Abstract
SCA1 is an adult-onset, dominantly inherited neurodegenerative disease caused by expansion of a glutamine repeat tract in ATXN1. Although the precise function of ATXN1 remains elusive, it appears to play a role in transcriptional repression. We find that mutant ATXN1 suppresses transcription of the neurotrophic and angiogenic factor VEGF. We also show that genetic or pharmacologic replenishment of VEGF mitigates SCA1 pathogenesis, suggesting a novel therapeutic strategy for this incurable disease.
Transcriptional derangement is the earliest pathogenic signature of SCA1 mouse models, inspiring the hypothesis that alterations of gene expression caused by ATXN1 are central to pathogenesis1-5. However, little is known about the direct targets of ATXN1-induced repression, particularly those genes that are misregulated in the vulnerable Purkinje cell population. Moreover, it is unclear whether correcting gene derangements can ameliorate SCA1.
Because Purkinje cells constitute less than 0.1% of cerebellar cells, we used laser capture microdissection (LCM) to enrich for these neurons so as to identify misregulated genes (Fig. 1a; Supplementary Fig. 1a). We used tissue from the SCA1 knock-in mice (henceforth SCA1 mice) that express an expanded version of ATXN1 with 154 glutamines, a model that closely mirrors human SCA16.
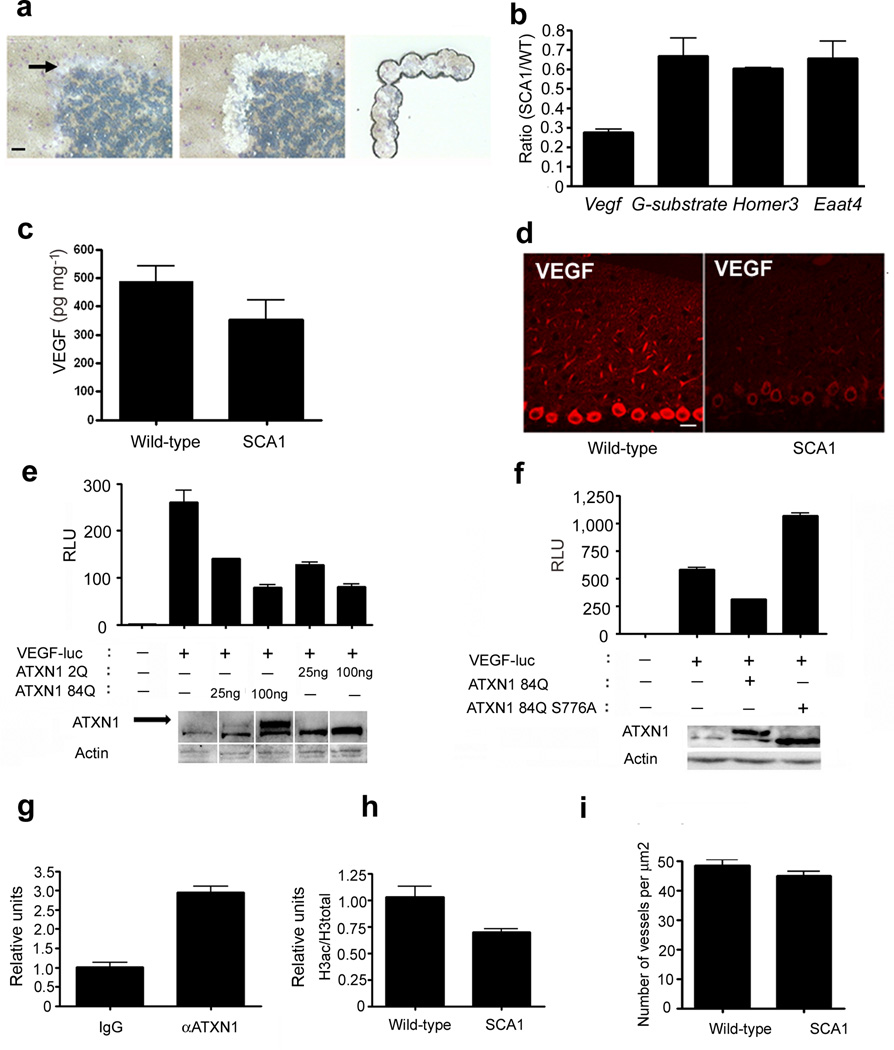
Figure 1. VEGF is downregulated in Purkinje cells affected by SCA1.
