Abstract
Trimethylation of histone H3 on lysine 27 (H3K27me3) is a repressive posttranslational modification mediated by the histone methyltransferase EZH2. EZH2 is a component of the polycomb repressive complex 2 and is overexpressed in many cancers. In B-cell lymphomas, its substrate preference is frequently altered through somatic mutation of the EZH2 Y641 residue. Herein, we identify mutation of EZH2 A677 to a glycine (A677G) among lymphoma cell lines and primary tumor specimens. Similar to Y641 mutant cell lines, an A677G mutant cell line revealed aberrantly elevated H3K27me3 and decreased monomethylated H3K27 (H3K27me1) and dimethylated H3K27 (H3K27me2). A677G EZH2 possessed catalytic activity with a substrate specificity that was distinct from those of both WT EZH2 and Y641 mutants. Whereas WT EZH2 displayed a preference for substrates with less methylation [unmethylated H3K27 (H3K27me0):me1:me2 kcat/Km ratio = 9:6:1] and Y641 mutants preferred substrates with greater methylation (H3K27me0:me1:me2 kcat/Km ratio = 1:2:13), the A677G EZH2 demonstrated nearly equal efficiency for all three substrates (H3K27me0:me1:me2 kcat/Km ratio = 1.1:0.6:1). When transiently expressed in cells, A677G EZH2, but not WT EZH2, increased global H3K27me3 and decreased H3K27me2. Structural modeling of WT and mutant EZH2 suggested that the A677G mutation acquires the ability to methylate H3K27me2 through enlargement of the lysine tunnel while preserving activity with H3K27me0/me1 substrates through retention of the Y641 residue that is crucial for orientation of these smaller substrates. This mutation highlights the interplay between Y641 and A677 residues in the substrate specificity of EZH2 and identifies another lymphoma patient population that harbors an activating mutation of EZH2.
Recent genome-wide sequencing studies have revealed several genes that are frequently altered in non-Hodgkin lymphomas, including EZH2, MLL2, MEF2B, CREBBP, and TP53 among others (1–3). Many of these genes mediate, either directly or indirectly, through the recruitment of cofactors, the array of posttranslational modifications observed on the amino-terminal tails of histones. Similar studies have implicated these and other epigenetic factors in transitional cell carcinoma of the bladder (e.g., UTX, ARID1A, MLL, MLL3), head and neck squamous cell cancers (e.g., EZH2, MLL2), and myeloid malignancies (e.g., IDH1/2, TET2, DNMT3A, EZH2) (4–7). The prevalence of genetic changes affecting transcription factors and chromatin-modifying genes highlights the central role of transcriptional dysregulation in tumorigenesis.
The EZH2 gene encodes a SET domain-containing lysine methyltransferase that, along with EED, SUZ12, RbAp48, and AEBP2, forms the polycomb repressive complex 2 (PRC2) (8, 9). EZH2 is responsible for the methylation of histone H3 on lysine 27 (H3K27), which is generally associated with transcriptional repression when present in the di- or trimethylated state (8–11). EZH2 is highly expressed in pro-B cells and progressively decreases in expression as cells progress into pre-B cells, immature B cells, and recirculating B cells (12). EZH2 expression is required in the bone marrow for progression of pro-B cells into pre-B cells and immature B cells, because genetic inactivation of EZH2 leads to an accumulation of cells at the pro–B-cell stage (12). However, if EZH2 is inactivated after the pro–B-cell stage, additional maturation steps are not hindered, suggesting that EZH2 functions early in B-cell differentiation (12). In fact, multiple groups have shown that EZH2 plays an important role in the maintenance of hematopoietic stem cells (HSCs) and progenitor cells (13, 14). In particular, EZH2 overexpression in HSCs leads to continued self-renewal capacity in serial transplantation models, suggesting that EZH2 contributes to repopulating potential and helps cells resist replicative stress (13).
EZH2 is frequently amplified and/or overexpressed in most solid tumor types (15); however, this does not appear to be the case in lymphomas, perhaps attributable to the high basal expression of EZH2 in normal proliferating B cells. Instead, EZH2 has been reported to harbor recurrent mutations of the tyrosine 641 (Y641) residue in 22% of germinal center B-cell (GCB) diffuse large B-cell lymphomas (DLBCLs) and 7% of follicular lymphomas (FLs) (3). Although initially reported to be a loss-of-function mutation (3), subsequent biochemical work demonstrated a unique gain-of-function activity for Y641 mutant EZH2 (16, 17). Although WT EZH2 exhibits a strong preference for unmethylated (H3K27me0) and monomethylated H3K27 (H3K27me1) substrates, the Y641 mutants observed in lymphomas (Y641F/N/S/H/C) exhibit profoundly increased activity for dimethylated H3K27 (H3K27me2), decreased activity for H3K27me1, and little to no activity for H3K27me0 (16, 17). Through the coordinated activities of WT and mutant EZH2, there is a global increase in trimethylation of H3K27 (H3K27me3) in Y641 mutant lymphomas concomitant with a decrease in H3K27me1 and H3K27me2 (16).
