Abstract
Antiangiogenic therapy has been thought to hold significant potential for the treatment of cancer. However, the efficacy of such treatments, especially in breast cancer patients, has been called into question, as recent clinical trials reveal only limited effectiveness of antiangiogenic agents in prolonging patient survival. New research using preclinical models further suggests that antiangiogenic agents actually increase invasive and metastatic properties of breast cancer cells. We demonstrate that by generating intratumoral hypoxia in human breast cancer xenografts, the antiangiogenic agents sunitinib and bevacizumab increase the population of cancer stem cells. In vitro studies revealed that hypoxia-driven stem/progenitor cell enrichment is primarily mediated by hypoxia-inducible factor 1α. We further show that the Akt/β-catenin cancer stem cell regulatory pathway is activated in breast cancer cells under hypoxic conditions in vitro and in sunitinib-treated mouse xenografts. These studies demonstrate that hypoxia-driven cancer stem cell stimulation limits the effectiveness of antiangiogenic agents, and suggest that to improve patient outcome, these agents might have to be combined with cancer stem cell-targeting drugs.
Keywords: antiangiogenesis, HIF-1α
Angiogenesis has been a long-standing therapeutic target in malignant tumors, an idea pioneered by Judah Folkman (1). Preclinical studies have shown that inhibition of the vascular endothelial growth factor (VEGF) pathway impedes tumor growth and, clinically, the VEGF-neutralizing antibody Avastin (bevacizumab) and VEGF receptor tyrosine kinase inhibitors (sorafenib and sunitinib) have been used as anticancer treatments in several tumor types including breast cancer (2). However, clinical and preclinical observations indicate that these therapies may have limited efficacy. Although these agents typically produce inhibition of primary tumor growth, lasting responses are rare, with only a moderate increase in progression-free survival and little benefit in overall survival (3). In addition, when antiangiogenic agents are administered on an intermittent schedule, such as with sunitinib (4 wk on, 2 wk off), tumor regrowth is sometimes seen during drug-free periods (4) or upon discontinuation of the treatment (5). In light of these limited clinical benefits demonstrated, a U.S. Food and Drug Administration panel has recently recommended that the approval of bevacizumab for treatment of advanced breast cancer be revoked. Interestingly, recent reports describe increased tumor invasiveness and metastasis in response to VEGF inhibitors or VEGF gene inactivation in preclinical mouse models of cancer (6, 7).
The administration of antiangiogenic agents has been shown to generate intratumoral hypoxia, and hypoxia has been shown to modulate each step in the metastatic process (8). Moreover, the transcription factors hypoxia-inducible factors 1 and 2 alpha (HIF-1α and HIF-2α) have been linked to the stimulation of cancer stem cells (CSCs) in glioblastoma (9–11). Because CSCs have tumor-initiating capabilities and a high metastatic potential (12), we hypothesized that hypoxia induced by administration of antiangiogenic agents might accelerate tumor growth and metastasis by increasing the CSC population. We demonstrate that administration of antiangiogenic agents such as the VEGF receptor tyrosine kinase inhibitor sunitinib and the anti-VEGF antibody bevacizumab increases the CSC population in breast cancer xenografts as a consequence of the generation of tumor hypoxia. The increase in CSCs in response to hypoxia was mediated through HIF-1α through the activation of the Wnt pathway via Akt/β-catenin signaling.
