. S6. Compared with hits TG4-155 and TG4-166, analogs TG4-290-1, TG4-292-1, and TG4-294-2 displayed improved potencies for EP2 receptor inhibition with KB = 2.1 nM, 1.8 nM, and 1.9 nM; in vitro therapeutic indexes (CC50/KB for EP2) of 73,800, 387,000, and 253,000; and selectivity indexes (KB for EP4/KB for EP2) of 2,600, 1,200, and 2,470 (Fig. 2 and Table 1).
Fig. 3.
Hit structure and analog design. Analogs were designed on the basis of the structure of hits TG4-155 and TG4-166.
Microglial EP2 Activation Induces COX-2.
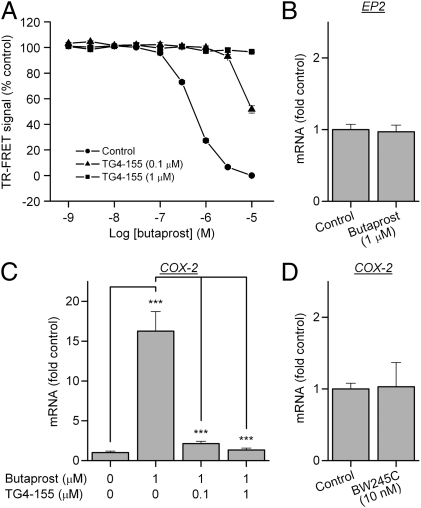
Microglia are regarded as the resident macrophages in the CNS and thus believed to play a pivotal role in inflammation-mediated neurodegeneration in various models of neurological disorders (24). COX-2 is well recognized as a major mediator of inflammation and neurotoxicity (7), and following seizures COX-2 up-regulation in neurons triggers an inflammatory reaction in the brain (13). The activation of EP2 receptors on microglia, presumably by PGE2 produced from neuronal COX-2, has been proposed to play an important role in brain inflammation (10, 16). To examine the activity of TG4-155 on native EP2 receptors, rat primary microglial cultures were preincubated with vehicle or increasing concentrations of TG4-155 for 30 min, followed by addition of butaprost in the presence of antagonist for 2 h. The cellular cAMP levels were evaluated by TR-FRET assay and the induction of COX-2 mRNA was measured by quantitative real-time PCR (qRT-PCR). Butaprost induced a substantial increase of cAMP level in rat primary microglia with an EC50 value of 0.5 μM, and TG4-155 displayed robust competitive inhibition of butaprost-induced EP2 activation in a concentration-dependent manner with potency equivalent to a Schild KB of 5 nM (Fig. 4A).
Fig. 4.
EP2 activation induces microglial activation. (A) Butaprost increased cAMP levels in rat primary microglial cultures with EC50 = 0.5 μM. TG4-155 showed robust inhibition of butaprost-induced cAMP accumulation in rat microglia in a concentration-dependent manner, with potency equivalent to a Schild KB = 5 nM. (B) EP2 activation in microglia by butaprost (1 μM) did not affect expression of the EP2 receptor itself in cultured microglia. (C) Butaprost (1 μM) induced COX-2 expression 16.3-fold above background in microglia, measured by quantitative real-time PCR (qRT-PCR). The COX-2 up-regulation was attenuated by TG4-155. (D) BW245C, a selective DP1 agonist, did not induce microglial COX-2 at 10 nM. Bars represent the mean ± SEM (n = 3–4). ***P < 0.001 by one-way ANOVA with posthoc Bonferroni.
