Abstract
CD175 or Tn antigen is a carbohydrate moiety of N-acetylgalactosamine (GalNAc)α1-O- linked to the residue of amino acid serine or threonine in a polypeptide chain. Despite the chemical simplicity of the Tn antigen, its antigenic structure is considered to be complex and the clear determinants of Tn antigenicity remain poorly understood. As a consequence, a broad variety of anti-Tn monoclonal antibodies (mAbs) have been generated. To further investigate the nature and complexity of the Tn antigen, we generated seven different anti-Tn mAbs of IgM and IgG classes raised against human Jurkat T cells, which are Tn-positive due to the low activity of T-synthase and mutation in specific chaperone Cosmc. The binding analysis of anti-Tn mAbs with the array of synthetic saccharides, glycopeptides and O-glycoproteins revealed unexpected differences in specificities of anti-Tn mAbs. IgM mAbs bound the terminal GalNAc residue of the Tn antigen irrespective of the peptide context or with low selectivity to the glycoproteins. In contrast, IgG mAbs recognized the Tn antigen in the context of a specific peptide motif. Particularly, JA3 mAb reacted to the GSPP or GSPAPP, and JA5 mAb recognized specifically the GSP motif (glycosylation sites are underlined). The major O-glycan carrier proteins CD43 and CD162 and isoforms of CD45 expressed on Jurkat cells were precipitated by anti-Tn mAbs with different affinities. In summary, our data suggest that Tn antigen–Ab binding capacity is determined by the peptide context of the Tn antigen, antigenic specificity of the Ab and class of the immunoglobulin. The newly generated anti-Tn IgG mAbs with the strong specificity to glycoprotein CD43 can be particularly interesting for the application in leukemia diagnostics and therapy.
Keywords: CD43, CD45, CD162, glycopeptide array, monoclonal antibodies, Tn antigen
Introduction
The Tn antigen was initially described as a carbohydrate antigen on the surface of red blood cells from patients with a very rare Tn-syndrome (Dausset et al. 1959; Berger 1999). The discovery of the Tn antigen expression in a variety of malignant disorders has opened up a new interest for this moiety as a cancer-associated marker (Springer 1995, 1997). Specific expression of the Tn antigen on the surface of malignant cells underlies the development of anti-cancer vaccine containing the Tn epitope (Springer 1997; Danishefsky and Allen 2000; Xu et al. 2004). Alternatively, passive administration of antibodies (Abs) directed against the Tn antigen had a potential effect in cancer therapy (Welinder et al. 2011). Anti-Tn mAbs were also shown to be effective as diagnostic tools for in vivo imaging of Tn-expressing tumors (Danussi et al. 2009). Despite a clear and defined structure of carbohydrate part of the Tn antigen, its real epitope architecture may have a more complex nature. Some of the reported Tn-specific Ab recognized only the GalNAcα monosaccharide (O'Boyle et al. 1996; Avichezer et al. 1997). For the other Abs, the binding was dependent on both the glycan and the peptide sequence (Reis et al. 1998). In addition, the clusters composed of two or three consecutive glycosylated serine/threonine (Ser/Thr) were also contributed in the formation of the Tn epitope (Nakada, Numata, et al. 1991; Nakada et al. 1993; Inoue et al. 1994; Tanaka et al. 1999; Osinaga et al. 2000).
Several carrier molecules of the Tn antigen, a number of different O-glycosylated proteins, have been described. For instance, in patients with Tn-syndrome, the Tn antigen was detected on glycophorin A and glycoprotein CD42b (Nurden et al. 1982). The different types of mucins served as the major carriers of the Tn antigen in carcinomas (Ju et al. 2011). The mouse fibrosarcoma cells Ag104A expressed the Tn antigen in the context of the tumor-associated glycoprotein podoplanin (Schietinger et al. 2006). The Tn antigen was detected on the proteoglycans, such as syndecan-3 (Akita et al. 2001), and sialophorin (CD43) was found to carry a major portion of the Tn antigen on the surface of the Jurkat leukemic cells (Nakada, Inoue, et al. 1991).
The Tn antigen has been reported to be expressed in 70–90% of breast, lung, prostate, and pancreatic tumors (Springer 1997; Sakai et al. 2010) but little is known about Tn expression on malignant cells of hematopoietic lineage. A few researchers have focused their Tn studies using patient-derived lymphoid tumors. Hence, Aller et al. (1996) have found that some patient's B-cell chronic lymphocytic leukemia cells were positive for the Tn antigen. Then, Lawrie et al. (2006) demonstrated the heterogeneous expression of the Tn antigen on the Reed–Sternberg cells isolated from classical Hodgkin's lymphomas. It should be mentioned that all of the previously generated anti-Tn Abs were obtained after the immunization with carcinoma cells (Hirohashi et al. 1985; Numata et al. 1990; Pancino et al. 1990), purified mucins (Reddish et al. 1997; Welinder et al. 2011) or synthetic GalNAc conjugated with bovine serum albumin (BSA) (Oppezzo et al. 2000; Danussi et al. 2009). No mAbs developed against lymphoid antigens (or cells) were extensively used to analyze the Tn reactivity in details.
Human Jurkat T cells bear a mutation in cosmc gene and have an abolished level of T-synthase activity (Piller et al. 1990; Ju et al. 2008). This results in the expression of 80% cellular glycans in a truncated GalNAcα-O form, i.e. Tn antigen positive (Nakada, Inoue, et al. 1991; Brown et al. 1996). Therefore, to study Tn carrier proteins related to the lymphoid cells, we generated mouse monoclonal Abs (mAbs) against Jurkat cells and selected four IgM and three IgG1 anti-Tn mAbs. The specificity of generated mAbs was analyzed by the binding capacity to the natural or synthetic glycans, glycopeptides and glycoproteins. We have determined the binding specificity of each Ab with the lymphocyte-associated O-glycan carrier proteins. The IgM mAbs were less affected by the peptide sequence for Tn recognition, but rather dependent on the clusterization of GalNAc-Ser/Thr in a polypeptide chain. In contrast to IgM, anti-Tn IgG mAbs recognized the Tn antigen in the context of specific peptide motifs, which were present in CD43 and CD45 proteins. Such specificity of generated IgG mAbs can potentially broaden the applicability of anti-Tn Abs in therapy of individuals with Tn-positive leukemia and lymphoma malignancies.
Results
Generation of anti-Tn mAbs
As demonstrated previously, T and Tn antigens were involved in cell–cell adhesion (Kishikawa et al. 1999; Glinsky et al. 2000). To explore the specificity and complexity of Tn antigen recognition, we generated a panel of new anti-Tn mAbs where BALB/c mice were immunized with live Jurkat cells. Anti-Tn hybridomas were selected after three rounds of a screening procedure. As the first step, the binding of mAbs to the surface of Jurkat cells was determined by immunofluorescence and flow cytometry (FACS) analysis. Second, in order to discriminate anti-Tn clones among all anti-Jurkat clones obtained, we analyzed the mAbs for the ability to induce the homotypic aggregation of Jurkat cells by using light scattering parameters of FACS (Aussel et al. 1995). Finally, the hybridomas recognizing surface antigens on Tn negative CEM T cells were excluded. After three hybridizations, we obtained seven clones that were positive for Tn+ Jurkat T cells, negative for Tn− CEM T cells and induced homotypic aggregation of Jurkat cells. The resulting stable clones were designated as JA1 (IgM), JA2 (IgG1), JA3 (IgG1), JA4 (IgM), JA5 (IgG1), JA6 (IgM) and JA7 (IgM). With the exception of Jurkat cells and human carcinoma cells MCF7, all mAbs listed above did not bind to the surface of many tested lymphoid cells: resting or ConA stimulated primary lymphocytes, neutrophils and cell lines CEM, HUT-78, MOLT-4, MT-2, Raji, Daudi, IM-9, Ramos, NALM-6, K-562, U-937, THP-1 and HeLa.
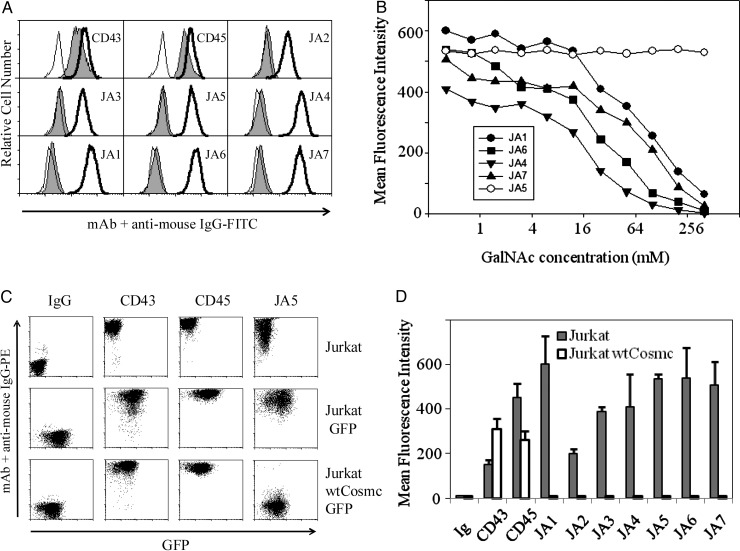
It has been reported that both Jurkat and MCF7 cells express the carbohydrate Tn antigen (Nakada, Inoue, et al. 1991). To determine whether cell surface antigens recognized by mAbs have a carbohydrate origin, we explored two methods mild periodate oxidation of cells (Woodward et al. 1985) and inhibition of Ab binding by soluble GalNAc. As shown in Figure 1A, the periodate oxidation of Jurkat cells resulted in the loss of binding of all generated Abs, while the binding of control mAbs MEM-59 (anti-CD43) and LT45 (anti-CD45) was not impaired. With the soluble GalNAc (Figure 1B), the binding of all IgM mAbs to the Jurkat cells was inhibited by GalNAc at 50 mM concentration and higher. In contrast, the binding of the IgG mAb to these cells was not affected by GalNAc at the concentration of 200 mM (Figure 1B, data are shown only for JA5). Thus, all tested mAbs of JA series were highly specific for Jurkat T cells and MCF7 carcinoma cells and recognized a carbohydrate-dependent epitope. However, the binding of IgG mAbs to the Jurkat cells was resistant to the high concentrations of a soluble GalNAc monosaccharide.
Fig. 1.
