Abstract
BACKGROUND:
Evidence is lacking to support the efficacy of lifestyle modification as first-line therapy in the clinical management of type 2 diabetes mellitus (T2DM) in adolescents.
METHODS:
A retrospective chart review of youth diagnosed with T2DM between 1999 and 2008 was conducted. The authors describe the percentage of youth presenting with glycosylated hemoglobin (HbA1c) of <9% who achieved/maintained target glycemic control (HbA1c ≤7.0%) with lifestyle monotherapy during the year following diagnosis.
RESULTS:
Among the 275 youth with T2DM, 38% (n=104) presented with an HbA1c <9% and were prescribed lifestyle monotherapy at diagnosis. Of the 80 youth who had sufficient follow-up data over 12 months, 54% successfully maintained target glycemic control solely with lifestyle management. The mean HbA1c score at diagnosis was lower in youth who where successful on lifestlye monotherapy compared with those who were not successful.
CONCLUSIONS:
A significant proportion of youth newly diagnosed with T2DM presenting with an HbA1c <9% effectively achieved/maintained target glycemic control with lifestyle recommendations alone for 12 months.
Keywords: Glycosylated hemoglobin, Lifestyle, Treatment, Type 2 diabetes, Youth
Abstract
HISTORIQUE :
On ne possède pas assez de preuves pour appuyer l’efficacité des modifications au mode de vie comme thérapie de première ligne afin de prendre en charge le diabète de type 2 (DT2) sur le plan clinique chez les adolescents.
MÉTHODOLOGIE :
Les chercheurs ont procédé à une analyse rétrospective des dossiers d’adolescents ayant un DT2 qui ont été diagnostiqués entre 1999 et 2008. Ils ont décrit le pourcentage d’adolescents dont l’hémoglobine glycosylée (HbA1c) était inférieure à 9 % et qui ont obtenu ou maintenu le contrôle ciblé de leur glycémie (HbA1c ≤7,0 %) grâce à une monothérapie liée au mode de vie au cours de l’année suivant le diagnostic.
RÉSULTATS :
Chez les 275 adolescents ayant un DT2, 38 % (n=104) avaient une HbA1c inférieure à 9 % et se sont fait proposer une monothérapie liée au mode de vie au moment du diagnostic. Chez les 80 adolescents qui disposaient de données de suivi suffisantes sur 12 mois, 54 % ont réussi à maintenir le contrôle de leur glycémie ciblée par la seule prise en charge de leur mode de vie. L’indice moyen d’HbA1c des jeunes qui parvenaient aux objectifs ciblés grâce à la monothérapie liée au mode de vie était plus faible que celui des jeunes qui n’y parvenaient pas.
CONCLUSIONS :
Une forte proportion d’adolescents qui venaient de se faire diagnostiquer un DT2 et dont l’HbA1c était inférieur à 9 % ont réussi à obtenir ou à maintenir le contrôle ciblé de leur glycémie seulement en respectant pendant 12 mois les recommandations liées au mode de vie.
The prevalence of type 2 diabetes mellitus (T2DM) in youth has increased dramatically over the past two decades (1), and now accounts for 40% to 80% of new cases of diabetes in some paediatric centres (2). There is little empirical evidence guiding the clinical management of this cohort due to the limited global experience with paediatric T2DM. Studies in adults with T2DM clearly demonstrate that lifestyle behaviour change effectively improves glycemic control (3–5); however, similar evidence is lacking in youth.
Published reports of clinical paediatric cohorts of T2DM suggest that only 6% to 12% of youth are able to achieve target glycemic control with lifestyle-based approaches alone (6–8). These numbers are biased, however, because studies are often underpowered, suffer from significant loss to follow-up and include youth presenting in significant metabolic decompensation (glycosylated hemoglobin [HbA1c] >9%) requiring insulin therapy. In the absence of empirical evidence, these results pervade consensus-based treatment paradigms (9) and lead to recommendations for drug therapy (10) despite inadequate evidence for the safety of long-term antihyperglycemic medications in youth. Current international clinical practice guidelines are predominately based on low-level evidence or consensus (9,11). In an effort to inform practice guidelines, the purpose of the present report was to describe our 10 years of clinical experience with prescribing lifestyle changes to treat hyperglycemia in youth with a diagnosis of T2DM.
