Abstract
Behavioral studies have suggested that food cues have stronger motivating effects in obese than in normal-weight individuals, which may be a risk factor underlying obesity. Previous cross-sectional neuroimaging studies have suggested that this difference is mediated by increased reactivity to food cues in parts of the reward system in obese individuals. To date, however, only a few prospective neuroimaging studies have been conducted to examine whether individual differences in brain activation elicited by food cues can predict differences in weight change. We used functional magnetic resonance imaging (fMRI) to investigate activation in reward-system as well as other brain regions in response to viewing high-calorie food vs. control pictures in 25 obese individuals before and after a 12-week psychosocial weight-loss treatment and at 9-mo follow-up. In those obese individuals who were least successful in losing weight during the treatment, we found greater pre-treatment activation to high-calorie food vs. control pictures in brain regions implicated in reward-system processes, such as the nucleus accumbens, anterior cingulate, and insula. We found similar correlations with weight loss in brain regions implicated by other studies in vision and attention, such as superior occipital cortex, inferior and superior parietal lobule, and prefrontal cortex. Furthermore, less successful weight maintenance at 9-mo follow-up was predicted by greater post-treatment activation in such brain regions as insula, ventral tegmental area, putamen, and fusiform gyrus. In summary, we found that greater activation in brain regions mediating motivational and attentional salience of food cues in obese individuals at the start of a weight-loss program was predictive of less success in the program and that such activation following the program predicted poorer weight control over a 9-mo follow-up period.
Keywords: obesity, fMRI, food cues, weight loss, weight maintenance, reward system
1. Introduction
Over the last 25 years, the prevalence of obesity has tripled in the United States (World Health Organization, 2003). The current environment in the United States has been labeled “obesogenic”, with a key feature being an overabundance of readily available highly palatable, high-calorie foods (Carnell and Wardle, 2008). Nevertheless, there is enormous variation in weight among adults in the population, with only some individuals becoming obese. One possibility is that certain individuals are particularly susceptible to effects of environmental stimuli that promote excessive food intake and weight gain (Carnell and Wardle, 2009). Exaggerated food-cue reactivity may not only be a factor in the development of obesity but may also contribute to its intractability, causing difficulty in losing weight and contributing to the prevalence of relapse after weight loss (e.g., Cornier, 2011; Jeffery et al., 2000; Kramer et al., 1989; Wadden et al., 1989).
Behavioral studies have provided evidence that differences in food cue reactivity contribute to differences in susceptibility to obesity (Rodin et al., 1989; Schachter and Gross, 1968). For example, studies of both children and adults have shown that the taste or smell of palatable snack food triggers overconsumption in obese individuals (Jansen et al., 2003) and that the magnitude of appetitive effects of the sight and smell of appetizing food is stronger in overweight compared to normal-weight individuals (Ferriday and Brunstrom, 2010; Tetley et al., 2006). Furthermore, this effect may be most pronounced for high-calorie foods, as suggested by reported relationships among BMI, food preferences, and food cravings. For example, several studies of food biases have indicated that preference for highly palatable, high-calorie foods is stronger among obese than among normal-weight individuals and can predict weight gain (Le Noury et al., 2002; Mela, 2001; Rissanen et al., 2002; Salbe et al., 2004). In addition, degree of craving for high-calorie foods is positively correlated with BMI (Burton et al., 2007). Taken together, these results suggest that food cues, especially high-calorie food cues, possess a greater than normal potency in driving food intake in obese individuals.
Neuroimaging studies investigating responses to visual food cues, such as pictures of food, have found increased activation in obese individuals within the reward system and associated brain regions, similar to the proposed circuitry for drugs of abuse (Del Parigi et al., 2003; Volkow and Wise, 2005; Volkow et al., 2008). We and others have used fMRI to compare obese and normal-weight individuals with regard to brain activation in response to high-calorie food pictures and found greater activation in obese individuals in a wide variety of structures within the classically defined reward system, such as ventral tegmental area (VTA), nucleus accumbens (NAc), and orbitofrontal cortex (OFC), as well as additional regions implicated in reward- and motivation-related processes, such as the insula, hippocampus, dorsal striatum, and anterior cingulate cortex (ACC) (Bruce et al., 2010; Martin et al., 2010; Rothemund et al., 2007; Stice et al., 2010a; Stoeckel et al., 2008).
The results cited above suggest that greater reward system reactivity to high-calorie food cues contributes to the development of obesity (e.g., Stice et al., 2011) and, as a corollary, may also cause greater difficulty in losing weight and in maintaining weight loss. Although there have been previous neuroimaging studies examining brain responses to pictures of food that signal impending food intake, or responses to food intake itself (e.g., Stice et al., 2008a, 2008b, 2009, 2011), to our knowledge, there have been only two published fMRI studies investigating whether activation of reward areas by visual food cues not signaling imminent food intake predicts subsequent body weight trajectory. Stice and colleagues (2010a) found that activation in reward areas to imagined intake of palatable food correlated positively with weight gain over the next year in some individuals, although negative correlations were found in those with genotypes suggestive of impaired dopamine signaling. The second study employed a go/no-go task that included pictures of appetizing, high-calorie foods on no-go trials and found a negative correlation between fMRI activation in the temporal operculum on such trials and subsequent one-year weight gain in adolescent girls (Batterink et al., 2010).
There is a growing body of research seeking to determine whether interventions aimed at weight loss can successfully change obese individuals’ reactivity to food cues. Some studies have shown that behavioral interventions aimed at reducing fat intake can decrease individuals’ motivation to consume high fat foods (Grieve and Vander Weg, 2003; Jansen et al., 2010; Ledikwe et al., 2007; Martin et al., 2011; Van Horn et al., 2005), raising the possibility of reduced reactivity to cues associated with such foods. Cross-sectional neuroimaging studies of successful dieters, or post-obese individuals, in comparison to normal-weight individuals, have yielded mixed results regarding responses of reward areas to visual or gustatory food cues (Cornier et al., 2009; Del Parigi et al., 2004). Other studies have shown that successful dieters, when compared to non-dieters, have greater Positron Emission Tomography (PET) activation in the dorsolateral PFC and OFC in response to meal consumption (Del Parigi et al., 2007) and have greater PET or functional Magnetic Resonance Imaging (fMRI) activation to high-calorie food pictures in prefrontal regions implicated in inhibitory control (Le et al., 2007; McCaffery et al., 2009), than currently obese individuals and never-obese healthy controls. Some authors suggest that this pattern of neural activity indicates enhanced inhibitory processing of food cues in order to promote successful weight-loss and weight maintenance (Del Parigi et al., 2007; McCaffery et al., 2009). This idea is strengthened by the results of an fMRI study by Hare and colleagues (2009), who studied normal-weight dieters classified as “self-controllers”, who considered both health and taste when choosing foods, vs. “non-self-controllers”, who considered only taste. When the “self-controllers” had to decide whether to respond “yes” or “no” to tasty but unhealthful foods (for later possible consumption), they had greater BOLD activation in the inferior frontal gyrus/Brodmann Area (BA) 9 during successful self-control trials. However, these cross-sectional studies are unable to examine brain changes as a function of successful dieters’ weight loss.
In the present study, we used a longitudinal design to examine neural activation to food pictures in obese participants before and after a 12-wk behavioral, psychosocial weight-loss program. We used fMRI to investigate differences in activation of reward areas, inhibitory control regions, and whole brain among participants in a weight-loss program who varied in success at losing weight and maintaining weight loss. We hypothesized that high activity of reward and associated areas in response to high-calorie food pictures would be predictive of relative lack of success in weight loss and maintenance of weight loss. In particular, we expected that (1) greater reward system activation to high-calorie food pictures prior to the weight-loss program would predict less weight loss during the 12-wk program, (2) greater post-treatment activation would predict poorer maintenance of weight loss over a 9-mo follow-up period, and (3) participants showing the greatest decreases in activation from pre- to post-treatment would show the greatest weight loss during the treatment and would be the most successful at maintaining their weight loss during the follow-up period. Conversely, we hypothesized that in the preceding analyses, greater activation or greater increases in activation in inhibitory control areas would be associated with greater weight loss and more effective post-treatment weight maintenance.
2. Materials and Methods
2.1 Participants
Participants were 25 obese (n = 21) and overweight (n = 4) adults (19 women, 6 men; mean age ± SD: 48.0 ± 10.91) and 13 normal-weight controls (8 women, 5 men; mean age: 45.2 ± 10.01) (Table 1). The obese and overweight participants were enrolled in the 12-wk EatRight Lifestyle Weight Management Program at the University of Alabama at Birmingham (UAB). Body Mass Index (kg/m2), a proxy measure of adiposity using height and weight, was used to classify participants as normal-weight (BMI < 25), overweight (BMI ≥ 25 and < 30) or obese (BMI ≥ 30). The initial BMI range of the obese participants in this study was 28.4 to 44.6 (mean ± SD: 32.86 ± 3.82). Because only 4 out of the 25 EatRight participants actually had a BMI less than 30, classifying them in the overweight category, for brevity we will refer to this group as obese. Obese participants were recruited at one of the first three (of 12) EatRight classes, so that their first fMRI imaging session could be performed before they had a chance to implement many of the lifestyle changes that EatRight promoted. They were informed that the purpose of the study was to determine how the brain reacts to pictures of food in people who are overweight or obese and whether changes in brain activation patterns occur after participation in a weight-loss program. Normal-weight control participants were recruited from UAB and the surrounding community using flyers and advertisements posted in the UAB Reporter newspaper.