Results
To determine whether antiangiogenic agents stimulate an increase in breast CSCs in vivo, we treated tumor-bearing mice with the multireceptor tyrosine kinase inhibitor sunitinib malate (Sutent; Pfizer). Previous studies have demonstrated strong growth inhibition of established primary tumors in mice treated with this agent (13). We compared the effect of sunitinib on tumors using both early and late treatment times. MDA-MB-231 and SUM159 human breast cancer cells were implanted in the mammary fat pads of non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice. Group A received vehicle control, and group B received sunitinib treatment (60 mg/kg daily) starting when tumors reached 4 mm in diameter (late treatment). Mice in group C were given continuous sunitinib therapy (60 mg/kg daily) starting the day after tumor implantation (early treatment). A sustained sunitinib therapy regimen of 60 mg/kg/d given continuously has previously been demonstrated to result in optimal tumor inhibition with minimal toxicity (14). As expected, significant inhibition of tumor growth was observed after sunitinib treatment of established tumors compared with controls (Fig. 1A). Sustained sunitinib therapy beginning 1 d after tumor implantation resulted in a delay in the onset of tumor formation as well as a decrease in tumor size (Fig. 1A). Staining for the endothelial marker CD31 revealed significantly fewer blood vessels in tumors from sunitinib-treated mice compared with controls (Fig. 1B and Fig. S1), which were smaller and less vascularized than the control tumors (Fig. 1C). We have previously demonstrated that a subpopulation of cells that displays stem cell properties can be isolated from normal human breast tissue and breast carcinomas, by virtue of their increased expression of aldehyde dehydrogenase (ALDH) activity as assessed by the Aldefluor assay (15). Many breast cancer cell lines, including MDA-MB-231, SUM159, and MCF-7 cells, also contain an Aldefluor+ population that displays stem cell properties in vitro and in NOD/SCID xenografts (12). We therefore determined the effects of sunitinib treatment on the proportion of Aldefluor+ cells in the mouse xenografts. Treatment with sunitinib for 35 d initiated after MDA-MB-231 tumors reached 4 mm in diameter significantly increased (P < 0.01) the percentage of Aldefluor+ tumor cells, by 4.8-fold (Fig. 1D). The percentage of Aldefluor+ cells from mice treated continuously beginning 1 d after implantation for 75 d (group C) was also significantly increased compared with the control, by 2.4-fold (P < 0.01). Sunitinib treatment also resulted in growth inhibition of SUM159 xenografts (Fig. 1A). When cells from SUM159 tumors treated continuously for 55 d were tested by the Aldefluor assay, there was a 4.6-fold increase (P < 0.05) in the proportion of Aldefluor+ cells.
Fig. 1.
Sunitinib malate induces hypoxia in breast tumors in vivo and results in an increase in the tumor stem/progenitor cell population. (A) MDA-MB-231 or SUM159 cells were injected into the inguinal fat pads of NOD/SCID mice. One day after injection, mice were given either vehicle (group A) or sunitinib (60 mg/kg) (group B). Group C received the vehicle control until tumors reached an average size of 4 mm in diameter, and then were administered sunitinib (60 mg/kg) daily. Data are shown as averages ± SD. n = 8–10 (MDA-MB-231); n = 4–6 (SUM159). Treated tumors were significantly smaller than control tumors at end point. *P < 0.01. (B) CD31 staining of blood vessels (green) and DAPI nuclear staining (blue) of tumors from a control mouse and a sunitinib-treated mouse. (Scale bars, 200 μm.) (C) Representative tumors displaying a lack of vasculature in sunitinib-treated mice compared with control tumors. (D) The percentage of ALDH+ cells in tumors was determined by Aldefluor assay. n = 8. *P < 0.05. (E) Serial dilutions of cells obtained from primary SUM159 tumors treated with control or sunitinib were implanted in secondary NOD/SCID mice. Primary tumors treated with sunitinib grew secondary tumors more rapidly than the control tumors. Data are shown as averages ± SD. n = 4–5. *P < 0.05.
Although the increase in the ALDH+ cell population in sunitinib-treated tumors suggests that this drug increases breast CSCs, the ability of residual cancer cells to initiate tumors upon reimplantation in secondary mice is a more definitive assay. We therefore assayed the ability of serial dilutions of cells isolated from the primary tumors to generate tumors when implanted in secondary NOD/SCID mice (Fig. 1E and Fig. S2). Tumor cells isolated from sunitinib-treated mice exhibited significantly increased tumor-initiating capacity and growth in secondary mice compared with cells isolated from control tumors. When 50,000 cells were injected, tumors grew equally well from control and sunitinib-treated primary tumors. However, when smaller numbers of cells were injected into secondary animals, those from sunitinib-treated mice showed a 2.5-fold increase for 5,000 cells (P < 0.05) and a 6-fold increase for 500 cells (P < 0.05) in tumor size compared with cells from control animals. The results from these Aldefluor assays and tumor regrowth experiments indicate that sunitinib increases the Aldefluor+, tumorigenic population of tumor cells.