METHODS
Study design and population
A retrospective chart review of youth with T2DM treated at the Diabetes Education Resource for Children and Adolescents (DERCA) in Winnipeg, Manitoba, was conducted. Diabetes was diagnosed using international criteria (11,12). Therefore, in the absence of symptoms, all patients had to demonstrate, on two occasions: a fasting blood glucose ≥7.0 mmol/L; and/or a random blood glucose of ≥11.1 mmol/L; or a blood glucose ≥11.1 mmol/L following a 75 g oral glucose challenge. Only one abnormal value was required in the presence of classic symptoms of hyperglycemia. The classification of T2DM was supported by clinical criteria and the absence of diabetes-associated autoantibodies (11–13). The DERCA is the only regional paediatric diabetes program providing services to the province of Manitoba and northwestern Ontario. Youth are seen by a paediatric endocrinologist, a dietitian and nurse educator three to four times annually. In 2007, an exercise specialist was added to the team. At each visit, youth are taught strategies to improve nutritional habits, increase physical activity and reduce sedentary/screen time.
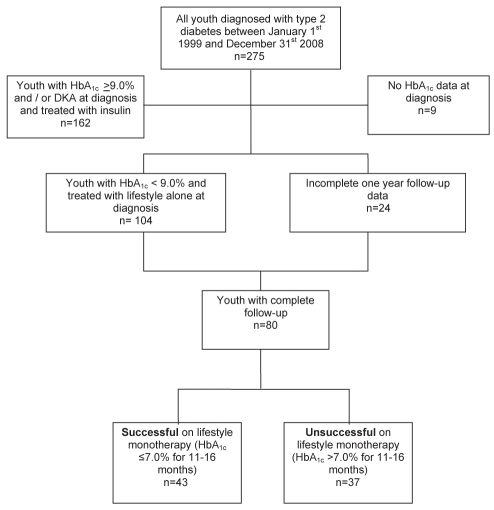
Charts of youth (<18 years of age) with T2DM treated between 1999 and 2008 were screened to identify those prescribed lifestyle monotherapy at diagnosis, with at least two clinic visits spanning one year of follow-up (Figure 1). Of the 275 charts available for review, nine were excluded because there was no HbA1c recorded in the chart. One hundred sixty-two youth were excluded because they had metabolic decompensation at diagnosis, with HbA1c ≥9% and/or diabetic ketoacidosis that required insulin therapy, reflecting clinical practice during the study period. This excluded group also included one youth with HbA1c of 8.9% at diagnosis who had insulin therapy started by the local health care team in a remote community for a fasting blood glucose of 20 mmol/L. Of the remaining 104 youth, 24 patients did not have complete one-year follow-up data (six were diagnosed <12 months before, six were transferred to adult care, seven did not have a clinic visit 11 to 16 months after diagnosis and five were lost to follow-up). Thus, 80 youth (60% female) met the inclusion criteria and had sufficient follow-up data to be included in the analysis. Youth included in the study had an HbA1c ≤9.1% and were prescribed lifestyle monotherapy. All HbA1c measurements were performed using the DCA 2000 (Bayer Diagnostics, USA) immunoassay at a single site, which is aligned to the Diabetes Complications and Control Trial.
Figure 1).