(a) Isolation of mouse cerebellar Purkinje cells using laser capture microdissection (LCM; 3 month old mice). Left: section before LCM; arrow points to the Purkinje cell layer; Middle: section after LCM with the Purkinje cell-layer cut out; Right: pure population of Purkinje cells on the retrieval cap. Scale bar = 50 µm. (b) Quantitative RT-PCR for Vegf on LCM/Purkinje cells samples from SCA1 mice compared to wild-type littermates (normalized to actin). The decrease in Vegf mRNA was more pronounced than that for three other genes previously described as downregulated in SCA1: G-substrate, Homer3 and Eaat4 1,2. P values using two-tailed t-test are as follows: Vegf, P = 0.0002; G-substrate, P = 0.035; Homer3, P = 0.002; and Eaat4, P = 0.047. Data represent 5 pairs of SCA1 and wild-type mice. (c) VEGF protein levels in cerebella of SCA1 knock-in mice compared to wild-type littermates. Each ELISA was done in duplicate and normalized to the weight of cerebella. n = 13 pairs of SCA1 and wild-type littermates 6 month of age or older. P value = 0.049; t-test. (d) VEGF staining of Purkinje cells in the cerebella of 10 month old SCA1 mouse and wild-type control (S-2 VEGF antibody; Santa Cruz). Scale bar = 25 µm. (e) Transfecting N2A cells with ATXN1-84Q or ATXN1-2Q causes a dose-dependent repression at the Vegf promoter using the luciferase reporter VEGF-luc. Western blot demonstrates ATXN1 transfected levels. Arrow points to the upper band representing ATXN1-84Q, while lower band is ATXN1-2Q. Data are representative of ten independent experiments done in duplicates, P < 0.05 for all ATXN1 overexpressing conditions compared to no ATXN1 (unpaired two-tailed t-test). (f) Mutating a key pathogenic phosphorylation residue, serine 776 to alanine abrogates ATXN1-induced repression of Vegf. Western blot shows the levels of transfected ATXN1-84Q and ATXN1-84Q S776A. Data are representative of five independent experiments, each performed in duplicate. P < 0.05 (unpaired two-tailed t-test) comparing ATXN1-84Q and ATXN1-84Q S776A. (g) ChIP on cerebellar lysates with antibody to ATXN1 and qPCR for the Vegf promoter (normalized to input using IgG as a reference). Data are representative of three independent experiments, P < 0.05 (unpaired two tailed t-test). (h) Histone acetylation at the Vegf promoter in SCA1 mice compared to wild-type littermates as shown by ChIP on cerebellar lysates with antibody to acetylated histones; qPCR data are normalized to input and total histone H3 levels, using wild-type mice as a reference. Data are representative of three independent experiments, P < 0.05 (unpaired two-tailed t–test). (i) Cerebella of 9 pairs of wild-type and SCA1 mice (older than 10 months) were examined for blood vessel density using collagen IV staining, P = 0.0015 (paired t-test). Data are represented as mean with error bars showing standard error in all figures.
Since the yield of RNA from LCM material was insufficient for microarray screening, we employed a PCR-based approach to test for expression of candidate genes involved in key neurodegenerative pathways. One of the genes that we found down-regulated encodes for vascular endothelial growth factor-A (VEGF), an angiogenic and trophic factor implicated in motor neuron disorders7–9(Fig 1b). Importantly, VEGF levels were decreased as early as postnatal day 30 (Supplementary Fig. 1b), well before any behavioral or pathological signs. In the cerebellum, VEGF is widely expressed in neurons, glia and endothelial cells10,11, with strong expression in Purkinje neurons (Fig. 1d, Supplementary Fig. 1c, d).
Low Vegf mRNA was accompanied by decreased VEGF protein in SCA1 cerebella (Fig. 1c, d). The decrease in VEGF was most prominent in Purkinje neurons (granule cells also demonstrated a trend toward lower Vegf mRNA levels and a significant decrease of VEGF protein; Supplementary Fig. 1e–h). We also found that Vegf mRNA levels were lower in a transgenic SCA1 mouse model that expresses mutant ATXN1 only in Purkinje neurons12 (Supplementary Fig. 1i).