To further confirm that disruption of the VEGF pathway leads to an increase in CSCs, we used bevacizumab, a humanized antibody to VEGF, to block angiogenesis in human breast cancer xenografts. MDA-MB-231 cells were implanted in the mammary fat pads of NOD/SCID mice. When tumors reached 4 mm in diameter, either vehicle control or 5 mg/kg of bevacizumab was administered twice weekly. Bevacizumab treatment effectively abrogated tumor growth (Fig. S3 A and B) and resulted in less vascularization (Fig. S1). There was approximately a twofold increase in the percentage of Aldefluor+ cells in tumors from bevacizumab-treated mice compared with control tumors (Fig. S3C). To determine whether the increase represents an increase in the absolute number of CSCs, cell viability was determined by trypan blue exclusion. On average, control tumors yielded 17 ± 6 million cells with 72% viability, whereas the sunitinib-treated tumors yielded 3 ± 1 million with 68% viability (Fig. S1B). Similarly, when bevacizumab was tested, control tumors had 31 ± 8 million cells with 93 ± 2% viability, whereas bevacizumab-treated tumors yielded 6 ± 2 million cells with 92 ± 3% viability (Fig. S3D). There was no significant difference between the viability of cells from control and drug-treated tumors. This suggests that both the absolute number and proportion of CSCs in these tumors increase in response to the antiangiogenic therapies.
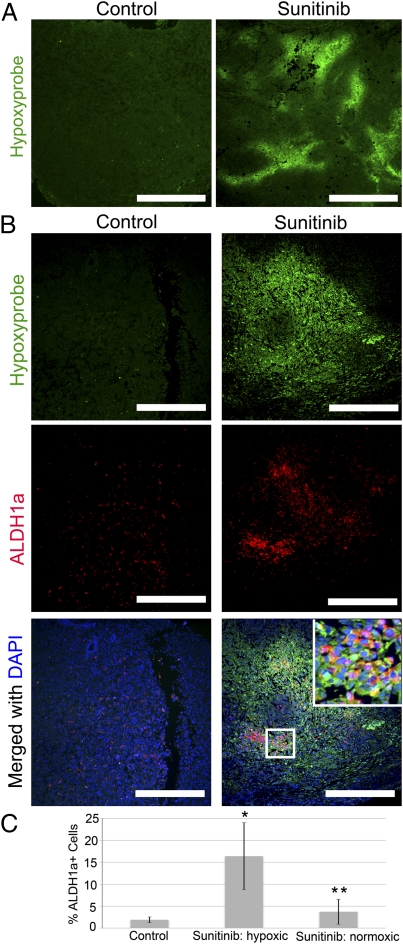
To gain additional insight into the mechanism by which hypoxia regulates breast CSC populations, we assessed the spatial relationship between ALDH1+ cells and areas of hypoxia within mammary tumors. Whereas tumors from control animals exhibited little or no hypoxia as determined by pimonidazole adduct (Hypoxyprobe) staining, tumors from sunitinib-treated mice displayed multiple areas of intense hypoxia (Fig. 2A). Hypoxyprobe positivity was found specific to zones of low oxygen because the most intense staining coincided alongside necrotic zones in both bevacizumab and sunitinib as well as very large control tumors with necrosis (Fig. S4). Double labeling with anti-ALDH1 antibody and pimonidazole adduct staining was carried out. Whereas ALDH1+ cells were scattered throughout tumors from control animals, we observed high-density areas of ALDH1+ cells within hypoxic regions of tumors from sunitinib-treated mice (Fig. 2B). Tumors from sunitinib-treated mice exhibited approximately an eightfold greater number of ALDH+ cells within hypoxic areas, but no significant difference within normoxic zones as determined by staining (Fig. 2C). This localization strongly suggests that the increase in Aldefluor+ tumor cells seen following sunitinib treatment occurs as a direct effect of the hypoxia induced by the angiogenic inhibition. Because sunitinib is a potent multikinase inhibitor, we further examined whether the effect of sunitinib on CSCs is mediated by the generation of tumor hypoxia or occurs via direct mechanisms. The antiproliferative effect of sunitinib in SUM159 and MDA-MB-231 was first evaluated in cells by methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay. Cell viability decreased as sunitinib concentration increased, with an IC50 of ∼30 μmol/L after 48 h (Fig. S5). The maximum plasma concentration achieved by 80 mg/kg of sunitinib in mice (higher than our 60-mg/kg dose) was reported to be 1.6 μM (16), below the concentration needed to inhibit cell viability in vitro, suggesting that the in vivo treatment does not select for CSC survival over non-CSCs. We next analyzed sunitinib-treated cells by Aldefluor assay to determine whether the drug directly affects the CSC population. In contrast to the increase in Aldefluor+ cells seen in vivo, the addition of 1 μmol/L did not have a significant effect on the Aldefluor+ cells compared with controls (Fig. S6). Bevacizumab does not have direct cytotoxic effects on tumor cells (17). Together, these findings support the hypothesis that antiangiogenic drugs stimulate the CSC population by generating hypoxia within tumors rather than through direct effects.
Fig. 2.