Anti-Tn mAbs recognize carbohydrate antigens on the surface of Jurkat cells. (A) Periodate oxidation affects mAb binding with Jurkat cells. Carbohydrates, but not protein epitopes, were destroyed by treatment of cells with 5 mM sodium m-periodate followed by neutralization with 50 mM sodium borohydride. Next, cells were stained with primary mAbs and secondary FITC labeled sheep anti-mouse Ig Ab (filled histograms). For comparison, untreated Jurkat cells were stained with primary and secondary Abs (open bold histograms) or with a secondary FITC-labeled Ab only (open thin histograms). (B) Increasing concentrations of soluble GalNAc progressively inhibit the binding of the IgM, but not JA5 IgG mAbs to the Jurkat cells. (C) wtCosmc expression abrogates binding of JA5 mAb to the Jurkat cells. Parental Jurkat cells transduced with IRES GFP (see Material and methods) express an epitope recognized by JA5 IgG mAb. In contrast to these cells, Jurkat cells transduced with wtCosmc IRES GFP are negative in JA5 staining. Carbohydrate-independent epitopes of CD43 and CD45 were detected by using MEM-59 and LT45 mAbs. Secondary goat anti-mouse PE-labeled Ab was used to evaluate the level of primary Ab binding. (D) Binding of JA mAbs to the Jurkat and Jurkat-wtCosmc cells was comparatively quantified by FACS and expressed in MFI with standard deviation error bars for triplicates.
Expression of wild-type cosmc in Jurkat cells abolishes the binding with anti-Tn mAbs
In order to further elucidate the saccharide origin of antigens recognized by Abs, we restored the O-linked glycan synthesis in Jurkat cells. These cells have been shown to bear mutation in T-synthase-specific chaperone gene cosmc resulting in the drastic decrease in T-synthase activity and consequently in the expression of the Tn antigen (Ju et al. 2008). Jurkat cells were stably transduced with a lentiviral vector containing a wild type (wt) Cosmc IRES green fluorescent protein (GFP) cassette or an IRES GFP cassette in the control. Then, GFP-positive cells were sorted by FACS. The purity of the GFP-positive cell population was more than 97%. Afterwards, Jurkat cells were stained with clones JA5, MEM-59 (anti-CD43), LT45 (anti-CD45) or isotype control mAb. As demonstrated in Figure 1C, Jurkat-wtCosmc cells were absolutely negative for staining with JA5 Ab (low right-hand graph), as well as for the other generated Tn mAbs (Figure 1D). Nevertheless, the levels of expression of heavily O-glycosylated proteins CD43 and CD45 were not altered upon the expression of either wtCosmc or GFP, as verified by staining with MEM-59 and LT45 mAbs correspondingly (two middle columns). These data indicated that generated mAbs recognized the Tn antigen, which was no longer exposed to the Abs, when the activity of T-synthase has been restored.
Carbohydrate-binding specificity of anti-Tn mAbs
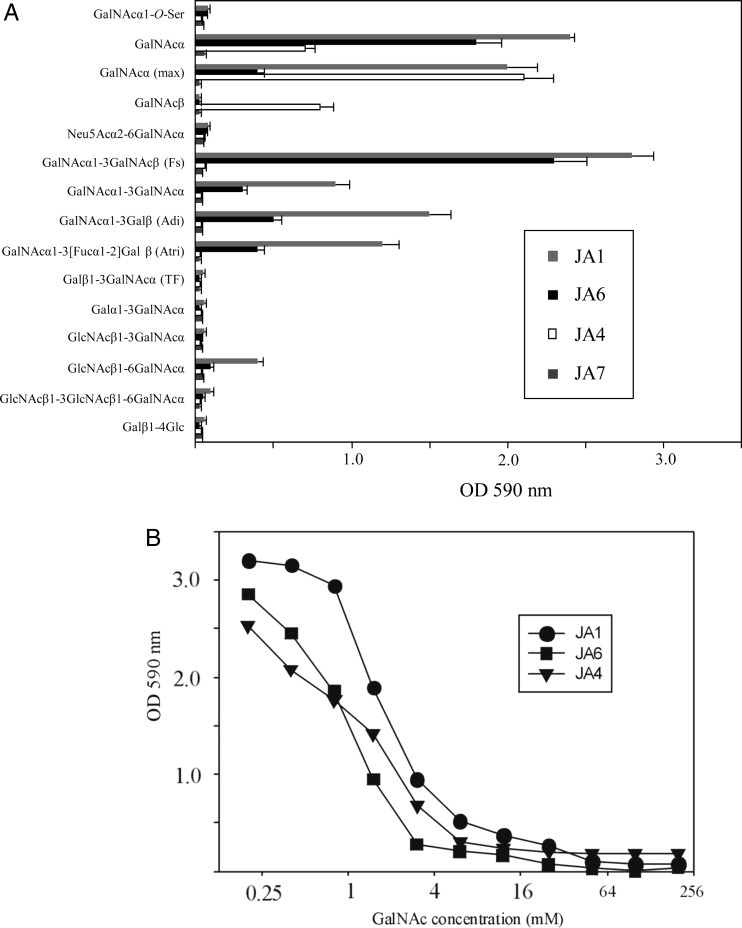
To explore fine carbohydrate specificity of generated mAbs, we tested the binding capacity of mAbs with a panel of Tn-related synthetic monovalent saccharides. Synthesized glycans were amine functionalized and immobilized on the amino-reactive glass slides (Blixt et al. 2004) or conjugated with polyacrylamide (PAA) and absorbed on the enzyme-linked immunosorbent assay (ELISA) plates (Shilova et al. 2005). None of the tested IgM or IgG showed binding to the glycans immobilized on the glass slides directly (data not shown) suggesting inadequate presentation of Tn-related structures for recognition. In contrast, three IgM mAbs showed binding with saccharides conjugated to PAA. All conjugates contained around 20 mol % of glycans, except maxGalNAcα, where the polymer was maximally saturated with the Tn saccharide (∼80 mol % accordingly to the high-performance liquid chromatography (HPLC) analysis of sample after acidic hydrolysis). As shown in Figure 2A, only the mAbs of IgM class, i.e. JA1, JA4 and JA6, exerted the binding activity to peptide-free glycans. The mAbs JA1 and JA6 had a similar, but not an identical, pattern of reactivity. They bound not only monosaccharide GalNAcα but other saccharides with a terminal residue of GalNAcα, such as Forssman (Fs) and blood group type A antigens. As the terminal GalNAcα residue was substituted by the other monosaccharide (Galβ, Neu5Acα or GlcNAcβ), the Abs lost the reactivity with glycans, particularly, with Thomsen–Friedenreich (TF) and sialylated GalNAcα. Interestingly, anti-Tn IgM mAbs bound Fs disaccharide GalNAcα1-3GalNAcβ with the higher affinity than the GalNAcα antigen. However, the binding of these mAbs with the analog of Fs disaccharide containing the internal α-residue instead of the β-anomeric form, GalNAcα1-3GalNAcα, was reduced in 2-fold, when compared with the GalNAcα. The reactivity of mAbs with terminal GalNAcα was still preserved in the case of the GalNAcα1-3Galβ disaccharide, or its fucosylated analog, blood group A trisaccharide. Thus, mAbs JA1 and JA6 may not be truly “anti-Tn Abs”, but rather be classified as anti-GalNAcα Abs. Unfortunately, the criteria for discrimination between anti-Tn and anti-GalNAcα Abs are not always fulfilled for the other well-known “anti-Tn Abs” (Ando et al. 2008). Unlike JA1 and JA6, JA4 mAb did not interact with the terminal GalNAcα residue in di- and trisaccharides, but demonstrated an ability to bind equally to the β- and α-anomers of GalNAc (Figure 2). In addition, while JA1 mAb bound GalNAcα (regular) and maxGalNAcα conjugates equally, JA4 reacted stronger with maxGalNAcα, but JA6 mAb had higher affinity to the GalNAcα. Surprisingly, none of the tested mAbs were reactive to GalNAcα linked to the PAA via the O-serine moiety. Perhaps, the free carboxyl group in the conjugate of GalNAcα-O-Ser may play a negative role for binding with the Ab. However, this was consistent with data reporting that the natural Abs from a large cohort of healthy donors also significantly prefer GalNAcα– to its Ser-containing analog immobilized on the printed glycan array (Huflejt et al. 2009). In summary, anti-Tn IgM mAbs preferentially recognized the terminal residue of GalNAcα.
Fig. 2.
(A) The binding of JA1, JA4, JA6 and JA7 mAbs with a panel of Tn-related synthetic saccharides. The 96-well plates were coated with 10 μg/mL of indicated saccharides conjugated with soluble PAA. The binding of mAbs was detected by using HRP-conjugated IgG. (B) Inhibition of the binding of mAb JA1, JA4 and JA6 with soluble GalNAc. The 96-well microplates were coated with 10 μg/mL of GalNAα1-3GalNAcβ-PAA (for JA1 and JA6 binding) or with 10 μg/mL of maxGalNAα-PAA (for JA4 binding). mAbs were mixed with indicated concentrations of free GalNacα prior to pipetting to the wells. The binding of mAbs to saccharides was evaluated as described in (A).
Inhibition of JA1, JA4 and JA6 reactivity with Tn antigen by monovalent GalNAc
In order to confirm the specificity of Ab binding in ELISA and to evaluate the range of its affinities, we used monomeric GalNAcα to inhibit the binding of JA1, JA4 and JA6 mAbs to glycan–PAA conjugates coated on an ELISA plate. Glycans with the highest binding activity to the corresponding mAb were selected to coat the plastic surface and perform the inhibition test. In particular, the inhibition was analyzed for the binding of JA1 and JA6 with GalNAcα1-3GalNAcβ–PAA, whereas JA4 mAbs were tested on maxGalNAcα–PAA. As shown in Figure 2B, IC50 values for the binding of mAbs JA1, JA6 and JA4 were approximately equivalent to 1 mM GalNAcα. These data indicated that binding affinities of anti-Tn IgM Abs to carbohydrate antigens were low, which was consistent with other reports (Brooks et al. 2010).
CD43, CD45 and CD162 are the major proteins carrying Tn antigen on Jurkat cells
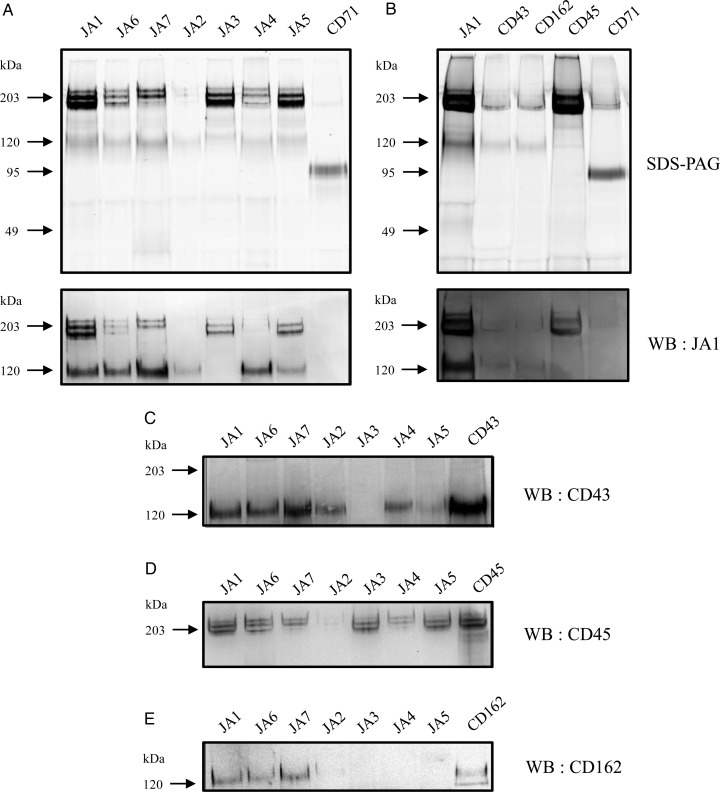
To identify proteins on the surface of Jurkat cells that were recognized by the generated anti-Tn mAbs, we precipitated antigens from the Rhodamine G6 (R6G)-labeled or -unlabeled cell lysates with mAbs and detected them by the fluorescent immunoprecipitation analysis (FIPA) or western blotting (WB), respectively. Immunoprecipitation (IP) of proteins from R6G-labeled Jurkat cell lysates in Triton X-100 solution revealed that all mAbs precipitated generally two groups of antigens, which migrated on the reduced sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) as a single band of 130 kDa and two or three closely related bands with molecular weights of 190–220 kDa (Figure 3A). The differences between anti-Tn mAbs related to the levels of precipitation of indicated bands. JA1, JA5, JA4, JA6 and JA7 mAbs precipitated 190–220 and 130 kDa proteins equally, whereas JA2 mAb predominantly pulled down a 130-kDa protein, and JA3 mAb precipitated exclusively proteins of 190–220 kDa. Likewise, a very similar pattern of protein recognition with mAbs studied was obtained on straight WB (data not shown).