These EZH2 Y641 mutations, along with EZH2 overexpression in many tumors, suggest that dysregulation of H3K27me3 is important in human tumorigenesis. Indeed, H3K27me3 levels correlate with progression-free survival in renal cell carcinoma (18) and with disease severity and poor tumor differentiation in esophageal squamous cell carcinoma (19). In addition to mutation of EZH2 Y641, alternative mechanisms for dysregulation of H3K27me3 include inactivating mutations of the H3K27 demethylase UTX (4, 20, 21) and overexpression of EZH2 attributable to multiple mechanisms, including decreased miR-101 levels (22, 23), aberrant E2F activity (24), and chromosomal amplification (25).
Through the investigation of global H3K27me3 levels in more than 100 cancer cell lines, we identified a unique EZH2 mutation at the A677 residue that is capable of increasing global H3K27me3 levels when exogenously expressed in cells. Characterization of this mutant protein revealed that exchange of A677 for glycine (A677G) leads to increased activity with H3K27me2 substrates similar to the Y641 EZH2 mutations. However, in contrast to the Y641 mutants, which lose activity with H3K27me0 substrates, this substitution retains critical interactions present in WT EZH2 leading to efficient utilization of all three methylation substrates (H3K27me0, H3K27me1, and H3K27me2). This mutation presents a unique approach for cells to dysregulate H3K27 methylation without requiring cooperation with WT EZH2 as is the case for Y641 EZH2 mutants.
Results
Aberrantly Elevated H3K27me3 Levels in Human Lymphoma Cell Lines.
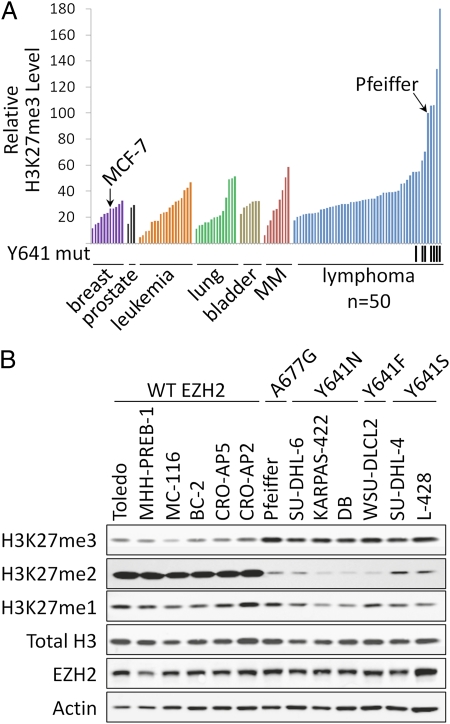
A variety of activating and inactivating mutations of EZH2 have been described in primary tumors derived from GCB DLBCLs, FLs, and myelodysplastic syndrome (1–3, 26–28). The end result of these mutations is increased or decreased methylation of H3K27 (16, 17, 26, 28). To characterize alterations of H3K27me3 in human cancer cell lines, we quantified global H3K27me3 and total histone H3 levels by ELISA in 111 cell lines from seven unique tumor types (Fig. 1A). The methylation-specific antibody was confirmed to be highly specific for H3K27me3 under these assay conditions through titration of full-length recombinant methylated histones and competition of signal from a protein lysate with methylated peptides (Fig. S1 A and B). Each of the tumor types examined exhibited a range of H3K27me3 levels, with several lymphoma cell lines possessing H3K27me3 levels two- to threefold higher than the highest nonlymphoma cell lines. Western blot analysis of protein lysates from several of these cell lines with antibodies specific for H3K27me3, H3K27me2, and H3K27me1 revealed an apparent imbalance between the methylation states of H3K27 (Fig. 1B and Fig. S2). Overall, lymphoma cell lines with elevated H3K27me3 appear to have increased trimethylation at the expense of dimethylation and monomethylation, because H3K27me2 levels and H3K27me1 levels, to a lesser extent, were reduced in these cell lines relative to those with lower global H3K27me3 levels.
Fig. 1.
A subset of lymphoma cell lines exhibits elevated H3K27me3 levels. (A) Global H3K27me3 levels (normalized to total H3) were determined for 111 cancer cell lines from seven different cancer types using H3K27me3 and total H3 ELISAs. Lymphoma cell lines harboring heterozygous Y641 mutations are indicated by black tick marks below the graph. MM, multiple myeloma. (B) Western blot analysis was performed with antibodies specific for H3K27me3, H3K27me2, H3K27me1, total histone H3, EZH2, and actin using protein lysates from a panel of lymphoma cell lines. Actin serves as a loading control. EZH2 mutation status as determined from full-length Sanger sequencing is indicated.