Sunitinib malate induces hypoxia in breast tumors in vivo, and ALDH1+ cells are concentrated in hypoxic regions. (A) Hypoxia in SUM159 tumors was detected by immunofluorescence staining of pimonidazole adducts in sections from control or sunitinib-treated animals. Staining shows pimonidazole immunodetection (green). (Scale bars, 800 μm.) (B) Staining shows pimonidazole (green) and ALDH1 (red) merged with DAPI-stained nuclei (blue). (Scale bars, 400 μm.) (Inset) Magnification: 5×. (C) Quantitation of ALDH1-positive cells in control tumors versus hypoxic and normoxic areas within sunitinib-treated tumors. Data are shown as averages ± SD. n = 5. *P < 0.05, **P < 0.01.
To elucidate the molecular mechanisms mediating hypoxia-induced CSC expansion, we determined whether we could simulate these effects in vitro. To test the effect of low oxygen on breast CSCs in vitro, three human mammary carcinoma cell lines were grown under 21% O2 (normoxia) or 1% O2 (hypoxia). Cells from these three lines were cultured at subconfluency for 2, 4, or 6 d and, after each time point, cells were assayed for ALDH activity by Aldefluor assay. As shown in Fig. 3, the percentage of Aldefluor+ cells in each of the cell lines increased approximately two- to threefold when cultured under low oxygen. This increase was seen in as little as 2 d of hypoxia treatment, and was sustained for the 6-d treatment period. To demonstrate that the effect of hypoxia on the Aldefluor+ population reflects an increase in the CSC population rather than a direct effect on ALDH expression, we analyzed ALDH1A1 mRNA expression in Aldefluor+ and Aldefluor− populations by RT-PCR. As shown in Fig. S7, hypoxia had no effect on ALDHA1 expression in Aldefluor+ or Aldefluor− populations. This indicates that the increase in the Aldefluor+ population induced by hypoxia reflects an actual increase in Aldefluor+ cells rather than merely in ALDH1A1 expression.
Fig. 3.
Hypoxia increases the Aldefluor+ stem cell population in breast cancer cell lines in vitro. MDA-MB-231, SUM159, and MCF-7 cells were grown under 21% O2 (normoxia) or 1% O2 (hypoxia) for 2–6 d. The percentage of ALDH+ cells was assessed using the Aldefluor assay (STEMCELL Technologies). Data are shown as averages ± SD. n = 3–5. *P < 0.05.
Cellular responses to low oxygen are principally regulated by the transcriptional activity of HIFs. We therefore investigated which of the HIF α subunits might mediate the increase in CSCs under hypoxia. Immunoblotting of Aldefluor+ and Aldefluor− cells grown under hypoxia shows a robust increase in HIF-1α (Fig. 4A). The increase in HIF-1α was most significant in the Aldefluor+ cells. HIF-2α was not detectable in either population. siRNA oligos directed against HIF-1α and/or HIF-2α were transfected into SUM159 cells, and the cells were then grown for 3 d under hypoxia. Complete knockdown of HIF-1α is shown in Fig. 4B. The CSC population in each sample was assessed by Aldefluor assay (Fig. 4C). Compared with untransfected cells or cells transfected with nontargeting siRNA oligos, knockdown of HIF-1α completely abrogated the increase of the Aldefluor+ population induced by hypoxia. As expected, knockdown of HIF-2α did not significantly block the effects of hypoxia, and knockdown of both HIFs did not decrease the CSC population any further than with HIF-1α siRNA alone. Although there was no increase in cell death following hypoxia or knockdown of HIF-1α (Fig. S8A), there were ∼30% fewer cells in untransfected or control siRNA-transfected cells grown under hypoxia (Fig. S8B), suggesting that cells grow at a slower rate under hypoxic conditions. When HIF-1α was knocked down, there was no longer a difference in the growth of the cells under hypoxia.
Fig. 4.
HIF-1α mediates increase of CSCs by hypoxia. Aldefluor+ and Aldefluor− cells were sorted by flow cytometry and plated. After 24 h, cells were placed under hypoxic conditions for 3 d, after which the levels of HIF proteins were assessed. (A) Immunoblotting shows only HIF-1α is expressed following 72 h of hypoxia. Cell lysate (0.5 μg) from HIF-2α–positive control was loaded next to 40 μg of SUM159 cell lysate to demonstrate the effectiveness of HIF-2α antibody. N, 21% O2; H, 1% O2. (B) SUM159 cells were transfected with siRNA oligos against HIF-1α or HIF-2α or nontargeting oligos for 24 h, and then placed under 1% or 21% O2 for 72 h. Immunoblotting demonstrates complete knockdown of HIF-1α after hypoxia treatment when HIF-1α siRNA was transfected. (C) The percentage of Aldefluor+ cells increased under hypoxia in untransfected cells and cells transfected with either control siRNA or HIF-2α siRNA oligos. Knockdown of HIF-1α blocked the stimulation of Aldefluor+ cells following hypoxia. Data are shown as averages ± SD. n = 3. *P < 0.05.