TABLE 1.
| Demographic and weight characteristics of overweight and obese participants (n = 25) | |
|---|---|
| Women; Men | 19 (76%); 6 (24%) |
| Caucasian; African American | 16 (64%); 9 (36%) |
| Age (years) | 48.04 ± 10.91a |
| Education (years) | 16.2 ± 2.20 |
| Initial BMI (kg/m2) | 32.86 ± 3.82 |
| Weight change from S1 to S2 (%)b | −3.46 ± 2.42 |
| Weight change from S2 to 1 yr (%)c | 0.75 ± 5.02 |
| Demographic and weight characteristics of control participants (n = 13) | |
|---|---|
| Women; Men | 8 (62%); 5 (38%) |
| Caucasian; African American | 9 (69%); 4 (31%) |
| Age (years) | 45.15 ± 10.01 |
| Education (years) | 16.46 ± 2.37 |
| BMI (kg/m2) | 22.64 ± 1.58 |
| Weight change from S1 to S2 (no program) (%)d | −0.80 ± 2.23 |
| Weight change from S2 to 1 yr (%)d | 1.60 ± 3.19 |
Mean ± Standard Deviation
Session 1 (S1) and Session 2 (S2)
n = 24
n = 11
Participants were excluded if they were left-handed (Edinburgh Handedness Inventory; Oldfield, 1971), and/or had an intelligence quotient < 80 on the Shipley Institute of Living Scale (SILS; Zachary, 1986). Participants were also excluded for having a chronic health or neurological condition, history of head injury or loss of consciousness for more than 5 min, history of psychosis, current or past alcohol or drug abuse problem, or being a cigarette smoker. As safety precautions, participants were also excluded for having ferromagnetic material in the body, being claustrophobic, or being pregnant. Also, because of our use of visual stimuli, participants were excluded for having worse than 20/40 vision not correctable with contact lenses or the non-ferromagnetic corrective glasses used at the magnet (range of ± 6 diopters). Participants were excluded for having blood pressure > 140/90 not controlled by medication, meeting the full criteria for any eating disorder (Eating Disorder Diagnostic Scale, EDDS; Stice et al., 2000, 2004), being concurrently enrolled in another weight-loss program, and/or any food preferences or allergies, as assessed in screening, inconsistent with our food pictures. For example, because many of the food pictures depicted meats, milk products, and chocolate, we excluded vegetarians as well as those who reported lactose intolerance or an allergic reaction to chocolate. Finally, we excluded immigrants for whom typical American foods were novel. Obese participants were also excluded for body weight of > 350 lbs (159 kg) and/or girth > 64 in (163 cm), as these exceeded the patient table and magnet bore limits. We included two obese participants who were on medication (metformin, glipizide, and/or lantus) to manage Type II diabetes. Because of the high prevalence of depression among individuals who are obese (Ohayon, 2007), we also included three obese participants who were being treated with anti-depressants (selective serotonin reuptake inhibitor), but ensured that they had been on the medication for more than 6 months and did not change medication between the first and second scanning sessions.
2.2 UAB’s EatRight Lifestyle Weight Management Program
The EatRight Lifestyle Weight Management Program is a 12-wk psychosocial weight loss program offered by the University of Alabama at Birmingham (UAB) Department of Nutrition Sciences. Classes meet once a week for 90 minutes and involve educational, motivational, and behavioral components. Topics include goal setting, cognitive restructuring, record keeping, label reading, portion control, stress management, and exercise. The overarching theme of the program is to encourage the replacement of high-energy-dense foods, those high in sugars and fats, by more high-bulk, low-energy-dense foods (Rolls et al., 2005). Registered nutritional specialists conduct the sessions, and medical supervision is provided by board-certified physicians. The program also includes a behavioral intervention component that focuses on cognitive factors that may play a role in creating barriers to achieving these lifestyle dietary changes. Although the program promotes a less sedentary lifestyle (e.g., taking the stairs instead of the elevator), it has no structured exercise component.
2.3 Stimuli
Visual stimuli consisted of 504 high-quality color photographs selected from previous studies (Stoeckel et al., 2007, 2008) with additional comparable photographs taken with a digital camera or scanned from popular food magazines. All images were converted to the same resolution (800 × 600) and luminance matched. The 504 pictures were randomly divided into two sets of 252 pictures, including 168 food images and 84 control images, for each of the pre- and post-scanning sessions. The 168 food images were further subdivided into 84 depicting high-calorie foods and 84 depicting low-calorie foods. The high-calorie food images consisted of an equal number of sweet foods, such as a slice of chocolate cake or cheesecake, and savory foods, such as a cheeseburger or french fries. The low-calorie food images consisted of low-fat foods, such as broiled fish or salad. The control images consisted of pictures of cars; these images were moderately engaging control stimuli that matched the low-calorie food images in ratings of pleasantness (Stoeckel et al., 2007). To minimize habituation, no image was shown more than once.
2.4 Procedure
After participants were recruited, initially screened for eligibility, and had given informed consent, they were scheduled for a laboratory session at which they completed measures to assess additional study requirements, such as computed BMI, maximum body girth, intellectual functioning (Shipley Institute of Living Scale, Zachary, 1986), and blood pressure. Participants were scheduled for two separate fMRI imaging sessions, one within the first 3 wk of starting the EatRight program and another within 1 wk after the program ended. Control participants were also imaged twice, 12 wk apart, but did not participate in the EatRight program.
Imaging sessions took place between 16:00 and 19:00. Following our standard protocol (Stoeckel et al., 2008), participants were instructed to eat a normal breakfast and then refrain from eating for 8 h before being imaged. Although 8 h is longer than the typical breakfast-to-lunch or lunch-to-breakfast eating gap, it is comparable to the gap between dinner or post-dinner snack and breakfast. Participants were also instructed to abstain from alcohol for 24 h prior to the imaging session, to avoid exercise on the day of the study, and to avoid caffeine for 3 h prior to imaging. Participants were called during the evening before each scan to remind them of the instructions. Before the scans, each participant completed a visual analog scale (VAS) measuring hunger (as in Stoeckel et al., 2008) and detailed the time and content of their last meal. Weight and blood pressure were measured, and each female participant was administered a urine-based pregnancy test to confirm that she was not pregnant.
Participants then entered the magnet for the functional MRI scans. Memory foam inserts were placed inside the head coil to limit participant head movement. Visual stimuli were presented in a block design format, with a total of six 3:15 min runs for both the pre- and post-treatment scanning sessions. Each run consisted of two pseudorandomly presented epochs each of car and low-calorie food images, and one epoch each of high-calorie sweet food and high-calorie savory food images (see Figure 1 in Stoeckel et al., 2008). Each individual epoch lasted for 21 s and included 7 individual images presented for 2.5 s followed by a 0.5 s gap. Each epoch was separated by 9 s of blank screen with a fixation cross. Each run began with 15 s of blank screen and ended with 9 s of blank screen. Each run consisted of 65 volumes for a total of 84 time points over the 6 runs for each main picture category. The stimuli were presented by a Dell Latitude D630 laptop computer using VPM software (Cook et al., 1987). The laptop was connected to a Sony LCD projector (VPLPX35) with a Navitar long-throw lens. The visual stimuli were presented on a projection screen behind the participant’s head at the rear of the scanner and viewed via a 45° single-surface rear-projecting mirror attached to the head coil. The images subtended visual angles of 10° × 13°.
FIGURE 1.
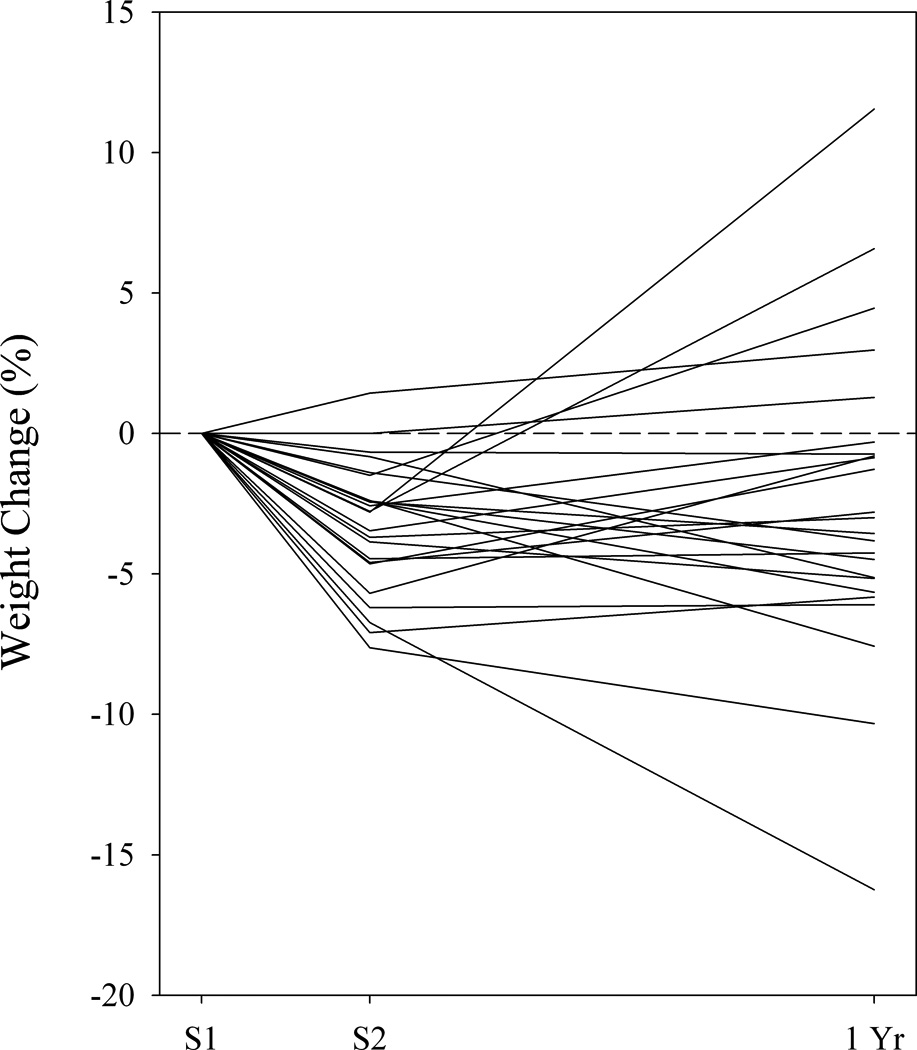
Weight trajectory, indexed by percent weight change, of the obese group at Session 1 (S1), immediately after the EatRight program ended at Session 2 (S2), and 9-mo after the EatRight Program ended, 1 yr from session 1 (1 Yr) (n = 24).
