Abstract
Hormonal control of sexual maturation is a common feature in animal development. A particularly dramatic example is the metamorphosis of insects, in which pulses of the steroid hormone ecdysone drive the wholesale transformation of the larva into an adult. The mechanisms responsible for this transformation are not well understood. Work in Drosophila indicates that the larval and adult forms are patterned by the same underlying sets of developmental regulators, but it is not understood how the same regulators pattern two distinct forms. Recent studies indicate that this ability is facilitated by a global change in the responsiveness of target genes during metamorphosis. Here we show that this shift is controlled in part by the ecdysone-induced transcription factor E93. Although long considered a dedicated regulator of larval cell death, we find that E93 is expressed widely in adult cells at the pupal stage and is required for many patterning processes at this time. To understand the role of E93 in adult patterning, we focused on a simple E93-dependent process, the induction of the Dll gene within bract cells of the pupal leg by EGF receptor signaling. In this system, we show that E93 functions to cause Dll to become responsive to EGF receptor signaling. We demonstrate that E93 is both necessary and sufficient for directing this switch. E93 likely controls the responsiveness of many other target genes because it is required broadly for patterning during metamorphosis. The wide conservation of E93 orthologs suggests that similar mechanisms control life-cycle transitions in other organisms, including vertebrates.
Keywords: Distal-less, Eip93F, epidermal growth factor receptor, ultraspiracle, heterochrony
Steroid hormones control metabolism, reproduction, and development in many organisms and have been linked to numerous human health problems. Steroids often control major transitions in the life cycle, regulating distinct cell responses in a stage-specific manner. Despite their global importance in development, relatively little is known about how steroid hormones control such stage-specific responses. One of the most dramatic life-cycle transitions driven by steroids is the metamorphosis of insects, in which there is a wholesale transformation of the larva into the adult. In Drosophila, metamorphosis is triggered by pulses of the steroid hormone 20-OH ecdysone (ecdysone) (reviewed in refs. 1–4). A complex of ecdysone bound to its nuclear receptor, a heterodimer of the ecdysone receptor (EcR) and the RXR ortholog Ultraspiracle (Usp), directly activates a small number of primary response genes, including E93, which in turn regulate many secondary response genes that function more directly in controlling cell fate. The role of ecdysone signaling in early events of metamorphosis, such as the death of larval cells and the morphogenesis of adult structures, has been studied extensively. However, mechanisms underlying the control of adult cell fates by ecdysone have been poorly characterized.
In adult cells, many genes seem to undergo a change in their response to specific signaling systems or transcription factors during metamorphosis. Such transitions are most clearly illustrated by recent work showing that targets of the Hox protein Ubx change dramatically at the prepupal and pupal stages (5). Studies of wing venation (6) and heart remodeling (7) provide additional clear examples, and studies of patterning during metamorphosis in the wing (8), eye (9), palp (10), and abdominal cuticle (11, 12) indicate similar shifts. Such global changes in target responsiveness likely explain how the same signaling systems and identity genes used to pattern the larva during embryogenesis are redeployed during metamorphosis to pattern the adult; however, it is not known how such transitions are controlled.
In this paper, we identify the ecdysone response gene E93 as a key determinant of target gene responsiveness during the pupal phase of metamorphosis. E93 is known to be required for the death of larval tissues, such as the midgut and salivary glands early in metamorphosis (13), and has been considered a dedicated regulator of cell death. However, we find that E93 is expressed extensively in imaginal (adult) cells during pupal development and is required for the patterning of many adult structures at this time. We focus on a relatively simple E93-dependent process: the induction of bracts (single-cell pigmented outgrowths) by bristles in the pupal leg. In this system, we show that the role of E93 is to render the target gene Distal-less (Dll) responsive to epidermal growth factor receptor (EGFR) signaling. E93 likely controls the pupal-specific responses of many other target genes because it is required broadly for patterning at the pupal stage. Our work establishes E93 as a temporal identity factor for adult cells in Drosophila. The conservation of steroid and cell-fate regulatory pathways in diverse taxa suggests that steroid hormones may control life-cycle transitions in vertebrates by similar mechanisms.
Results
Nonsense Alleles of E93 Are Defective in Patterning the Adult During Metamorphosis.
In a screen for E93 mutant alleles, Lee et al. (13) identified two complementation groups in the E93 genomic region (93F) whose alleles cause lethality at the pupal stage. Alleles of one group cause death early in pupal development and are defective in the death of larval salivary gland and midgut cells. This group was identified as E93 because one of its alleles (E931) contains a nonsense change at codon 995 of the 1,165-codon E93 sequence. No changes in the E93 coding sequence were identified for the other two alleles in this group (E932 and E933), but both cause a reduction in the level of E93 transcripts.
The second complementation group identified contains three mutant alleles. These alleles fail to complement the P-element insertion l(3)ry93 (14) as well as Tp(3)Vno (15), a complex rearrangement having one breakpoint in 93F. Homozygotes for these alleles die just before emergence of the adult and show numerous defects affecting the eyes, antennae, palps, labellum, wings, legs, and abdomen (Fig. 1 A–H). Strikingly, all of the defects found are in structures that are patterned largely or entirely during metamorphosis. This specificity prompted us to investigate the second complementation group further.