The Akt/Wnt/β-catenin pathway has been reported to be a key regulator of breast CSC self-renewal (18), and activation of β-catenin by hypoxia has been shown to enhance metastatic potential of cancer cells (19). In addition, HIF-1α was reported to enhance β-catenin signaling in hypoxic embryonic stem cells (20). For these reasons, we investigated whether hypoxia induces Akt/Wnt/β-catenin signaling in human breast cancer cells. SUM159 and MCF-7 cells were grown under normoxic or hypoxic conditions for 2 d, and total and activated (phosphorylated) Akt and β-catenin levels were determined by Western blot. Although total amounts of Akt were unchanged, hypoxia treatment resulted in an increase in activated phospho-Akt in both cell lines (Fig. 5A). When β-catenin levels were assessed in SUM159, total amounts of the protein were unchanged following hypoxia, but an increase in the activated phospho-S552 form of β-catenin was detected. Despite an increase in phospho-Akt, no change was detected in β-catenin levels in MCF-7 cells, suggesting that Akt may not signal via the Wnt/β-catenin pathway in this cell line.
Fig. 5.
The β-catenin pathway is stimulated in response to hypoxia. (A) SUM159 and MCF-7 cells were grown under 1% (H) or 21% (N) O2 for 24 h. Immunoblotting was carried out for total and phospho-Akt and total and phospho-β-catenin. (B) SUM159 cells infected with the pGreenFire LEF/TCF lentivirus reporter were sorted by flow cytometry into GFP+ and GFP− populations, grown under 1% or 21% O2 for 20 h, and assayed for luciferase activity. GFP− cells displayed significantly higher luciferase activity in response to hypoxia. Data are shown as averages ± SD. n = 6. *P < 0.01. (C) Immunoblot for phospho-β-catenin in SUM159 cell extracts following transfection with control siRNA or HIF-1α siRNA and 3-d hypoxia treatment. (D) Representative immunofluorescent staining for β-catenin in SUM159 xenografts from control or sunitinib-treated mice. Note the primarily cytoplasmic staining in cells from control tumors and intense nuclear staining in cells near necrotic regions (N) of sunitinib-treated tumors. (Scale bars, 100 μm.)
To provide further evidence for cross-talk between Akt signaling and the Wnt pathway, we used an LEF-1/TCF reporter system to monitor β-catenin transcriptional activity. SUM159 cells were infected with an LEF-1/TCF lentiviral reporter driving GFP and luciferase. Cells were first sorted into GFP+ and GFP− populations by flow cytometry to isolate cells in which β-catenin signaling was active or inactive under normoxia. Equal numbers of cells were grown for 20 h under normoxia or hypoxia, and the transcriptional activity of β-catenin was determined by luciferase assay. As expected, luciferase reporter activity was ∼40-fold higher in the GFP+ cells compared with GFP− cells under normoxic conditions (Fig. 5B). Whereas no significant difference was detected in GFP+ cells following hypoxia treatment, luciferase activity was increased by approximately twofold in GFP− cells following hypoxia treatment. To determine whether the activation of β-catenin following hypoxia treatment required the presence of HIF-1α, we probed cell extracts following HIF-1α siRNA knockdown for phospho-S552-β-catenin. Indeed, no activation was found after hypoxia when HIF-1α was absent (Fig. 5C), demonstrating that HIF-1α is upstream of the β-catenin response.
To extend these observations to the mouse models, we examined the expression and localization of β-catenin in SUM159 tumors from control and sunitinib-treated mice by immunohistochemistry. All tumors showed the presence of β-catenin staining. As shown in Fig. 5D, β-catenin was primarily detected in the cytoplasm of tumor cells from control mice. In contrast, cells within sunitinib-treated tumors displayed distinct nuclear localization of β-catenin, especially prominent in hypoxic areas surrounding necrotic regions. Together, these experiments suggest that activation of the Akt/β-catenin signaling cascade may modulate the hypoxic response in CSCs induced by antiangiogenic agents.