We next asked whether ATXN1 is directly responsible for down-regulation of Vegf mRNA. To test whether ATXN1 modulates Vegf promoter activity, we used a Vegf luciferase reporter assay13. Both expanded (ATXN1-84Q) and wild-type (ATXN1-2Q) ATXN1 repressed Vegf in a dose-dependent manner (Fig. 1e). The ability of even wild-type ataxin to cause repression is in keeping with the observation that overexpression of wild-type ATXN1 can induce pathology in animal models, and the notion that SCA1 can in part be caused by a gain of ATXN1 normal function14. Mutating a phosphorylation site critical for ATXN1 toxicity (serine 776 to alanine; S776A)15 disrupted VEGF repression (Fig. 1f). Thus ATXN1 repression of the Vegf promoter correlates with its toxicity in vivo. Consistent with these reporter experiments, VEGF levels were reduced in primary cerebellar neurons from SCA1 mice (Supplementary Fig. 2a). We next performed chromatin immunoprecipitation (ChIP) using an ATXN1-specific antibody to test for ATXN1 occupancy of the Vegf promoter. We found that the Vegf promoter (but not the promoter of a closely related family member Vegfc8) was indeed enriched in ChIP lysates (Fig. 1g, Supplementary Fig. 2b). The low expression of Vegf in SCA1 mice was associated with hypoacetylation at the Vegf promoter (Fig. 1h); furthermore, inhibitors of histone deacetylases relieved ATXN-1 84Q-induced repression at the Vegf promoter (Supplementary Fig. 2c). These results suggest a role for histone acetylation in ATXN1-induced repression.
Since VEGF is an angiogenic factor, diminished VEGF could contribute to cerebellar dysfunction by limiting angiogenesis. We observed a significant decrease in cerebellar microvessel density and total vessel length in SCA1 mice (Fig. 1i, Supplementary Fig. 3a–d). We also detected evidence for hypoxia in SCA1 cerebella using pimonidazole (a chemical that can detect the effects of hypoxia) (Supplementary Fig. 3e, f).
In addition to its role in angiogenesis, VEGF is also a neurotrophic factor. Thus, inadequate VEGF levels could also be deleterious by limiting neurotrophic support to cerebellar neurons. We tested the effects of reduced VEGF levels on mixed cerebellar neuronal cultures that express VEGF and its receptor VEGF R2 (the predominant VEGF receptor in neurons) (Supplementary Figs. 4, 5 and 6). We demonstrated that cerebellar neurons are susceptible to a decrease in VEGF signaling using VEGF R2 tyrosine kinase inhibitors and a neutralizing VEGF antibody. These experiments suggest that low VEGF levels compromise the growth and survival of not only Purkinje neurons, but also other cerebellar neurons through a reduction in neurotrophic support.