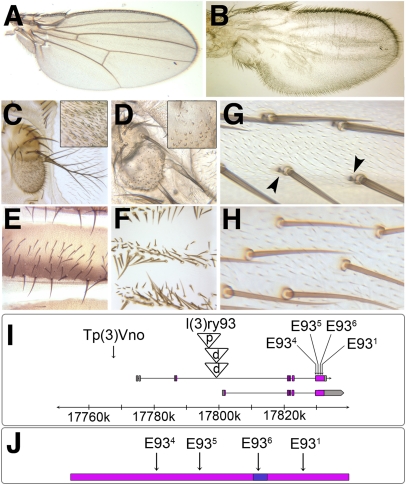
Fig. 1.
E93 phenotypes and alleles. (A–H) Wild-type (A, C, E, and G) and E934 mutant (B, D, F, and H) structures. In the mutant, wing veins are absent (B), most antennal sensilla are absent (Insets in C and D), abdominal tergites show mirror symmetry (F), and bracts (arrowheads in G) are absent in the leg (H). (I) Locations of E93 alleles. E93 encodes two isoforms: E93-A (1,165 aa) and E93-B (1,188 aa) that differ at their N-termini; the residue numbers cited in the text are for E93-A. (J) E93 alleles in relation to the DNA-binding (Pipsqueak) domain (blue). For the expression of E93 in imaginal tissues during metamorphosis, see Fig. S1.
We localized l(3)ry93 to ∼2 kb upstream of an apparent E93 promoter, suggesting that the second complementation group might also be allelic to E93, which proved to be the case: The alleles of this group (designated E934, E935, and E936) are nonsense changes at E93 codons 360, 545, and 783, respectively (Fig. 1 I and J). In addition, we localized the 93F breakpoint of Tp(3)Vno to 7.5 kb upstream of an alternate E93 promoter. These findings indicate that E93 is not a dedicated regulator of larval cell death, as previously thought (13), but it also functions in patterning the adult.
Consistent with the spectrum of defects present in homozygotes for the E934–6 alleles, we find that E93 is expressed very broadly in developing adult tissues during metamorphosis (Fig. S1) but is not expressed in larvae (Fig. S2) [refs. 16 and 17 (http://flybase.org/reports/FBgn0013948.html)]. E93 is activated in imaginal cells at ∼12 h after puparium formation (APF), approximately coincident with the molt of the prepupa to the pupa. E93 is expressed throughout the imaginal discs and abdominal histoblasts, which together produce the cuticle of the entire adult exoskeleton. Expression is also seen in the imaginal cells of the midgut and in a subset of cells in the brain and heart. Although E93 expression within the imaginal discs is initially uniform, expression becomes highly differentiated as development proceeds. At least within the leg and labellum, strongly expressing cells correlate with structures (bracts and pseudotracheae) that depend on E93 for their formation. E93 staining in imaginal cells remains prominent until cuticle secretion prevents antibody penetration (∼45 h APF). Early in metamorphosis, E93 is also expressed in the larval midgut and salivary gland, where it is known to drive cell death (13, 16).
E93 Is Required for Dll Activation in the Bract Pathway.
To begin to understand the role of E93 in adult cells, we focused on a relatively simple E93-dependent process: the induction of bracts by EGFR signaling. Bracts are pigmented single-cell outgrowths located immediately proximal to most bristles in the leg (Fig. 1 G and H). During metamorphosis, one or more cells of the bristle lineage secrete EGFR ligand, which induces an adjacent epidermal cell to develop as a bract (18, 19). Analysis of genetic mosaics reveals that E93+ is required within the signal-receiving (bract) cell, and not within cells of the bristle lineage, during bract induction (Fig. 2 A and B). Bract fate requires the transcription factor Dll, which is up-regulated within bract cells (Fig. 3 A and B) at ∼24 h APF and is expressed in these cells until adulthood (19–21). Beginning at 15–18 h APF, E93 is also up-regulated in bract cells (Fig. S1J and Fig. 3). Expression is sustained in these cells until at least 45 h APF. In some tissues where bracts do not form, apparently equivalent bristle-associated cells up-regulate E93 (Fig. S1O). The fates of these “bract-equivalent” cells are not known. Curiously, in the leg, E93 is also up-regulated in the bristle and socket cells of the bristle lineage (Fig. S1J and Fig. 3 A, B, and F). Here expression is lost at ∼30 and 40 h APF, respectively. The function of E93 in these cells is not known.
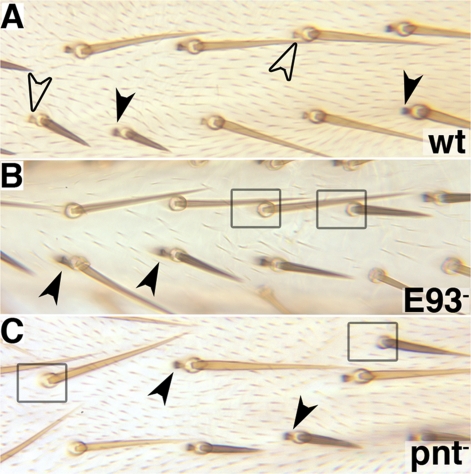
Fig. 2.
E93+ and pnt+ are required within bract cells but not bristle cells. Mitotic recombination clones marked by yellow cuticle and Bsb+. (A) The clone is otherwise wild type. Yellow bracts are found in association with both yellow and black bristles (open arrowheads), and black bracts are found in association with both yellow and black bristles (solid arrowheads), demonstrating that bracts are not clonally related to bristles. (B) The clone is homozygous for E934. Black (E934/+) bracts are found in association with both yellow and black bristles (solid arrowheads), but yellow (E934/E934) bracts are never seen, indicating that E93+ is required within bract cells for their development. Many yellow bristles and nearby black bristles lack bracts (boxes), presumably because the adjacent cells are homozygous for E934. (C) A clone homozygous for pnt−. The features are identical to those described in B.