Fig. 3.
Analysis of protein carriers of Tn antigens by IP and WB. Proteins from Jurkat cells labeled with R6G were precipitated with indicated mAbs, separated by 8% SDS–PAGE and visualized by fluorescent scanner (A and B, top panels). The proteins from the gel were transferred to the nitrocellulose membrane and stained with JA1 (A and B, bottom panels), CD43 (C), CD45 (D) or CD162 (E) mAbs.
To identify proteins precipitated with anti-Tn mAbs, two bands of 190–220 and 130 kDa after precipitation with JA7 mAb were excised, trypsinized in-gel and analyzed by mass spectrometry (MS). MS identified one CD45 peptide in heavy bands and a number of peptides in lower bands, which corresponded to the CD43 antigen. Based on the electrophoretic mobility of precipitated antigens, we proposed that the 130-kDa protein may consist of two O-glycosylated antigens, CD43 and CD162, which are expressed on Jurkat cells. The group of proteins with molecular weights of 190–220 kDa can be visibly separated into three distinct bands, among which the bands of 190 and 205 kDa were the most intensive. These bands may represent four isoforms of the CD45 molecule: CD45ABC, CD45AB, CD45BC and CD45B (McKenney et al. 1995; Sgroi et al. 1995), which are also O-glycosylated. The protein with a molecular weight of 220 kDa very likely corresponded to CD45ABC. The isoforms CD45BA and CD45BC have a similar molecular weight and can be visualized as a single band of 205 kDa, while CD45B migrates on a gel as a 190-kDa protein. The isoform CD45R0 is expressed on Jurkat cells at a low level (McKenney et al. 1995) and, therefore, was not detected in WB. To confirm our predictions, the R6G-labeled proteins precipitated with JA1 mAb were ran on the gel in parallel with the previously characterized mAbs anti-CD43 (clone MEM-59), anti-CD45 (clone LT45), anti-CD162 (clone TC2) and anti-CD71 (as control), and the electrophoretic mobility of proteins was compared in FIPA (Figure 3B, top graph). To further identify the proteins precipitated with anti-Tn mAbs, we analyzed the cross-reactivity of anti-Tn mAbs with commercial mAbs. Precipitated proteins from the gel (Figure 3A, top panels) were transferred to the blotting membrane and stained with JA1 mAb (Figure 3A, lower panels), anti-CD43 mAb (Figure 3C), anti-CD45 mAb (Figure 3C) or anti-CD162 mAb (Figure 3C). JA1 mAb, which had the broadest Tn reactivity than all the other Abs, cross-detected all of the precipitated proteins including all three bands (isoforms) of the CD45 antigen (Figure 3A). In our experiments, all anti-Tn mAbs precipitated an antigen of ∼130 kDa, which corresponded to CD43 and/or CD162. IP followed by WB analysis with CD43, CD45 and CD162 mAbs determined that JA1, JA6 and JA7 IgM mAbs had the broad reactivity and recognized all three antigens. In contrast, JA2 exclusively precipitated CD43, JA3 reacted only with CD45 and JA4 and JA5 had the dual reactivity to both CD43 and CD45. However, these mAbs were negative in binding to CD162. CD99, another heavily O-glycosylated protein, which is expressed on the surface of Jurkat cells, was not detected in JA1 immunoprecipitates neither by FIPA nor by WB analysis (data not shown). Thus, using two methods, IP coupled to WB detection and MS identification, we have proved the binding of the new Abs to the CD43, CD45 and CD162 antigens expressed on Jurkat cells.
Analysis of anti-Tn Ab reactivities with glycopeptides from CD43
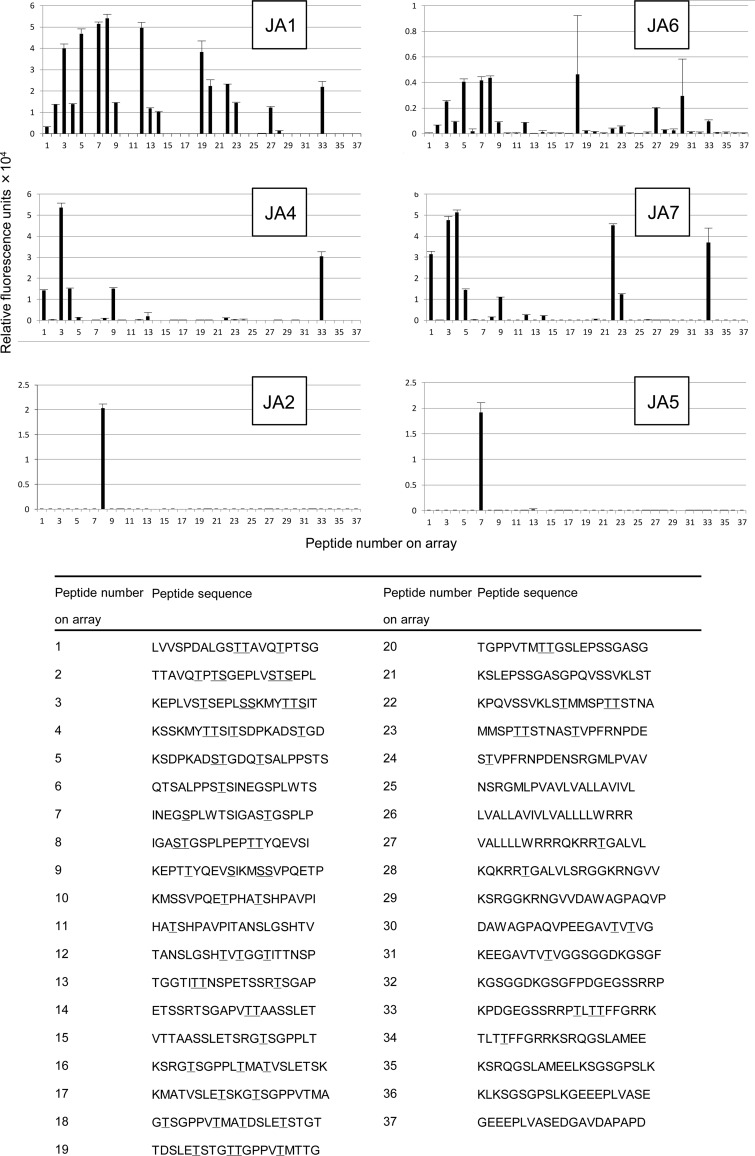
To analyze the binding sites of anti-Tn mAbs in the molecule CD43, we synthesized an array of glycopeptides from the CD43 protein, the major O-glycan carrier of leukocytes. Glycopeptide synthesis with on-chip enrichment was carried out as described previously (Blixt et al. 2010). Every peptide consisted of 20 amino acids in average and overlapped with half of their sequence with the next peptides on the array. All or some of the residues of Ser or Thr in the peptides were modified with GalNAcα. Six peptides from CD43 did not have the sites of O-glycosylation and served as negative controls. All glycopeptides were test-printed and screened for the binding with Vicia villosa lectin B4 (VVL) to verify maximal immobilization on a chip. As shown in Figure 4, all IgM mAbs, i.e. JA1, JA4, JA6 and JA7, displayed a broad reactivity to the CD43 glycopeptides. The binding of anti-Tn IgM mAbs was mostly independent of the peptide sequence adjacent to the modified Ser or Thr. Nevertheless, IgM mAbs seemed to prefer bis-GalNAc peptides in CD43 sequences. For instance, JA4 and JA7 reacted with limited numbers of glycopeptides having only bis-GalNAc. JA1 bound 12 of 14 bis-GalNAc and 3 of 14 single-GalNAc peptides. However, the binding of JA6 with single- and bis-GalNAc peptides was the same. Unlike IgM, IgG mAbs exerted very specific epitope recognition properties. JA2 mAb exclusively bound with the glycopeptide IGASTGSPLPEPTTYQEVSI (glycosylation sites are underlined) and JA5 selectively reacted with the glycopeptide INEGSPLWTSIGASTGSPLP. JA3 did not react with any of 37 arrayed CD43 peptides (data not shown).
Fig. 4.
Binding of anti-Tn mAbs to the CD43 glycopeptide chip. Indicated glycopeptides were spotted on the protein microarray chip. Then, the microchip was covered with one of the indicated mAb. The binding of mAbs to the glycopeptide chip was detected by a secondary anti-mouse IgM-Cy3-labeled Ab and a microarray scanner. Mean values of relative fluorescence for each glycopeptide ± SD in error bars are presented.
Reactivities of anti-Tn mAbs with non-CD43 glycopeptides
To further elucidate the peptide reactivity of anti-Tn mAbs, we evaluated all mAbs on another array of ∼1700 (glyco)peptides from the different O-glycosylated proteins produced in the lab as described (Blixt et al. 2010). The results in Table I demonstrated that the Tn antigen arise from a set of O-linked proteins: mucins, α1-chain from the C region of IgA1, ceruloplasmin, podoplanin, CD46 and some others. Unlike preferential binding of JA1 mAb to the bis-GalNAc glycopeptides from CD43, this mAb reacted with more single GalNAc-modified peptides from non-CD43 proteins. IgG mAbs again demonstrated the high specificity to bind peptides. Thus, JA3 selectively reacted with two peptides: one from MUC24 (CD164) and the other belonged to glycoprotein G-2 from the human herpesvirus 2. The common motifs for these peptides were GSPP or GSPAPP. JA5 additionally bound to the CD43-like peptide recognized glycopeptides from MUC24 (CD164), inhibin β and two peptides from the HSV-2 virus, which had an identical motif GSP. JA2 was very specific for CD43 glycopeptide and none of the other 1700 (glyco)peptides were detected.