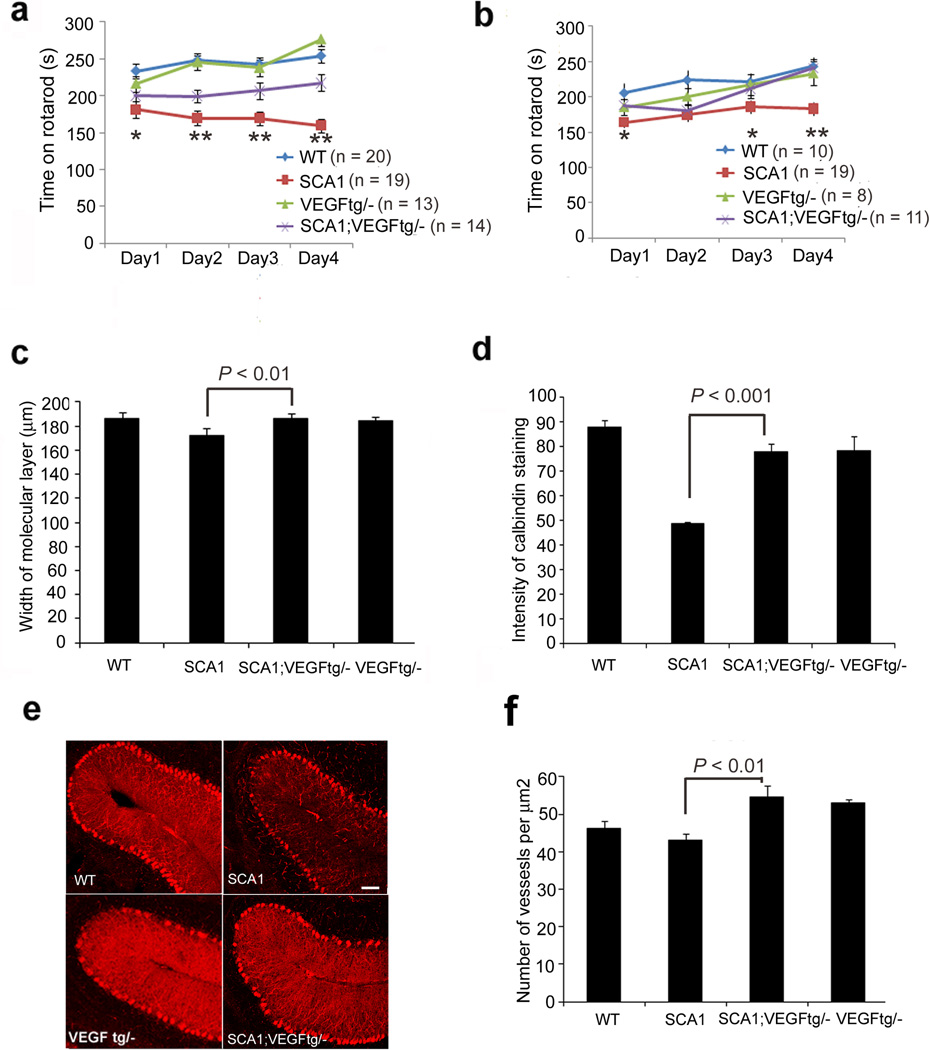
We then examined whether replenishing VEGF improves the SCA1 phenotype in mice. We mated the SCA1 mice to a transgenic VEGF line that overexpresses human VEGF in neurons starting at embryonic day 14 (Supplementary Fig. 7a, b and16). Genetically increasing VEGF enhanced the Rotarod performance of mice at 13 weeks and 6 months, and improved SCA1 cerebellar pathology as measured by calbindin staining intensity (calbindin specifically labels Purkinje cells) and molecular layer thickness (Fig. 2a–e). In addition, VEGF overexpression increased cerebellar microvessel density (Fig. 2f).
Figure 2. VEGF overexpression ameliorates the SCA1 phenotype.
Rotarod performance of (a) 13 week-old and (b) 6-month old SCA1 mice. n = number of mice of each genotype. Data were analyzed using two-way ANOVA followed by the Bonferroni post hoc test. *P < 0.05 (day 1) and ** P < 0.001 for (days 2, 3 and 4) for 13 weeks; *P < 0.05 for days 1 and 3 and ** P < 0.001 for day 4, comparing SCA1 mice to SCA1 mice with one copy of the Vegf transgene. SCA1 pathology in 36 week old mice as determined by (c) width of the molecular layer and (d) intensity of calbindin staining. Data represent one of 8 (width) or 5 (intensity) experiments using independent litters to generate data for 4 experimental genotypes. Data were analyzed using one-way ANOVA followed by the Bonferroni post hoc test. (e) Representative confocal images of 36-week old mice of the indicated genotypes stained with calbindin specific antibody (Sigma). (f) Cerebella of three quadriplicates of wild-type, SCA1 mice, VEGF tg/–, and SCA1 VEGF tg/– (older than 10 months) were examined for the number of blood vessels using collagen IV staining. P < 0.05 determined using one-way ANOVA with Bonferroni post hoc test. Data are represented as mean with error bars showing standard error in all figures.
We next tested whether pharmacological delivery of recombinant VEGF is beneficial after disease onset. Since VEGF cannot cross the blood brain barrier, we used an intracerebroventricular route to deliver VEGF (Supplementary Fig. 7c, d). Mirroring our genetic rescue experiments, we observed improved Rotarod performance along with amelioration in pathology (Supplementary Fig. 8).