Discussion
Hypoxia Increases the Population of Breast CSCs.
Using the Aldefluor assay to identify populations enriched for CSCs, we determined that growing human breast cancer cell lines under mild hypoxic conditions resulted in an increase in the CSC population. Furthermore, we demonstrate that this effect is mediated by HIF-1α. This is consistent with previous reports that knockdown of HIF-1α reduces migration potential and formation of tumor spheres in glioma cells (21), expansion of CD133+ CSCs in glioblastoma (9), and tumorigenicity of renal cell carcinoma (22).
We further demonstrate that the increase in CSCs following hypoxic stress is at least partly regulated by the Akt/β-catenin signaling pathway. We previously demonstrated that Akt activation increases breast CSC self-renewal through stimulation of the Wnt pathway (18). Hypoxia increases levels of both phospho-Akt and phospho-S552-β-catenin in SUM159 cells. Moreover, nuclear translocation of β-catenin was detected in tumor cells from sunitinib-treated mice, particularly in hypoxic regions near necrotic areas. In accordance with our findings, inhibition of the Akt pathway was demonstrated to reduce hypoxia-driven CD133+ glioma cell expansion (9). HIF proteins have been reported to interact with the β-catenin pathway in multiple ways depending on the cell type. HIF-1α modulates Wnt/β-catenin signaling in hypoxic embryonic stem cells and neural stem cells by enhancing β-catenin activation and increasing expression of the downstream effectors LEF-1 and TCF-1 (20). With respect to cancer cells, HIF-1α and HIF-2α are reported to have potentially antagonistic effects on β-catenin signaling. A recent study showed that HIF-2α binds β-catenin and enhances the transcriptional activity of β-catenin/TCF by recruiting p300 in HEK293 cells (23). However, HIF-1α has been shown to bind to and inhibit β-catenin–T-cell factor 4 (TCF-4) complex formation and transcriptional activity in colorectal cancer cells (24). Although at first glance these findings seem contradictory, they may simply reflect the differences between activities of HIF-1α in stem cells versus nonstem cells, because reported cancer studies only examined the bulk population of cells rather than activity in the rarer CSC population. The lack of Wnt activation in MCF-7 cells, which are a luminal breast cancer cell type, is consistent with previous reports that CSCs in luminal breast cancers may be regulated by alternate pathways such as Notch (25).
Induction of HIF proteins may also be involved in epithelial-to-mesenchymal transition (EMT). This could be another potential mechanism for hypoxia-induced increase in the CSC population. Recent reports have linked EMT to stem cell characteristics in both normal and tumor cells (26–28), and hypoxia has been demonstrated to induce an EMT-like phenotype in cancer cells (29). The possibility of tumor cell plasticity and an EMT-induced stem cell-like phenotype in response to tumor hypoxia thus warrants further examination.
Defining a Hypoxic Niche for Breast Cancer Stem Cells.
We investigated the in vivo effects of hypoxia on CSCs induced by the antiangiogenic agents sunitinib and bevacizumab in mice bearing human breast cancer xenografts. As expected, pimonidazole staining revealed extensive areas of hypoxia in tumors from drug-treated, but not control mice. Concomitant with the increase in hypoxia, the percentage of CSCs within these tumors also increased, as determined by Aldefluor assay and reimplantation studies. Tumors treated continuously from day 1 had an initial delay in tumor formation. However, tumors stopped growing once they reached an average size of ∼3 mm, suggesting that these tumors cannot continue growing beyond that size without the formation of new vasculature. Because the antiangiogenic effects on CSCs are related to the hypoxia generated in the tumor microenvironment, this effect is increased in larger tumors that develop areas of hypoxia. Additionally, immunohistochemical staining revealed that concentrated populations of ALDH1+ stem cells were predominantly localized in hypoxic regions within tumors from sunitinib-treated mice. The increase in CSCs following treatment with bevacizumab provides additional evidence that blockade of the VEGF pathway, whether via a VEGF RTKI or an anti-VEGF antibody, increases CSCs through the generation of tumor hypoxia. Our results are consistent with a previous report that in vivo treatment with the mouse VEGFR2-targeting antibody DC101 increases the secondary sphere-forming capacity of cells from glioma xenografts (30). The existence of a hypoxic niche for breast CSCs is in agreement with reports that several types of normal tissue stem cells reside in hypoxic niches. In particular, a hypoxic microenvironment regulates hematopoietic stem cells within bone marrow, where the least differentiated cells reside in the least oxygenated areas (31–33). Our results are also in agreement with findings of a hypoxic CSC niche within glioblastoma tumors (10). Although an enrichment of CSCs in hypoxic microenvironments seems to contradict findings that CSCs are often detected near blood vessels (10, 34, 35), this juxtaposition may be explained by the intimate interactions between CSCs and vascular endothelial cells. In fact, emerging evidence has implicated a number of vascular-derived factors that can regulate CSCs (36, 37). In addition, tumor neovasculature often develops rapidly, resulting in structural and functional abnormalities ultimately leading to reduced oxygen transport. Thus, it may be possible for CSCs to be concurrently in close proximity to tumor vasculature and still be exposed to low oxygen levels.