Table I.
Reactivity of anti-Tn mAbs with non-CD43 glycopeptides
| mAb | Protein | Glycopeptide |
|---|---|---|
| JA1 | Mucin 1 | VTSAPDTRPAPGSTAPPAHG |
| Mucin-13 | IPIPTAADSESTTNVNSLAT | |
| SESTTNVNSLATSDIITASS | ||
| ITASSPNDGLITMVPSETQS | ||
| NFPETASTTANTPFPTATSP | ||
| TATSPAPPIISTHSSSTIPT | ||
| SPNDGLITMVPSETQSNNEM | ||
| ATNQGNSADAVTTTETATSG | ||
| Mucin-16 | VNGFTHQTSAPNTSTPGTST | |
| SAPNTSTPGTSTVDLGTSGT | ||
| THQTSAPNTSTPGTSTVDLG | ||
| TSTPGTSTVDLGTSGTPSSL | ||
| SPTSAGPLLVPFTLNFTITN | ||
| Mucin-17 | VSTMPVVSSEASTHSTTPVD | |
| Ig α-1 chain C region | VPSTPPTPSPSTPPTPSPSA | |
| Ceruloplasmin | NPQSRSVPPSASHVAPTETF | |
| Receptor-binding cancer antigen expressed on SiSo cells | KLSGDQITLPTTVDYSSVPK | |
| Podoplanin | EGASTGQPEDDTETTGLEGG | |
| VTPGTSEDRYKSGLTTLVAT | ||
| Glycoprotein G-2 (Human herpesvirus 2) | THATPRPTTPGPQTTPPGPA | |
| Receptor tyrosine-protein kinase erbB-2 | TGTDMKLRLPASPETHLDML | |
| LAMP1 protein | PSPTTAPPAPPSPSPSPVPK | |
| JA2 | CD43 | IGASTGSPLPEPTTYQEVSI |
| JA3 | CD164 (MUC24) | PTYEPKTVTTGSPPVPEAHS |
| Glycoprotein G-2 (Human herpesvirus 2) | GPADAPPGSPAPPPPEHRGG | |
| JA4 | Mucin 1 | VTSAPDTRPAPGSTAPPAHG |
| Mucin-13 | AADTTETNFPETASTTANTA | |
| Mucin-16 | LLYSGCRLTLLRPEKNGAAT | |
| Mucin-17 | GTSTVDLGTSGTPSSLPSPT | |
| MUC18 | TTPVDTSTPVTTSTEASSSP | |
| PDSNTTTGLSTSTASPHTRA | ||
| Ig α-1 chain C region | VPSTPPTPSPSTPPTPSPSA | |
| Ceruloplasmin | SASHVAPTETFTYEWTVPKE | |
| R-PTPα | GLICVSANNATTVAPSVGIT | |
| Podoplanin | VTPGTSEDRYKSGLTTLVAT | |
| JA5 | CD43 | INEGSPLWTSIGASTGSPLP |
| Inhibin β | AAGWLGPEAWGSPTPPPTPA | |
| Endolyn CD164 | PTYEPKTVTTGSPPVPEAHS | |
| Glycoprotein G-2 (human herpesvirus 2) | GPADAPPGSPAPPPPEHRGG | |
| Glycoprotein G-2 (human herpesvirus 2) | NPPNSDVVFPGGSPVAQYCY | |
| JA6 | Mucin 1 | VTSAPDTAPAPGSTAPPAHG |
| Mucin-13 | NFPETASTTANTPFPTATSP | |
| Mucin-17 | STTPVDTSTPVTTSTEASSS | |
| CD46 | LPPSSTKPPALSHSVSTSST | |
| STKPPALSHSVSTSSTTKSP | ||
| PKCLKVLPPSSTKPPALSHS | ||
| PPALSHSVSTSSTTKSPASS | ||
| Ceruloplasmin | YYSPNYNPQSRSVPPSASHV | |
| TRAIL receptor 3 | SPGTPAPAAEETMNTSPGTP | |
| Interleukin 6 signal transducer | PEDTASTRSSFTVQDLKPFT | |
| JA7 | Mucin 1 | VTSAPDTAPAPGSTAPPAHG |
| Podoplanin | EGASTGQPEDDTETTGLEGG | |
| DTETTGLEGGVAMPGAEDDV | ||
| CEL protein | RYWTLTYLALPTVTDQEATP |
Amino acid residues modified with GalNAcα are underlined.
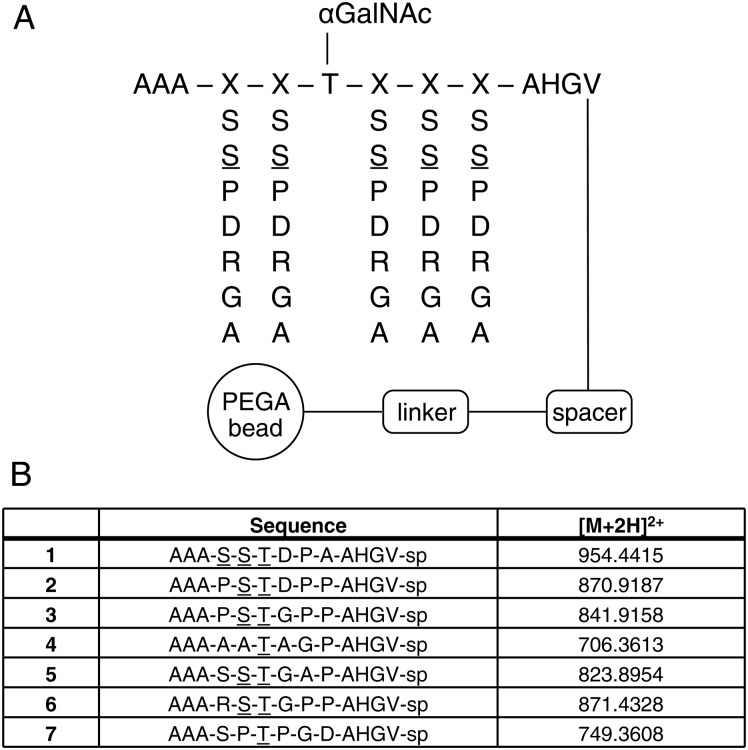
Moreover, we previously developed a one-bead-one-compound (OBOC) random glycopeptide library for screening of mAbs and serum auto-Abs (Kracun et al. 2010). The library contains ∼16,000 unique Tn peptides composed of randomized amino acids as shown in Figure 5. Staining with JA2 mAb at 1:1000 dilution generated seven positive types of beads that were cleaved and sequenced by MS2. All seven glycopeptides identified had more or less sequence similarities to the CD43 glycopeptide IGASTGSPLPEPTTYQEVSI demonstrating highly specific mAb reactivity.
Fig. 5.
Binding of JA2 to an OBOC random Tn-peptide library. (A) Design of the glycopeptide library with randomized amino acids (S, S, P, D, R, G and A) at position denoted as X flanking a fixed GalNAcα-Thr. Underlined (_) amino acids denote a GalNAcα glycosylated sites in figure. (B) Identified JA2-positive Tn-peptide sequences with designated masses.
In summary, analysis of the Ab binding with a glycopeptide microarray revealed a low selectivity of Tn recognition for IgM mAbs and a highly specific recognition of a Tn-peptide motif by IgG mAbs.
Reactivities of anti-Tn mAbs with mutated MUC1 tandem-repeat glycopeptides
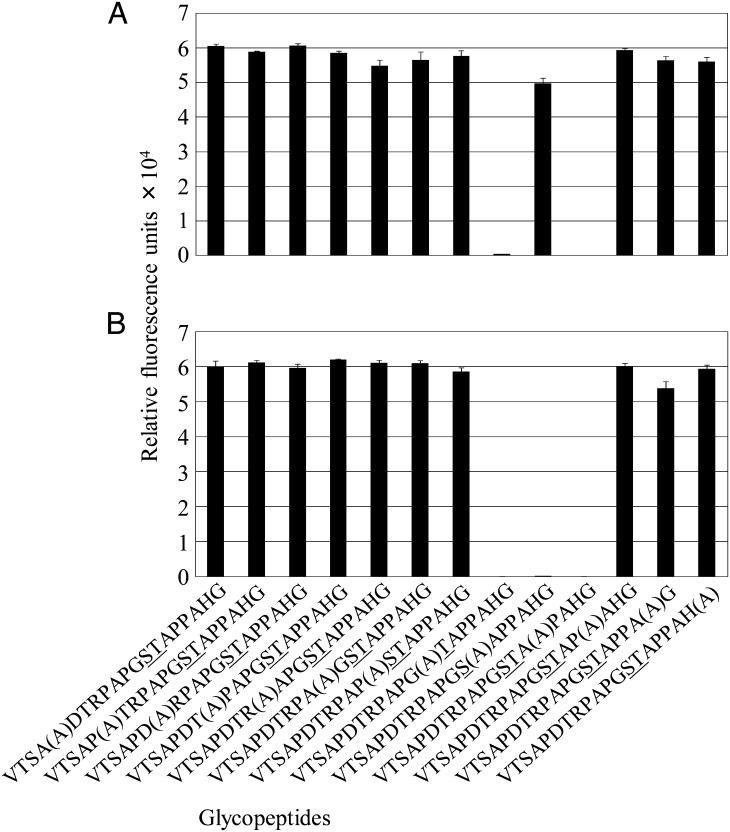
To elucidate whether some amino acid residues have influenced the binding activity of IgM anti-Tn mAbs, we exploited the method of the alanine-mutation walk. For the mutation, we selected glycopeptide representing MUC1 tandem repeats, since all generated IgM mAbs reacted with it. The alanine-mutation walk analysis showed that JA1 binding was abrogated, when GalNAc-Ser, but not GalNAc-Thr was mutated to alanine (Figure 6A). Similar results were obtained for JA6 reactivity (data not shown). In contrast, the binding capacities of mAbs JA7 (Figure 6B) and JA4 (data not shown) were sensitive to the substitution of both Ser and Thr to Ala in the GalNAc peptide. Interestingly, Pro residue at the +3 position turned out to be a critical for binding of all tested Abs with glycopeptides. All the other amino acid residue mutations had no influence on epitope formation and mAb recognition. In summary, the Ser, Thr and Pro residues in the STAP motif of the MUC1 peptide are important for anti-Tn mAb binding.
Fig. 6.
Binding of JA1 (A) and JA7 (B) to an MUC1 alanine mutation scan glycopeptide chip. Indicated glycopeptides were spotted on the protein microarray chip. Then, the chip was covered with one of the mAbs. The binding of mAbs with a glycopeptide chip was detected by a secondary anti-mouse IgM-Cy3-labeled Ab and a microarray reader. Mean values of relative fluorescence ± standard deviation of mean (SEM) were measured as represented bars for each glycopeptide. A in parentheses indicates alanine mutation, and underlined (_) amino acids indicate GalNAcα glycosylated sites.