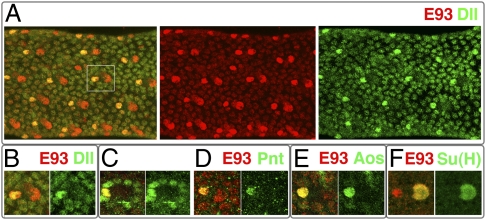
Fig. 3.
Gene expression in bracts. (A) E93 and Dll expression in a 30-h APF pupal tibia. At this stage, E93 is up-regulated in bract and socket cells, whereas Dll is up-regulated only in bract cells. (B–E) Coexpression of E93 with Dll (B), Pnt (C and D), and aos-lacZ (E) within bract cells. The images are from 30-h APF (B and C) and 36-h APF (D and E) pupal legs. (F) Coexpression of E93 and the socket cell marker Su(H) in a 30-h APF pupal leg.
In many developmental contexts, including bract induction, EGFR signaling is mediated by Ras pathway activation of the transcription factor Pointed (Pnt) (22, 23). Like E93+ and Dll+ (19), we find that pnt+ is required within bract cells, but not within bristle lineage cells, for bract formation (Fig. 2C). During bract induction, Pnt is initially up-regulated in all cells adjacent to bristle lineage cells (Fig. 3C), suggesting that EGFR ligand is secreted radially by these cells. However, Pnt expression is highest in the bract cell and is soon lost from all but this proximal cell (Fig. 3D). The argos (aos) gene, a common target of EGFR signaling (24), is also expressed within bract cells (Fig. 3E) as well as within E93-expressing bract-equivalent cells located elsewhere.
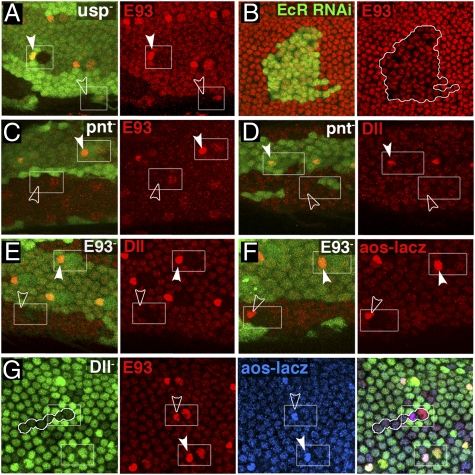
To understand the regulatory interactions that take place within bract cells, we first addressed how E93 is activated. To determine whether E93 expression in developing adult tissues depends on steroid signaling, we examined clones of cells mutant for usp, which encodes a component of the ecdysone receptor (25, 26). usp− clones completely lack imaginal E93 expression. Within bract cells in particular, the requirement for usp+ is clearly autonomous; usp+ bract cells adjacent to usp− socket cells express E93, whereas usp− bract cells adjacent to usp+ socket cells do not (Fig. 4A). In addition, reduced E93 expression is often seen within clones knocked down for EcR expression by RNAi (27) (Fig. 4B). Up-regulation of E93 in bract cells also depends on pnt+; pnt− clones lose this up-regulation (Fig. 4C) but do not affect the general expression of E93 in the leg or the up-regulation of E93 in the bristle and socket cells. These observations establish that ecdysone signaling induces E93 expression in all adult cells during metamorphosis, whereas Pnt is required for the up-regulation of E93 specifically within bract cells. To test the possibility that loss of expression of E93 in usp− bract cells is caused by a failure of EGFR signaling, we examined usp− clones for expression of the known EGFR signaling target aos-lacZ. Although the results are variable, many such clones express aos-lacZ at normal levels in bract cells (Fig. S3), indicating that ecdysone and EGFR signaling act independently to promote E93 expression in these cells.
Fig. 4.
Regulatory interactions in the bract pathway. (A) E93 expression is absent within usp− clones, marked by the absence of GFP, in a pupal leg. The requirement for usp+ in bract cells is autonomous. (B) E93 expression is reduced within a clone in the pupal wing knocked down for EcR expression by RNAi. (C and D) pnt− clones show loss of bract expression of both E93 (C) and Dll (D). (E and F) E934 clones lose bract expression of Dll (E) but not aos-lacZ (F). (G) A Dll− leg clone. Both E93 and aos-lacZ are expressed normally within the clone. Open arrowheads, bract cells inside clones; solid arrowheads, bract cells outside clones.
To place E93 in the bract pathway, we examined clones homozygous for E934. Such clones show loss or strong reduction of Dll expression in bract cells (Fig. 4E), demonstrating that E93 lies upstream of Dll. However, expression of aos-lacZ is unaffected (Fig. 4F), indicating that E93 lies downstream of pnt+ and the EGFR signaling pathway. Consistent with a simple linear pathway, pnt− clones lose bract expression of Dll (Fig. 4D), aos-lacZ (Fig. S3), and E93 (Fig. 4C), whereas Dll− clones have no effect on expression of E93 or aos-lacZ (Fig. 4G).
E93 Controls the Responsiveness of Dll to EGFR Signaling.