Following the scans, participants completed a memory task in which they were asked to sort 60 laminated color pictures of foods and cars into two piles, those seen in the magnet and those not seen. Half of the images were a subset of those presented in the magnet, and the other half were novel. The purpose of this task was to assess whether participants had attended to the images while in the magnet. Also, participants rated a subset of the food images for appetitive motivation and emotional valence (Stoeckel et al., 2008), results of which are not included here. All but one obese participant returned for a brief lab visit 9 mo later, that is, 1 yr after the start of the EatRight class, for a final body weight measurement. Participants were financially compensated for their participation at the initial laboratory session, the pre- and post-treatment scanning sessions, and the 1-yr follow-up visit. All procedures were reviewed and approved by UAB's Institutional Review Board for Human Use.
2.5 MRI Data Acquisition
Structural and functional MRI data were collected using a Philips Achieva 3 Tesla ultra-short (1.57 m) bore magnet, housed in UAB's Cardiovascular MRI facility. During the time of the study, the Cardiovascular MRI facility upgraded the scanner from a 6-channel to an 8-channel head coil, so that half of our participants were imaged using the 6-channel and half with the 8-channel head coil; subsequent data analyses indicated no effect of statistically controlling this variable. For each participant, the same head coil was used for both scanning sessions. For structural imaging, high resolution T1-weighted scans were acquired using a 160-slice 3D T1 Turbo-field Echo (TFE) volume sequence with T1 = 400ms, TR = 9.9 ms, TE = 4.6 ms, flip angle = 8°, FOV = 24 cm, 256 × 256 matrix size, and 1 mm slice thickness. These were used to exclude any participants with gross brain abnormality. Functional MR images were acquired using single-shot gradient echo T2*-weighted echo-planar imaging (EPI) with blood oxygenation level dependent (BOLD) contrast. We used TR = 3 s, TE = 30 ms, an 85° flip angle, a scan resolution of 80 × 79, reconstructed to 128 × 128, with a 230 × 149 × 230 mm FOV. Each volume comprised 30, 4 mm axial slices with a 1 mm interslice gap and a voxel size of 3.75 × 3.75 × 5 mm. The first 2 scans were discarded to allow for the BOLD signal to stabilize. The start of each run of visual stimuli was manually synchronized with the MRI data acquisition.
2.6 fMRI Data Analysis
Data were preprocessed and analyzed using SPM8 (Wellcome Department of Cognitive Neurology, University College London, London, UK) run within Matlab (7.9; Mathworks, Inc.). Functional images were slice time-corrected to the onset of the 15th slice (middle slice) and spatially realigned using INRIAlign, a motion correction algorithm unbiased by local signal changes (Freire et al., 2002). During realignment, a mean functional image was computed for each run and then matched to the EPI template provided within SPM8. Data were then spatially normalized to standard Montreal Neurological Institute (MNI) brain space and spatially smoothed using a three-dimensional Gaussian kernel of 6 mm full-width at half-maximum (FWHM), resulting in a resampled in-plane resolution of 2 × 2 × 2 mm voxels. The functional data were then passed through a 1/128 Hz high-pass filter in order to remove low-frequency artifacts, as well as temporally filtered using an autoregressive (AR(1)) model. No participant’s data set failed to meet the movement inclusionary criteria, which were that within-run movement before correction did not exceed 2 mm in translational movement and 2° in rotational movement.
Single-subject contrast maps were generated within the context of the General Linear Model (GLM) on a voxel by voxel basis as implemented in SPM8. Separate regressors were created for high-calorie food, low-calorie food, and car images by convolving the time course of activation, using a boxcar function, with the canonical hemodynamic response function (HRF). Fixed-effects analyses were performed for each individual participant for both imaging sessions, examining the variance in regional BOLD response to high-calorie foods vs. cars and high-calorie foods vs. low-calorie foods. These analyses allowed us to identify regions that were more activated when the participant was viewing high-calorie food images than the low-calorie food or control (car) images. There were no significant differences overall between activation in response to high-calorie foods and low-calorie foods, and activation to low-calorie foods did not differ between groups, so the high-calorie food versus car contrasts were emphasized in regression analyses related to weight change.
Second level (group) analyses were performed using a random-effects model of the beta images from the single-subject contrast maps. The contrast maps were smoothed prior to analysis with a three-dimensional Gaussian kernel of 6 mm FWHM, achieving a smoothing of 7.25 mm FWHM at the second level. In addition, we generated union masks (using the ImCalc function within SPM8) of the single-subject contrast maps for the pre-treatment scanning session (Session 1) vs. post-treatment scanning session (Session 2) data for each participant. These higher level subtractions allowed us to identify regions in which high-calorie food images became more or less effective in eliciting activation after treatment. For these analyses, two control participants, both men, had to be excluded because they did not return for the second scanning session. Additional between-group comparisons were run to determine whether the contrast estimates for both the sessions separately and Session 1 vs. Session 2 were significantly different between the obese participants and controls.
The analyses of primary interest were multiple regressions relating activation for the high-calorie foods vs. cars contrast to percent weight change using data from the obese group only. In all analyses, initial BMI was included as a covariate. Specifically, the analyses were: (1) activation at Session 1 vs. 12-wk weight change, (2) Session 1 minus Session 2 activation vs. 12-wk weight change, (3) Session 2 activation vs. weight change from the end of the program until the final weight measurement, subsequently referred to as 9-mo weight change, and (4) Session 1 minus Session 2 activation vs. 9-mo weight change. In the two analyses of 9-mo weight change, weight change during the program (12-wk change) was added as an additional covariate. Finally, because of previous prospective studies of participants with a wide range of BMI’s (Batterink et al., 2010, Stice et al., 2010a), we analyzed Session 1 activation in the control group vs. 12-wk weight change and Session 2 activation vs. 9-mo change (see Supplementary Material).
Statistical parametric maps were derived from the resulting t values associated with each voxel. Statistical maps were superimposed on normalized T1-weighted images from SPM8. For cluster-level inference, extent threshold was set such that FDR ≤ 0.05 for number of contiguous voxels with p ≤ 0.01. Voxel-level inference used a threshold corrected at FWE ≤ 0.05.
We performed both regions of interest (ROI) and whole-brain analyses. ROI analyses were used to improve statistical power and address our a priori hypotheses. In those regions subsequently referred to as reward ROI’s, we hypothesized that activation would be positively correlated with weight change. That is, those individuals who were least successful at losing weight would have greater activation in regions implicated in reward processes. These ROI’s included areas within the classically defined reward system as well as functionally related areas found to be activated in similarly designed fMRI studies (e.g., Rothemund et al., 2007; Stoeckel et al., 2008). All reward ROI’s were bilateral and included anterior cingulate cortex (ACC), amygdala, caudate/putamen, hippocampus, insula, medial prefrontal cortex (MPFC), nucleus accumbens (NAc), and ventral tegmental area/substantia nigra (VTA/SN). We predicted that activation in inhibitory control ROI’s would be negatively correlated with weight change. That is, those individuals who were most successful at losing weight would have greater activation in regions implicated in inhibitory control processes. These ROI’s included superior frontal gyrus (SFG), middle frontal gyrus (MFG), and inferior frontal gyrus (IFG), as other neuroimaging studies have found frontal regions to be activated in post-obese, successful weight-loss maintainers (DelParigi et al., 2007; Le et al., 2007; McCaffery et al., 2009) or in dieters exercising self-control about food choices (Hare et al., 2009). Motor cortex (Brodmann areas 4 and 6) was excluded from these ROI’s. Both sets of ROIs were defined structurally using templates from the WFU Pickatlas toolbox within SPM8 using the AAL or Talairach Daemon atlases (Lancaster et al., 2000; Maidjian et al., 2004). The exceptions included the nucleus accumbens, for which we used a predefined sphere (see Stoeckel et al., 2008), and the VTA/SN, for which a composite ROI was created using a predefined sphere for the VTA (see Stoeckel et al., 2008) and the SN as defined in the WFU Pickatlas.
3. Results
3.1 Demographics, Weight Change and Behavioral Assessments
Our participants’ characteristics reflected those generally represented within the EatRight Program (Table 1), that is, mostly female (76% in our sample), Caucasian (64%), and middle-aged (mean ± SD = 48.0 ± 10.9 yr) (cf. Greene et al., 2006; Cox et al., 2007). No statistically significant differences between the control and obese groups were observed for age (t(36) = −0.79, p = 0.433), years of education (t(36) = 0.34, p = 0.737), race (χ2 (1) = 0.10, p = 0.747), or gender (χ2(1) = 0.31, p = 0.579).
As expected, the obese participants, who attended the EatRight program classes, lost significantly more weight relative to their initial weights than the controls (control mean: −0.80%, obese mean: −3.46%, t(34) = 3.11, p = 0.004). Within the obese group, the differences in body weight between Session 1 and Session 2 were highly significant (t(24) = 6.76, p < 0.0001); 8% remained stable, 88% lost weight, and 4% gained weight (Figure 1), and were consistent in magnitude with the pre- to post-treatment weight changes that have been previously reported for this program (Svetkey et al., 2008; Ard et al., 2010). Within the control group, on the other hand, there was no significant change in body weight from Session 1 to Session 2 (paired t-test, t(10) = 1.12, p = 0.29); 9% remained approximately weight stable (±1%), 64% lost weight, and 27% gained weight. In addition, in the obese group, the percent change in body weight at the end of the 12-wk program was not significantly different between Caucasians and African Americans (CA mean: −4.05, AA mean: −2.40, t(23) = 1.70, p = 0.11), or between men and women (mean for men: −2.90, mean for women: −3.64, t(23) = 0.64, p = 0.53), nor was it related to age (r = −0.22, p = 0.29). In the obese group (n = 24), mean (± SD) weight change in the 9 mo from Session 2 to the 1-yr weighing was +.75 ± 5.02% (range = −10.20 to +14.76%), with 17% remaining approximately stable (±1%), 38% losing additional weight after treatment ended, and 46% gaining weight (Figure 1). In the control group (n = 11), over the same period, mean (± SD) weight change was +1.60 ± 3.19%, with 27% remaining stable, 18% losing weight, and 55% gaining weight (not shown).