Discussion
One of the most important tools in studies of the Tn antigen represents mAbs. Different laboratories generated a number of different mAbs designated as “anti-Tn” (Hirohashi et al. 1985; Takahashi et al. 1988; Numata et al. 1990; Avichezer et al. 1997; Oppezzo et al. 2000; Kannagi and Hakomori 2001; Ando et al. 2008; Welinder et al. 2011). At the 7th Conference on Human Leucocyte Differentiation Antigens, these Abs were assigned as CD175 (Karsten 2002; Cao et al. 2008). Obviously, CD175 is not homogenous, because it combines a group of mAbs that differ by their fine specificity toward the both carbohydrate and peptide moieties of the Tn antigen (O'Boyle et al. 1996; Reis et al. 1998) and by the ability to recognize antigenic clusters of adjacent or spatially separated O-glycans (Shimizu and Shaw 1993). As a consequence of complexity of the Tn antigen and the diversity of mAb specificity, the levels of expression and the distribution of the Tn antigen on the surface of cell targets reported by the different laboratories have been found to be controversial (Li et al. 2009). To describe the antigenic determinants, which are important for the reactivity of CD175 mAbs with the Tn antigen expressed on lymphoid cells, we established a panel of anti-Tn mAbs and studied their fine specificity. The reactivities of Abs were evaluated by using a set of different targets: Tn-positive natural proteins, synthetic conjugates of GalNAcα or other Tn-related saccharides with non-peptide polymers or peptide oligomers from CD43, MUC1 and other O-glycosylated proteins. The analysis of reactivities of generated mAbs suggested that the Tn antigen determinants are really diverse.
The ability of an anti-Tn mAb to recognize a peptide motif determines its strong specificity toward a certain O-glycosylated protein and, consequently, defines the value of the Ab for cancer diagnostic and therapy. Among all of the generated anti-Tn mAbs, three IgG1 potentially met such requirements. They neither bound conjugates of Tn and Tn-related saccharides with PAA nor most of the glycopeptides tested. However, they exerted specificity to recognize the Tn antigen in a certain peptide motif and bind proteins containing this motif. Testing of mAbs on a microarray display of 1700 glycopeptides derived from different O-glycosylated proteins showed that JA3 mAb reacted only with two glycopeptides containing similar motifs GSPP or GSPAPP. JA5 mAb specifically recognized the GSP motif in five independent experiments. JA2 exclusively bound glycopeptide from CD43 (IGASTGSPLPEPTTYQEVSI), which had two double and quite different clusters of glycosylation. The binding experiment to a randomized Tn-peptide library further suggested that the major recognition motif for anti-Tn IgGs was the ASTGSP sequence. The similar high-protein selectivity of some anti-Tn Abs has been reported before. For example, PMH1 (IgM) mAb recognized GalNAc only in the MUC2 glycopeptide (Reis et al. 1998), mAb SM3 (IgG1) selectively recognized the PDTR motif (Dokurno et al. 1998; Moller et al. 2002) and mAbs 5E5 and 2D9 (both IgG1) (Tarp et al. 2007) reacted specifically to GSTAPP and GSTAPP motifs, respectively (Blixt et al. 2010) both from the MUC1 tandem-repeat domain. Anti-Tn mAb 237 (IgG2a) specific for the motif GTKPP from podoplanin was reported by Brooks et al. (2010). Thus, we have supplemented a list of peptide-specific anti-Tn mAbs and reported new IgG1 mAbs specifically recognizing cancer-associated truncated (i.e. Tn-positive) glycoforms of CD43 and CD45 with no cross-reactivity toward the normally glycosylated proteins. As previously shown by NMR, O-glycosylated peptides have specific open-rigid-like structure of peptide backbone, which is predominantly sustained by the first residue of GalNAcα-O- linked to Thr/Ser and retained after O-glycan elongation (Coltart et al. 2002). Hence, it is logical to propose that anti-Tn IgG mAbs can recognize such structures without the immediate binding to GalNAc moiety. In this case, “anti-peptide” mAbs have to bind proteins from cells with normal O-glycan extension, such as Jurkat-wtCosmc cells. However, in our study, none of IgG mAbs stained these cells. This result suggests that GalNAcα moiety is likely included in the recognition epitope of JA2, JA3 and JA5 mAbs, However, the final conclusion about the specificity of the anti-Tn IgG mAbs can be made by the X-ray analysis of antigen–Ab crystal structures, as was demonstrated for anti-Tn mAb 237 (Brooks et al. 2010).
Unlike IgG mAbs, the binding of JA1, JA4 and JA6 mAbs to the Tn and Tn-related saccharides conjugated with PAA indicated that IgM mAbs may recognize the Tn antigen independently of the polypeptide sequence. However, testing them on glycans conjugated with a PAA polymer at the different densities revealed that JA4 reactivity was dependent on the formation of Tn clusters, but the binding of JA1 and JA6 were obviously cluster-independent. These data consisted with the other results obtained for these mAbs on the alanine-scanning mutation array. CD43 and non-CD43 glycopeptide microarrays did not confirm the Tn-cluster-dependent binding of IgM mAbs, except of JA7, which did preferentially bound targets with tandem GalNAc. Traditionally, the capacity of anti-Tn Abs to bind glycopeptides with two or three consecutive GalNAc moieties explained by the recognition of several Tn units as a single cluster, so-called “minimum recognized structure” (Nakada, Inoue, et al. 1991; Nakada, Numata, et al. 1991; Nakada et al. 1993; Inoue et al. 1994). However, there can be a different interpretation of such an observation. It is known that the clusterization of Tn units renders the polypeptide chain to a rigid and elongated conformation (Live et al. 1999) and results into the maximal exposure of GalNAc (Coltart et al. 2002). Thus, the anti-Tn mAb may directly bind with a single residue of GalNAc, and the other residues in the carbohydrate cluster would just be necessary for the maintaining of an open rigid-like structure of the protein carrier. In accordance with this model, VVL can bind glycopeptides via single GalNAc, based on the X-ray structure of VVL (Babino et al. 2003), but nevertheless the affinity of its binding was demonstrated to depend on Tn cluster formation (Kato et al. 2008). Interestingly, none of the Abs reacted with Tn-spacered antigens monovalently immobilized onto a glycan microarray (Blixt et al. 2004), but reacted with Tn-spacered antigens conjugated to PAA further supporting the cluster effect.
All generated IgM mAbs were characterized by a myriad of glycopeptides, which they reacted with. Although the peptide sequence had the least effect on IgM binding, the MUC1 tandem-repeat glycopeptide array showed the role of Pro at the +3 position relative to Ser-O-GalNAc. The molecular modeling indicates that O-3 in O-GalNAc can potentially form a hydrogen bond with the NH group of the amino acid residue at the +3 position (Butenhof and Gerken 1993). That may impede the Tn epitope exposure for recognition by the mAb, but not in the case of Pro, which does not have such a group to make a hydrogen bond. In addition to Pro at +3, scanning mutation showed that JA1 and JA6 bound single GalNAc coupled with Ser, but not with the Thr residue. Based on these data, we propose that the SXXP motif which is broadly represented in proteins CD43 and CD162 and all isoforms of CD45 can serve as a more favorable recognition structure for JA1 and JA6 mAbs. These results together with data showing that JA1, JA4 and JA6 bound GalNAc conjugated to PAA suggested that it is unlikely that peptide motifs create a Tn recognition epitope for IgM mAbs, but rather interfere with the Ab in GalNAc binding.
As the leukemic Jurkat cells are deficient in T-synthase activity, they are expected to carry the Tn antigen on all O-glycosylated proteins expressed on the cell surface. The Tn antigen on the Jurkat cells was reported previously to be associated largely with the CD43 antigen (Ando et al. 2008). We have confirmed the reactivity of our anti-Tn mAbs with CD43 and, for the first time, demonstrated the presence of the Tn antigen on CD45 and CD162. All listed antigens belong to mucin-like glycoproteins. Mucins are characterized by the high frequency of Thr, Ser and Pro in the polypeptide sequence, which are mostly O-glycan modified and have a rod-like shape of the external domain (Shimizu and Shaw 1993). Another O-glycosylated protein expressed on Jurkat cells, CD99, was not detected in precipitates with any of our mAbs. Unfortunately, structural data for CD99 are currently not available and this antigen does not share any similarity with known groups of proteins (Kreppel et al. 2005). The sequence of the CD99 extracellular domain contains only a single residue of Thr, which is potentially O-glycosylated. Perhaps, a single site of O-glycosylation in the CD99 protein cannot unfold this molecule in a rod-like shape and leaves the Tn epitope masked.
In summary, we generated and characterized seven mAbs specific for the Tn antigen, associated with carrier proteins CD43, CD45 and CD162 (Table II). Three anti-Tn IgG mAbs selectively recognized specific peptide sequences from CD43 and CD45, which virtually represent tumor-associated glycopeptide antigens. Consequently, these newly generated mAbs may not only extend the studies of Tn antigenicity, but more importantly be useful in diagnostic and Ab therapy of hematopoietic cancers.
Table II.
Summary datasheet of anti-Tn mAb reactivities
| mAb | GalNAcα-PAA |
O-glycosylated proteins of Jurkat cells |
Specificity | ||||
|---|---|---|---|---|---|---|---|
| CD43 | CD45ABC gp220 | CD45AB/BC gp205 | CD45B gp190 | CD162 | |||
| JA1 | + | + | + | + | + | + | Terminal GalNAcα1 |
| JA2 | − | + | − | − | − | − | ASTGSP |
| JA3 | − | − | + | + | + | − | GSPP or GSPAPP |
| JA4 | + | + | + | + | − | − | GalNAcα1 and GalNAcβ1 |
| JA5 | − | + | + | + | + | − | GSP |
| JA6 | + | + | + | + | + | + | Terminal GalNAcα1 |
| JA7 | − | + | + | + | − | + | GalNAcα-O-Ser/Thr |
Underlined are the residues of amino acids, which were linked to GalNAcα monosaccharide.
Materials and methods
Cells and mAbs
Human cell lines (Jurkat, CEM, HUT-78, MOLT-4, MT-2, Raji, Daudi, IM-9, Ramos, NALM-6, K-562, U-937, THP-1, HeLa, MCF7 and 293T) were purchased from ATCC (Manassas, VA). All cell lines except HeLa, MCF7 and 293T were maintained in the RPMI 1640 medium supplemented with 10% fetal calf serum. Cell lines HeLa, MCF7 and 293T were cultured in modified Dulbecco's modified eagles medium (DMEM) supplemented with 10% fetal calf serum. Peripheral blood mononuclear cells of healthy donors were isolated by a Ficoll density gradient centrifugation. The Abs LT45 (IgG2a, anti-CD45), H7 (IgG1, anti-CD45), 4E3 (IgG2b, anti-CD71) and TC2 (IgG2a, anti-CD162) were produced in our laboratory earlier (Filatov et al. 2007). The Ab MEM-59 (IgG1, anti-CD43) was purchased from Exbio (Czech Republic).