Mutation of the A677 Residue of EZH2 to Glycine in a Lymphoma Cell Line with Aberrantly Elevated H3K27me3.
Based on recent findings demonstrating an H3K27 hypertrimethylation phenotype in lymphoma cells harboring mutation of the Y641 residue of EHZ2 to either phenylalanine (F), asparagine (N), serine (S), histidine (H), or cysteine (C), we hypothesized that the lymphoma cell lines with the highest levels of H3K27me3 may harbor activating mutations in EZH2. Sanger sequencing of all cell lines for the Y641 codon revealed activating mutations for six of the top seven lymphoma types when ranked by global H3K27me3 levels (Fig. 1A).
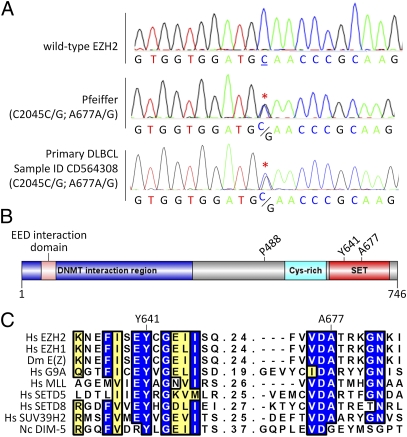
The one cell line with high H3K27me3 levels that was not mutated at Y641 was the Pfeiffer cell line. That cell line was established in 1992 from the pleural effusion of a patient in the leukemic phase of DLBCL (29). Sanger sequencing of genomic DNA for all EZH2 coding exons revealed a heterozygous C-to-G mutation at nucleotide 2045, leading to a nonsynonymous mutation of the A677 residue to a glycine (A677G) (Fig. 2A). Sequence analysis of cDNA revealed that both WT and mutant alleles are expressed (Fig. S3). This residue falls within the catalytic SET domain of EZH2 and is located in exon 18 two exons downstream of the Y641 residue (Fig. 2B). This residue is highly conserved across multiple species and multiple histone methyltransferases (Fig. 2C), indicating that it may play an essential role in the function of EZH2.
Fig. 2.
The Pfeiffer lymphoma cell line harbors a heterozygous A677G mutation in EZH2. (A) Chromatograms from Sanger sequencing of EZH2 in a WT control sample, the Pfeiffer DLBCL cell line, and a primary DLBCL patient sample (sample ID CD564308). Heterozygous nonsynonymous missense mutation of C2045C/G (red asterisks) translates to A677A/G. Nucleotide and amino acid residue numbering is based on the NM_001203247 EZH2 cDNA transcript. (B) EZH2 domain architecture (UniProt Q15910). Sites of nonsynonymous mutations identified in lymphoma cell lines and primary tumors in this study are highlighted. (C) Alignment of human EZH2 with human EZH1, the fly ortholog E(z), and six other related SET domain-containing histone lysine methyltransferases showing that Y641 and A677 are highly conserved. Blue shading represents identical residues, and yellow shading represents conserved residues. In order for a column to be shaded, seven of nine residues must be conserved/identical.
Occurrence of the A677G EZH2 Mutation in Primary Lymphoma Samples.
To establish whether the mutation identified in the Pfeiffer cell line occurs in primary human lymphomas, this residue was sequenced in a panel of 41 lymphoma specimens. This panel consisted of 30 DLBCLs, six FLs, one mantle cell lymphoma, one extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue, one splenic marginal zone lymphoma, and two Waldenström macroglobulinemia or lymphoplasmacytic lymphomas (Table S1). In addition to four occurrences of the Y641 mutation (1 Y641N, 1 Y641F, 1 Y641H, and 1Y641C), nonsynonymous missense mutations were observed at P488 (P488S) and A677 (A677G). The A677 mutation was heterozygous and occurred in a stage IIE DLBCL obtained from a 74-y-old woman (Fig. 2A and Table S1). These data confirm that the A677G mutation occurs in primary human lymphoma and is not simply an artifact of cell culture.
EZH2 A677G Mutation Confers Biochemical Activity Independent of H3K27 Methylation State.