Flow chart of cohort selection into subgroups, by glycosylated hemoglobin (HbA1c) at 11 to 16 month follow-up, as successful with lifestyle monotherapy (HbA1c ≤7.0%) or unsuccessful with lifestyle monotherapy (HbA1c >7.0%). DKA Diabetic ketoacidosis
Protocol and data extraction
The 80 eligible charts were divided into two groups based on HbA1c levels during the 12-month period after diagnosis: patients who achieved and maintained target HbA1c of ≤7.0% (11,14) on lifestyle therapy alone and those who did not achieve or maintain target glycemic control for 12 months on lifestyle monotherapy. Demographic data (age, sex and self-defined ethnicity) were extracted from charts, as well as weight, height, blood pressure, HbA1c and fasting glucose. Each chart was reviewed to confirm how the diagnosis of T2DM was made before referral to the DERCA. The study protocol was approved by the Human Research Ethics Review Committee, Faculty of Medicine, University of Manitoba, in accordance with the Declaration of Helsinki.
Statistical analysis
Data are expressed as mean ± SD. Student’s t test was used to test for group-wise differences in continuous characteristics and χ2 testing was used for categorical variables. Group-wise differences in the 12-month change in dependant variables were assessed using repeated measures ANOVA. The body mass index (BMI) z-score was not normally distributed; therefore, group differences were assessed using the Mann-Whitney U test. Multiple logistic regression was used to test for predictors of successful achievement/maintenance of target glycemia with lifestyle therapy alone. χ2 testing was used to test for differences in success rates based on thresholds of baseline HbA1c or fasting plasma glucose. All statistical analyses were performed using SPSS version 16.0 (IBM Corporation, USA). P<0.05 was considered to be significant.
RESULTS
Characteristics of each group at diagnosis and follow-up are presented in Table 1. Youth excluded from the analyses due to incomplete follow-up data (n=24) presented with a mean HbA1c of 7.0%±0.9% and did not differ in demographics or clinical measures from youth included in the study. Among the 80 eligible youth, 43 (54%) achieved or maintained target HbA1c ≤7.0% during a period of 12 months following diagnosis with lifestyle therapy alone. This group of patients was characterized by a lower HbA1c at diagnosis (P<0.001) and had a higher percentage of males than the unsuccessful group. No other significant differences in clinical variables collected at diagnosis were observed between the groups. The duration of follow-up was similar between youth who were in the successful group versus the unsuccessful group (13.5±2.0 versus 13.9±1.9 months, P not significant).
TABLE 1.
Baseline and one-year characteristics and biochemical measures of patients categorized by success with lifestyle management
| Variable |
Successful |
Unsuccessful |
Between groups P |
|||
|---|---|---|---|---|---|---|
| Baseline (n=43) | 1 year | Baseline (n=37) | 1 year | Baseline | Change over 1 year | |
| Age, years (range)* | 13.0 (6.8–16.5) | – | 12.5 (7.0–17.0) | – | 0.33 | – |
| Male n (%) | 22 (51) | – | 13 (27%) | – | 0.04 | – |
| Ethnicity (FN/C/other) | 37/3/3 | – | 34/1/2 | – | – | |
| BMI z-score | 2.2±0.7 | 2.1±0.7 | 2.0±0.8 | 1.8±0.8 | 0.18 | 0.17 |
| SBP z-score | 1.6±1.3 | 1.3±1.1 | 1.3±1.2 | 1.2±1.5 | 0.27 | 0.463 |
| DBP z-score | 0.7±0.6 | 0.5±0.7 | 0.9±0.7 | 0.5±0.8 | 0.11 | 0.16 |
| HbA1c, % | 6.8±1.0† | 6.1±0.6 | 7.5±0.8† | 8.7±1.7 | 0.004 | <0.001‡ |
| FPG¶ | 7.0±2.4 | 6.3±1.2 | 8.3±2.9 | 10.5±4.6 | 0.053 | <0.001‡ |
Data presented as mean ± SD, unless otherwise indicated.