Generation of mAbs
BALB/c mice were injected intraperitoneally three times with 107 Jurkat cells. Spleen cells were fused with Sp2/0 mouse myeloma cells according to the standard techniques and pipetted into 96-well tissue culture plates. After 2 weeks, hybridoma culture supernatants were harvested and tested for staining of Jurkat cells by indirect immunofluorescence using a FACScan flow cytometer (BD Biosciences, San Diego, CA). The positive cultures were cloned.
Immunofluorescence staining and flow cytometry
For immunofluorescent staining, a pellet containing 1 × 5·105 cells was mixed with 25 μL of unlabeled primary Abs and incubated at 4°C for 30 min. The cells were washed twice with phosphate-buffered saline (PBS) and precipitated by centrifugation at 320 × g for 5 min. Then, the cells were incubated with fluorescein isothiocyanate (FITC)-labeled secondary Abs, i.e. F(ab′)2 fragments of sheep Abs to mouse Ig (Sigma, St. Louis, MO), or goat anti-mouse PE-labeled Ab (Santa Cruz Biotechnology, Santa Cruz, CA) and washed subsequently. The stained cells were analyzed using FACScan flow cytometer (BD Biosciences). The levels of fluorescence were measured and expressed as a mean intensity of fluorescence (MFI). The cells treated only with the secondary Abs were used as a negative control. In some experiments, we used cells fixed with 4% formaldehyde before immunofluorescent staining.
Plasmids and transfections
The lentiviral expression plasmid pUCHR Cosmc IRES GFP encoding human cosmc gene was constructed by subcloning of C1GALT1C1 (Cosmc) ORF from pCMV SPORT6 C1GALT1C1 plasmid (Open Biosystem, Lafayette, CO) into pUCHR IRES GFP bicistronic vector (Mazurov et al. 2010) using EcoRI/XmaI sites of restriction. To generate virus like particles (VLPs), human kidney cells 293T were co-transfected with the Cosmc vector, HIV-1 packaging plasmid pCMVΔ8.2R (Naldini et al. 1996) and pCMV VSV-G plasmid encoding protein G from vesicular stomatitis. Transfection was carried out with the LF2000 transfection reagent (Invitrogen, Carlsbad, CA) at a plasmid ratio 6:4:1, respectively. Forty-eight hours later undiluted supernatants containing VLPs were used to infect Jurkat cells. Cells stably expressing wtCosmc were then enriched by using FACSAria sorting instrument (BD Biosciences) and directly utilized in experiments.
Periodate oxidation
Jurkat cells fixed in 4% paraformaldehyde were treated with 5 mM sodium m-periodate in 50 mM sodium acetate buffer (pH 4.5) containing 100 mM NaCl for 30 min. Aldehyde groups generated by periodate oxidation were reduced with 50 mM sodium borohydride in PBS for 30 min (Woodward et al. 1985). All treatments were performed at room temperature in the dark with solutions made ex tempore.
Enzyme-linked immunosorbent assay
The Ab reactivity against synthetic saccharides was evaluated by using ELISA. Microplates (MaxiSorp, Nunc, Roskilde, Denmark) were coated with 10 μg/mL of conjugate of saccharides with PAA in 50 mM NaHCO3 buffer (pH 9.6) overnight, at 4°C. After extensive washing with PBS, wells were blocked with 100 μL of 1% BSA in PBS for 1 h. Then, mAbs (hybridoma supernatants) were pipetted into the microplate and incubated for 1 h at room temperature. After washing with PBS-Tween (PBST), the horseradish peroxidase (HRP) conjugate of goat anti-mouse IgG (Bio-Rad, Hercules, CA; diluted 1:3000) was added to the wells for 1 h. The microplate was washed 3–4 times with PBST. The reaction was developed by applying the tetramethylbenzidine substrate for 20–30 min and stopped with 5% sulfuric acid. The resulting color development was detected at 490 nm using Multiskan MCC/340 MK II (Titertek, Flow Laboratories, McLean, VA). To evaluate the inhibitory effect of soluble GalNAc on Ab–antigen binding, the primary mAbs were mixed, first, with GalNAc solubilized in PBS at the different concentrations and then pipetted into the microplate.
Fluorescent IP analysis
The labeling of cells with amino reactive fluorescent dye R6G (Sintol) was performed as described previously (Filatov et al. 2007). Briefly, 4 × 107 cells were washed with PBS (pH 7.4) three times and resuspended in 1 mL of PBS (pH adjusted to 8.0). Then, 50 μL of R6G succinimide ester stock solution in dimethyl sulfoxide (DMSO) (3 mg/mL) was added to the cells twice with 10 min interval. The reaction was stopped with 10 mM glycine in PBS (pH 7.4) followed by washing of cells with ice-cold PBS three times. Cells were lysed in 1 mL of buffer containing 20 mM Tris–HCl (pH 8.0), 1% Triton X-100, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA) and 1 mM phenylmethylsulfonyl fluoride (PMSF) for 30 min at 4°C.
Cell debris was pelleted at 20,000 × g for 10 min at 4°C. The lysates were cleared by rotation with normal mouse IgG covalently linked to CNBr-Sepharose at 4°C for 1 h and then with Protein A agarose beads (Pierce, Rockford, IL) loaded with normal mouse IgG for another 1 h. Precipitations of precleared lysates with specific Abs were carried out by using Protein A agarose beads preloaded with a rabbit anti-mouse serum first and then with the mouse mAb. Samples were precipitated under rotation overnight at 4°C. Afterwards, the beads were washed four times in the lysis buffer. The proteins were eluted by heating beads in the SDS sample buffer at 80°C for 5 min and separated by 8% PAGE under reducing conditions. After electrophoresis, the proteins on a gel were visualized using Molecular Imager FX Pro fluorescence scanner (Bio-Rad).
Immunoblotting
Proteins from the gel were transferred to the nitrocellulose blotting membrane using Mini Trans-Blot apparatus (Bio-Rad) according to the manufacturer's instruction. The blotting membranes were blocked with 5% (w/v) dry non-fat milk in PBS containing 0.1% Tween 20 (PBST) for 1 h and then stained with primary Abs for 1–2 h in milk PBST. The membranes were washed three times with PBST and probed with the secondary HRP-conjugated anti-mouse Abs (Bio-Rad). Blots were washed again with PBST three times and immunoreactive bands were detected with Immobilon™ Western ECL reagent (Millipore) on Molecular Imager ChemiDoc XRS instrument (Bio-Rad).
Glycopeptide array
Most peptides and Tn-peptide microarrays were synthesized as described previously (Blixt et al. 2010), and some were purchased from Schafer-N (Copenhagen, Denmark). Up to 1700 peptides and glycopeptides were available for the study. The sites of glycosylation were confirmed by MS analysis. For some peptides, an additional N-terminal lysine residue was introduced to improve its conjugation to the slide. Printing of the microarray slides was performed using a BioRobotics MicroGrid II spotter (Genomics Solution) with a 0.21-mm pitch using Stealth 3B Micro Spotting Pins (Telechem International ArrayIt Division). Scanning of the slides was performed on a ProScanArray HT Microarray Scanner (PerkinElmer) followed by image analysis with ProScanArray Express 4.0 software (PerkinElmer). Data were analyzed and plotted using Microsoft® Excel or GraphPad Prism software.
After printing, the microarrays were blocked with the blocking buffer (50 mM ethanolamine, 0.05 M Na2B4O7, pH 8.5) for 1 h at room temperature. The slides were then washed three times with PBST (0.5%). The slides were stained with the JA mAbs at a 1:1000 dilution in staining buffer for 1 h at room temperature without shaking. After washing the slides three times with PBST (0.5%), they were stained with anti-mouse-IgG-Cy3 at a 1:1000 dilution in staining buffer for 1 h at room temperature. The slides were then washed three times with PBST (0.5%) spun dry and scanned.
Glycopeptide OBOC library
From the random Tn-glycopeptide bead library, synthesized as described previously, an aliquote (MeOH, 3 mL bead slurry) of ∼30,000 beads were taken and washed with water (10 volumes). Beads were stained with the JA2 Ab diluted 1:1000 with PBS staining buffer (0.5 M NaCl, 3 mM KCl, 1.5 mM KH2PO4, 6.5 mM Na2HPO4, 1% BSA, 1% Triton X-100, pH 7.4) for 1 h with shaking at room temperature. The beads were then washed three times with PBST (0.5%) and stained with a secondary Ab (anti-mouse-IgG-AP) at a 1:500 dilution in PBS-staining buffer for 1 h with shaking at room temperature. After additional washing [PBST (0.5%)], ready-to-use BCIP/NBT substrate for alkaline phosphatase (10 mL) was added and the suspension was incubated at room temperature for 15 min. After color developed, the reaction was stopped by washing the beads with MeOH 2 times. PBST (0.5%) was added and beads were transferred to a plastic Petri dish and the positively stained beads were picked out by hand using a low-volume pipette. To the selected beads, 50 μL of a reduction cocktail [6.6 mM NaBH4 and 6 mM I2 in tetrahydrofuran (THF)] was added and incubated for 20 min without shaking at room temperature. The reduction cocktail was removed and 5 μL of the cleavage cocktail were added trifluoroacetic acid (TFA:TES:water = 95:2:3) and incubated for 30 min after which the liquid was left to evaporate overnight.
Electrospray-ionization MS was performed on a linear ion trap-Orbitrap hybrid instrument (LTQ-Orbitrap XL, Thermo-Scientific, Bremen, Germany) equipped for multistage fragmentation (MSn) via conventional collision-induced dissociation (CID) higher energy CID in an external octopole collision cell and electron-transfer dissociation using fluoranthene anions generated in an external chemical ionization source, with the capability of supplemental activation in the LTQ ion trap. The instrument was controlled using Thermo LTQ Orbitrap XL Tune Plus 2.5.5 (Thermo Fischer Scientific, Waltham, MA). Acquired spectra were processed and analyzed using Xcalibur Qual Browser 2.0.7 (Thermo Fischer Scientific). The samples were analyzed as described previously (Kracun et al. 2010).
Funding
This research was supported by Russian Foundation for Basic Research (project No. 09-04-00318); the grant of Presidium of Russian Academy of Sciences “Molecular and Cell Biology”; the Carlsberg Foundation; the Benzon Foundation; the Velux Foundation; the Danish Research Council; the Danish Agency for Science; Technology and Innovation (FTP); NIH PO1 CA052477 (NIH 1U01CA128437-01); EU FP7-HEALTH-2007-A 201381, EU FP7/2007-2013-EuroGlycoArrays 215536, and University of Copenhagen Programme of Excellence.
Conflict of interest
None declared.