Antiangiogenic Therapy and Cancer Stem Cells.
The findings presented here not only broaden our understanding of the role of hypoxia in CSC biology, but also have significant clinical implications. Angiogenesis has been a long-standing therapeutic target in cancer. Currently, there are three major antiangiogenic therapies targeting the VEGF signaling pathway approved for clinical use. In most cancer types, use of these therapeutics results in growth inhibition of primary tumors and a moderate increase in progression-free survival. Unfortunately, responses are often transitory, and patients relapse with more invasive metastatic disease resulting in little to no benefit in overall survival (3, 38). In addition, recent reports have described enhancement of tumor invasiveness and metastasis in response to different classes of VEGF inhibitors or VEGF gene inactivation in various preclinical mouse models of cancer (6, 7). Of importance, breast CSCs have been linked to tumor invasion and metastasis (12). Interestingly, pretreatment of mice with sunitinib before tumor inoculation was shown to increase metastasis of breast cancer cells, suggesting that this observed effect may result from a “host” response to the drug (6). Indeed, manipulation of the tumor microenvironment likely induces a range of responses, in both the tumor as well as the host. We propose that an increase in the CSC population would contribute to the overall aggressiveness of a tumor. Our findings that treatment with the antiangiogenic agents sunitinib or bevacizumab leads to an increase in CSCs provides a potential explanation for the limited clinical effectiveness of antiangiogenic agents. If this is the case, then improving the clinical efficacy of antiangiogenic treatments will require the concurrent use of CSC-targeting agents.
Materials and Methods
Cell Culture and Reagents.
Culture media and conditions are described in SI Materials and Methods. Sunitinib malate (Sutent) was a kind gift from Pfizer. The drug was suspended in vehicle containing carboxymethylcellulose sodium [United States Pharmacopeia (USP); 0.5% wt/vol], NaCl (USP; 1.8% wt/vol), Tween 80 [National Formulary (NF); 0.4% wt/vol], benzyl alcohol (NF; 0.9% wt/vol), and deionized water adjusted to pH 6.0. Sunitinib was prepared weekly and kept at 4 °C. Bevacizumab (Genentech) was diluted in saline solution.
Animal Studies.
All mouse experimentation was conducted in accordance with standard operating procedures approved by the University Committee on the Use and Care of Animals at the University of Michigan. NOD/SCID mice were purchased from The Jackson Laboratory. Cancer cells were injected into the mammary fat pads of mice (2 × 106 MDA-MB-231, 1 × 105 SUM159). Drug treatment and tumor digestion methodology is described in SI Materials and Methods.
Aldefluor Assay and Flow Cytometry.
The Aldefluor assay was carried out according to the manufacturer's (STEMCELL Technologies) guidelines. Briefly, cells were suspended in Aldefluor assay buffer containing an ALDH substrate, bodipy-aminoacetaldehyde, at 1.5 μM, and incubated for 45 min at 37 °C. To distinguish between ALDH+ and ALDH− cells, a fraction of cells was incubated with a 10-fold excess of an ALDH inhibitor, diethylamino-benzaldehyde. This results in a significant decrease in fluorescence intensity of ALDH+ cells and was used to compensate the flow cytometer.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded tissue was sectioned, dewaxed, and rehydrated through graded alcohol. Sections were heated to 98 °C for 40 min in 0.01 M sodium citrate buffer (pH 6.0) for antigen retrieval. For specific methodology, see SI Materials and Methods.
Immunoblotting.
Cells were lysed in RIPA buffer, boiled, subjected to SDS/PAGE, and transferred to PVDF (Pierce). Blots were blocked with 5% milk or 2% BSA (for phospho-specific antibodies) and incubated with primary antibodies overnight at 4 °C. Blots were washed and incubated with secondary antibodies (GE Healthcare) and detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce). See SI Materials and Methods for more information. Densitometric analysis was carried out using ImageJ software (National Institutes of Health).
siRNA.