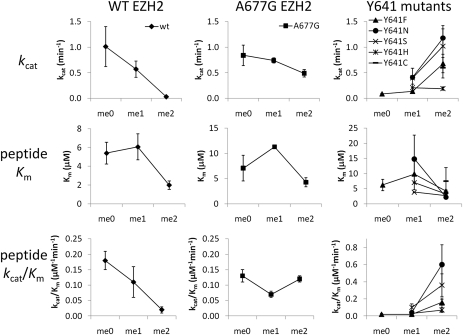
Given the hypertrimethylation phenotype in cells bearing the A677G mutation and the spatial proximity of A677 to Y641 in our structural models (vide infra), we tested in vitro whether A677G EZH2 displayed an altered substrate specificity similar to Y641 mutants (Fig. 3 and Table S2). When comparing turnover and catalytic efficiency with the H3K27 peptide substrates (kcat and kcat/Km, respectively), WT EZH2 loses activity when progressively more methyl groups are incorporated into H3K27 (i.e., H3K27me0 > H3K27me1 > H3K27me2), with the ratio of catalytic efficiencies for H3K27me0, H3K27me1, and H3K27me2 being 9:6:1. In contrast, all Y641 mutant enzymes that were evaluated (Y641F/N/S/H/C) displayed the opposite trend, with the H3K27me2 peptide being used most efficiently (H3K27me0:me1:me2 kcat/Km ratio = 1:2:13). These substrate preferences are consistent with recently reported data demonstrating H3K27me0:me1:me2 kcat/Km ratios of 13:4:1 and 1:2:22 for WT and Y641F EZH2, respectively (16). The A677G EZH2 complex, on the other hand, displayed a profile different from both WT and Y641 mutants. A677G EZH2 used all three substrates with nearly equal efficiency (H3K27me0:me1:me2 kcat/Km ratio = 1.1:0.6:1) and at a rate comparable to the highest turnover rate observed with the WT enzyme (kcat = 0.84 min−1 for A677G with H3K27me0 vs. 1.01 min−1 for WT with H3K27me0). When nucleosomes purified from HeLa cells were evaluated, the activity of A677G EZH2 was slightly higher than WT EZH2 and the Y641 mutants (kcat = 0.19 min−1 for A677G vs. 0.11 min−1 for WT and 0.15 min−1 for Y641S), likely attributable to the ability of the A677G EZH2 complex to act on a greater proportion of the HeLa histones, which are heterogeneously modified at H3K27.
Fig. 3.
A677G EZH2 mutant exhibits a unique substrate specificity. Enzyme turnover number [kcat (min−1)], the substrate concentration at which the reaction rate was half of Vmax [Km (μM)], and catalytic efficiency [kcat/Km (μM−1⋅min−1)] were evaluated for recombinant five-member PRC2 complexes containing WT, A677G, or Y641 mutant EZH2 using peptides from histone H3 AA21–44 with no methylation (K27me0), monomethylation (K27me1), or dimethylation (K27me2) at the lysine 27 position. Briefly, EZH2 (20 nM) was combined with varying concentrations of peptide and [3H]-SAM before quenching with unlabeled SAM during the linear portion of their progress curves. Reactions were then captured using filter plates, scintillation mixture was added, and signal was detected with a TopCount liquid scintillation counter (PerkinElmer). Error bars represent the standard deviation from replicate experiments.
To determine whether the A677G mutation might affect EZH2 specificity toward other histone substrates, we also evaluated WT and mutant EZH2 complexes using a library of 602 peptides representing sequences within histones H2A, H2B, H3, or H4 and possessing up to five posttranslational modifications, such as lysine and/or arginine methylation; lysine acetylation; or phosphorylation of serine, tyrosine, and/or threonine (Dataset S1). This global analysis revealed that the A677G mutant EZH2 did not enhance activity for any other histone sites compared with WT EZH2 (Fig. S4A). In addition, when comparing the A677G mutant with the Y641N mutant, it was observed that their only common activity was with a peptide containing H3K27me2 (Fig. S4B). Thus, the A677G EZH2 complex is unique in that it has both retained WT activities with unmodified and monomethylated substrates and acquired the neomorphic activity observed in Y641 EZH2 mutants with dimethylated substrates.
Expression of A677G EZH2 Is Sufficient to Drive Hypertrimethylation of H3K27.