Abbreviations
BSA, bovine serum albumin; CID, collision-induced dissociation; DMEM, Dulbecco's modified eagles medium; DMSO, dimethyl sulfoxide; ELISA, enzyme-linked immunosorbent assay; FACS, flow cytometry; FIPA, fluorescent immunoprecipitation analysis; FITC, fluorescein isothiocyanate; Fs, Forssman; GalNAc, N-acetylgalactosamine; HRP, horseradish peroxidase; GFP, green fluorescent protein; HPLC, high-performance liquid chromatography; IP, immunoprecipitation; mAb, monoclonal antibody; MFI, mean intensity of fluorescence; MS, mass spectrometry; OBOC, one-bead-one-compound; PAA, polyacrylamide; PAGE, polyacrylamide gel electrophoresis; PBS, phosphate-buffered saline; PBST, PBS-Tween; R6G, 5-(and-6)-carboxyrhodamine 6G succinimidyl ester; SDS, sodium dodecyl sulfate; Ser, serine; TES, N-Tris(hydroxymethyl)methyl-2-aminoethane sulfonic acid); TF, Thomsen–Friedenreich; TFA, trifluoroacetic acid; THF, tetrahydrofuran; Thr, threonine; Tn, GalNAca1-O-Ser/Thr; VVL, Vicia villosa lectin; WB, western blotting; wt, wild type.
References
- Akita K, Fushiki S, Fujimoto T, Munesue S, Inoue M, Oguri K, Okayama M, Yamashina I, Nakada H. Identification of the core protein carrying the Tn antigen in mouse brain: Specific expression on syndecan-3. Cell Struct Funct. 2001;26:271–278. doi: 10.1247/csf.26.271. doi:10.1247/csf.26.271. [DOI] [PubMed] [Google Scholar]
- Aller CT, Kucuk O, Springer GF, Gilman-Sachs A. Flow cytometric analysis of T and Tn epitopes on chronic lymphocytic leukemia cells. Am J Hematol. 1996;52:29–38. doi: 10.1002/(SICI)1096-8652(199605)52:1<29::AID-AJH5>3.0.CO;2-8. doi:10.1002/(SICI)1096-8652(199605)52:1<29::AID-AJH5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ando H, Matsushita T, Wakitani M, Sato T, Kodama-Nishida S, Shibata K, Shitara K, Ohta S. Mouse-human chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat cells in vitro and in vivo. Biol Pharm Bull. 2008;31:1739–1744. doi: 10.1248/bpb.31.1739. doi:10.1248/bpb.31.1739. [DOI] [PubMed] [Google Scholar]
- Aussel C, Mahmoudi AH, Bernard G, Breittmayer JP, Bernard A. Sphingosine, oleylamine and stearylamine inhibit both CD11a/CD18-dependent and -independent homotypic aggregation: Demonstration by cytofluorimetry. Immunol Lett. 1995;47:175–180. doi: 10.1016/0165-2478(95)00073-3. doi:10.1016/0165-2478(95)00073-3. [DOI] [PubMed] [Google Scholar]
- Avichezer D, Springer GF, Schechter B, Arnon R. Immunoreactivities of polyclonal and monoclonal anti-T and anti-Tn antibodies with human carcinoma cells, grown in vitro and in a xenograft model. Int J Cancer. 1997;72:119–127. doi: 10.1002/(sici)1097-0215(19970703)72:1<119::aid-ijc17>3.0.co;2-e. doi:10.1002/(SICI)1097-0215(19970703)72:1<119::AID-IJC17>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Babino A, Tello D, Rojas A, Bay S, Osinaga E, Alzari PM. The crystal structure of a plant lectin in complex with the Tn antigen. FEBS Lett. 2003;536:106–110. doi: 10.1016/s0014-5793(03)00037-1. doi:10.1016/S0014-5793(03)00037-1. [DOI] [PubMed] [Google Scholar]
- Berger EG. Tn-syndrome. Biochim Biophys Acta. 1999;1455:255–268. doi: 10.1016/s0925-4439(99)00069-1. [DOI] [PubMed] [Google Scholar]
- Blixt O, Clo E, Nudelman AS, Sorensen KK, Clausen T, Wandall HH, Livingston PO, Clausen H, Jensen KJ. A high-throughput O-glycopeptide discovery platform for seromic profiling. J Proteome Res. 2010;9:5250–5261. doi: 10.1021/pr1005229. doi:10.1021/pr1005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. doi:10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CL, Schietinger A, Borisova SN, Kufer P, Okon M, Hirama T, Mackenzie CR, Wang LX, Schreiber H, Evans SV. Antibody recognition of a unique tumor-specific glycopeptide antigen. Proc Natl Acad Sci USA. 2010;107:10056–10061. doi: 10.1073/pnas.0915176107. doi:10.1073/pnas.0915176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, Shuford WW, Wang WC, Nadler SG, Bailey TS, Marquardt H, Mittler RS. Characterization of a CD43/leukosialin-mediated pathway for inducing apoptosis in human T-lymphoblastoid cells. J Biol Chem. 1996;271:27686–27695. doi: 10.1074/jbc.271.44.27686. doi:10.1074/jbc.271.44.27686. [DOI] [PubMed] [Google Scholar]
- Butenhof KJ, Gerken TA. Structure and dynamics of mucin-like glycopeptides. Examination of peptide chain expansion and peptide-carbohydrate interactions by stochastic dynamics simulations. Biochemistry. 1993;32:2650–2663. doi: 10.1021/bi00061a025. doi:10.1021/bi00061a025. [DOI] [PubMed] [Google Scholar]
- Cao Y, Merling A, Karsten U, Goletz S, Punzel M, Kraft R, Butschak G, Schwartz-Albiez R. Expression of CD175 (Tn), CD175s (sialosyl-Tn) and CD176 (Thomsen-Friedenreich antigen) on malignant human hematopoietic cells. Int J Cancer. 2008;123:89–99. doi: 10.1002/ijc.23493. doi:10.1002/ijc.23493. [DOI] [PubMed] [Google Scholar]
- Coltart DM, Royyuru AK, Williams LJ, Glunz PW, Sames D, Kuduk SD, Schwarz JB, Chen XT, Danishefsky SJ, Live DH. Principles of mucin architecture: Structural studies on synthetic glycopeptides bearing clustered mono-, di-, tri-, and hexasaccharide glycodomains. J Am Chem Soc. 2002;124:9833–9844. doi: 10.1021/ja020208f. doi:10.1021/ja020208f. [DOI] [PubMed] [Google Scholar]
- Danishefsky SJ, Allen JR. From the laboratory to the clinic: A retrospective on fully synthetic carbohydrate-based anticancer vaccines frequently used abbreviations are listed in the appendix. Angew Chem Int Ed Engl. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. doi:10.1002/(SICI)1521-3773(20000303)39:5<836::AID-ANIE836>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Danussi C, Coslovi A, Campa C, Mucignat MT, Spessotto P, Uggeri F, Paoletti S, Colombatti A. A newly generated functional antibody identifies Tn antigen as a novel determinant in the cancer cell-lymphatic endothelium interaction. Glycobiology. 2009;19:1056–1067. doi: 10.1093/glycob/cwp085. doi:10.1093/glycob/cwp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J, Moullec J, Bernard J. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (anti-Tn) Blood. 1959;14:1079–1093. [PubMed] [Google Scholar]
- Dokurno P, Bates PA, Band HA, Stewart LM, Lally JM, Burchell JM, Taylor-Papadimitriou J, Snary D, Sternberg MJ, Freemont PS. Crystal structure at 1.95 A resolution of the breast tumour-specific antibody SM3 complexed with its peptide epitope reveals novel hypervariable loop recognition. J Mol Biol. 1998;284:713–728. doi: 10.1006/jmbi.1998.2209. doi:10.1006/jmbi.1998.2209. [DOI] [PubMed] [Google Scholar]
- Filatov AV, Krotov GI, Zgoda VG, Volkov Y. Fluorescent immunoprecipitation analysis of cell surface proteins: A methodology compatible with mass-spectrometry. J Immunol Methods. 2007;319:21–33. doi: 10.1016/j.jim.2006.09.014. doi:10.1016/j.jim.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Glinsky VV, Huflejt ME, Glinsky GV, Deutscher SL, Quinn TP. Effects of Thomsen-Friedenreich antigen-specific peptide P-30 on beta-galactoside-mediated homotypic aggregation and adhesion to the endothelium of MDA-MB-435 human breast carcinoma cells. Cancer Res. 2000;60:2584–2588. [PubMed] [Google Scholar]
- Hirohashi S, Clausen H, Yamada T, Shimosato Y, Hakomori S. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC-LU-35 and -81 expressed in cancer of blood group O or B individuals: Its identification as Tn antigen. Proc Natl Acad Sci USA. 1985;82:7039–7043. doi: 10.1073/pnas.82.20.7039. doi:10.1073/pnas.82.20.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huflejt ME, Vuskovic M, Vasiliu D, Xu H, Obukhova P, Shilova N, Tuzikov A, Galanina O, Arun B, Lu K, et al. Anti-carbohydrate antibodies of normal sera: Findings, surprises and challenges. Mol Immunol. 2009;46:3037–3049. doi: 10.1016/j.molimm.2009.06.010. doi:10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nakada H, Tanaka N, Yamashina I. Tn antigen is expressed on leukosialin from T-lymphoid cells. Cancer Res. 1994;54:85–88. [PubMed] [Google Scholar]
- Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE, et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 2008;68:1636–1646. doi: 10.1158/0008-5472.CAN-07-2345. doi:10.1158/0008-5472.CAN-07-2345. [DOI] [PubMed] [Google Scholar]
- Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 2011;50:1770–1791. doi: 10.1002/anie.201002313. doi:10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R, Hakomori S. A guide to monoclonal antibodies directed to glycotopes. Adv Exp Med Biol. 2001;491:587–630. doi: 10.1007/978-1-4615-1267-7_38. doi:10.1007/978-1-4615-1267-7_38. [DOI] [PubMed] [Google Scholar]
- Karsten U. CD175 Workshop Panel report. In: Mason D, Andre P, Bensussan A, Buckley C, Civin C, Clark E, de Haas M, Goyert S, Hadam M, Hart D, et al., editors. Leucocyte Typing VII, White Cell Differentiation Antigens. Oxford (UK): Oxford University Press; 2002. pp. 201–202. [Google Scholar]
- Kato K, Takeuchi H, Ohki T, Waki M, Usami K, Hassan H, Clausen H, Irimura T. A lectin recognizes differential arrangements of O-glycans on mucin repeats. Biochem Biophys Res Commun. 2008;371:698–701. doi: 10.1016/j.bbrc.2008.04.120. doi:10.1016/j.bbrc.2008.04.120. [DOI] [PubMed] [Google Scholar]