The pre-scan mean hunger rating was 96.5 on the 0–150 VAS rating scale, and was thus generally consistent with the moderate hunger that would be experienced after an 8-h fast. There were no significant between-group differences on the hunger ratings. The average percentage of pictures correctly identified as having been seen during each fMRI scan was greater than what would be expected by chance, with chance being 50% (Session 1 mean: 61.7%, t(35) = 10.07, p < 0.0001, Session 2 mean: 59.0%, t(35) = 8.08, p < 0.0001), suggesting that the participants were paying attention to the pictures while in the scanner. For both the controls and obese participants, there were no differences in memory performance scores from Session 1 to Session 2 (t(35) = 0.89, p = 0.38). Also, there were no between-group differences in memory scores at either session (p’s > .45).
3.2 Session 1 fMRI Results
In the obese group, reward ROI analysis revealed greater activation to high-calorie food images compared to control (car) images in the insula, amygdala, and ACC (Table 2). Whole-brain analysis, in the obese group, of activation in response to high-calorie food images vs. control images revealed many regions activated, including reward associated regions, such as the insula, ACC, and amygdala (also significant by ROI analysis), as well as regions such as the inferior parietal lobule (IPL), middle occipital cortex, postcentral gyrus, inferior temporal gyrus (ITG), and IFG (Table 2). Within the control group, reward ROI analysis revealed no regions more strongly activated by high-calorie food images than by control images. Whole brain analysis revealed that high-calorie food images elicited greater activation than control images in a cluster that included the tail of the caudate nucleus and in a number of cortical regions, such as the inferior occipital cortex, IFG, and superior parietal lobule (SPL) (Table 2). Thus, the control group showed fewer areas of activation in reward-associated regions than the obese group. Nevertheless, the qualitative differences between the obese and control groups observed in their within-group analyses were not apparent in the between-group comparison. Group analyses revealed no significant group differences for the high-calorie foods vs. control contrast.
TABLE 2.
Within-group comparisons for the obese group (n = 25) and control group (n = 13), contrasting greater activation to the high-calorie food images than the car images at session 1.
| Group | Reward ROI Analysis | BAa | Hemb | kc | xd | Y | z | t | p, FWE correctede |
|---|---|---|---|---|---|---|---|---|---|
| Obese | Insula | L | 113 | −36 | −2 | −2 | 5.54 | 0.046 | |
| R | 233 | 38 | −6 | 6 | 4.40 | 0.003 | |||
| Amygdala | L | 45 | −20 | −2 | −22 | 4.52 | 0.036 | ||
| Anterior Cingulate Cortex | R | 149 | 2 | 36 | 14 | 4.11 | 0.014 | ||
| Whole Brain Analysis | BA | Hem | k | x | Y | z | t | p, FDR correctedf | |
| Insula | L | 152 | −36 | −2 | −2 | 5.54 | 0.037 | ||
| R | 657 | 38 | 4 | −18 | 4.98 | 0.00002 | |||
| Amygdala | L | 232g | −20 | −2 | −22 | 4.62 | 0.012 | ||
| Anterior Cingulate Cortex | 32 | R | 199 | 2 | 36 | 14 | 4.11 | 0.016 | |
| Middle Cingulate Cortex | L | 346 | −6 | −6 | 28 | 4.41 | 0.001 | ||
| Inferior Parietal Lobule | 40 | L | 745 | −52 | −32 | 42 | 4.35 | 0.000008 | |
| Middle Occipital Cortex | L | 203 | −26 | −86 | 14 | 4.35 | 0.016 | ||
| Inferior Temporal Gyrus | 19,37 | L | 4051 | −50 | −70 | −8 | 8.06 | 6.51 × 10−20 | |
| Superior Temporal Gyrus | L | 232g | −36 | 4 | −20 | 4.58 | 0.012 | ||
| Inferior Frontal Gyrus | 47 | L | 232g | −26 | 12 | 18 | 3.93 | 0.012 | |
| 45 | L | 202 | −50 | 36 | 4 | 4.86 | 0.016 | ||
| Perirhinal Cortex | 35 | R | 476 | 26 | −22 | 24 | 3.92 | 0.0002 | |
| Postcentral Gyrus | R | 429h | 52 | −28 | 42 | 4.88 | 0.00004 | ||
| 1,2 | R | 429h | 66 | −20 | 34 | 4.24 | 0.00004 | ||
| Premotor Cortex | 6 | R | 429h | 62 | −18 | 42 | 4.23 | 0.00004 | |
| Group | Whole Brain Analysis | BA | Hem | k | x | Y | z | t | p, FDR corrected |
| Control | Caudate (Tail) | R | 298 | 20 | −32 | 14 | 6.68 | 0.003 | |
| Inferior Occipital Cortex | L | 6149i | −46 | −64 | −14 | 8.99 | 1.20 × 10−27 | ||
| R | 6149i | 40 | −88 | 10 | 8.08 | 1.20 × 10−27 | |||
| Inferior Frontal Gyrus | 47 | L | 255 | −46 | 30 | 10 | 5.56 | 0.006 | |
| R | 322 | 48 | 34 | −2 | 5.63 | 0.003 | |||
| Superior Parietal Lobule | 7 | R | 566 | 34 | −56 | 56 | 4.80 | 0.00008 | |
| Superior Temporal Gyrus | L | 291 | −36 | 10 | −18 | 5.48 | 0.003 | ||
| Precuneus | 7 | L | 329 | −26 | −70 | 32 | 5.55 | 0.003 | |
| Postcentral Gyrus | L | 306 | −54 | −24 | 26 | 4.49 | 0.003 | ||
| Precentral Gyrus | R | 199 | 48 | 14 | 8 | 3.57 | 0.016 | ||
| Thalamus | L | 245 | −8 | −28 | 0 | 4.90 | 0.006 | ||
Brodmann area
Hemisphere: R, right, L, left
Number of contiguous voxels with p < 0.01
x, y, and z coordinates in MNI space
Family-wise error corrected at the peak voxel level
False discovery rate corrected at the individual cluster level
Regions of activation encompassed within the same cluster
3.3 Changes in fMRI Activation from Session 1 to Session 2
For the obese group, the reward ROI analysis revealed less activation to high-calorie food pictures in the MPFC at Session 2 than at Session 1 (Table 3). Whole-brain analysis revealed additional clusters in IPL, precuneus (PrC), posterior cingulate cortex, premotor cortex (Brodmann Area 6), and angular gyrus (Table 3). For the obese participants, there were no regions with greater activation to high-calorie food pictures in Session 2 compared to Session 1. Within the control group, there were no significant differences in activation between Session 1 and Session 2 for the high-calorie foods vs. control contrast. A between-group comparison using the IFG ROI mask revealed a significant Group × Session interaction for the left IFG (FDR = 0.019; not shown). However, analysis of contrast estimates from the peak voxel revealed that activation in the obese group was not significantly different for Session 1 vs. Session 2 and that the interaction was carried by a significant decrease in activity in Session 2 in the controls.
TABLE 3.
Decreased activation from session 1 to session 2 for the high-calorie food vs. control contrast in the obese group (n = 25).
| Reward ROI Analysis | BA | Hem | k | x | y | z | t | p, FWE corrected |
|---|---|---|---|---|---|---|---|---|
| Medial Prefrontal Cortex | 32 | R | 120a | 4 | 22 | 42 | 5.75 | 0.011 |
| L | 120a | −6 | 30 | 40 | 5.44 | |||
| Whole Brain Analysis | BA | Hem | k | x | y | z | t | p, FDR corrected |
| Medial Prefrontal Cortex | 32 | R | 221b | 4 | 22 | 42 | 5.75 | 0.023 |
| L | 221b | −6 | 30 | 40 | 5.44 | 0.006 | ||
| Inferior Parietal Lobule | 40 | R | 253 | 54 | −38 | 40 | 5.38 | 0.018 |
| Precuneus | 7 | R | 227 | 18 | −72 | 46 | 4.98 | 0.023 |
| R | 407 | 22 | −50 | 48 | 4.01 | 0.004 | ||
| Posterior Cingulate Cortex | 23 | L | 279 | −2 | −32 | 28 | 5.12 | 0.014 |
| 31 | R | 6 | −34 | 40 | 3.53 | |||
| Premotor Cortex | 6 | R | 344 | 44 | 4 | 42 | 4.48 | 0.006 |
| Angular Gyrus | R | 177 | 40 | −74 | 30 | 3.14 | 0.047 | |
Regions of activation encompassed within the same cluster
Other conventions as in Table 2
3.4 Relationship Between fMRI Activation at Session 1 and 12-wk Weight Change in Obese Participants
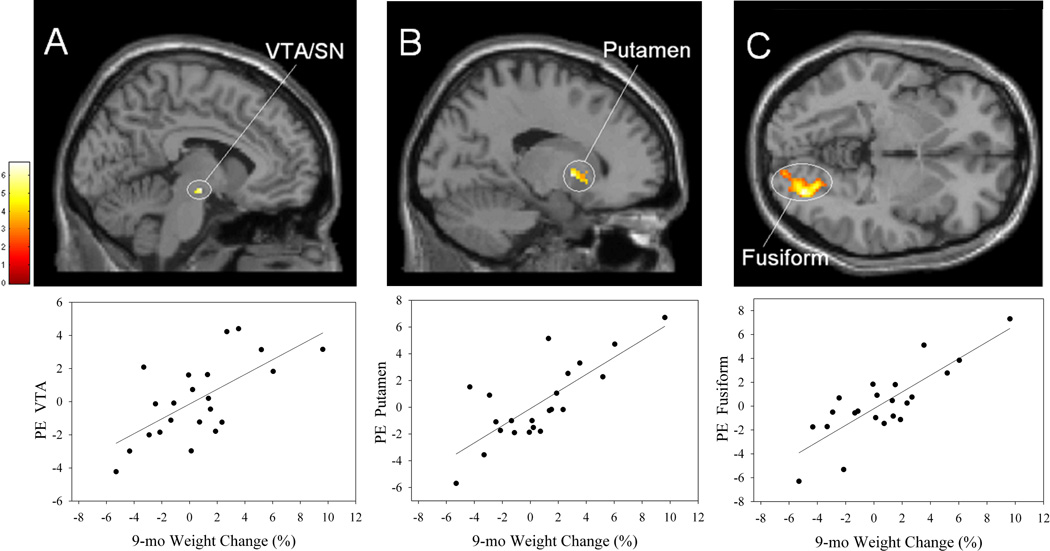
Multiple regression analysis was performed to determine whether weight loss during the EatRight Program was related to activation to high-calorie foods vs. control images at the initial scan. Reward ROI analysis showed a significant positive correlation within the obese group between weight change and activation of the NAc at the peak voxel level (Table 4; Figure 2A). Whole-brain analysis revealed positive correlations in clusters which entirely or partly involved three reward areas (ACC, frontal operculum, and insula; Figure 2B), three visual processing areas in the occipital lobe (calcarine cortex, Figure 2C; superior occipital cortex, lingual gyrus), areas mediating attentional processes (Brodmann Area 8 within the MFG, Figure 2B; superior parietal lobule, SPL, Figure 2C; and inferior parietal lobule, IPL) (Lane et al., 1999; Pessoa et al., 2002), as well as middle temporal gyrus (MTG) and cerebellum (Table 4). As indicated in the Figure 2 scatterplots, these and, in fact, all significant correlations, reflect continuous functions relating activation and weight loss. In all analyses in which outliers were identified (using SYSTAT 11), the correlations were not substantially affected by their removal and remained significant. Parenthetically, when similar analyses were performed using the low-calorie food versus control contrast, the only significant result was a positive correlation with activation in the substantia nigra (from ROI analysis for VTA/SN; MNI coordinates of 16, −22, −6). The pattern of correlations indicates that less success in losing weight was associated with greater activation in brain areas listed in Table 4 in response to high-calorie food images. No significant negative correlations were revealed in whole-brain or reward ROI analyses, nor in inhibitory control ROI’s.