Refers to age (range) at diagnosis;
Significant difference between groups at baseline (P<0.05);
Significant difference between groups for change over 1 year (P<0.05);
Not all youth had a 12-month fasting plasma glucose tested; 64 complete pairs were available for comparison. BMI Body mass index; C Caucasian; FN First Nations; SBP Systolic blood pressure; DBP Diastolic blood pressure; FPG Fasting plasma glucose
As per the study design, the change in HbA1c was significantly different between groups at one-year follow-up (−0.7%±1.0% versus +1.3%±1.9%, P<0.001). While the BMI z-score decreased in both groups, the change in the BMI z-score was significant only in the unsuccessful group (P=0.07 for successful group versus P=0.03 for unsuccessful group).
Further analyses were conducted to determine whether a baseline HbA1c or fasting plasma glucose threshold could identify those youth in which lifestyle monotherapy resulted in diminished success rates (Table 2). Youth were categorized into groups based on baseline HbA1c and fasting plasma glucose. There was no threshold of HbA1c <9% at diagnosis or fasting plasma glucose that could be used to predict success.
TABLE 2.
The 12-month change in glycemic control according to gylcosylated hemoglobin (HbA1c) and fasting plasma glucose at diagnosis
| Range of HbA1c at diagnosis, % | n | Mean HbA1c at one year | Between groups, P | Successful, n (%) | Unsuccessful, n (%) | Between groups, P |
| <7 | 39 | 6.9±1.6 | 0.08 | 26 (66.7) | 13 (33.3) | 0.46 |
| 7–7.9 | 21 | 7.2±1.5 | 13 (61.9) | 8 (38.1) | ||
| 8–9.1 | 20 | 8.0±2.3 | 10 (50) | 10 (50) | ||
| Fasting plasma glucose range, mmol/L | n* | Mean HbA1c at one year | Between groups, P | Successful, n (%) | Unsuccessful, n (%) | Between groups, P |
| <6.0 | 18 | 7.1±2.3 | 0.23 | 13 (72.2) | 5 (27.8) | 0.10 |
| 6–6.9 | 14 | 6.9±1.1 | 10 (71.4) | 4 (28.6) | ||
| 7–7.9 | 11 | 7.3±2.1 | 7 (63.6) | 4 (36.4) | ||
| 8–8.9 | 5 | 8.5±1.9 | 2 (40.0) | 3 (60.0) | ||
| 9–9.9 | 4 | 9.2±1.2 | 0 (0.0) | 4 (100.0) | ||
| >10 | 12 | 7.8±1.8 | 6 (50.0) | 6 (50.0) | ||
Data presented as mean ± SD, unless otherwise indicated.
Not all youth had a 12-month fasting plasma glucose tested; 64 complete pairs were available for comparison
Youth were categorized into one of five groups according to how the diagnosis of T2DM was made. The majority of youth (n=51, 63.7%) were identified through medical screening tests during a routine appointment or medical visit unrelated to diabetes. Nine youth (11.3%) presented to a medical centre with symptoms of hyperglycemia. Twelve youth (15%) were diagnosed because a caregiver brought the youth to a medical centre to be tested for diabetes. Three youth (3.8%) had caregivers test their blood sugar on home glucose monitors because of known risk (eg, ethnicity, family history of diabetes, obesity), and brought the youth to medical attention following a high blood glucose reading at home. Five youth (6.3%) were found to have hyperglycemia at community screening programs (one at a community pow wow and four at a school screening event). All youth were confirmed to have T2DM at the DERCA using the Canadian Diabetes Association criteria (11) as described above.
Finally, preliminary analyses were conducted to explore the effect of adding an exercise specialist to the team in 2007. No significant difference in change in HbA1c was observed with the presence of an exercise specialist in the clinic from 2007 to 2008 (Table 3). However, only 13 subjects diagnosed from 2007 to 2008 met inclusion criteria, likely underpowering this subanalysis.
TABLE 3.