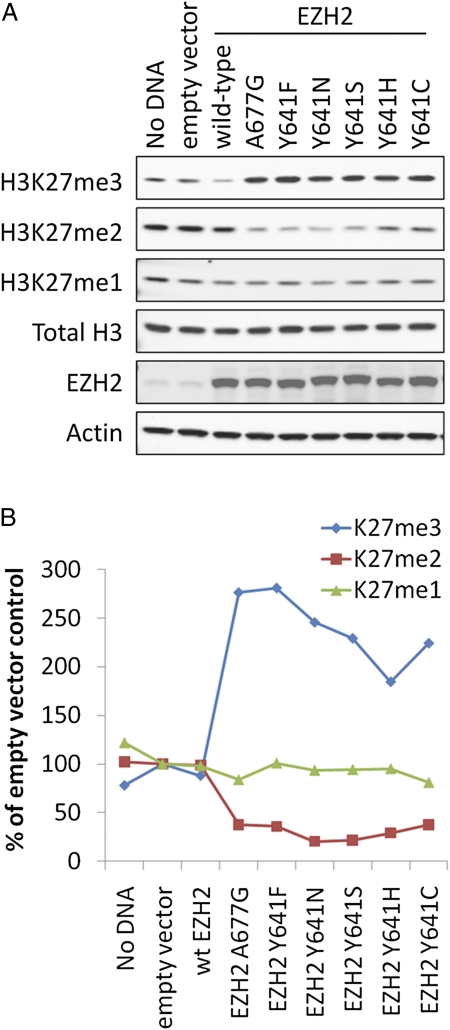
To explore the effect of the A677G and Y641 EZH2 mutants on histone methylation levels, WT and mutant versions of EZH2 were transiently expressed in cells before evaluation of global H3K27me3 levels. MCF-7 cells were selected for this analysis because they are WT for EZH2, exhibit relatively low levels of H3K27me3 (26% of Pfeiffer H3K27me3 levels; Fig. 1A), and are easily transfected. Consistent with the biochemical data, exogenous expression of either A677G or Y641 mutant EZH2 increased H3K27me3 levels 1.9- to 2.8-fold relative to the empty vector control (Fig. 4 A and B). H3K27me2, on the other hand, was depleted in cells expressing A677G or Y641 mutant EZH2. H3K27me1 levels were not significantly affected by expression of either WT or mutant EZH2. These observations are consistent with those from EZH2 WT and mutant lymphoma cell lines, where the A677G and Y641 EZH2 mutant cell lines exhibit increased H3K27me3, decreased H3K27me2, and modest effects on H3K27me1 (Fig. 1B). These data demonstrate that expression of A677G EZH2 is sufficient to induce a global hypertrimethylation of the H3K27 residue similar to Y641 mutants.
Fig. 4.
Exogenous expression of A677G EZH2 stimulates trimethylation of H3K27 at the expense of H3K27me2. (A) MCF-7 breast cancer cells were transiently transfected with mammalian expression constructs encoding either a WT or mutant form of EZH2. In addition, transfection reagent alone (No DNA) and empty vector treatments were included as controls. Cells were lysed 72 h after transfection, and whole-cell protein extracts were assessed for levels of H3K27me3, H3K27me2, H3K27me1, total histone H3, EZH2, and actin via Western blotting as described in SI Materials and Methods. Actin and total histone H3 were included as loading controls. (B) Average values of H3K27me3, H3K27me2, or H3K27me1 normalized to total histone H3 from at least two replicates. Values are presented as a percentage of the empty vector control sample.
Discussion
Disruption of the normal patterning of H3K27me3 occurs in human cancers (30–32) and appears to be required for the proliferation of some lymphoma cells because knockdown of EZH2 in the SU-DHL-4 DLBCL cell line results in growth arrest at the G1/S transition (33). Recent studies have demonstrated that one common mechanism for the H3K27 hypertrimethylation phenotype in lymphoma is mutation of the EZH2 Y641 residue (1, 3, 16, 17). Herein, we have reported the identification and characterization of a unique EZH2 mutation equally capable of increasing global H3K27me3 in human lymphoma cells.
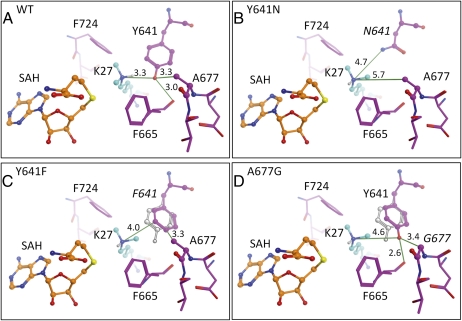
In the absence of an EZH2 crystal structure, a homology model was constructed to understand the distinct substrate specificities of WT, A677G, and Y641 mutant forms of EZH2. The models were based on the protein sequence of EZH2 and a crystal structure of GLP/EHMT1 bound to a histone H3 peptide substrate containing H3K9me2 (Fig. 5A). The homology model of WT EZH2 is consistent with the biochemical data, demonstrating that it primarily catalyzes mono- and dimethylation, but not trimethylation, of H3K27 (Fig. 3 and Table S2). Similar to other methyltransferases, such as Set7/9, the hydroxyl group of the highly conserved EZH2 Y641 residue appears to optimally orient the unmethylated and monomethylated forms of the H3K27 substrate for methyl transfer through a hydrogen bond with the ε-amine group of H3K27 (34, 35). However, with ∼3.3 Å between the hydroxyl oxygen of Y641 and the ε-amine group of H3K27me2, the model shows very little room for the dimethylated lysine to rotate into position to accept a third methyl group. Therefore, the Y641 residue of EZH2 appears to have a dual purpose, participating in the orientation of the unmethylated and monomethylated lysine and, at the same time, sterically restricting activity with a dimethylated substrate.