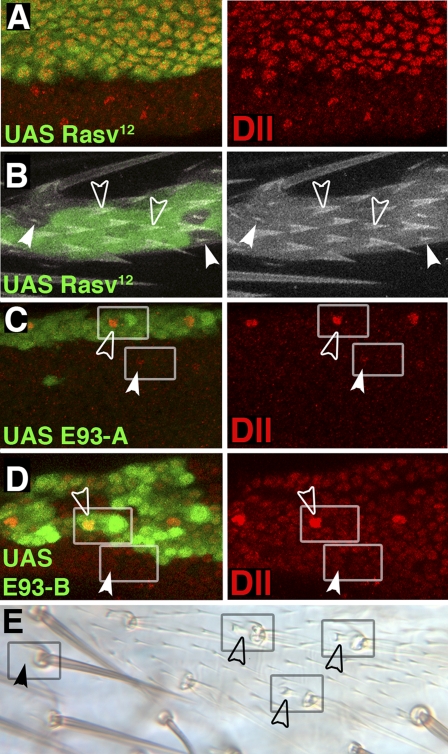
Although the above results are consistent with E93 acting in a linear pathway for bract specification, the results of ectopic expression experiments reveal that the situation is more complex. To activate EGFR signaling ectopically, clones of cells expressing activated Ras (RasV12) (28) during pupal development were generated. Such RasV12-expressing clones show autonomous activation of bract fate and Dll expression in all epidermal cells in the leg (Fig. 5 A and B), demonstrating that EGFR signaling is instructive in specifying which cells are to become bracts. Ectopic RasV12 does not activate Dll or bract formation in an E93 mutant background (Fig. S4). To test the effects of ectopic E93, we generated clones expressing E93 at the pupal stage within animals that otherwise lack E93 function (Fig. 5 C–E). Such clones expressing either of the known E93 isoforms (Fig. 1I) in the leg show rescue of bracts in their normal location proximal to each bristle but do not induce ectopic bracts. Activation of Dll in these clones is also limited to bract cells in their normal location (Fig. 5 C and D). These observations demonstrate that the precise pattern of E93 expression is not critical and that E93 must function permissively in the bract pathway to confer upon Dll the ability to respond to EGFR signaling. The differing roles of EGFR signaling and E93 are incompatible with their action in a simple linear pathway and indicate instead that these factors work in parallel to activate Dll during bract specification.
Fig. 5.
EGFR signaling is instructive, whereas E93 is permissive during bract induction. (A and B) Clones expressing RasV12 activate Dll (A) and bract formation (B) autonomously. Open arrowheads indicate bracts, and solid arrowheads indicate normal trichomes. (C and D) Clones expressing E93-A (C) or E93-B (D) in an E93 mutant background rescue Dll expression within bract cells (open arrowheads) but do not activate Dll elsewhere. Weak E93-independent expression of Dll is seen in some bract cells outside of the clones (solid arrowheads in C). (E) A clone expressing E93-A marked by yellow shows rescue of bract development proximal to leg bristles (open arrowheads).
E93 Is both Necessary and Sufficient for Switching the Responsiveness of Dll.
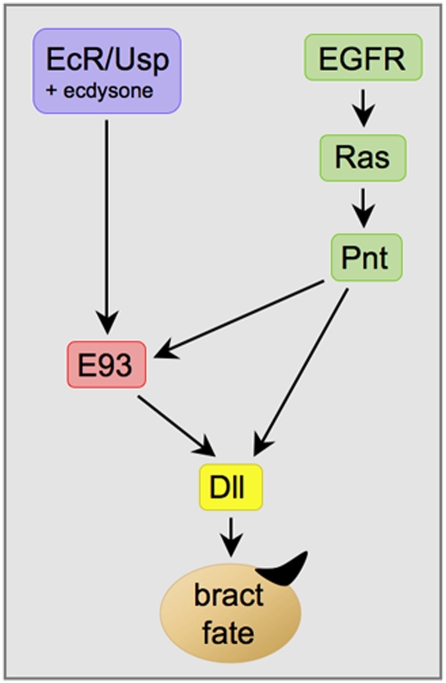
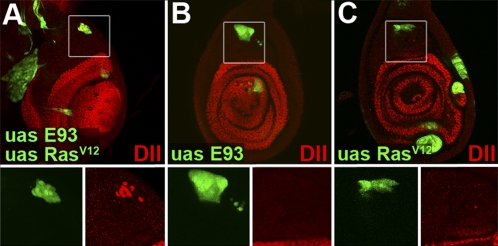
Our data indicate that Dll is activated in the pupal leg by the combined action of EGFR signaling, which specifies which cells are to activate Dll, and E93, which controls the time when Dll can be activated (Fig. 6). A prediction of this model is that developing leg cells in larvae would activate Dll prematurely if exposed to both EGFR signaling and E93. To test this prediction, we compared clones of cells in larval leg discs that express both RasV12 and E93 to clones expressing either RasV12 or E93 alone (Fig. 7): 35 of 41 clones expressing both RasV12 and E93 in the femur region showed activation of Dll, whereas none of the clones expressing only RasV12 (n = 35) or only E93 (n = 37) expressed Dll. These findings strongly support the hypothesis that E93 serves as a temporal competence factor that renders Dll responsive to EGFR signaling. Our results also demonstrate that E93 is both necessary and sufficient for conferring pupal-specific temporal identity to developing adult cells.
Fig. 6.
Model for activation of Dll during bract induction. See text for details.
Fig. 7.
