Abstract
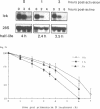
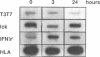
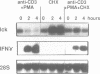
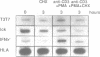
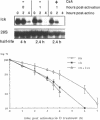
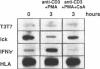
The p56lck tyrosine kinase is most likely to be involved in signal transduction of T lymphocyte activation. After full activation through the TcR/CD3 complex lck mRNA is transiently down-modulated. This down-modulation was due to an early decrease of both transcription and stability of the lck mRNA. To study the involvement of transcriptional and post-transcriptional factors in this regulations, we have analysed the effect of cycloheximide, a protein synthesis inhibitor, on the steady-state of the lck mRNA. Cycloheximide superinduced lck mRNA by increasing its stability, although cycloheximide concomitantly decreased lck transcription. This suggests that the constitutive level of lck mRNA observed prior to activation is controlled by transcriptional activator(s) and post-transcriptional destabilizing factor(s). Second, lck mRNA down-modulation observed after full activation was inhibited by cycloheximide. It increased lck mRNA stability whereas lck transcription remained low. Therefore, full activation might increase the synthesis and/or activity of destabilizing factor(s). Cyclosporin A also inhibited the down-modulation of lck mRNA by increasing its transcription with no effect on its stability. Since, lck mRNA down-modulation was always associated with lymphokine mRNA induction, and since CsA blocks both lymphokine transcription and lck decrease of transcription, this indicates that these genes might share common regulatory pathways leading to their inverse transcriptional regulation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Chou H. S., Loh D. Y. A conserved sequence in the T-cell receptor beta-chain promoter region. Proc Natl Acad Sci U S A. 1988 May;85(10):3551–3554. doi: 10.1073/pnas.85.10.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbu V., Dautry F. Northern blot normalization with a 28S rRNA oligonucleotide probe. Nucleic Acids Res. 1989 Sep 12;17(17):7115–7115. doi: 10.1093/nar/17.17.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani P. M., Robb A., Hess A. D. Cyclosporin A binding to calmodulin: a possible site of action on T lymphocytes. Science. 1985 Apr 19;228(4697):337–339. doi: 10.1126/science.3885394. [DOI] [PubMed] [Google Scholar]
- Danielian S., Fagard R., Alcover A., Acuto O., Fischer S. The lymphocyte-specific protein tyrosine kinase p56lck is hyperphosphorylated on serine and tyrosine residues within minutes after activation via T cell receptor or CD2. Eur J Immunol. 1989 Dec;19(12):2183–2189. doi: 10.1002/eji.1830191202. [DOI] [PubMed] [Google Scholar]
- Emmrich F. Cross-linking of CD4 and CD8 with the T-cell receptor complex: quaternary complex formation and T-cell repertoire selection. Immunol Today. 1988 Oct;9(10):296–300. doi: 10.1016/0167-5699(88)91320-5. [DOI] [PubMed] [Google Scholar]
- Fischer S., Fagard R., Gacon G., Genetet N., Piau J. P., Blaineau C. Stimulation of tyrosine phosphorylation in lectin treated human lymphocytes. Biochem Biophys Res Commun. 1984 Nov 14;124(3):682–689. doi: 10.1016/0006-291x(84)91012-x. [DOI] [PubMed] [Google Scholar]
- Garvin A. M., Pawar S., Marth J. D., Perlmutter R. M. Structure of the murine lck gene and its rearrangement in a murine lymphoma cell line. Mol Cell Biol. 1988 Aug;8(8):3058–3064. doi: 10.1128/mcb.8.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. D., Colombani P. N. Cyclosporin-resistant and -sensitive T-lymphocyte subsets. Ann Inst Pasteur Immunol. 1987 Jul-Aug;138(4):606–611. doi: 10.1016/s0769-2625(87)80131-9. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S., Gross G., Horowitz M., Givol D. Promoter and enhancer elements in the rearranged alpha chain gene of the human T cell receptor. EMBO J. 1987 Nov;6(11):3307–3312. doi: 10.1002/j.1460-2075.1987.tb02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Lewis D. B., Cooke M. P., Mellins E. D., Gearn M. E., Samelson L. E., Wilson C. B., Miller A. D., Perlmutter R. M. Lymphocyte activation provokes modification of a lymphocyte-specific protein tyrosine kinase (p56lck). J Immunol. 1989 Apr 1;142(7):2430–2437. [PubMed] [Google Scholar]
