Abstract
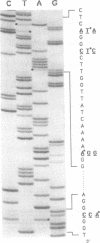
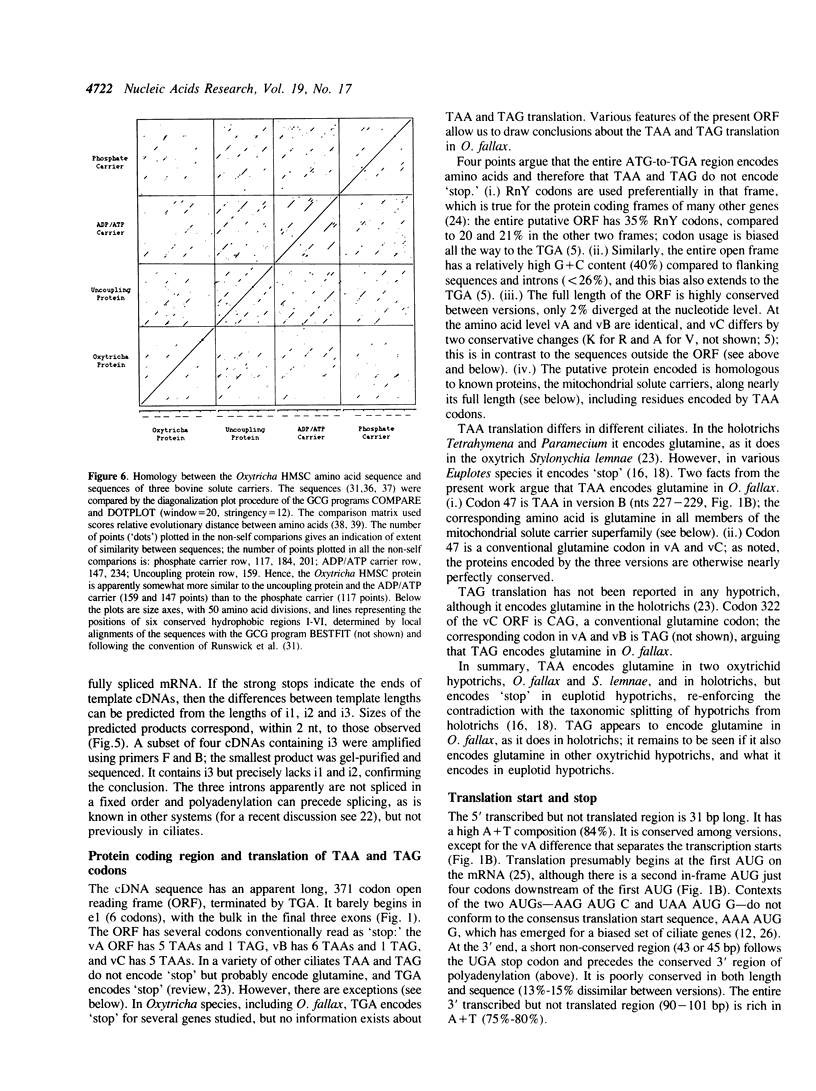
Hypotrichous ciliated protozoa, such as Oxytricha fallax, produce tiny chromosomes during generation of the transcriptionally active macronucleus. The 81-MAC family of macronuclear chromosomes is produced by alternative DNA processing, such that the chromosomes share a common region of 1.6 kbp. Transcription of a 1.3 kb mRNA from the common region has been analyzed. Transcription starts very near the telomere (34 bp), in a 23 bp region of pure A + T DNA. Polyadenylation sites are very near the other telomere (26 bp), also in a region of nearly pure A + T DNA. Three introns are clustered in the first third of the gene. Intron removal can follow polyadenylation, and the order of removal is not fixed. All three known sequence versions of the 81-MAC chromosomes are represented in the mRNA pool, with no evidence of any further versions. The A + T sequences surrounding the transcription starts and polyadenylation sites are conserved among versions. Introns have conserved 5' and 3' ends and a putative branch-point sequence (YYRAT), but otherwise are highly diverged and are AT-rich. A single long open reading frame, interrupted by the three introns, encodes a homolog of known mitochondrial solute carriers, and contains the codon TAA, which does not encode 'stop,' but a conserved glutamine; TAG appears also to encode glutamine. The results significantly enlarge the small data set of transcription start and polyadenylation sites, of intron features, and of translation signals for hypotrichs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquila H., Link T. A., Klingenberg M. Solute carriers involved in energy transfer of mitochondria form a homologous protein family. FEBS Lett. 1987 Feb 9;212(1):1–9. doi: 10.1016/0014-5793(87)81546-6. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Kahn R. W., Sadler L. A. Phylogenetic relationships among Tetrahymena species determined using the polymerase chain reaction. J Mol Evol. 1990 Mar;30(3):290–297. doi: 10.1007/BF02099999. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Sadler L. A. Characterization of the promoter region of Tetrahymena genes. Nucleic Acids Res. 1990 Jan 25;18(2):323–329. doi: 10.1093/nar/18.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartinhour S. W., Herrick G. A. Three different macronuclear DNAs in Oxytricha fallax share a common sequence block. Mol Cell Biol. 1984 May;4(5):931–938. doi: 10.1128/mcb.4.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteilla L., Bouillaud F., Forest C., Ricquier D. Nucleotide sequence of a cDNA encoding bovine brown fat uncoupling protein. Homology with ADP binding site of ADP/ATP carrier. Nucleic Acids Res. 1989 Mar 11;17(5):2131–2131. doi: 10.1093/nar/17.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann K. K., Helftenbein E. Nucleotide sequence and expression of two beta-tubulin genes in Stylonychia lemnae. J Mol Biol. 1987 Dec 20;198(4):643–653. doi: 10.1016/0022-2836(87)90207-5. [DOI] [PubMed] [Google Scholar]