Premature activation of Dll by coexpression of E93 and RasV12. (A) Clones in a third-instar larval leg disk coexpressing RasV12 and E93. A clone in the femur region (Inset) expresses Dll. (B and C) Clones in the femur region expressing E93 alone (B) or RasV12 alone (C) do not express Dll (Insets).
Discussion
In this paper, we show that the ecdysone-induced transcription factor E93 specifies pupal-specific target gene responsiveness in adult cells. Relatively little is known about how ecdysone signaling controls adult development at the pupal stage. A few reports have addressed the control of adult cuticle synthesis in the pupa by the ecdysone response gene HR38 (29, 30). However, our study addresses how ecdysone signaling controls adult cell fates at this stage. We focus on a simple and well-characterized patterning event in the pupa: the EGFR-dependent induction of bracts by bristles in the leg. We show that E93 functions as a temporal identity determinant in this process, conferring responsiveness to EGFR signaling upon the Dll gene, which specifies bract fate. The specification of temporal identity by E93 parallels the control of spatial identity by Hox and other selector genes; for some targets, at least, both types of determinant confer competence to respond to specific signaling pathways (31–34). Although temporal identity genes have been known for many years in Caenorhabditis elegans (2), the mode of action suggested here (large-scale alteration of target gene response) has not previously been considered.
The temporal identity function of E93 is clearly executed by one or both of the known E93 protein isoforms because ectopic expression of either isoform rescues bract formation in E93 mutants, and alleles that truncate these isoforms are defective in this function. How the larval cell-death function of E93 is executed is much less clear. Cell clones homozygous for the E931–3 alleles all show normal cuticular patterning (Fig. S5), indicating that all specifically affect larval cell death. It has been difficult to understand why the E934–6 alleles (nonsense changes at codons 360, 545, and 783, respectively) almost fully complement E931 (a nonsense change at codon 995). One possibility is that the cell-death function of E93 is executed by a currently unknown product encoded in part by the region containing the E931 change. Resolution of this issue will likely require identification of the sequence changes in the E932 and E933 alleles.
The E93 protein contains a helix–turn–helix DNA binding domain of the Pipsqueak family (35). This 54-aa domain is highly conserved within the E93 family of orthologs and, for the honey bee ortholog (Mblk-1), has been shown to bind DNA (36). With one exception, all E93 orthologs contain nuclear receptor interaction motifs (LXXLL motifs) (37). E93 contains three such motifs, suggesting that it binds target enhancers in concert with the EcR or other nuclear receptors induced by ecdysone signaling (3). In addition, all members of the E93 family contain an interaction motif (PXDLS/TXK/R) (38) for the corepressor C-terminal binding protein (CtBP). The best studied of the E93 orthologs is mammalian ligand-dependent corepressor (LCoR). LCoR was identified as a protein that interacts with hormone-bound estrogen receptor α (39) but was subsequently shown to interact with a range of ligand-bound nuclear receptors. In most cases, LCoR acts as a corepressor, recruiting both histone deacetylases and CtBP. However, at some targets it acts with CtBP to promote transcription (40). Structural similarities suggest that E93 will prove to function similarly.
The biological roles of the E93 family have not been well characterized. The C. elegans ortholog (MBR-1) is required for the pruning of excess neurites during the larval stages (41), and Mblk-1 is expressed within the mushroom bodies, regions of the brain thought to be involved in learning, memory, and sensory integration (36). In humans, the roles of LCoR are not yet known, but its paralog LCoR-Like has been associated with control of height (42, 43). Interestingly, two of the insect orthologs of E93 identified are from hemimetabolous species, in which there is no pupal stage. Study of the expression and function of these orthologs promises to shed light on the evolutionary origins of insect metamorphosis.
In our analysis of bract induction, we show that E93 plays a key role in integrating temporal (ecdysone) and spatial (EGFR) signals. This integration occurs at two levels (Fig. 6). First, E93 itself receives inputs from both signals in its expression in bract cells. Ecdysone signaling is required for all imaginal expression of E93 during metamorphosis, whereas EGFR signaling is required only for up-regulation of E93 in bract cells. Second, integration occurs at the level of the Dll gene. Here EGFR signaling plays an instructive role in specifying which epidermal cells express Dll, whereas E93 plays a permissive role, causing Dll to become responsive to EGFR signaling. The requirement for both signals ensures that Dll is activated at only the right place and time.
There are two likely ways that Dll could integrate inputs from EGFR signaling and E93. First, E93 and Pnt could both bind to the Dll bract enhancer and cooperate to activate transcription. A second possibility is suggested by the finding that activity of Mblk-1 is modulated by phosphorylation by MAPK, a component of the EGFR signaling pathway (44). If E93 were similarly modified, signal integration could be achieved simply by the direct activation of Dll by phosphorylated E93. Although we cannot rule out this possibility, we think it unlikely for three reasons: First, unlike Mblk-1, E93 has no MAPK consensus phosphorylation sites; second, this mechanism provides no obvious role for Pnt, which is required for Dll activation; and third, E93 is not absolutely essential for activation of Dll (Fig. 5C). Resolution of which mechanism is used will require identification of an enhancer that requires both E93 and EGFR signaling for its activation.
Several of the defects present in E93 mutants result from failures in processes that are regulated by EGFR signaling. In addition to bract induction, these processes include the patterning of wing veins and the pigment cells of the eye (Fig. S6). A key step in wing vein development is the activation of the dpp gene in the wing vein primordia during metamorphosis by EGFR signaling (6). We find that this activation largely fails in E934 mutants (Fig. S6), consistent with a role for E93 in rendering dpp competent to respond to EGFR signaling at the pupal stage. Although these observations suggest a particularly close relationship between E93 and EGFR signaling, other defects in E93 mutants, such as the loss of chemosensory sensilla and patterning abnormalities in the abdominal cuticle, are not clearly related to EGFR signaling and likely result from a failure of target genes to respond to other signaling pathways or transcription factors during metamorphosis.