EP2 receptor activation on microglia causes induction of proinflammatory cytokines and other mediators (25). We studied EP2-mediated induction of COX-2 because induction is rapid and large in comparison with other inflammatory mediators. EP2 activation by 1 μM butaprost for 2 h did not affect expression of the EP2 receptor itself in microglia (Fig. 4B), but increased COX-2 expression 16.3-fold (Fig. 4C). The up-regulation of COX-2 by microglial EP2 was returned to the basal level by 100 nM TG4-155, but not below basal (Fig. 4B). It is unlikely that the DP1 receptor is involved in this inhibitory effect on COX-2 induction for the following reasons. First, 1 μM butaprost does not activate DP1 (26); second, TG4-155 inhibits DP1 by <50% at 100 nM (Fig. S3); third, the DP1 agonist, BW245C (26), does not induce COX-2 mRNA at a concentration selective for DP1 under the same conditions (Fig. 4D); finally, a 2-h assay incubation would be insufficient time to produce significant COX-2 protein (if indeed the mRNA is translated in microglia) and hence the DP1 agonist, PGD2, from the induced mRNA. These considerations lead to the conclusion that TG4-155 blocks endogenous as well as recombinant EP2 and confirm a role for the EP2 signaling pathway in microglia.
Delayed Administration of EP2 Antagonist Reduces Neuronal Injury Induced by Status Epilepticus.
Up-regulation of COX-2 in neuronal tissue after a seizure or cerebral ischemia usually contributes to neuronal injury. However, the downstream COX-2 signaling pathways involved in brain injury are not well known. Creation of potent antagonists of the human prostaglandin EP2 receptor provides a new opportunity to determine whether this key prostaglandin receptor plays a role in pathological conditions. Thus, we determined the effect of EP2 inhibition by these compounds after pilocarpine-induced SE in mice. First, we evaluated the potency of TG4-155 on mouse EP2 receptor because of sequence differences between human and mouse EP2. TG4-155 displayed robust inhibition of PGE2-induced cAMP accumulation in C6G cells stably expressing mouse EP2 receptor (C6G-mEP2) in a dose-dependent manner with a KB value of 4.7 nM, which is similar to that in human EP2 (Fig. S7). TG4-155 displayed a bioavailability of 61% (i.p. route compared with i.v.), a plasma half-life of 0.6 h, and a brain/plasma ratio of 0.3 in mice after i.p. administration (Fig. S8). Despite its short plasma half-life we felt it was worthwhile to test the effect of TG4-155 in an in vivo mouse model of epilepsy because COX-2 up-regulation after seizures is rapid.
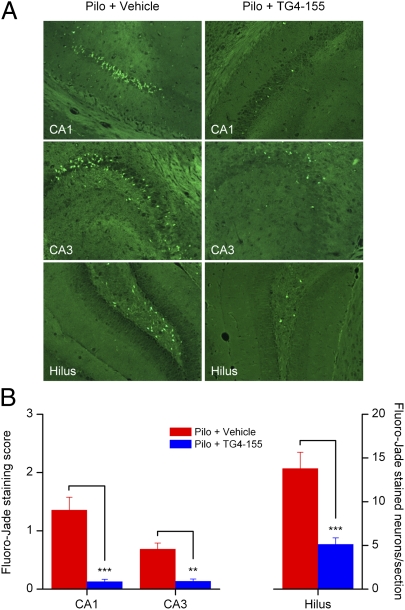
Pilocarpine-induced SE was allowed to proceed for 1 h and then terminated by pentobarbital (SI Materials and Methods). The mice were then randomized and TG4-155 or vehicle was administered (5 mg/kg, i.p.) 1 h after termination of SE and then again 12 h later. The first injection was timed to overlap the early peak of neuronal COX-2 induction; given the brain half-life and the amount injected, the brain concentration of TG4-155 is predicted to be higher than the mouse KB for >3 h (Fig. S8). Animals (10 in each group) were euthanized and the brains were collected 24 h after SE. To evaluate brain injury of mice, coronal brain sections (8 μm, three to nine sections per mouse throughout the hippocampus) were stained with Fluoro-Jade (0.001%, wt/vol) and scored for degenerating neurons in hippocampal subregions CA1, CA3, and dentate hilus (Fig. S9). Pilocarpine-induced SE caused substantial neurodegeneration in hippocampus 24 h after SE (Fig. 5A), whereas no positive staining was detected in animals from the control groups. Administration of TG4-155 significantly reduced SE-induced neurodegeneration scores by 91% (P < 0.001) in CA1, by 80% (P < 0.01) in CA3, and by 63% (P < 0.001) in hilus (Fig. 5B). The involvement of DP1 in the effect of TG4-155 should be very limited because TG4-155 had a concentration of ∼69 nM in the mouse brain at 1 h after administration (Fig. S8) and thus would have had at most a very brief effect on DP1 (Fig. S3). Moreover, DP1 inhibition is proconvulsive in the pentylenetetrazol model (27), arguing against a prominent role for DP1 inhibition in the neuroprotection produced by TG4-155. However, DP1 activation can be neuroprotective in models of excitotoxicity and ischemia (28, 29), so whether DP1 activation by PGD2 contributes to seizure-induced neuronal injury requires additional study. Further studies are also needed to optimize the TG4-155 treatment protocol (dose and dose intervals) to determine whether neuroprotection is sustained at longer time points (e.g., several weeks after SE) and to determine whether TG4-155 displays a direct anticonvulsant effect. However, these results provide strong support for involvement of the EP2 receptor in neuronal injury following a prolonged seizure.