TABLE 4.
Significant positive correlations between percent 12-wk weight change during the EatRight Program and activation in session 1 for the obese participants’ contrast of high-calorie food vs. control images (n = 25).
| Reward ROI Analysis | BA | Hem | k | x | Y | z | t | p, FWE corrected |
|---|---|---|---|---|---|---|---|---|
| Nucleus Accumbens | R | 40 | 2 | 6 | −10 | 4.53 | 0.018 | |
| Whole Brain Analysis | BA | Hem | k | x | Y | z | t | p, FDR corrected |
| Insula | 38 | R | 242a | 44 | 12 | −16 | 4.00 | 0.016 |
| Frontal Operculum | 45 | R | 242a | 46 | 18 | 6 | 3.44 | 0.016 |
| Middle Cingulate Cortex | 32 | L | 211b | −2 | 10 | 42 | 4.11 | 0.026 |
| R | 211b | 4 | 4 | 42 | 3.54 | 0.026 | ||
| Anterior Cingulate Cortex | L | 211b | −8 | 18 | 28 | 2.95 | 0.026 | |
| Superior Parietal Lobule | L | 350 | −20 | −56 | 56 | 4.90 | 0.004 | |
| R | 303 | 18 | −58 | 54 | 4.94 | 0.007 | ||
| Inferior Parietal Lobule | 40 | L | 165 | −46 | −36 | 42 | 3.54 | 0.047 |
| Middle Frontal Gyrus | 8 | R | 185 | 44 | 10 | 52 | 4.43 | 0.036 |
| Calcarine Cortex | 31 | R | 498 | 2 | −68 | 16 | 5.03 | 0.001 |
| Lingual Gyrus | L | 192 | −12 | −64 | −4 | 4.48 | 0.035 | |
| Superior Occipital Cortex | L | 259 | −24 | −76 | 26 | 4.75 | 0.013 | |
| Cerebellum | L | 345 | −6 | −28 | −30 | 4.56 | 0.004 | |
| Middle Temporal Gyrus | 21 | R | 177 | 60 | −44 | −2 | 4.16 | 0.039 |
Regions of activation encompassed within the same cluster
Other conventions as in Table 2
FIGURE 2.
Top row, brain areas showing significant positive correlations between percent weight change during the 12-wk EatRight Program and activation at session 1 to high-calorie food pictures > control pictures in the obese participants (n = 25) in the (A) nucleus accumbens (NAc); (B) left anterior cingulate cortex (ACC), right middle frontal gyrus (MFG), right insula (Ins); and (C) bilateral superior parietal lobule (SPL), right calcarine cortex (Calc). A identified from the reward ROI analyses, and B and C identified from the whole brain analyses. Activation is overlaid on the SPM8 single-subject T1 template. The bar indicates t values. Bottom row, scatterplots of activation (PE, parameter estimate) with regression lines of one of the regions activated in the brain image above versus 12-wk weight change. For illustrative purposes, A, r = .61; B, r = .68; C, r = .68.
3.5 Relationship Between the Difference in fMRI Activation at Sessions 1 and 2 and 12-wk Weight Change
Multiple regression with Session 1 minus Session 2 activation as a regressor revealed no significant correlations with weight change in ROI or whole-brain analyses.
3.6 Relationship Between fMRI Activation at Session 2 and Weight Maintenance in Obese Participants
Multiple regression analysis sought to determine whether maintenance of treatment-reduced weight loss over the 9 mo post-treatment period was related to activation at Session 2. Preliminary analyses revealed two outliers with regard to 9-mo weight change (Figure 1), which were ≥ 1 σ beyond their nearest neighbors in the distribution of weight change scores. These scores were excluded from this analysis because they would have exerted inordinate leverage in regression calculations.
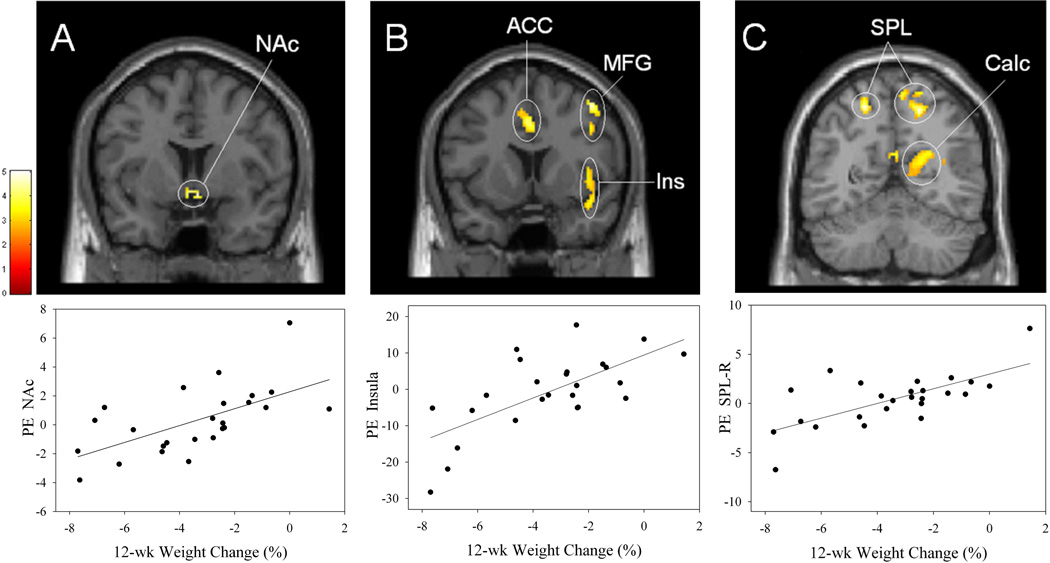
As shown in Table 5, reward ROI analysis revealed positive correlations between 9-mo weight change and activation within VTA/SN (Figure 3A) and putamen (Figure 3B). Whole-brain analysis revealed significant positive correlations with activation in numerous clusters. These clusters included three within reward areas (putamen, insula, and hippocampus), three within visual processing areas (superior occipital gyrus, cuneus, and fusiform gyrus; Figure 3C) and one in an area implicated in attention, the IPL. No significant negative correlations were found. To ensure that our findings did not depend on decisions about outliers, we also performed robust regression analyses (least median of squares procedure in SYSTAT 11) on data from all 24 obese participants (i.e., with the two weight-change outliers included) and using contrast estimates from peak voxels listed in Table 5. In all cases, this procedure found strong, positive relationships between activation and 9-mo weight change. Finally, there were no significant results in SPM when regression analyses related the low-calorie food versus control contrast to 9-mo weight change.
TABLE 5.
Significant positive correlations between 9-mo percent weight change following the EatRight Program and activation in session 2 for the obese participants’ contrast of high-calorie food vs. control images (n = 22).
| Reward ROI Analysis | BA | Hem | k | x | y | z | t | p, corrected |
|---|---|---|---|---|---|---|---|---|
| Putamen | L | 334 | −16 | 6 | 0 | 5.18 | 0.014a | |
| VTA/SNb | L | 12 | −6 | −12 | −10 | 4.05 | 0.024c | |
| Whole Brain Analysis | BA | Hem | k | x | y | z | t | p, FDR corrected |
| Putamen | R | 270d | 34 | −18 | −2 | 4.16 | 0.005 | |
| Insula | R | 270d | 32 | −20 | 18 | 4.15 | 0.005 | |
| Hippocampus | R | 270d | 32 | −24 | −8 | 3.64 | 0.005 | |
| Fusiform Gyrus | R | 223 | 30 | −80 | −4 | 6.69 | 0.013 | |
| Superior Occipital Gyrus | 19 | R | 204e | 36 | −86 | 26 | 4.30 | 0.017 |
| Cuneus | 19 | R | 204e | 28 | −86 | 26 | 3.18 | 0.017 |
| Inferior Parietal Lobule | 40 | L | 273 | −44 | −36 | 36 | 4.14 | 0.005 |
| Cerebellum | R | 156 | 6 | −80 | −22 | 6.42 | 0.043 | |
| R | 159 | 28 | −80 | −20 | 4.57 | 0.043 | ||
| Midbrain | L | 357 | −8 | −10 | −10 | 4.90 | 0.001 | |
| Inferior Frontal Gyrus | L | 823 | −48 | 26 | 14 | 4.88 | 9.00 × 10−7 | |
| Middle Temporal Gyrus | R | 204e | 38 | −74 | 22 | 6.41 | 0.017 | |
Cluster-level, FDR corrected
Ventral Tegmental Area/Substantia Nigra
Voxel-level, FWE corrected
Regions of activation encompassed within the same cluster
Other conventions as in Table 2
FIGURE 3.