- Marth J. D., Lewis D. B., Wilson C. B., Gearn M. E., Krebs E. G., Perlmutter R. M. Regulation of pp56lck during T-cell activation: functional implications for the src-like protein tyrosine kinases. EMBO J. 1987 Sep;6(9):2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Altman A. Do CD4 and CD8 control T-cell activation via a specific tyrosine protein kinase? Immunol Today. 1989 Jun;10(6):189–192. doi: 10.1016/0167-5699(89)90322-8. [DOI] [PubMed] [Google Scholar]
- Norment A. M., Lonberg N., Lacy E., Littman D. R. Alternatively spliced mRNA encodes a secreted form of human CD8 alpha. Characterization of the human CD8 alpha gene. J Immunol. 1989 May 1;142(9):3312–3319. [PubMed] [Google Scholar]
- Paillard F., Sterkers G., Bismuth G., Gomard E., Vaquero C. Lymphokine mRNA and T cell multireceptor mRNA of the Ig super gene family are reciprocally modulated during human T cell activation. Eur J Immunol. 1988 Oct;18(10):1643–1646. doi: 10.1002/eji.1830181028. [DOI] [PubMed] [Google Scholar]
- Paillard F., Sterkers G., Vaquero C. Transcriptional and post-transcriptional regulation of TcR, CD4 and CD8 gene expression during activation of normal human T lymphocytes. EMBO J. 1990 Jun;9(6):1867–1872. doi: 10.1002/j.1460-2075.1990.tb08312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Ziegler S. F., Garvin A. M., Pawar S., Cooke M. P., Abraham K. M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1989 Feb;948(3):245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Reem G. H., Cook L. A., Vilcek J. Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science. 1983 Jul 1;221(4605):63–65. doi: 10.1126/science.6407112. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Reinherz E. L. Multiple nuclear proteins bind upstream sequences in the promotor region of a T-cell receptor beta-chain variable-region gene: evidence for tissue specificity. Proc Natl Acad Sci U S A. 1987 Jan;84(1):232–236. doi: 10.1073/pnas.84.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Takadera T., Leung S., Gernone A., Koga Y., Takihara Y., Miyamoto N. G., Mak T. W. Structure of the two promoters of the human lck gene: differential accumulation of two classes of lck transcripts in T cells. Mol Cell Biol. 1989 May;9(5):2173–2180. doi: 10.1128/mcb.9.5.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero C., Sanceau J., Sondermeyer P., Falcoff R. Kinetics of messenger accumulation coding for IFN gamma, related to modifications in the poly(A) RNA population of activated human lymphocytes. Nucleic Acids Res. 1984 Mar 26;12(6):2629–2640. doi: 10.1093/nar/12.6.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero C., Sancéau J., Catinot L., Andreu G., Falcoff E., Falcoff R. Translation of mRNA from phytohemagglutinin-stimulated human lymphocytes: characterization of interferon mRNAs. J Interferon Res. 1982;2(2):217–228. doi: 10.1089/jir.1982.2.217. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Horak E. M., Bookman M. A., Bolen J. B. Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T-cell activation. Mol Cell Biol. 1988 Oct;8(10):4353–4361. doi: 10.1128/mcb.8.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Adler H. T., Sefton B. M. Two lck transcripts containing different 5' untranslated regions are present in T cells. Mol Cell Biol. 1987 Dec;7(12):4407–4413. doi: 10.1128/mcb.7.12.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Buss J. E., Patschinsky T., Hunter T., Sefton B. M. Characterization of the protein apparently responsible for the elevated tyrosine protein kinase activity in LSTRA cells. Mol Cell Biol. 1984 Dec;4(12):2705–2713. doi: 10.1128/mcb.4.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskocil R., Weiss A., Imboden J., Kamin-Lewis R., Stobo J. Activation of a human T cell line: a two-stimulus requirement in the pretranslational events involved in the coordinate expression of interleukin 2 and gamma-interferon genes. J Immunol. 1985 Mar;134(3):1599–1603. [PubMed] [Google Scholar]