Fig. 5.
EP2 inhibition reduced neuronal injury after SE. (A) Neurodegeneration in hippocampi from animals treated with vehicle or TG4-155 was assessed by Fluoro-Jade staining 24 h after SE. No positive staining was detected in control mice treated with vehicle or TG4-155. (B) Quantification of neurodegenerating neurons in hippocampal subregions CA1, CA3, and dentate hilus. Coronal brain sections from 10 animals in each group were examined with a fluorescence microscope. Neuron injury in CA1 and CA3 was quantified by averaging the injury scores from three to nine sections per mouse (in each section: 0, <3 Fluoro-Jade–positive cells; 1, 3–30 cells; 2, 31–100 cells; 3, extensive Fluoro-Jade staining), exemplified in Fig. S9. Neuron injury in the hilus was evaluated by counts of Fluoro-Jade–positive cells per section (n = 10 mice per group, **P < 0.01, ***P < 0.001; one-way ANOVA and posthoc Bonferroni with selected pairs for CA1 and CA3, t test for hilus). Data are shown as mean ± SEM.
Discussion
Sustained COX-2 activation in CNS can result in PGE2-mediated brain inflammation and injury (2, 4, 5, 7, 20). For example, the EP1 prostaglandin receptor was reported to mediate COX-2–directed neurotoxicity in ischemic stroke (6), and EP2 accelerates the progression of experimental amyotrophic lateral sclerosis (10). EP2 is emerging as a principal mediator of brain inflammation after injury. Genetic ablation of prostanoid receptors has been useful but is complicated by the possibility of developmental and other homeostatic adjustments (9). Small molecules as selective modulators for prostaglandin receptors would be a valuable complement to genetic strategies. We now report discovery of a group of compounds that act as potent competitive antagonists of the human, mouse, and rat prostaglandin EP2 receptors. These compounds contain a 3-aryl-acrylamide scaffold and are structurally distinct from PF-04418948, a highly selective EP2 antagonist recently reported by Pfizer (21). The selectivity of PF-04418948 for EP2 over DP1 is higher than that of TG4-155; however, PF-04418948 was not tested as an inhibitor of COX-1 or COX-2, and its brain penetration was not reported. Identification of selective and potent antagonists of the EP2 receptor provides an opportunity to address the pathological functions of this key prostaglandin receptor to reach a more detailed understanding of the COX-2 cascade in disease.
The EP2 antagonists we report here have robust potency, good selectivity, and brain penetration. The most potent compounds showed competitive antagonism with KB values of 2 nM against human EP2 in Schild regression analyses (Fig. 2 and Table 1). These EP2 inhibitors produced a similar effect on the butaprost and PGE2 dose–response curves, indicating that their inhibition is not agonist specific (Fig. 1C), and they were effective on native EP2 receptors expressed by microglia (Fig. 4A). The results from secondary assays exclude possible effects of these compounds up- or downstream of the EP2 receptor. Their lack of effect on EP4 and β2-adrenergic receptors rules out a direct action on the Gαs protein, adenylate cyclase, phosphodiesterase, and the TR-FRET assay itself (Fig. S1 and Fig. 1 D and E). Both EP2 and EP4 receptors are Gαs coupled and elevate the cytoplasmic cAMP level in response to PGE2 binding, which has made it difficult to distinguish EP2 from EP4 functions in conditions involving induced PGE2 formation. The high selectivity (up to 4,730-fold) of these antagonists for EP2 over EP4 (Fig. 1D and Table 1) will make it feasible to address which PGE2 receptor subtype is involved in Gαs-mediated inflammatory conditions. Furthermore, these compounds displayed very little or no detectable activity at other tested prostanoid receptors except the DP1 receptor, against which TG4-155 was 7-fold less potent than at EP2 (Fig. S3).