ON-TARGETplus siRNA pools against HIF-1α or HIF-2α and a nontargeting pool were from Thermo Scientific. SUM159 cells were transfected using Dharmafect Reagent 1 (Thermo Scientific) overnight; media were then changed and cells were incubated under 1% O2 for 72 h before being subjected to Aldefluor assay.
Reporter Assay.
pGreenFire TCF/LEF lentivirus reporter was obtained from System Biosciences. mCMV pGreenFire reporter lacking a β-catenin/TCF binding site was used as a control to select GFP+ cells. Cells were sorted by flow cytometry into 96-well plates and incubated for 20 h under 1% or 21% O2. Luciferase activity was measured using the ONE-Glo Luciferase Assay Kit from Promega.
Supplementary Material
Acknowledgments
We thank Dr. J. Christensen (Pfizer) for providing sunitinib; the University of Michigan Flow Cytometry and Vector Cores; and Tahra Luther, Hsiu-Fang Lee, and Denise Poirier for their assistance. This work was supported by Breast Cancer Research Foundation Grants N012653 and W011541, National Institutes of Health Grants CA-R01 129765 and CA-101860, and The Taubman Research Institute.
Footnotes
Conflict of interest statement: M.S.W. is a consultant for Pfizer and OncoMed Pharmaceuticals and holds equity in OncoMed Pharmaceuticals.
This article is a PNAS Direct Submission. K.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018866109/-/DCSupplemental.
References
- 1.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 3.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein HJ, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 5.Johannsen M, et al. Can tyrosine kinase inhibitors be discontinued in patients with metastatic renal cell carcinoma and a complete response to treatment? A multicentre, retrospective analysis. Eur Urol. 2009;55:1430–1438. doi: 10.1016/j.eururo.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pàez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: Master regulators of metastasis. Clin Cancer Res. 2010;16:5928–5935. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soeda A, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 10.Seidel S, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 α. Brain. 2010;133:983–995. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 11.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charafe-Jauffret E, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18(Suppl 10):x3–x10. doi: 10.1093/annonc/mdm408. [DOI] [PubMed] [Google Scholar]
- 14.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haznedar JO, et al. Single- and multiple-dose disposition kinetics of sunitinib malate, a multitargeted receptor tyrosine kinase inhibitor: Comparative plasma kinetics in non-clinical species. Cancer Chemother Pharmacol. 2009;64:691–706. doi: 10.1007/s00280-008-0917-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 18.Korkaya H, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/β-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, et al. Activation of β-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res. 2010;16:2740–2750. doi: 10.1158/1078-0432.CCR-09-2610. [DOI] [PubMed] [Google Scholar]
- 20.Mazumdar J, et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Méndez O, et al. Knock down of HIF-1α in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol Cancer. 2010;9:133. doi: 10.1186/1476-4598-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu K, et al. Silencing of HIF-1α suppresses tumorigenicity of renal cell carcinoma through induction of apoptosis. Cancer Gene Ther. 2010;17:212–222. doi: 10.1038/cgt.2009.66. [DOI] [PubMed] [Google Scholar]
- 23.Choi H, Chun YS, Kim TY, Park JW. HIF-2α enhances β-catenin/TCF-driven transcription by interacting with β-catenin. Cancer Res. 2010;70:10101–10111. doi: 10.1158/0008-5472.CAN-10-0505. [DOI] [PubMed] [Google Scholar]
- 24.Kaidi A, Williams AC, Paraskeva C. Interaction between β-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 25.Bouras T, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong D, et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang MH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 29.Cannito S, et al. Epithelial-mesenchymal transition: From molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12:1383–1430. doi: 10.1089/ars.2009.2737. [DOI] [PubMed] [Google Scholar]
- 30.Folkins C, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 31.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J. 2001;81:685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophys J. 2001;81:675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–1378. doi: 10.4161/cc.7.10.5954. [DOI] [PubMed] [Google Scholar]
- 35.Christensen K, Schrøder HD, Kristensen BW. CD133 identifies perivascular niches in grade II–IV astrocytomas. J Neurooncol. 2008;90(2):157–170. doi: 10.1007/s11060-008-9648-8. [DOI] [PubMed] [Google Scholar]
- 36.Gilbertson RJ, Rich JN. Making a tumour's bed: Glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 37.Krishnamurthy S, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes DF. Bevacizumab treatment for solid tumors: Boon or bust? JAMA. 2011;305:506–508. doi: 10.1001/jama.2011.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.