Top row, brain areas showing significant positive correlations between percent 9-mo weight change following the EatRight Program and activation at session 2 to high-calorie food pictures > control pictures in the obese participants (n = 22) in the (A) ventral tegmental area/substantia nigra (VTA/SN), (B) putamen, and (C) fusiform gyrus. A and B identified from the reward ROI analyses, and C identified from the whole brain analyses. Bottom row, corresponding scatterplots (A, r = .66; B, r = .77; C, r = .82). Conventions as in Figure 2.
3.7 Relationship Between the Difference in fMRI Activation at Sessions 1 and 2 and Weight Maintenance in Obese Participants
Multiple regression analysis revealed a significant negative correlation between 9-mo weight change and the Session 1 minus Session 2 difference in activation in the insula reward ROI (Table 6). Whole-brain analysis revealed negative correlations in the IFG and a large cluster with its peak voxel in the thalamus/midbrain (Table 6). Thus, participants whose activation in these areas at Session 2 was relatively low compared to Session 1 tended to be most successful at weight maintenance in the 9 mo after treatment. There were no significant positive correlations.
TABLE 6.
Significant negative correlations between 9-mo percent weight change following the EatRight Program and session 1 minus session 2 activation for the obese participants’ contrast of high-calorie food vs. control images (n = 22).
| Reward ROI Analysis | BA | Hem | k | x | y | z | t | p, FDR corrected |
|---|---|---|---|---|---|---|---|---|
| Insula | L | 107 | −36 | −2 | 8 | 4.42 | 0.011 | |
| Whole Brain Analysis | BA | Hem | k | x | y | z | t | p, FDR corrected |
| Thalamus/Midbrain | R | 1377a | 12 | −24 | −6 | 5.28 | 1.00 × 10−10 | |
| Putamen | L | 1377a | −30 | −12 | −6 | 5.05 | 1.00 × 10−10 | |
| L | 1377a | −18 | 18 | −6 | 4.80 | 1.00 × 10−10 | ||
| Inferior Frontal Gyrus | L | 411 | −42 | 26 | 14 | 4.52 | 0.001 | |
Regions of activation encompassed within the same cluster
Other conventions as in Table 2
4. Discussion
In our obese participants, we found similar results for prediction of post-treatment weight loss from pre-treatment brain activation to high-calorie food pictures and for prediction of 9-mo follow-up weight maintenance from post-treatment brain activation. In both cases, there were multiple clusters within reward areas, as well as areas in which activity has been shown to be modulated in relation to attentional salience of visual stimuli. In all clusters, greater activation predicted a poorer outcome: less weight loss or, for some individuals, weight gain. In addition, more successful weight management was associated with greater decreases in activation from pre- to post-treatment in the insula and putamen, as well as midbrain/thalamus and IFG. Comparable regression analyses performed using the contrast of low-calorie foods vs. control images revealed only one significant cluster for activation at Session 1 and 12-wk weight change, and no correlations between activation at Session 2 and 9-mo weight maintenance, suggesting that our results were specific to high-calorie foods. No significant correlations were found relating pre- to post-treatment changes in activation to weight loss during treatment. To our knowledge, this is the first prospective neuroimaging study that has found that cue-elicited brain activation can predict how successful obese individuals will be in losing weight and in subsequent weight maintenance. The observed pattern of activations is consistent with the conclusion that greater activation in brain regions mediating motivational and attentional salience of food cues is detrimental to weight control among obese individuals.
We also found evidence consistent with the hypothesis that participation in a weight-loss treatment results in a decrease in activity in regions involved in reward (MPFC; Ochner et al., 2011) or attentional (IPL; Rosenbaum et al., 2008) processes. Indeed, Rosenbaum et al. (2008) found a decrease in brain activation following weight loss in the MFG, IPL, and fusiform gyrus, although they also found increased activation after weight loss in regions implicated in reward processes. As discussed below, these outcomes raise the possibility that the changes we observed represent neuroadaptations resulting from participation in a weight-loss treatment. However, at this point, we are unable to comment on whether these changes reflect a cause or consequence of weight loss.
4.1 Prediction of Weight Loss and Weight Gain by Brain Activation to High-Calorie Food Pictures
Several reward ROI’s were among the brain areas that were more activated by high-calorie food images in those obese individuals who were least successful in losing weight. Indeed, our results are congruent with those of other neuroimaging experiments providing evidence for an “orexigenic network” that encompasses such regions as the insula, VTA, NAc, and ACC, which are more active during hunger and fasting and are thought to motivate one to consume calorically-dense foods (e.g., Del Parigi et al., 2002; LaBar et al., 2001; Uher et al., 2006). Animal studies have further defined the roles of reward areas in mediating appetitive behavior. For example, the NAc and its dopaminergic input from the VTA have also been implicated in reward-seeking behavior, including enabling motor movement towards, and attributing value and salience to, a reward (for review, see Nicola, 2007). In regards to reward-seeking behavior and food, the NAc has been found to promote food-seeking behaviors and increase appetitive motivation (Taha and Fields, 2005).
Two of the reward ROI’s that emerged repeatedly in our analyses as regions predicting body weight trajectory were the insula and the striatum, especially the putamen. High levels of pre-treatment activity in the anterior insula and adjacent frontal operculum predicted poor weight loss during treatment; high post-treatment activation of the insula predicted less weight loss over the 9-mo follow-up period; and a relative decrease in activation of the insula from pre- to post-treatment predicted better weight maintenance. Similar prediction of weight maintenance was afforded by bilateral activation of the putamen at post-treatment and the difference in activation from pre- to post-treatment. These regions have been found to respond to pictures of food or anticipated ingestion of palatable food (e.g., Bruce et al., 2010; O’Doherty et al., 2002; Pelchat et al., 2004; Rothemund et al., 2007; Stoeckel et al., 2008; Uher et al., 2006), and their responses to food cues are augmented by hunger (Siep et al., 2009). Indeed, the insula includes primary taste cortex, with greater activation in response to sweet and salty tastes (Spetter et al., 2010) and in response to flavors indicating nutrients (Rudenga et al., 2010). The striatum and insula have also been implicated in both food and drug craving (Garavan et al., 2000; Garavan, 2010; Naqvi et al., 2007; Naqvi and Bechara, 2010; Pelchat et al., 2004). Moreover, in scans performed prior to smoking cessation treatment, high activation in regions including the insula and putamen by smoking cues was predictive of relapse (Janes et al., 2010). In addition, greater activation to alcohol-related visual cues in the putamen (as well as ACC) was predictive of relapse in abstinent alcoholics (Grüsser et al., 2004). Therefore, it is possible that higher cue reactivity in the putamen and insula/operculum contributed to food cravings in our participants, which were counterproductive to success in subsequent weight loss and maintenance.
The studies most similar to ours are those by Stice and colleagues (2008a, 2010a, 2011), who have used fMRI to investigate future body weight trajectory as a function of brain activation in response to both food intake and food cues, although not in dieters. They have reported that activity in the putamen and frontal operculum could predict weight change over the succeeding year in female high school students with a mean BMI within the normal-weight range (24.6), although the direction of the relationship was moderated by genotype (Stice et al., 2010a). In those who lacked the TaqIA A1 allele of the dopamine D2 receptor (DRD2) gene, greater weight gain was predicted by greater activation of the putamen and frontal operculum, which is congruent with our results. A similar predictive relationship was also revealed for frontal opercular activity in individuals lacking the DRD4-7R allele. On the other hand, the signs of these relationships were in the opposite direction for those with the DRD2-A1 or DRD4-7R alleles. Thus, they argue that the significance of the responsiveness of reward circuitry to food cues is conditional according to a given individual’s level of dopamine signaling. It is an open question as to whether this moderating effect of genotype on spontaneous weight change in adolescents would also be observed in adults and/or those who are explicitly engaged in a weight-loss program. In an fMRI study that used an attention task with food stimuli, Yokum et al. (2011) found that the BMI of adolescent girls was positively correlated with activation in regions related to food reward, such as the anterior insula/frontal operculum and lateral OFC, and in regions related to attention, such as the superior parietal lobule. Activation in lateral OFC was also positively correlated with increase in BMI over one year.
Interestingly, the cerebellum also showed greater pre-treatment activation in those individuals who lost the least amount of weight. Among its other roles, the cerebellum has been implicated in motivation. For example, studies have found the cerebellum to be activated during cue-induced cocaine craving (Bonson et al., 2002), alcohol odor cues (Schneider et al., 2001), as well as in response to food stimuli (Killgore et al., 2003; Tataranni et al., 1999). Anderson et al. (2006) suggest that the cerebellum may also be involved in what they term a “hyperattentive state”. The cerebellum receives input from parietal, frontal, and visual brain regions implicated in attention, and selective attention to drug cues may underlie drug cravings and subsequent reward-seeking motor behaviors (Franken, 2003; Anderson et al., 2006). Indeed, the obese individuals in our study who were least successful at losing weight also showed greater activation in a number of brain regions implicated in attention.
Positive correlations between activation to high-calorie food vs. control images and subsequent weight change were found in a number of brain regions implicated in directing visual attention – IPL, SPL, and Brodmann Area (BA) 8 in the MFG (Behrmann et al., 2004; Fan et al., 2005; Shulman et al., 2009) – or in visual areas, where activity is enhanced by attention – fusiform gyrus, lingual gyrus, calcarine cortex, and superior occipital cortex (Bradley et al., 2003; Lang et al., 1998). Behavioral studies of attention, using eye-tracking and visual probe tasks, have found greater attentional bias, or selective attention, for food images in hungry normal-weight individuals (Piech et al., 2010) and in normal-weight individuals scoring high on a self-report measure of eating in response to external food cues (Brignell et al., 2008). Furthermore, hungry overweight or obese individuals have increased directed attention to food images or faster reaction times to food images and, after viewing such food images, show increased food intake compared to hungry normal-weight controls (Nijs et al., 2010; Yokum et al., 2011). Additionally, obese individuals continue to have sustained increased attention to food images even when sated (Castellanos et al., 2009).