Almost certainly, E93 acts in concert with other factors to confer metamorphosis-specific competence to target genes. The residual expression of Dll in bract cells seen in our E93 mutants (Fig. 5C) implies the existence of such additional factors. Moreover, E93 is not expressed in the first 12 h of metamorphosis (the prepupal period), so shifts in target specificity occurring at this stage must be directed by other factors. Several ecdysone response genes are active at this time.
Our work establishes that E93 is both necessary and sufficient to render the Dll gene responsive to EGFR signaling during metamorphosis. Shifts in target gene specificity directed by E93 likely account in part for how the same selector genes and signaling systems used during embryonic patterning are redeployed during metamorphosis to pattern the adult. Similar mechanisms may operate in humans during the hormonally directed changes of puberty. In addition, shifts in the target specificity of EGFR signaling directed by steroid hormones may play an important role in cancers that depend on both signals, such as many cancers of the breast.
Materials and Methods
Antibody Staining.
Pupae were split middorsally, fixed, and rehydrated essentially as described in ref. 45. Legs and wings were freed of pupal membranes by using number 5 Dumont forceps and stained as described in ref. 46. Antibodies used were mouse anti-E93 antibodies (this study; described in Antibody Production), mouse anti–β-gal (Promega), chicken anti–β-gal (Immunology Consultant Laboratories), mouse anti-Dll (47), rabbit anti-Pnt P1 (48), and rat anti-Su(H) (49). Secondary antibodies were Cy3 anti-mouse, FITC anti-rabbit, and FITC anti-rat (all from Jackson ImmunoResearch) as well as FITC anti-chicken (Immunology Consultant Laboratories). Images were captured on a Nikon A1 confocal microscope.
Antibody Production.
The coding sequence for E93-A residues Asn999–His1125 was cloned into pET28a, and expression was induced in BL21(DE3) cells. Proteins were purified by Ni chromatography followed by overnight dialysis in PBS. Mice were immunized six times with 10 μg of protein at 1-mo intervals. All experiments were approved by and conform to the standards of the Washington University Animal Studies Committee.
Mitotic Recombination Clones.
Mutant alleles were placed in cis to appropriate Flippase recognition target (FRT) elements (X, FRT18E; 2R, FRT42D; and 3R, FRT82B), and clones were generated with hsFLP122 or hsFLP38. The markers yellow+ and Blunt short bristles (Bsb) were used for scoring clones in the adult cuticle, and Ubiquitin-GFP insertions were used for scoring clones in pupae.
Ectopic Expression Clones.
Clones expressing UAS constructs were generated by Flippase recombination enzyme (FLP)-mediated excision of yellow+ from Act>yellow+>GAL4 insertions. hsFLP12 was used as a source of FLP, and clones were identified by expression of UAS-GFP. To limit expression to the pupal stage, animals carrying tub-GAL80ts (50) were raised at 17 °C until pupation and then shifted to 30° for 24 h before fixation.
Mutant Alleles and UAS Constructs.
UAS–E93 lines were generated by cloning E93-A and E93-B cDNAs into pUAST and recovering germ-line transformants. Although UAS–E93-A lines have been reported previously (13), sequencing revealed that the initiating AUG is mutated in these original lines. All other UAS lines used are from the Bloomington Drosophila Stock Center. Also used were usp2, pntΔ88, and DllSA1, all null alleles. RNAi knockdown of EcR was achieved by using UAS–EcR line 104 (27) in combination with UAS–Dicer2. Expression of aos was monitored by using the enhancer trap aos-lacZ05845 (Bloomington Drosophila Stock Center).
Localization of l(3)ry93.
Initial attempts to localize l(3)ry93 by inverse PCR revealed that the ry11 P element present is inserted into a Doc transposable element, which is itself inserted within another Doc element. To bypass the Doc sequences and identify the flanking genomic sequence, we digested genomic DNA from the l(3)ry93 strain with PstI and then circularized the resulting fragments by ligation. Because PstI cuts just downstream of the 5′ primer sites of ry11, but does not cut in the Doc element, circles should be formed that include the 5′ end region of ry11 ligated to unique genomic DNA, with an intervening region of Doc sequence. Most of the internal Doc DNA was removed by a second digest with the 4-base cutter MspI, and the resulting small fragments were ligated once again. These small circles were then subject to inverse PCR with ry11 5′ primers, yielding unique sequence from E93. The insertion site lies between nucleotides 17799285 and 17799286 on 3R (Drosophila melanogaster genome release R5.30).
Localization of Tp(3)Vno.
Tp(3)Vno is a complex transposition of material from 89E through 94A1 into 96F, with the new order 61–89E|94A1–96F11/14|93F–94A|89E–93F|96F11/14–100F. The site of insertion at 96F had previously been localized to a 1.4-kb XhoI–EcoRI fragment lying within the Enhancer of Split complex (51); inverse PCR with primers from this fragment allowed us to identify adjacent sequences from 93F in the insertion. The 93F break lies at position 17767296, 7.5 kb upstream of the E93-RB transcription start. The proximal side of 93F in the insertion has not been sequenced.