The mechanisms of COX induction and the roles of EP2 receptor activation in seizure-induced neuroinflammation and neurodegeneration are clearly multifactorial and likely cell specific. Studies of neuron-specific conditional knockouts of COX-2 demonstrate that neuronal induction of COX-2 triggers or exacerbates brain inflammation and neurodegeneration after pilocarpine (13). Microglial COX-1 is an alternative source of PGE2 that might also play a role in brain inflammation (30, 31). As brain macrophages, microglia are a major mediator of immune responses in CNS and are effectors of brain inflammation and neurodegeneration in various models of neurological disorders (24). We hypothesize that after seizures neuronal COX-2 produces PGE2, which activates EP2 receptors on microglia, accelerating the innate immune response after SE and triggering secondary neurodegeneration. At the same time, activation of EP2 receptors on neurons, via a PKA-dependent pathway, appears to be neuroprotective in acute models of NMDA-induced excitotoxicity and ischemia (3, 11, 12). The dual role of EP2 receptors, mediating both neuroprotection and neurodegeneration perhaps by different cell types and different pathways (32), complicates exploitation of EP2 as a therapeutic target. The balance between opposing NMDA-mediated cell injury and promoting injurious inflammation will likely be different for different neurologic disorders.
SE in man is associated with substantial mortality and morbidity that involve brain injury and inflammation. We demonstrate that inhibition of the EP2 receptor after SE was terminated significantly reduces neurodegeneration in mice assessed 24 h later (Fig. 5). This beneficial effect was not derived from conventional COX blockade because TG4-155 does not target COX-1 or COX-2 (Fig. S3) and was unlikely to involve the weaker inhibition of DP1 because the TG4-155 brain levels reached were too low. Neuroprotection by EP2 inhibition reveals a role for this key prostaglandin receptor in progression of seizure-induced neurodegeneration, possibly via induction of inflammatory mediators in microglia. The effect of EP2 antagonists on brain inflammation in other chronic neurologic disorders such as Alzheimer's and Parkinson's diseases awaits study.
Materials and Methods
Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) cAMP Assay.
cAMP was measured with a cell-based homogeneous TR-FRET method (Cisbio Bioassays). The assay is based on generation of a strong FRET signal upon the interaction of two molecules: an anti-cAMP antibody coupled to a FRET donor (Cryptate) and cAMP coupled to a FRET acceptor (d2). Endogenous cAMP produced by cells competes with labeled cAMP for binding to the cAMP antibody and thus reduces the FRET signal. Please see SI Materials and Methods for details.
Rat Primary Microglial Culture.
Cortices from newborn Sprague−Dawley rats were carefully dissected, triturated, and washed. Cortical cells were cultured for 14 d with medium changed every 2−3 days. The loosely attached microglia were dislodged from the underlying astrocyte monolayer and collected. The cells were resuspended and plated on BD Primaria culture dishes or plates (BD Biosciences). Nonadherent cells were removed by changing the medium after 30−60 min. The adherent microglia were incubated for 24 h, underwent serum starvation for 24 h, and were then ready for use. Please see SI Materials and Methods for details.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR).
Rat primary microglial cultures were preincubated for 30 min with vehicle or test compound, followed by treatment with 1 μM butaprost or 10 nM BW245C for 2 h. Total RNA was isolated using TRIzol (Invitrogen) with the PureLink RNA Mini Kit (Invitrogen) from cultured cells. First-strand complementary DNA (cDNA) synthesis was performed with SuperScript II Reverse Transcriptase (Invitrogen). qRT-PCR was performed by using iQ SYBR Green Supermix (Bio-Rad Laboratories) in the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories). Please see SI Materials and Methods for details.