Indeed, directed attention is thought to be influenced by an individual’s emotional and motivational state (Appelhans, 2009), with greater attentional resources given to motivationally salient stimuli, including food cues (Mogg et al., 1998). The fronto-parietal attention network, of which the MFG, SPL, and IPL are components, is suggested to be involved in top-down attentional control of the visual cortex, with a bias towards more emotionally-primed, or salient, stimuli (e.g., Pessoa et al., 2002). Neuroimaging studies have found greater activation of the visual cortex, including the calcarine cortex, in response to emotional pictures compared to neutral pictures (Lane et al., 1997), and emotional pictures produce functional activation in the occipital lobe and right IPL and SPL (Lang et al., 1998). Studies that have assessed motivational processes have found that increased incentive value of both food images (Mohanty et al., 2008) and monetary reward (Pessoa and Engelmann, 2010; Stice et al., 2011) is associated with increased activation in visual (e.g., calcarine sulcus, fusiform gyrus, and occipital cortices) and attentional (e.g., parietal cortex) brain regions. Paralleling our results, studies of drug addictions have found that greater activation in the IPL to drug cues immediately after initiation of abstinence can predict subsequent relapse in methamphetamine-dependent individuals (Paulus et al., 2005), cocaine-dependent individuals (Kosten et al., 2006) and nicotine-dependent individuals (Janes et al., 2010).
We found no support for the hypothesis that greater activation of putative inhibitory-control regions by high-calorie foods would be associated with better treatment outcome. Activation in most of our inhibitory control areas was unrelated to weight loss or maintenance, and less successful 9-mo weight maintenance was in fact predicted by greater post-treatment activation in left IFG. As a possible explanation for our failure to find activation in most inhibitory control areas related to weight loss or maintenance, prior studies relating activation in frontal inhibitory areas to successful weight maintenance (DelParigi et al., 2007; McCaffery et al., 2009) used participants with a history of substantially greater weight loss and longer-term maintenance than participants in the current study.
In addition, although the IFG has been implicated in inhibitory control processes (e.g., Swick et al., 2008; Hare et al., 2009), it may mediate other cognitive processes as well (e.g., Chouinard et al., 2009; Coull et al., 1996). For example, activity in IFG has been reported to positively correlate with participants’ valuation of food items during a decision task (Plassman et al., 2007; Hare et al., 2008). Moreover, anterior (although right side) IFG has been found to exhibit positive functional connectivity with ventromedial prefrontal cortex, a region also thought to encode choice value (Hare et al., 2009). Thus, our finding that greater activation of IFG by high-calorie food pictures was associated with poorer weight maintenance may be a reflection of the effect of placing high value on foods high in caloric density.
4.2 Treatment-related Changes in Brain Activation to High-Calorie Food Pictures
Our between-session results revealed differences in brain activation to high-calorie food images from pre- to post-treatment. In the obese group, activation to high-calorie food pictures decreased in the MPFC, premotor cortex (BA6), IPL, PrC, and posterior cingulate cortex (PCC). In contrast to a previous report in those who lost weight (Rosenbaum et al., 2008), no regions showed significantly increased activity. Our control group showed no differences in activation between sessions. On the basis of these comparisons, we suggest that the observed decreases in the obese group did, in fact, result from their weight-loss treatment. Admittedly, because our research design was not intended to be a controlled treatment outcome study, this conclusion must remain tentative. Nonetheless, it is interesting that several of the regions showing between-session decrease in activity have been implicated in reward (MPFC) and attention (IPL and PrC). Activation of the MPFC by high-calorie food pictures has been reported in studies of obese individuals (Stoeckel et al., 2008) and normal-weight controls (Killgore et al., 2003), and this region appears to be involved in processing the relative value of an anticipated reward or its subjective value (Amodio and Frith, 2006; Sripada et al., 2010). The MPFC also shows increased functional activation to images high in emotional arousal and positive valence, suggesting the MPFC’s involvement in emotional processing of visual stimuli and promotion of appetitive behaviors (Dolcos et al., 2004). The MFG (BA8) and IPL are both components of a fronto-parietal attention network, as mentioned previously (Pessoa et al., 2002). The PrC has also been suggested as involved in attention, specifically in “unfocused attention” or attention to introspective information not directly task related, such as hunger sensations (Stawarczyk et al., 2011). Of especial interest, Ochner et al. (2011) found decreased brain activation to high-calorie food cues in individuals 1-mo post-bariatric surgery in regions such as the MPFC, IPL, and PrC. Thus, it would be intriguing if further studies could substantiate that a cognitive/behavioral weight-loss treatment can, in effect, target regions such as these.
4.3 Comparison of Present Between-group Results with Those of Previous Studies
Comparison of our present results for obese versus normal-weight participants, at Session 1, to those of previous studies, in particular, Stoeckel et al. (2008), revealed some differences. For example, we did not find significant differences between low-calorie and high-calorie food images for either the obese or control group. More importantly, unlike Stoeckel and colleagues, we did not observe group differences to the high-calorie food vs. control contrast when the obese and control groups were compared at Session 1. Although our within-group results suggested that the obese group had greater overall brain activation and we found a larger number of reward system structures activated in obese participants than in controls, this difference was not significant in the between-group analysis. Differences in patterns of activation between the studies could be due to the greater heterogeneity of our current sample. Stoeckel et al. (2008) had a very homogenous sample of young adult women all in the follicular phase of their menstrual cycle. Our sample, on the other hand, was much more heterogeneous, including both men and women and a wide range of ages, particularly older individuals. Indeed, the addition of men may have diluted our results compared to those of previous findings (Stoeckel et al., 2008), as other studies assessing activation differences to high-calorie food images between men and women consistently find women to have greater activation to high-calorie food images than men in a number of brain regions, including prefrontal cortex, insula, cingulate cortex, and fusiform gyrus (Cornier et al., 2010; Frank et al., 2010; Killgore and Yurgelun-Todd, 2010). Also, the mean age of our participants was considerably greater than in previous studies (e.g., Bruce et al., 2010; Rothemund et al., 2007; Stice et al., 2011; Stoeckel et al., 2008), and the older age of our current sample may be an important contributor as to why we did not replicate previous findings. A previous study comparing individuals of different ages found that older adults showed less activation in reward areas, as well as other regions, during reward anticipation and at the time of reward delivery (Dreher et al., 2008). Admittedly, we did not find significant correlations between age and activation of reward areas. However, another potentially important factor that is likely to be correlated with age is chronicity of obesity, which was likely to have been much greater in our participants than in studies with younger samples. Evidence is accumulating that overconsumption and weight gain can blunt the activation of reward areas by food (Colantuoni et al., 2001; Johnson and Kenny, 2010; Stice et al., 2010b; Val-Laillet et al., 2011; Wilcox et al., 2010). Thus, the lack of group differences in activation in the current study may have been a consequence of the age and weight histories of our obese participants.
An implication of the discussion above is that, even though hyperreactivity of reward circuitry may be important for the development of obesity, it may not be required for maintenance of obesity over a long period of time. However, combining the outcomes of our group comparison and our correlational analyses leads to the view that, even in the absence of greater-than-control reactivity to food cues in the chronically obese, less may be more with regard to weight loss and subsequent weight maintenance. That is, whatever other factors contribute to the continuation of chronic obesity, low reward and attention-related reactivity may be conducive to good treatment outcome.
4.4 Caveats
It is important to note some of the limitations of this study. In general, we had considerable heterogeneity in our obese and control groups, in that our participants varied in age, ethnicity, and gender, which may have introduced variance that obscured further differences. In addition, three of the obese participants were on anti-depressants and two had Type II diabetes. However, this heterogeneity could also be considered a strength of the study, as our sample was more reflective of the general population. It should be noted that we did not find any significant correlations between age, gender, or ethnicity and weight-loss. In addition, because of the constraint of scanning our obese participants as soon as possible after the beginning and end of the 12-wk weight-loss program, we were unable to image three of our 19 female participants in the same phase of the menstrual cycle at pre- and post-treatment, and menstrual phase has been shown to affect neural reward-associated activation (Dreher et al., 2007). We were also unable to assess gender differences in functional activation because of our small sample of men (obese group: n = 6, control group: n = 5).
Another limitation is that we relied solely on change in BMI as a measure of weight loss. Although promoting more exercise is part of the EatRight program, its main emphasis is on changes in diet. While it is conceivable that some weight loss could have been masked by gains in muscle mass, we do not believe this to be the case. After the second imaging session, participants were asked about exercise practices during the 12-wk weight loss program. No participant reported engaging in an exercise regimen, and all participants reported that they felt their weight loss was due to dietary changes, not exercise. Another limitation is that we had no measure of compliance to the requested 8-hr pre-scan fasting other than self-report data. Finally, we studied individuals in a fasted state only, and it would be interesting in future studies to compare results obtained with individuals in both a fasted and sated state.
5. Conclusions
In summary, we found that activation suggestive of greater reward- and attention-related activation to high-calorie food pictures in obese individuals at the start of a weight-loss program was predictive of less success in the program, and such activation following the program predicted poorer weight control over a 9-mo follow-up period. These patterns of activation may mediate or reflect heightened emotional and attentional salience to food images, leading to an increased sensitivity to food cues and perpetuation of overeating, making such individuals more resistant to weight loss and more prone to relapse. Our study also found that obese participants had decreased activation to high-calorie food pictures from pre- to post-treatment in regions implicated in reward and attentional processes, which may reflect the influence of the weight-loss program on brain response. If further studies substantiate these results, it would suggest that treatment outcomes could be enhanced by approaches that especially target brain regions involved in reward processing and attentional control. Overall, our results suggest that a pattern of heightened functional activation in brain regions implicated in both reward and attention may be a risk factor for increased susceptibility to obesogenic cues in the environment, and, subsequently, greater difficultly in both losing weight and maintaining weight-loss following treatment.
Highlights.
Assessed brain activation in obese individuals pre- and post-weight-loss treatment.
Activation to high-calorie food stimuli predicted less weight loss post-treatment.
Activation post-treatment predicted poorer weight control over 9-mo follow-up.
Decreased activation from pre- to post-treatment may reflect treatment influence.
Results suggest pattern of activation may be a risk factor for poor weight control.