Exploratory analysis of effect of adding an exercise specialist to the diabetes team in 2007
| Baseline HbA1c, % | 1 year follow-up HbA1c, % | Between group difference in change in HbA1c, P | |
|---|---|---|---|
| 1999–2006 (pre-exercise specialist) | 7.2±1.0 | 7.5±1.9 | 0.112 |
| 2007–2008 (with exercise specialist) | 6.9±0.9 | 6.6±1.2 | – |
Data presented as mean ± SD, unless otherwise indicated. HbA1c Glycosylated hemoglobin
DISCUSSION
The present retrospective chart review of a large Canadian paediatric cohort of youth with T2DM (15), revealed important observations relevant to the initial management of T2DM in youth. Optimal glycemic control with lifestyle monotherapy was achieved or maintained for one year in more than 50% of youth with an HbA1c <9% at diagnosis, independent of changes in body weight. Baseline HbA1c was significantly lower in those youth who were successful with lifestyle monotherapy.
Our findings contradict previous studies, which report that approximately 10% of youth with T2DM are able to achieve or maintain target glycemic control with lifestyle therapy alone (6, 16,17). Factors possibly contributing to this discrepancy include our exclusion of youth presenting with metabolic decompensation at diagnosis (HbA1c >9%), because clinical practice at our centre during the study period was to initiate insulin at diagnosis in youth presenting with an HbA1c >9%. If all youth from our centre diagnosed with T2DM between 1999 to 2008 with one year follow-up data regardless of initial HbA1c were included in the analysis (data not shown), 18% were able to achieve target glycemic control with lifestyle management alone after one year, a rate still higher than previously reported.
Differences in the emphasis and resources allocated to families and communities for lifestyle education between reports could explain disparate success rates. Other studies (17) have reported significant loss to follow-up (>50%), whereas less than 10% of youth in the present study were missing follow-up data at 12 months. Finally, because T2DM is more common in Manitoba than other regions of Canada (15), T2DM may be detected earlier in youth due to higher screening rates and thus may be at an earlier stage of metabolic decompensation. In accordance with this theory, our data suggest that youth diagnosed at milder stages of dysglycemia (ie, with a lower HbA1c level) are most amenable to lifestyle treatment. However, even within our highest category of HbA1c and fasting glucose at diagnosis, 50% of youth achieved target glycemic control on lifestyle monotherapy at one year postdiagnosis. Therefore, it appears that lifestyle monotherapy is beneficial in youth with HbA1c <9% at diagnosis, and future prospective or randomized controlled trials will be necessary to delineate the individual factors that contribute to success.
Of note, the majority of youth in the present study were asymptomatic at diagnosis and were diagnosed following medical screening during a routine or unrelated medical visit, highlighting the importance of screening youth at high risk for developing T2DM. Parental vigilance also played a large role in the detection of T2DM in youth; nearly 20% of youth were brought to a medical centre because a caregiver recognized signs and/or symptoms of T2DM. There were no widespread community efforts at diabetes screening during the period covered by the present study, and only five youth were identified by this approach.
We are unable to explain why a higher percentage of males were observed in the successful group. Future research is warranted to determine whether success with lifestyle monotherapy is sex dependent.
Although the present study was not designed to address the addition of an exercise specialist to the diabetes team, exploratory analyses were conducted. While the trends in change in HbA1c over the year following diagnosis might suggest a positive effect of this addition to clinical outcomes, larger prospective or randomized trials are needed to evaluate this important question. The present study design and sample size precluded us from fully evaluating the effect of adding an exercise specialist to the diabetes education team, free from the effects of confounders.
Limitations
Data for the present study were collected retrospectively from patient charts and, therefore, were susceptible to information bias and missing values. Factors unrelated to those recorded in clinical charts may additionally influence successful management of glycemia, in particular socioeconomic status. The large percentage of First Nations youth, representative of our patient population, may limit generalization of the findings.