- Csank C., Taylor F. M., Martindale D. W. Nuclear pre-mRNA introns: analysis and comparison of intron sequences from Tetrahymena thermophila and other eukaryotes. Nucleic Acids Res. 1990 Sep 11;18(17):5133–5141. doi: 10.1093/nar/18.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Douglas S. E., Murphy C. A., Spencer D. F., Gray M. W. Cryptomonad algae are evolutionary chimaeras of two phylogenetically distinct unicellular eukaryotes. Nature. 1991 Mar 14;350(6314):148–151. doi: 10.1038/350148a0. [DOI] [PubMed] [Google Scholar]
- Fink G. R. Pseudogenes in yeast? Cell. 1987 Apr 10;49(1):5–6. doi: 10.1016/0092-8674(87)90746-x. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand M. S. Statistical analysis of mammalian pre-mRNA splicing sites. Nucleic Acids Res. 1989 Aug 11;17(15):6369–6382. doi: 10.1093/nar/17.15.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R. Sigma factors from E. coli, B. subtilis, phage SP01, and phage T4 are homologous proteins. Nucleic Acids Res. 1986 Aug 26;14(16):6745–6763. doi: 10.1093/nar/14.16.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. S., Jahn C. L. Actin, tubulin and H4 histone genes in three species of hypotrichous ciliated protozoa. Gene. 1989 Jan 30;75(1):93–107. doi: 10.1016/0378-1119(89)90386-7. [DOI] [PubMed] [Google Scholar]
- Harper D. S., Jahn C. L. Differential use of termination codons in ciliated protozoa. Proc Natl Acad Sci U S A. 1989 May;86(9):3252–3256. doi: 10.1073/pnas.86.9.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helftenbein E., Müller E. Both alpha-tubulin genes are transcriptionally active in Stylonychia lemnae. Curr Genet. 1988 May;13(5):425–432. doi: 10.1007/BF00365664. [DOI] [PubMed] [Google Scholar]
- Herrick G., Cartinhour S. W., Williams K. R., Kotter K. P. Multiple sequence versions of the Oxytricha fallax 81-MAC alternate processing family. J Protozool. 1987 Nov;34(4):429–434. doi: 10.1111/j.1550-7408.1987.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Herrick G., Hunter D., Williams K., Kotter K. Alternative processing during development of a macronuclear chromosome family in Oxytricha fallax. Genes Dev. 1987 Dec;1(10):1047–1058. doi: 10.1101/gad.1.10.1047. [DOI] [PubMed] [Google Scholar]
- Hicke B. J., Celander D. W., MacDonald G. H., Price C. M., Cech T. R. Two versions of the gene encoding the 41-kilodalton subunit of the telomere binding protein of Oxytricha nova. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1481–1485. doi: 10.1073/pnas.87.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S., Bowen J. K., Bannon G. A., Gorovsky M. A. Unusual features of transcribed and translated regions of the histone H4 gene family of Tetrahymena thermophila. Nucleic Acids Res. 1987 Jan 12;15(1):141–160. doi: 10.1093/nar/15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T., Proudfoot N. J. A beginning to the biochemistry of polyadenylation. Trends Genet. 1988 Sep;4(9):243–245. doi: 10.1016/0168-9525(88)90028-5. [DOI] [PubMed] [Google Scholar]