Our findings demonstrate an important role for VEGF in SCA1 pathogenesis and suggest that reversal of low VEGF levels offers the potential for meaningful treatment in patients with SCA1. In addition, these findings advance our understanding of SCA1 neurodegeneration in several ways. First, they suggest a cross-talk between the degenerating nervous system and the vascular system, an unexplored area in SCA1 research. Second, our results may explain the alterations in energy metabolism and decreased oxygen consumption identifiable by functional imaging in SCA117. Third, our work on VEGF provides clues to develop biomarkers to monitor disease progression (such as VEGF itself, or sequelae of VEGF signaling in blood or cerebrospinal fluid of patients). Finally, since ataxia pathways interact in pathogenic hubs18 our results could prove relevant to other ataxias and possibly even other neurodegenerative syndromes.
Supplementary Material
ACKNOWLEDGEMENTS
We thank H. Zoghbi and H. Orr for generously providing reagents and the SCA1 mouse models, and S. Leibovich and P. D’Amore for VEGF luciferase reporter constructs. We also thank K. Gobeske for assistance with the intracerebroventricular delivery methods, A. Ma for help with pathological analyses, and V. Brandt for editorial assistance. This work was funded by U.S. National Institutes of Health grants K02 NS051340; R21 NS060080, and R01 NS062051 (P.O), a National Organization for Rare Disorders grant (P.O), and a National Ataxia Foundation grant (P.O.). M.C. received funding from the U.S. National Institutes of Health training grant T32. The authors wish to dedicate this manuscript to fellow scientist Dr. Tim Spann who is currently fighting amyotrophic lateral sclerosis.
Footnotes
AUTHOR CONTRIBUTIONS
P.O., A.K. and M.C. conceived the study and designed the experiments. M.C. and J.P. conducted and analyzed the experiments. H.M. provided and characterized the VEGF transgenic mice. P.O., M.C., and A.K. wrote the paper, and J.P. helped with revising the manuscript.
BIBLIOGRAPHY
- 1.Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci. 2000;3:157–163. doi: 10.1038/72101. [DOI] [PubMed] [Google Scholar]
- 2.Serra HG. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum Mol Genet. 2004;13:2535–2543. doi: 10.1093/hmg/ddh268. [DOI] [PubMed] [Google Scholar]
- 3.Gatchel JR. The insulin-like growth factor pathway is altered in spinocerebellar ataxia type 1 and type 7. Proc Natl Acad Sci U S A. 2008;105:1291–1296. doi: 10.1073/pnas.0711257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 5.Tsai CC. Ataxin 1, a SCA1 neurodegenerative disorder protein, is functionally linked to the silencing mediator of retinoid and thyroid hormone receptors. Proc Natl Acad Sci U S A. 2004;101:4047–4052. doi: 10.1073/pnas.0400615101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watase K, et al. A Long CAG Repeat in the Mouse Sca1 Locus Replicates SCA1 Features and Reveals the Impact of Protein Solubility on Selective Neurodegeneration. Neuron. 2002;34:905–919. doi: 10.1016/s0896-6273(02)00733-x. [DOI] [PubMed] [Google Scholar]
- 7.Oosthuyse B, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 8.Lambrechts D, Carmeliet P. VEGF at the neurovascular interface: therapeutic implications for motor neuron disease. Biochim BioPhys Acta. 2006;1762:1109–1121. doi: 10.1016/j.bbadis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Sopher BL. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 2004;41:687–699. doi: 10.1016/s0896-6273(04)00082-0. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz de Almodovar C. Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1. J Neurosci. 30:15052–15066. doi: 10.1523/JNEUROSCI.0477-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and-2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev. 2001;108:45–57. doi: 10.1016/s0925-4773(01)00471-3. [DOI] [PubMed] [Google Scholar]
- 12.Burright EN, et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–948. doi: 10.1016/0092-8674(95)90273-2. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan M, Pinhal-Enfield G, Hao I, Leibovich SJ. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter. Mol Biol Cell. 2007;18:14–23. doi: 10.1091/mbc.E06-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Funez P, et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature. 2000;408:101–106. doi: 10.1038/35040584. [DOI] [PubMed] [Google Scholar]
- 15.Chen HK. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003;113:457–468. doi: 10.1016/s0092-8674(03)00349-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, et al. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2005;128:52–63. doi: 10.1093/brain/awh325. [DOI] [PubMed] [Google Scholar]
- 17.Oz G, et al. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 25:1253–1261. doi: 10.1002/mds.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim J. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell. 2006;125:801–814. doi: 10.1016/j.cell.2006.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.