CONCLUSIONS
Optimal glycemic control for up to one year after diagnosis is possible with lifestyle monotherapy in youth with T2DM. Improvements in glycemic control in the successful group were made following regular education and counselling during outpatient clinic and community outreach visits, and were independent of changes in body weight. It is plausible that higher rates of success with lifestyle monotherapy may be realized with a more intensive lifestyle program similar to that provided in the Diabetes Prevention Project and Look Ahead Trial (18,19). In light of the present data, randomized control trials of lifestyle monotherapy for achieving glycemic control in youth with T2DM are warranted. The present report provides data to inform the design of these trials and evidence that careful attention to lifestyle modification is an important clinical target for the management of youth with T2DM.
Acknowledgments
The authors are grateful for the assistance provided by Pat Bobko and Joanne Hamilton. They also thank Catherine MacDonald for her insightful review of the manuscript.
Footnotes
FUNDING SUPPORT: Establishment grants from the Manitoba Institute of Child Health and Manitoba Health Research Council (Dr McGavock), a Scholar Award from the Canadian Diabetes Association (Dr McGavock); Clinical Fellowships from Canadian Institutes of Health Research (Dr Wittmeier), and Manitoba Institute of Child Health and Manitoba Health Research Council (Dr Wicklow).
DECLARATION OF COMPETING INTERESTS: The authors have no competing interests to declare.
REFERENCES
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 3.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann Intern Med. 2007;147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 5.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 6.Sheild JP, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with Type 2 diabetes. Arch Dis Child. 2009;94:206–9. doi: 10.1136/adc.2008.143313. [DOI] [PubMed] [Google Scholar]
- 7.McQuaid S, O’Gorman DJ, Yousif O, et al. Early-onset insulin-resistant diabetes in obese Caucasians has features of typical type 2 diabetes, but 3 decades earlier. Diabetes Care. 2005;28:1216–8. doi: 10.2337/diacare.28.5.1216. [DOI] [PubMed] [Google Scholar]
- 8.Peterson K, Silverstein J, Kaufman F, Warren-Boulton E. Management of type 2 diabetes in youth: An update. Am Fam Physician. 2007;76:658–64. [PubMed] [Google Scholar]
- 9.Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ. ISPAD Clinical Practice Consensus Guidelines 2006–2007. Type 2 diabetes mellitus in the child and adolescent. Pediatr Diabetes. 2008;9:512–26. doi: 10.1111/j.1399-5448.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- 10.Zeitler P. Considerations regarding the diagnosis and treatment of childhood type 2 diabetes. Postgrad Med. 2010;122:89–97. doi: 10.3810/pgm.2010.05.2146. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32:S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellers E, Eisenbarth G, Young TK, Dean HJ. Diabetes-associated autoantibodies in aboriginal children. Lancet. 2000;355:1156. doi: 10.1016/S0140-6736(00)02067-5. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ. Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Suppl 12):17–32. doi: 10.1111/j.1399-5448.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 15.Amed S, Dean HJ, Panagiotopoulos C, et al. Type 2 diabetes, medication-induced diabetes, and monogenic diabetes in Canadian children: A prospective national surveillance study. Diabetes Care. 2010;33:786–91. doi: 10.2337/dc09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinstein G, Muzumdar R, Aponte L, Vuguin P, Saenger P, Di-Martino-Nardi J. Presentation and 5-year follow-up of type 2 diabetes mellitus in African-American and Caribbean-Hispanic adolescents. Horm Res. 2003;60:121–26. doi: 10.1159/000072523. [DOI] [PubMed] [Google Scholar]
- 17.Reinehr T, Schober E, Roth CL, Wiegand S, Holl R. Type 2 diabetes in children and adolescents in a 2-year follow-up: Insufficient adherance to diabetes centres. Horm Res. 2008;69:107–13. doi: 10.1159/000111814. [DOI] [PubMed] [Google Scholar]
- 18.Zeitler P, Epstein L, Grey M, et al. Treatment options for type 2 diabetes in adolescents and youth: A study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]