Animals and Treatment.
C57BL/6 mice (8–12 wk old) were injected with pilocarpine (280 mg/kg, i.p.) to induce status epilepticus (SE). SE was allowed for 1 h and terminated by pentobarbital (30 mg/kg, i.p.). Mice were then randomized by assignment to a random number stream and received two doses of vehicle or TG4-155 (5 mg/kg, i.p.) at 1 and 12 h after SE termination. Mice were euthanized under deep isoflurane anesthesia 24 h after SE and brains were collected for histology. Please see SI Materials and Methods for details.
Neuropathology.
Fixed mouse brains (n = 10 per group) were sectioned (8 μm) coronally. Neurodegeneration in the hippocampus was assessed by Fluoro-Jade staining as previously described (13). Fluoro-Jade staining in each of three to nine sections (median: six sections) per mouse between bregma −1.22 and −3.52 was quantified in CA1 and CA3 by scoring criteria (0, <3 Fluoro-Jade–positive cells per section; 1, 3–30 cells; 2, 31–100 cells; 3, extensive Fluoro-Jade staining), exemplified in Fig. S9. The average injury score for each mouse was a continuous variable that ranged from 0.11 to 2.60 and was used to compare the degree of injury in mice treated with vehicle or TG4-155. The injury scores passed the Kolmogorov–Smirnov test for normality. Neuronal injury in the dentate hilus was measured by counts of Fluoro-Jade–positive cells in each section. Please see SI Materials and Methods for details.
Statistical Analysis.
Data were plotted with Origin (OriginLab). Statistical analyses were performed using Prism (GraphPad Software) with one-way ANOVA and posthoc Bonferroni or t test as appropriate. P < 0.05 was considered to be statistically significant. Data were normalized to control (vehicle) values and presented as mean ± SEM.
Acknowledgments
We appreciate the excellent technical assistance of Renee Shaw, Clinton Maddox, Iestyn Lewis, and Brian Revennaugh. This work was supported by Epilepsy Foundation Postdoctoral Research Fellowship 219142 (to J.J.); National Institutes of Health Grant R21NS074169 (to R.D.); and the CounterACT Program, Office of the Director, National Institutes of Health (OD) and the National Institute of Neurological Disorders and Stroke (NINDS), Grant U01NS058158 (to R.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Accession: The screening data reported in this paper have been deposited in the PubChem database, http://pubchem.ncbi.nlm.nih.gov (assay ID no. 1422).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120195109/-/DCSupplemental.
References
- 1.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcheselli VL, Bazan NG. Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J Biol Chem. 1996;271:24794–24799. doi: 10.1074/jbc.271.40.24794. [DOI] [PubMed] [Google Scholar]
- 3.McCullough L, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama M, et al. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iadecola C, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano T, et al. Prostaglandin E2 EP1 receptors: Downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 7.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 8.Abraham NS, El-Serag HB, Hartman C, Richardson P, Deswal A. Cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction and cerebrovascular accident. Aliment Pharmacol Ther. 2007;25:913–924. doi: 10.1111/j.1365-2036.2007.03292.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirata T, Narumiya S. Prostanoid receptors. Chem Rev. 2011;111:6209–6230. doi: 10.1021/cr200010h. [DOI] [PubMed] [Google Scholar]
- 10.Liang X, et al. The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol. 2008;64:304–314. doi: 10.1002/ana.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang J, et al. Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci USA. 2010;107:2307–2312. doi: 10.1073/pnas.0909310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol. 2005;57:758–761. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- 13.Serrano GE, et al. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011;31:14850–14860. doi: 10.1523/JNEUROSCI.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Zhang J, Breyer RM, Chen C. Altered hippocampal long-term synaptic plasticity in mice deficient in the PGE2 EP2 receptor. J Neurochem. 