Supplementary Material
Acknowledgments
We thank J. Skeath, E. Sánchez-Herrero, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Center for flies and antibodies; C. S. Thummel and C.-Y. Lee for their support in the early stage of mutant characterization; I. Rebay and M. Muskavitch for discussions; J. Skeath and Y. Ben-Shahar for discussions and review of the manuscript; and T. Fortier and P. Kiefel for technical support. This work was supported by National Institutes of Health Grants GM032318 (to I.D.) and GM079431 (to E.H.B.). E.H.B. is a member of the University of Massachusetts Diabetes and Endocrinology Research Center and was supported by Grant DK32520.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117559109/-/DCSupplemental.
References
- 1.Thummel CS. Flies on steroids—Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996;12:306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 2.Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell. 2001;1:453–465. doi: 10.1016/s1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 3.King-Jones K, Thummel CS. Nuclear receptors—A perspective from Drosophila. Nat Rev Genet. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa Y, Henrich VC. Arthropod nuclear receptors and their role in molting. FEBS J. 2009;276:6128–6157. doi: 10.1111/j.1742-4658.2009.07347.x. [DOI] [PubMed] [Google Scholar]
- 5.Pavlopoulos A, Akam M. Hox gene Ultrabithorax regulates distinct sets of target genes at successive stages of Drosophila haltere morphogenesis. Proc Natl Acad Sci USA. 2011;108:2855–2860. doi: 10.1073/pnas.1015077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Celis JF. Pattern formation in the Drosophila wing: The development of the veins. Bioessays. 2003;25:443–451. doi: 10.1002/bies.10258. [DOI] [PubMed] [Google Scholar]
- 7.Monier B, Astier M, Sémériva M, Perrin L. Steroid-dependent modification of Hox function drives myocyte reprogramming in the Drosophila heart. Development. 2005;132:5283–5293. doi: 10.1242/dev.02091. [DOI] [PubMed] [Google Scholar]
- 8.Martín-Blanco E, et al. A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. Development. 1999;126:5739–5747. doi: 10.1242/dev.126.24.5739. [DOI] [PubMed] [Google Scholar]
- 9.Freeman M. Cell determination strategies in the Drosophila eye. Development. 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- 10.Lebreton G, Faucher C, Cribbs DL, Benassayag C. Timing of Wingless signalling distinguishes maxillary and antennal identities in Drosophila melanogaster. Development. 2008;135:2301–2309. doi: 10.1242/dev.017053. [DOI] [PubMed] [Google Scholar]
- 11.Kopp A, Duncan I. Anteroposterior patterning in adult abdominal segments of Drosophila. Dev Biol. 2002;242:15–30. doi: 10.1006/dbio.2001.0529. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence PA, Casal J, Struhl G. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development. 2002;129:2749–2760. doi: 10.1242/dev.129.11.2749. [DOI] [PubMed] [Google Scholar]
- 13.Lee C-Y, et al. E93 directs steroid-triggered programmed cell death in Drosophila. Mol Cell. 2000;6:433–443. doi: 10.1016/s1097-2765(00)00042-3. [DOI] [PubMed] [Google Scholar]
- 14.Berg CA, Spradling AC. Studies on the rate and site-specificity of P element transposition. Genetics. 1991;127:515–524. doi: 10.1093/genetics/127.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grell EH. New mutants report. Drosoph Inf Serv. 1959;33:94. [Google Scholar]
- 16.Baehrecke EH, Thummel CS. The Drosophila E93 gene from the 93F early puff displays stage- and tissue-specific regulation by 20-hydroxyecdysone. Dev Biol. 1995;171:85–97. doi: 10.1006/dbio.1995.1262. [DOI] [PubMed] [Google Scholar]
- 17.Tweedie S, et al. FlyBase: Enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 2009;37:D555–D559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Alamo D, Terriente J, Díaz-Benjumea FJ. Spitz/EGFr signalling via the Ras/MAPK pathway mediates the induction of bract cells in Drosophila legs. Development. 2002;129:1975–1982. doi: 10.1242/dev.129.8.1975. [DOI] [PubMed] [Google Scholar]
- 19.Held LI., Jr Bristles induce bracts via the EGFR pathway on Drosophila legs. Mech Dev. 2002;117:225–234. doi: 10.1016/s0925-4773(02)00212-5. [DOI] [PubMed] [Google Scholar]
- 20.Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125:4483–4493. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- 21.Gorfinkiel N, Morata G, Guerrero I. The homeobox gene Distal-less induces ventral appendage development in Drosophila. Genes Dev. 1997;11:2259–2271. doi: 10.1101/gad.11.17.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- 23.Gabay L, et al. EGF receptor signaling induces pointed P1 transcription and inactivates Yan protein in the Drosophila embryonic ventral ectoderm. Development. 1996;122:3355–3362. doi: 10.1242/dev.122.11.3355. [DOI] [PubMed] [Google Scholar]
- 24.Golembo M, Schweitzer R, Freeman M, Shilo B-Z. argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development. 1996;122:223–230. doi: 10.1242/dev.122.1.223. [DOI] [PubMed] [Google Scholar]