Fig. 5.
Y641 and A677G EZH2 mutations alter the lysine binding pocket to affect H3K27 substrate specificity. A homology model of WT EZH2 was generated using the crystal structure of GLP/EHMT1 bound to an H3K9me2 peptide substrate as described in SI Materials and Methods. Modeled structures of the active site region in WT (A), Y641N (B), Y641F (C), and A677G (D) EZH2 are depicted. For the Y641F and A677G mutant models, the lowest energy rotamer for the 641 residue (F or Y, respectively) was selected after rotating the dimethylated lysine into an orientation optimal for trimethylation. In C and D, the WT Y641 orientation is shown in a gray outline to highlight the alternative low-energy conformations adopted by the 641 residue in these mutants. Key heavy atom distances (measurement unit = Å) are indicated with green lines. S-adenosyl-homocysteine (SAH) is colored with orange carbons, EZH2 residues are colored with magenta carbons, and the dimethylated H3K27 is colored with cyan carbons.
This model further predicts that the mutation of Y641 to a significantly smaller residue, such as asparagine (Y641N), would result in a larger lysine tunnel (H3K27me2 ε-amine–to-N641 side-chain distance of at least 4.7 Å) and loss of the critical tyrosine hydroxyl-to–ε-amine hydrogen bond (Fig. 5B). These changes presumably weaken stabilization of the highly flexible unmodified or monomethylated lysine, whereas the larger lysine tunnel permits the dimethylated lysine to rotate into position for the third methyl transfer. Although the Y641N, Y641S, and Y641C mutations dramatically decrease the size of the residue at position 641, the mutation of Y641 to a phenylalanine (Y641F) is relatively conservative, with only loss of the tyrosine hydroxyl group (Fig. 5C). Modeling of the Y641F EZH2 suggests that loss of the hydroxyl group may allow the phenylalanine residue to adopt an alternative low-energy conformation in which it rotates away from the lysine tunnel toward the A677 residue. This second conformation likely generates sufficient space (H3K27me2 ε-amine–to-F641 side-chain distance of at least 4.0 Å) for the dimethylated lysine to orient optimally for the third methyl transfer. However, with the loss of the stabilizing hydrogen bond that occurs between the tyrosine hydroxyl group and the lysine substrate, the Y641F mutant exhibits greatly reduced activity with unmodified lysines. This interpretation is consistent with data presented in this report and by others (16, 17) demonstrating that Y641 mutants do not efficiently use unmethylated lysines yet have acquired robust activity with dimethylated substrates (Fig. 3 and Table S2).
Interestingly, although the structural model for WT EZH2 suggests that the highly conserved A677 residue does not interact directly with either H3K27 or [3H]-S-adenosyl-methionine (SAM), it is in close proximity to the hydroxyl group of Y641 with a distance of ∼3.3 Å (Fig. 5A). It is therefore predicted that mutating A677 to a smaller glycine residue permits Y641 to adopt an alternative low-energy conformation similar to that predicted for the phenylalanine in the Y641F mutant (Fig. 5 C and D). This alternative conformation for Y641 produces sufficient space (at least 4.6 Å) between the hydroxyl oxygen of Y641 and the ε-amine group of H3K27me2 to permit the lysine tail to rotate into position for trimethylation. However, in contrast to the Y641F mutant, the A677G mutant retains the tyrosine hydroxyl group at position 641, thus preserving the stabilizing interaction between Y641 and unmodified lysine substrates. This model therefore suggests that the retention of the Y641 residue, combined with the ability of this residue to adopt an alternative conformation in the A677G mutant, contributes to the efficient methylation of unmodified, mono- and dimethylated substrates (Fig. 3 and Table S2).
Although the EZH2 Y641 mutation occurs in 22% of GCB DLBCLs and 7% of FLs (3), our study indicates that mutation of the EZH2 A677 to glycine is a fairly rare event. We observed this mutation in 1 of 50 lymphoma cell lines and 1 of 41 primary lymphoma samples. Morin et al. (1) also recently observed a single case of DLBCL (a 63-y-old woman with stage IAE) with an A677G mutation among 127 samples that were assessed by RNA-seq, exome-seq, and/or genome-seq. Although a matched normal sample was not available for the A677G EZH2 mutant lymphoma specimen reported in this study, the mutation was reported to be somatic in the case reported by Morin et al. (1), and it is not reported as a normal sequence variant in the dbSNP database or the 1000 Genomes project. Thus, although a more extensive study with focused genotyping of the A677 codon will be required to establish the true incidence of this alteration, these initial data suggest that the frequency of this mutation is likely below 2–3%.