- Hunter D. J., Williams K., Cartinhour S., Herrick G. Precise excision of telomere-bearing transposons during Oxytricha fallax macronuclear development. Genes Dev. 1989 Dec;3(12B):2101–2112. doi: 10.1101/gad.3.12b.2101. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Molecular aspects of the adenine nucleotide carrier from mitochondria. Arch Biochem Biophys. 1989 Apr;270(1):1–14. doi: 10.1016/0003-9861(89)90001-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- LeMaire M. F., Thummel C. S. Splicing precedes polyadenylation during Drosophila E74A transcription. Mol Cell Biol. 1990 Nov;10(11):6059–6063. doi: 10.1128/mcb.10.11.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W. Codon usage in Tetrahymena and other ciliates. J Protozool. 1989 Jan-Feb;36(1):29–34. doi: 10.1111/j.1550-7408.1989.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Miceli C., La Terza A., Melli M. Isolation and structural characterization of cDNA clones encoding the mating pheromone Er-1 secreted by the ciliate Euplotes raikovi. Proc Natl Acad Sci U S A. 1989 May;86(9):3016–3020. doi: 10.1073/pnas.86.9.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara O., Dorit R. L., Gilbert W. One-sided polymerase chain reaction: the amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5673–5677. doi: 10.1073/pnas.86.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C. Metazoan phylogeny. Reassessing relationships. Nature. 1990 Mar 15;344(6263):199–200. doi: 10.1038/344199a0. [DOI] [PubMed] [Google Scholar]
- Powell S. J., Medd S. M., Runswick M. J., Walker J. E. Two bovine genes for mitochondrial ADP/ATP translocase expressed differences in various tissues. Biochemistry. 1989 Jan 24;28(2):866–873. doi: 10.1021/bi00428a069. [DOI] [PubMed] [Google Scholar]
- Ribas-Aparicio R. M., Sparkowski J. J., Proulx A. E., Mitchell J. D., Klobutcher L. A. Nucleic acid splicing events occur frequently during macronuclear development in the protozoan Oxytricha nova and involve the elimination of unique DNA. Genes Dev. 1987 Jun;1(4):323–336. doi: 10.1101/gad.1.4.323. [DOI] [PubMed] [Google Scholar]
- Runswick M. J., Powell S. J., Nyren P., Walker J. E. Sequence of the bovine mitochondrial phosphate carrier protein: structural relationship to ADP/ATP translocase and the brown fat mitochondria uncoupling protein. EMBO J. 1987 May;6(5):1367–1373. doi: 10.1002/j.1460-2075.1987.tb02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. C. Method to determine the reading frame of a protein from the purine/pyrimidine genome sequence and its possible evolutionary justification. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1596–1600. doi: 10.1073/pnas.78.3.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer V. L., Wobbe C. R., Struhl K. A wide variety of DNA sequences can functionally replace a yeast TATA element for transcriptional activation. Genes Dev. 1990 Apr;4(4):636–645. doi: 10.1101/gad.4.4.636. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Zarrilli R., Oates E. L., McBride O. W., Lerman M. I., Chan J. Y., Santisteban P., Ursini M. V., Notkins A. L., Kohn L. D. Sequence and chromosomal assignment of a novel cDNA identified by immunoscreening of a thyroid expression library: similarity to a family of mitochondrial solute carrier proteins. Mol Endocrinol. 1989 Sep;3(9):1498–1505. doi: 10.1210/mend-3-9-1498. [DOI] [PubMed] [Google Scholar]