- Kishikawa T, Ghazizadeh M, Sasaki Y, Springer GF. Specific role of T and Tn tumor-associated antigens in adhesion between a human breast carcinoma cell line and a normal human breast epithelial cell line. Jpn J Cancer Res. 1999;90:326–332. doi: 10.1111/j.1349-7006.1999.tb00751.x. doi:10.1111/j.1349-7006.1999.tb00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracun SK, Clo E, Clausen H, Levery SB, Jensen KJ, Blixt O. Random glycopeptide bead libraries for seromic biomarker discovery. J Proteome Res. 2010;9:6705–6714. doi: 10.1021/pr1008477. doi:10.1021/pr1008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppel F, Gackowski J, Schmidt E, Kochanek S. Combined genetic and chemical capsid modifications enable flexible and efficient de- and retargeting of adenovirus vectors. Mol Ther. 2005;12:107–117. doi: 10.1016/j.ymthe.2005.03.006. doi:10.1016/j.ymthe.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lawrie CH, Marafioti T, Hatton CS, Dirnhofer S, Roncador G, Went P, Tzankov A, Pileri SA, Pulford K, Banham AH. Cancer-associated carbohydrate identification in Hodgkin's lymphoma by carbohydrate array profiling. Int J Cancer. 2006;118:3161–3166. doi: 10.1002/ijc.21762. doi:10.1002/ijc.21762. [DOI] [PubMed] [Google Scholar]
- Li Q, Anver MR, Butcher DO, Gildersleeve JC. Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol Cancer Ther. 2009;8:971–979. doi: 10.1158/1535-7163.MCT-08-0934. doi:10.1158/1535-7163.MCT-08-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Live DH, Williams LJ, Kuduk SD, Schwarz JB, Glunz PW, Chen XT, Sames D, Kumar RA, Danishefsky SJ. Probing cell-surface architecture through synthesis: An NMR-determined structural motif for tumor-associated mucins. Proc Natl Acad Sci USA. 1999;96:3489–3493. doi: 10.1073/pnas.96.7.3489. doi:10.1073/pnas.96.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurov D, Ilinskaya A, Heidecker G, Lloyd P, Derse D. Quantitative comparison of HTLV-1 and HIV-1 cell-to-cell infection with new replication dependent vectors. PLoS Pathog. 2010;6:e1000788. doi: 10.1371/journal.ppat.1000788. doi:10.1371/journal.ppat.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney DW, Onodera H, Gorman L, Mimura T, Rothstein DM. Distinct isoforms of the CD45 protein-tyrosine phosphatase differentially regulate interleukin 2 secretion and activation signal pathways involving Vav in T cells. J Biol Chem. 1995;270:24949–24954. doi: 10.1074/jbc.270.42.24949. doi:10.1074/jbc.270.42.24949. [DOI] [PubMed] [Google Scholar]
- Moller H, Serttas N, Paulsen H, Burchell JM, Taylor-Papadimitriou J. NMR-based determination of the binding epitope and conformational analysis of MUC-1 glycopeptides and peptides bound to the breast cancer-selective monoclonal antibody SM3. Eur J Biochem. 2002;269:1444–1455. doi: 10.1046/j.1432-1033.2002.02787.x. doi:10.1046/j.1432-1033.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- Nakada H, Inoue M, Numata Y, Tanaka N, Funakoshi I, Fukui S, Mellors A, Yamashina I. Epitopic structure of Tn glycophorin A for an anti-Tn antibody (MLS 128) Proc Natl Acad Sci USA. 1993;90:2495–2499. doi: 10.1073/pnas.90.6.2495. doi:10.1073/pnas.90.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada H, Inoue M, Tanaka N, Numata Y, Kitagawa H, Fukui S, Yamashina I. Expression of the Tn antigen on T-lymphoid cell line Jurkat. Biochem Biophys Res Commun. 1991;179:762–767. doi: 10.1016/0006-291x(91)91882-d. doi:10.1016/0006-291X(91)91882-D. [DOI] [PubMed] [Google Scholar]
- Nakada H, Numata Y, Inoue M, Tanaka N, Kitagawa H, Funakoshi I, Fukui S, Yamashina I. Elucidation of an essential structure recognized by an anti-GalNAc alpha-Ser(Thr) monoclonal antibody (MLS 128) J Biol Chem. 1991;266:12402–12405. [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. doi:10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata Y, Nakada H, Fukui S, Kitagawa H, Ozaki K, Inoue M, Kawasaki T, Funakoshi I, Yamashina I. A monoclonal antibody directed to Tn antigen. Biochem Biophys Res Commun. 1990;170:981–985. doi: 10.1016/0006-291x(90)90488-9. doi:10.1016/0006-291X(90)90488-9. [DOI] [PubMed] [Google Scholar]
- Nurden AT, Dupuis D, Pidard D, Kieffer N, Kunicki TJ, Cartron JP. Surface modifications in the platelets of a patient with α-N-acetyl-d-galactosamine residues, the Tn-syndrome. J Clin Invest. 1982;70:1281–1291. doi: 10.1172/JCI110727. doi:10.1172/JCI110727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Boyle KP, Markowitz AL, Khorshidi M, Lalezari P, Longenecker BM, Lloyd KO, Welt S, Wright KE. Specificity analysis of murine monoclonal antibodies reactive with Tn, sialylated Tn, T, and monosialylated (2→6) T antigens. Hybridoma. 1996;15:401–408. doi: 10.1089/hyb.1996.15.401. doi:10.1089/hyb.1996.15.401. [DOI] [PubMed] [Google Scholar]
- Oppezzo P, Osinaga E, Tello D, Bay S, Cantacuzene D, Irigoin F, Ferreira A, Roseto A, Cayota A, Alzari P, et al. Production and functional characterization of two mouse/human chimeric antibodies with specificity for the tumor-associated Tn-antigen. Hybridoma. 2000;19:229–239. doi: 10.1089/02724570050109620. doi:10.1089/02724570050109620. [DOI] [PubMed] [Google Scholar]
- Osinaga E, Bay S, Tello D, Babino A, Pritsch O, Assemat K, Cantacuzene D, Nakada H, Alzari P. Analysis of the fine specificity of Tn-binding proteins using synthetic glycopeptide epitopes and a biosensor based on surface plasmon resonance spectroscopy. FEBS Lett. 2000;469:24–28. doi: 10.1016/s0014-5793(00)01248-5. doi:10.1016/S0014-5793(00)01248-5. [DOI] [PubMed] [Google Scholar]
- Pancino GF, Osinaga E, Vorauher W, Kakouche A, Mistro D, Charpin C, Roseto A. Production of a monoclonal antibody as immunohistochemical marker on paraffin embedded tissues using a new immunization method. Hybridoma. 1990;9:389–395. doi: 10.1089/hyb.1990.9.389. doi:10.1089/hyb.1990.9.389. [DOI] [PubMed] [Google Scholar]
- Piller V, Piller F, Fukuda M. Biosynthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J Biol Chem. 1990;265:9264–9271. [PubMed] [Google Scholar]
- Reddish MA, Jackson L, Koganty RR, Qiu D, Hong W, Longenecker BM. Specificities of anti-sialyl-Tn and anti-Tn monoclonal antibodies generated using novel clustered synthetic glycopeptide epitopes. Glycoconj J. 1997;14:549–560. doi: 10.1023/a:1018576224062. doi:10.1023/A:1018576224062. [DOI] [PubMed] [Google Scholar]
- Reis CA, Sorensen T, Mandel U, David L, Mirgorodskaya E, Roepstorff P, Kihlberg J, Hansen JE, Clausen H. Development and characterization of an antibody directed to an α-N-acetyl-D-galactosamine glycosylated MUC2 peptide. Glycoconj J. 1998;15:51–62. doi: 10.1023/a:1006939432665. doi:10.1023/A:1006939432665. [DOI] [PubMed] [Google Scholar]
- Sakai K, Yuasa N, Tsukamoto K, Takasaki-Matsumoto A, Yajima Y, Sato R, Kawakami H, Mizuno M, Takayanagi A, Shimizu N, et al. Isolation and characterization of antibodies against three consecutive Tn-antigen clusters from a phage library displaying human single-chain variable fragments. J Biochem. 2010;147:809–817. doi: 10.1093/jb/mvq014. doi:10.1093/jb/mvq014. [DOI] [PubMed] [Google Scholar]
- Schietinger A, Philip M, Yoshida BA, Azadi P, Liu H, Meredith SC, Schreiber H. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 2006;314:304–308. doi: 10.1126/science.1129200. doi:10.1126/science.1129200. [DOI] [PubMed] [Google Scholar]
- Sgroi D, Koretzky GA, Stamenkovic I. Regulation of CD45 engagement by the B-cell receptor CD22. Proc Natl Acad Sci USA. 1995;92:4026–4030. doi: 10.1073/pnas.92.9.4026. doi:10.1073/pnas.92.9.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilova NV, Galanina OE, Pochechueva TV, Chinarev AA, Kadykov VA, Tuzikov AB, Bovin NV. High molecular weight neoglycoconjugates for solid phase assays. Glycoconj J. 2005;22:43–51. doi: 10.1007/s10719-005-0280-y. doi:10.1007/s10719-005-0280-y. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Shaw S. Cell adhesion. Mucins in the mainstream. Nature. 1993;366:630–631. doi: 10.1038/366630a0. doi:10.1038/366630a0. [DOI] [PubMed] [Google Scholar]
- Springer GF. T and Tn pancarcinoma markers: Autoantigenic adhesion molecules in pathogenesis, prebiopsy carcinoma-detection, and long-term breast carcinoma immunotherapy. Crit Rev Oncog. 1995;6:57–85. doi: 10.1615/critrevoncog.v6.i1.50. [DOI] [PubMed] [Google Scholar]
- Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. doi:10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Metoki R, Hakomori S. Immunoglobulin G3 monoclonal antibody directed to Tn antigen (tumor-associated alpha-N-acetylgalactosaminyl epitope) that does not cross-react with blood group A antigen. Cancer Res. 1988;48:4361–4367. [PubMed] [Google Scholar]
- Tanaka N, Nakada H, Inoue M, Yamashina I. Binding characteristics of an anti-Siaα2-6GalNAcα-Ser/Thr (sialyl Tn) monoclonal antibody (MLS 132) Eur J Biochem. 1999;263:27–32. doi: 10.1046/j.1432-1327.1999.00401.x. doi:10.1046/j.1432-1327.1999.00401.x. [DOI] [PubMed] [Google Scholar]
- Tarp MA, Sorensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, Clausen H. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology. 2007;17:197–209. doi: 10.1093/glycob/cwl061. doi:10.1093/glycob/cwl061. [DOI] [PubMed] [Google Scholar]
- Welinder C, Baldetorp B, Borrebaeck C, Fredlund BM, Jansson B. A new murine IgG1 anti-Tn monoclonal antibody with in vivo anti tumour activity. Glycobiology. 2011;21:1097–1107. doi: 10.1093/glycob/cwr048. [DOI] [PubMed] [Google Scholar]
- Woodward MP, Young WW, Jr, Bloodgood RA. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. doi:10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gendler SJ, Franco A. Designer glycopeptides for cytotoxic T cell-based elimination of carcinomas. J Exp Med. 2004;199:707–716. doi: 10.1084/jem.20031865. doi:10.1084/jem.20031865. [DOI] [PMC free article] [PubMed] [Google Scholar]