Considering that the end result of these two mutations may be quite similar (i.e., increased H3K27me3), it is at first somewhat surprising that these mutations occur at such different rates. However, this discrepancy might be explained, in part, by the spectrum of possible activating mutations at each site. To date, mutation of Y641 to any of five different residues (F, N, S, C, or H) has been reported to increase activity with an H3K27me2 substrate (refs. 1, 3, 16, 17, 36 and this study). This increased activity has been attributed to the exchange of Y641 for smaller residues, which permit the larger H3K27me2 substrate to rotate into a position for methyl transfer. The A677G mutation appears to increase the dimensions of the lysine tunnel similarly by permitting an alternate conformation of Y641 through exchange of alanine for a smaller amino acid; however, because alanine is already the second smallest amino acid, it may only be exchanged for a glycine. At the nucleotide level, only one of nine single-nucleotide mutations within the A677 codon will result in a glycine residue. In contrast, five of nine single-nucleotide mutations within the Y641 codon generate a gain-of-function mutant. Thus, the apparently low incidence of the EZH2 A677G mutation may be attributable in part to the extremely limited number of possible alterations for this particular site.
The fact that the SET domain is highly conserved across orthologous and homologous methyltransferases readily permits translation of findings from one methyltransferase to another. For example, the effect of changes at the Y641 residue of EZH2 is predictable based on mutational analyses of other SET domain methyltransferases whose biochemical properties have been more extensively studied. The human SET7/9 methyltransferase normally monomethylates H3K4; however, when the SET7/9 Y245 residue (the equivalent of EZH2 Y641) is mutated to alanine, the mutant can no longer modify an H3K4me0 substrate but, instead, gains the ability to di- and trimethylate an H3K4me1 peptide substrate (37). Similarly, exchange of the Y641 equivalent in the H3K9 methyltransferase G9a (Y1067) for phenylalanine converts the enzyme from a mono- and dimethyltransferase to a trimethyltransferase (38).
This study examines the biochemical and cellular activity of the conserved A677 residue of EZH2. Interestingly, the structurally related SET domain-containing DIM-5 from Neurospora crassa has a glycine at the equivalent position (Fig. 2C) and has been reported to perform all three methylation events on its H3K9 substrate (39, 40). Thus, it appears that the alanine at residue 677 of EZH2, and likely equivalent residues in other SET domain methyltransferases, plays an important role in the regulation of substrate specificity without being in direct contact with the substrate. This interplay between Y641 and A677 in EZH2 highlights just one of the many important mechanisms that have likely evolved to regulate the substrate and product specificities of lysine methyltransferases.
Materials and Methods
All cell lines were obtained from either the American Type Culture Collection or Deutsche Sammlung von Mikroorganismen und Zellkulturen and maintained in the recommended cell culture media. Primary tissue and DNA samples were obtained from OriGene (Table S1). Global histone modification levels were determined with ELISA or Western blot methods using antibodies specific for total histone H3, H3K27me1, H3K27me2, or H3K27me3 (Figs. S1 and S2). The full-length EZH2 sequence was determined for genomic DNA and cDNA using primers described in Table S3 and standard Sanger sequencing methods. MCF-7 cells were transiently transfected with WT and mutant EZH2 expression constructs using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Biochemical studies were performed with peptide and HeLa nucleosomes using [3H]-SAM and purified recombinant five-member PRC2 complexes containing WT or mutant forms of EZH2 (Fig. S5 and Table S4). Structural models of EZH2 were built using GLP/EHMT1 bound to an H3K9me2 peptide substrate (Protein Data Bank ID code 2RFI) as a primary template and structurally compared with other related SET domain-containing histone lysine methyltransferases with determined crystal structures. Additional detailed information is available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We acknowledge Daniel Fornwald for the production of baculovirus, Amy Taylor for assistance with protein purification, Harjeet Van Der Keyl for assistance with construct generation, Gilbert Scott for MS support, and Don Fisher for coordinating reagent generation.
Footnotes
Conflict of interest statement: All authors are employees of GlaxoSmithKline.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116418109/-/DCSupplemental.