- 25.Yao T-P, et al. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- 26.Hall BL, Thummel CS. The RXR homolog Ultraspiracle is an essential component of the Drosophila ecdysone receptor. Development. 1998;125:4709–4717. doi: 10.1242/dev.125.23.4709. [DOI] [PubMed] [Google Scholar]
- 27.Colombani J, et al. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 28.Lowy DR, Willumsen BM. Function and regulation of RAS. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 29.Bruey-Sedano N, et al. The Drosophila ACP65A cuticle gene: Deletion scanning analysis of cis-regulatory sequences and regulation by DHR38. Genesis. 2005;43:17–27. doi: 10.1002/gene.20150. [DOI] [PubMed] [Google Scholar]
- 30.Cui H-Y, Lestradet M, Bruey-Sedano N, Charles J-P, Riddiford LM. Elucidation of the regulation of an adult cuticle gene Acp65A by the transcription factor Broad. Insect Mol Biol. 2009;18:421–429. doi: 10.1111/j.1365-2583.2009.00889.x. [DOI] [PubMed] [Google Scholar]
- 31.Bondos SE, Tan XX. Combinatorial transcriptional regulation: The interaction of transcription factors and cell signaling molecules with homeodomain proteins in Drosophila development. Crit Rev Eukaryot Gene Expr. 2001;11 (1–3):145–171. [PubMed] [Google Scholar]
- 32.Guss KA, Nelson CE, Hudson A, Kraus ME, Carroll SB. Control of a genetic regulatory network by a selector gene. Science. 2001;292:1164–1167. doi: 10.1126/science.1058312. [DOI] [PubMed] [Google Scholar]
- 33.Grienenberger A, et al. Tgfβ signaling acts on a Hox response element to confer specificity and diversity to Hox protein function. Development. 2003;130:5445–5455. doi: 10.1242/dev.00760. [DOI] [PubMed] [Google Scholar]
- 34.Walsh CM, Carroll SB. Collaboration between Smads and a Hox protein in target gene repression. Development. 2007;134:3585–3592. doi: 10.1242/dev.009522. [DOI] [PubMed] [Google Scholar]
- 35.Siegmund T, Lehmann M. The Drosophila Pipsqueak protein defines a new family of helix-turn-helix DNA-binding proteins. Dev Genes Evol. 2002;212:152–157. doi: 10.1007/s00427-002-0219-2. [DOI] [PubMed] [Google Scholar]
- 36.Park J-M, Kunieda T, Takeuchi H, Kubo T. DNA-binding properties of Mblk-1, a putative transcription factor from the honeybee. Biochem Biophys Res Commun. 2002;291:23–28. doi: 10.1006/bbrc.2002.6397. [DOI] [PubMed] [Google Scholar]
- 37.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 38.Vo N, Fjeld C, Goodman RH. Acetylation of nuclear hormone receptor-interacting protein RIP140 regulates binding of the transcriptional corepressor CtBP. Mol Cell Biol. 2001;21:6181–6188. doi: 10.1128/MCB.21.18.6181-6188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White JH, Fernandes I, Mader S, Yang X-J. Corepressor recruitment by agonist-bound nuclear receptors. Vitam Horm. 2004;68:123–143. doi: 10.1016/S0083-6729(04)68004-6. [DOI] [PubMed] [Google Scholar]
- 40.Palijan A, et al. Ligand-dependent corepressor LCoR is an attenuator of progesterone-regulated gene expression. J Biol Chem. 2009;284:30275–30287. doi: 10.1074/jbc.M109.051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kage E, et al. MBR-1, a novel helix-turn-helix transcription factor, is required for pruning excessive neurites in Caenorhabditis elegans. Curr Biol. 2005;15:1554–1559. doi: 10.1016/j.cub.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 42.Soranzo N, et al. Meta-analysis of genome-wide scans for human adult stature identifies novel loci and associations with measures of skeletal frame size. PLoS Genet. 2009;5:e1000445. doi: 10.1371/journal.pgen.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J-J, et al. Identification of 15 loci influencing height in a Korean population. J Hum Genet. 2010;55:27–31. doi: 10.1038/jhg.2009.116. [DOI] [PubMed] [Google Scholar]
- 44.Park J-M, Kunieda T, Kubo T. The activity of Mblk-1, a mushroom body-selective transcription factor from the honeybee, is modulated by the Ras/MAPK pathway. J Biol Chem. 2003;278:18689–18694. doi: 10.1074/jbc.M300486200. [DOI] [PubMed] [Google Scholar]
- 45.Gompel N. Madison: Univ of Wisconsin; 2003. Immunostaining on Drosophila pupal abdominal epidermis. http://www.molbio.wisc.edu/carroll/methods.html. [Google Scholar]
- 46.Kankel MW, Duncan DM, Duncan I. A screen for genes that interact with the Drosophila pair-rule segmentation gene fushi tarazu. Genetics. 2004;168:161–180. doi: 10.1534/genetics.104.027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 1998;12:1290–1303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez AD, Shi W, Wilson BA, Skeath JB. pannier and pointedP2 act sequentially to regulate Drosophila heart development. Development. 2003;130:3015–3026. doi: 10.1242/dev.00488. [DOI] [PubMed] [Google Scholar]
- 49.Gho M, Lecourtois M, Géraud G, Posakony JW, Schweisguth F. Subcellular localization of Suppressor of Hairless in Drosophila sense organ cells during Notch signalling. Development. 1996;122:1673–1682. doi: 10.1242/dev.122.6.1673. [DOI] [PubMed] [Google Scholar]
- 50.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 51.Preiss A, Hartley DA, Artavanis-Tsakonas S. The molecular genetics of Enhancer of split, a gene required for embryonic neural development in Drosophila. EMBO J. 1988;7:3917–3927. doi: 10.1002/j.1460-2075.1988.tb03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.