Supplementary Material
Acknowledgements
The study was supported by NIDDK R21DK075685 grant to James Cox and GCRC grant M01-RR00032 from the National Center for Research Resources. The authors wish to thank Jamy Ard (medical director of EatRight), Matthew Edinger, Jan den Hollander, Barbara Gower, Robert Knowlton, and Luke Stoeckel for their help at different stages of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Maas LC, Frederick B, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF, Kaufman MJ. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharm. 2006;31:1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: Implications for dieting and obesity. Obesity. 2009;17:640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- Ard JD, Cox TL, Zunker C, Wingo BC, Jefferson WK, Brakhage C. A study of a culturally enhanced EatRight dietary intervention in a predominately African American workplace. J Public Health Manag Pract. 2010;16:E1–E8. doi: 10.1097/PHH.0b013e3181ce5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. NeuroImage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharm. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons WK, Desai P. Activation of the visual cortex in motivated attention. Behav Neurosci. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Brignell C, Griffiths T, Bradley BP, Mogg K. Attentional and approach biases for pictorial food cues. Influence of external eating. Appetite. 2008;52:299–306. doi: 10.1016/j.appet.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond) 2010;34:1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P, Smit HJ, Lightowler HJ. The influence of restrained and external eating patterns on overeating. Appetite. 2007;49:191–197. doi: 10.1016/j.appet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Carnell S, Wardle J. Appetite and adiposity: a behavioral susceptibility model of obesity. Am J Clin Nutr. 2008;88:22–29. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- Carnell S, Wardle J. Appetitive traits in children. New evidence for associations with weight and a common, obesity-associated genetic variant. Appetite. 2009;53:260–263. doi: 10.1016/j.appet.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Whitwell RL, Goodale MA. The lateral-occipital and the inferior-frontal cortex play different roles during the naming of visually presented objects. Hum Brain Mapp. 2009;30:3851–3864. doi: 10.1002/hbm.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychol. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Cook EW, III, Atkinson LS, Lang PG. Stimulus control and data acquisition for IBM PCs and compatibles. Psychophysiology. 1987;24:726–727. [Google Scholar]
- Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA. Is your brain to blame for weight regain? Physiol Behav. 2011;104:608–612. doi: 10.1016/j.physbeh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TL, Malpede CZ, Desmond RA, Faulk LE, Myer RA, Henson CS, Heimburger DC, Ard JD. Physical activity patterns during weight maintenance following a low-energy density dietary intervention. Obesity. 2007;15:1226–1232. doi: 10.1038/oby.2007.144. [DOI] [PubMed] [Google Scholar]
- Del Parigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E, Tataranni PA. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci. 2002;967:389–397. [PubMed] [Google Scholar]
- Del Parigi AD, Chen K, Salbe AD, Reiman E, Tataranni PA. Are we addicted to food? Obesity Res. 2003;11:493–495. doi: 10.1038/oby.2003.68. [DOI] [PubMed] [Google Scholar]
- Del Parigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- Del Parigi AD, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes. 2007;31:440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Caneza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. NeuroImage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci U S A. 2008;105:15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. “I just can't help myself”: effects of food-cue exposure in overweight and lean individuals. Int J Obes. 2010;35:142–149. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, Fritsche A, Preissl H. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatr. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Struct Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Greene LF, Malpede CZ, Henson CS, Hubbert KA, Heimburger DC, Ard JD. Weight maintenance 2 years after participation in a weight loss program promoting low-energy density foods. Obesity. 2006;14:1795–1801. doi: 10.1038/oby.2006.207. [DOI] [PubMed] [Google Scholar]
- Grieve FG, Vander Weg MW. Desire to eat high- and low-fat foods following a low-fat dietary intervention. Nutr Educ Behav. 2003;35:98–102. doi: 10.1016/s1499-4046(06)60046-8. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharm. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boon B, Mulkens S, Roefs A. Overweight children overeat after exposure to food cues. Eat Behav. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Jansen A, Stegerman S, Roefs A, Nederkoorn C, Hovermans R. Decreased salivation to food cues in formerly obese successful dieters. Psychother Psychosom. 2010;79:257–258. doi: 10.1159/000315131. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Epstein LH, Wilson GT, Drewnowski A, Stunkard AJ, Wilson GT, Wing RR. Long-term maintenance of weight loss: current status. Health Psych. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Kilgore WD, Yurgelun-Todd DA. Sex differences in cerebral responses to images of high versus low-calorie food. Neuroreport. 2010;31:354–358. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharm. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kramer FM, Jeffery RW, Foster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: patterns of weight gain among men and women. Int J Obesity. 1989;13:124–136. [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of picture. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Le DS, Pannacciulli N, Chen K, Salbe AD, Del Parigi A, Hill JO, Wing RR, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr. 2007;86:573–579. doi: 10.1093/ajcn/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledikwe JH, Ello-Martin J, Pelkman CL, Birch LL, Mannino ML, Rolls BJ. A reliable, valid questionnaire indicates that preference for dietary fat declines when following a reduced-fat diet. Appetite. 2007;49:74–83. doi: 10.1016/j.appet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Le Noury J, Lawton CL, Blundell JE. Food choice and hedonic responses: differences between overweight and lean high-fat phenotypes. Int J Obesity. 2002;26:S125. [Google Scholar]
- Maidjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach Atlas. NeuroImage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RM, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- Martin CK, Rosenbaum D, Han H, Geiselman PJ, Wyatt HR, Hill JO, Brill C, Bailer B, Miller BV, III, Stein R, Klein S, Foster GD. Change in food cravings, food preferences, and appetite during a low-carbohydrate and low-fat diet. Obesity. 2011 doi: 10.1038/oby.2011.62. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, Cohen R, Wing RR. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela DJ. Determinants of food choice: relationships with obesity and weight control. Obesity Res. 2001;9:249S–255S. doi: 10.1038/oby.2001.127. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Hyare H, Lee S. Selective attention to food-related stimuli in hunger: Are attentional biases specific to emotional and psychopathological states, or are they also found in normal drive states? Behav Res Ther. 1998;36:227–237. doi: 10.1016/s0005-7967(97)00062-4. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54:243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Ochner CN, Kwok Y, Conceição E, Pantazatos SP, Puma LM, Carnell S, Teixeira J, Hirsch J, Geliebter A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of depression and its treatment in the general population. J Psychiatr Res. 2007;41:207–213. doi: 10.1016/j.jpsychires.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychol. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food craving activation during fMRI. NeuroImage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Frontiers in Neuroscience. 2010;4:1–8. doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piech RM, Pastorino MT, Zald DH. All I saw was the cake. Hunger effects on attentional capture by visual food cues. Appetite. 2010;54:579–582. doi: 10.1016/j.appet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Plassman H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen A, Hakala P, Lissner L, Mattlar C-E, Koskenvuo M, Ronnemaa T. Acquired preference for dietary fat and obesity: a study of weight-discordant monozygotic twin pairs. Int J Obesity. 2002;26:973–977. doi: 10.1038/sj.ijo.0802014. [DOI] [PubMed] [Google Scholar]
- Rodin J, Schank D, Striegel-Moore R. Psychological features of obesity. Med Clin North Am. 1989;73:47–66. doi: 10.1016/s0025-7125(16)30691-5. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105:S98–S103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund YC, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Rudenga K, Green B, Nachtigal D, Small DM. Evidence for an integrated oral sensory module in the human anterior ventral insula. Chem Senses. 2010;35:693–703. doi: 10.1093/chemse/bjq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbe AD, Del Parigi A, Pratley RE, Drewnowski A, Tataranni PA. Taste preferences and body weight changes in an obesity-prone population. Amer J Clin Nutrition. 2004;79:372–378. doi: 10.1093/ajcn/79.3.372. [DOI] [PubMed] [Google Scholar]
- Schachter S, Gross LP. Manipulated time and eating behavior. J Pers Soc Psychol. 1968;10:98–106. doi: 10.1037/h0026285. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Hönig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DLW, Snyder AZ, McAvoy MP. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Spetter MS, Smeets PA, de Graaf C, Viergever MA. Representation of sweet and salty taste intensity in the brain. Chem Senses. 2010;35:831–840. doi: 10.1093/chemse/bjq093. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Gonzalez R, Luan Phan K, Liberzon I. The neural correlates of intertemporal decision-making: Contributions of subjective value, stimulus type, and trait impulsivity. Hum Brain Mapp. 2010;32:1637–1648. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D'Argembeau A. Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PLoS One. 2011;6:e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Telch CF, Rizvi SL. Development and validation of the Eating Disorder Diagnostic Scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess. 2000;12:123–131. doi: 10.1037//1040-3590.12.2.123. [DOI] [PubMed] [Google Scholar]
- Stice E, Fisher M, Martinez E. Eating disorder diagnostic scale: additional evidence of reliability and validity. Psychol Assess. 2004;16:60–71. doi: 10.1037/1040-3590.16.1.60. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008a;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008b;117:924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry reponsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010a;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010b;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Cox JE, Cook EW, III, Weller RE. Motivational state modulates the hedonic value of food images differently in men and women. Appetite. 2007;48:139–144. doi: 10.1016/j.appet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, Vollmer WM, Gullion CM, Funk K, Smith P, Samuel-Hodge C, Myers V, Lien LF, Laferriere D, Kennedy B, Jerome GJ, Heinith F, Harsha DW, Evans P, Erlinger TP, Dalcin AT, Coughlin J, Charleston J, Champagne CM, Bauck A, Ard JD, Aicher K. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci. 2005;25:1193–1202. doi: 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley AC, Brunstrom JM, Griffiths P. Individual differences in food cue reactivity. Appetite. 2006;47:278. doi: 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Val-Laillet D, Layec S, Guerin S, Meurice P, Malbert C. Changes in brain activity after a diet-induced obesity. Obesity. 2011;19:749–756. doi: 10.1038/oby.2010.292. [DOI] [PubMed] [Google Scholar]
- Van Horn L, Obarzanek E, Friedman LA, Gernhofer N, Barton B. Children’s adaptation to a fat-reduced diet: the Dietary Intervention Study in Children (DISC) Pediatrics. 2005;115:1723–1733. doi: 10.1542/peds.2004-2392. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes. 1989;13 suppl 2:39–46. [PubMed] [Google Scholar]
- Wilcox CE, Braskie MN, Kluth JT, Jagust WJ. Overeating behavior and striatal dopamine with 6-[F]-Fluoro-L-m-tyrosine PET. J Obes. 2010;2010 doi: 10.1155/2010/909348. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Obesity and overweight. 2003 Retrieved from http://www.who.int/hpr/NHP/docs/gs_obesity.pdf.
- Zachary RA. Shipley Institute of Living Scale. Revised Manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.