References
- 1.Morin RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bödör C, et al. EZH2 Y641 mutations in follicular lymphoma. Leukemia. 2011;25:726–729. doi: 10.1038/leu.2010.311. [DOI] [PubMed] [Google Scholar]
- 3.Morin RD, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181–185. doi: 10.1038/ng.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gui Y, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fathi AT, Abdel-Wahab O. Mutations in epigenetic modifiers in myeloid malignancies and the prospect of novel epigenetic-targeted therapy. Adv Hematol. 2012;2012:469592. doi: 10.1155/2012/469592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueroa ME, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller J, et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 10.Cao R, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 11.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Su IH, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4(2):124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 13.Kamminga LM, et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107:2170–2179. doi: 10.1182/blood-2005-09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majewski IJ, et al. Opposing roles of polycomb repressive complexes in hematopoietic stem and progenitor cells. Blood. 2010;116:731–739. doi: 10.1182/blood-2009-12-260760. [DOI] [PubMed] [Google Scholar]
- 15.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647(1-2):21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Sneeringer CJ, et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc Natl Acad Sci USA. 2010;107:20980–20985. doi: 10.1073/pnas.1012525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yap DB, et al. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood. 2011;117:2451–2459. doi: 10.1182/blood-2010-11-321208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogenhofer S, et al. Global histone H3 lysine 27 (H3K27) methylation levels and their prognostic relevance in renal cell carcinoma. BJU Int. 2012;109(3):459–465. doi: 10.1111/j.1464-410X.2011.10278.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, et al. Abnormal histone acetylation and methylation levels in esophageal squamous cell carcinomas. Cancer Invest. 2011;29:548–556. doi: 10.3109/07357907.2011.597810. [DOI] [PubMed] [Google Scholar]
- 20.Dalgliesh GL, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Haaften G, et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41:521–523. doi: 10.1038/ng.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman JM, et al. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 23.Varambally S, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bracken AP, et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saramäki OR, Tammela TL, Martikainen PM, Vessella RL, Visakorpi T. The gene for polycomb group protein enhancer of zeste homolog 2 (EZH2) is amplified in late-stage prostate cancer. Genes Chromosomes Cancer. 2006;45:639–645. doi: 10.1002/gcc.20327. [DOI] [PubMed] [Google Scholar]
- 26.Makishima H, et al. Novel homo- and hemizygous mutations in EZH2 in myeloid malignancies. Leukemia. 2010;24:1799–1804. doi: 10.1038/leu.2010.167. [DOI] [PubMed] [Google Scholar]
- 27.Nikoloski G, et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42:665–667. doi: 10.1038/ng.620. [DOI] [PubMed] [Google Scholar]
- 28.Ernst T, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42:722–726. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- 29.Gabay C, Ben-Bassat H, Schlesinger M, Laskov R. Somatic mutations and intraclonal variations in the rearranged Vkappa genes of B-non-Hodgkin's lymphoma cell lines. Eur J Haematol. 1999;63:180–191. doi: 10.1111/j.1600-0609.1999.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 30.Cai MY, et al. High expression of H3K27me3 in human hepatocellular carcinomas correlates closely with vascular invasion and predicts worse prognosis in patients. Mol Med. 2011;17(1-2):12–20. doi: 10.2119/molmed.2010.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng AS, et al. EZH2-mediated concordant repression of Wnt antagonists promotes β-catenin-dependent hepatocarcinogenesis. Cancer Res. 2011;71:4028–4039. doi: 10.1158/0008-5472.CAN-10-3342. [DOI] [PubMed] [Google Scholar]
- 32.Gal-Yam EN, et al. Frequent switching of Polycomb repressive marks and DNA hypermethylation in the PC3 prostate cancer cell line. Proc Natl Acad Sci USA. 2008;105:12979–12984. doi: 10.1073/pnas.0806437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velichutina I, et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood. 2010;116:5247–5255. doi: 10.1182/blood-2010-04-280149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Rizzo PA, et al. SET7/9 catalytic mutants reveal the role of active site water molecules in lysine multiple methylation. J Biol Chem. 2010;285:31849–31858. doi: 10.1074/jbc.M110.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couture JF, Dirk LM, Brunzelle JS, Houtz RL, Trievel RC. Structural origins for the product specificity of SET domain protein methyltransferases. Proc Natl Acad Sci USA. 2008;105:20659–20664. doi: 10.1073/pnas.0806712105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wigle TJ, et al. The Y641C mutation of EZH2 alters substrate specificity for histone H3 lysine 27 methylation states. FEBS Lett. 2011;585:3011–3014. doi: 10.1016/j.febslet.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Xiao B, et al. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003;421:652–656. doi: 10.1038/nature01378. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, et al. Structural biology of human H3K9 methyltransferases. PLoS ONE. 2010;5:e8570. doi: 10.1371/journal.pone.0008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, et al. Structural basis for the product specificity of histone lysine methyltransferases. Mol Cell. 2003;12(1):177–185. doi: 10.1016/s1097-2765(03)00224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamaru H, et al. Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat Genet. 2003;34:75–79. doi: 10.1038/ng1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.