2009;108:295–304. doi: 10.1111/j.1471-4159.2008.05766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang X, et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer's disease. J Neurosci. 2005;25:10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin J, et al. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007 doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiao HY, et al. TNF-alpha/IFN-gamma-induced iNOS expression increased by prostaglandin E2 in rat primary astrocytes via EP2-evoked cAMP/PKA and intracellular calcium signaling. Glia. 2007;55:214–223. doi: 10.1002/glia.20453. [DOI] [PubMed] [Google Scholar]
- 18.Hösl K, et al. Spinal prostaglandin E receptors of the EP2 subtype and the glycine receptor alpha3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain. 2006;126:46–53. doi: 10.1016/j.pain.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Reinold H, et al. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115:673–679. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: A matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 21.af Forselles KJ, et al. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP2 receptor antagonist. Br J Pharmacol. 2011;164:1847–1856. doi: 10.1111/j.1476-5381.2011.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang J, Ganesh T, Du Y, Quan Y, Dingledine R. Novel selective antagonists reveal yin-yang nature of the prostaglandin EP2 receptor in brain injury. Soc Neurosci Abstract Viewer and Itinerary Planner. 2010 Available at http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=855950d5-ec4f-4899-819b-b706b8881f31&cKey=6c4117d5-9e19-485e-b5bd-03b3f3abb107&mKey=%7bE5D5C83F-CE2D-4D71-9DD6-FC7231E090FB%7d. [Google Scholar]
- 23.Jiang J, et al. Novel small molecule antagonists reveal a role of prostaglandin EP2 receptor in seizure-related neuronal injury and inflammation. Soc Neurosci Abstract Viewer and Itinerary Planner. 2011 Available at http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=aceb149d-00af-4c74-9a09-69314e726c7d&cKey=eefb3ecf-dbb0-476f-bc8e-3441a37bf194&mKey={8334BE29-8911-4991-8C31-32B32DD5E6C8} [Google Scholar]
- 24.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 25.Li P, et al. Expression of cyclooxygenase-1/-2, microsomal prostaglandin-E synthase-1 and E-prostanoid receptor 2 and regulation of inflammatory mediators by PGE(2) in the amoeboid microglia in hypoxic postnatal rats and murine BV-2 cells. Neuroscience. 2009;164:948–962. doi: 10.1016/j.neuroscience.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Abramovitz M, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 27.Akarsu ES, Mamuk S, Comert A. Inhibition of pentylenetetrazol-induced seizures in rats by prostaglandin D2. Epilepsy Res. 1998;30:63–68. doi: 10.1016/s0920-1211(97)00092-2. [DOI] [PubMed] [Google Scholar]
- 28.Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J Neurochem. 2005;92:477–486. doi: 10.1111/j.1471-4159.2004.02870.x. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi H, et al. Prostaglandin D2 protects neonatal mouse brain from hypoxic ischemic injury. J Neurosci. 2007;27:4303–4312. doi: 10.1523/JNEUROSCI.0321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deininger MH, Schluesener HJ. Cyclooxygenases-1 and -2 are differentially localized to microglia and endothelium in rat EAE and glioma. J Neuroimmunol. 1999;95:202–208. doi: 10.1016/s0165-5728(98)00257-4. [DOI] [PubMed] [Google Scholar]
- 31.Yermakova AV, Rollins J, Callahan LM, Rogers J, O'Banion MK. Cyclooxygenase-1 in human Alzheimer and control brain: Quantitative analysis of expression by microglia and CA3 hippocampal neurons. J Neuropathol Exp Neurol. 1999;58:1135–1146. doi: 10.1097/00005072-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Quan Y, Jiang J, Dingledine R. Signaling pathways for EP2 receptors regulate microglial activation. Society Neurosci Abstract Viewer and Itinerary Planner. 2011 Available at http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=3b5856f9-dbbd-4ef6-9834-e8a538e84bf5&cKey=aed366a6-adeb-4d0d-ad4d-106fb01ca5eb&mKey=%7b8334BE29-8911-4991-8C31-32B32DD5E6C8%7d. [Google Scholar]