Abstract
Receptor ligands, identified as antagonists, based on the absence of stimulation of signaling, can rarely stimulate receptor internalization. d-Tyr-Gly-[(Nle28,31,d-Trp30)CCK-26–32]-2-phenylethyl ester (d-Trp-OPE) is such a ligand that binds to the cholecystokinin (CCK) receptor and stimulates internalization. Here, the molecular basis of this trafficking event is explored, with the assumption that ligand binding initiates conformational change, exposing an epitope to direct endocytosis. Ligand-stimulated internalization was studied morphologically using fluorescent CCK and d-Trp-OPE. d-Trp-OPE occupation of Chinese hamster ovary cell receptors stimulated internalization into the same region as CCK. Arrestin-biased action was ruled out using morphological translocation of fluorescent arrestin 2 and arrestin 3, moving to the membrane in response to CCK, but not d-Trp-OPE. Possible roles of the carboxyl terminus were studied using truncated receptor constructs, eliminating the proline-rich distal tail, the serine/threonine-rich midregion, and the remainder to the vicinal cysteines. None of these constructs disrupted d-Trp-OPE-stimulated internalization. Possible contributions of transmembrane segments were studied using competitive inhibition with peptides that also had no effect. Intracellular regions were studied with a similar strategy using coexpressing cell lines. Peptides corresponding to ends of each loop region were studied, with only the peptide at the carboxyl end of the third loop inhibiting d-Trp-OPE-stimulated internalization but having no effect on CCK-stimulated internalization. The region contributing to this effect was refined to peptide 309–323, located below the recognized G protein-association motif. While a receptor in which this segment was deleted did internalize in response to d-Trp-OPE, it exhibited abnormal ligand binding and did not signal in response to CCK, suggesting an abnormal conformation and possible mechanism of internalization distinct from that being studied. This interpretation was further supported by the inability of peptide 309–323 to inhibit its d-Trp-OPE-stimulated internalization. Thus the 309–323 region of the type 1 CCK receptor affects antagonist-stimulated internalization of this receptor, although its mechanism and interacting partner are not yet clear.
Keywords: G protein-coupled receptors, receptor internalization, antagonist
with expression on essentially every excitable cell in the body, G protein-coupled receptors (GPCRs) represent the largest family of membrane receptors. They represent very attractive potential drug targets and, in fact, are the targets of more than one-third of currently approved drugs. Most of these drugs have been developed seeking classical agonists or antagonists, based on a relatively simple understanding of the physiology of these signaling and regulatory systems. Recently, however, drugs that represent “biased” agonists have been approved; these drugs have a subset of the biological effects of the natural agonist (3). For GPCRs, the most common “bias” has been the stimulation of a G protein-mediated pathway without concomitant activation of arrestin (G protein bias) or activation of arrestin independent of recognized G protein coupling and activation (arrestin bias) (23, 48). Since these receptors are recognized as being “shape-shifting” proteins with prominent allosteric effects (23), it is easy to understand the concept of different ligands inducing distinct active conformations that facilitate the exclusive coupling with a G protein or with arrestin.
The present study goes beyond the traditional concept of G protein-biased and arrestin-biased agonism and focuses on the possible impact of a ligand inducing a conformational change in the receptor that results in a recognizable change in function that is independent of either of these molecular associations. Here, a ligand that has been identified as an antagonist, on the basis of the absence of ligand-induced signaling events and the ability of the ligand to competitively inhibit natural agonist action (29), has been recognized to stimulate the internalization of the occupied receptor (44). The peptide being utilized is d-Tyr-Gly-[(Nle28,31,d-Trp30)CCK-26–32]-2-phenylethyl ester (to be identified as d-Trp-OPE) (44). d-Trp-OPE is a variant of the peptide first described by J. Martinez and called JMV-179 [t-butyloxycarbonyl-Tyr(SO3)-Met-Gly-d-Trp-Nle-Asp-2-phenylethyl ester] (29); d-Trp-OPE can be radio-iodinated for direct ligand binding analysis (44) and has a free amino terminus that is available for modification, such as attachment of a fluorophore for morphological tracking (20, 44).
GPCR internalization often proceeds via clathrin-coated pits and receptor-mediated endocytosis (6, 12, 33). The type 1 cholecystokinin (CCK) receptor (CCK1R) is no exception (46). Agonist-stimulated internalization of this receptor is known to occur predominantly through this pathway (45, 46). While this receptor is known to be phosphorylated on serine and threonine residues within the third intracellular loop and carboxyl-terminal tail regions in response to natural agonist stimulation (24, 37, 38), this is not a required mechanism for its ligand-stimulated internalization (42). It has been clearly documented that antagonists of this receptor do not stimulate its phosphorylation (44); yet some, such as d-Trp-OPE, are capable of stimulating internalization. The possible roles for arrestin molecules in the internalization of the CCK1R have not previously been studied.
We attempt to elucidate the molecular basis for the internalization of CCK1R stimulated by d-Trp-OPE. We established that this occurs without translocation of arrestin 2 or arrestin 3, and we examine the regions of this receptor that might be involved in protein interactions key for directing this receptor into the endocytic pathway. These efforts successfully localized an important region within the carboxyl-terminal end of the third intracellular loop of this receptor. Presumably, this region becomes accessible for molecular interactions upon conformational change induced by d-Trp-OPE binding, with disruption of such interactions interfering with the internalization process.
MATERIALS AND METHODS
Materials.
Ham's F-12 medium was obtained from Invitrogen (Carlsbad, CA); fetal clone II cell culture medium supplement was purchased from Hyclone Laboratories (Logan, UT). All other reagents were analytical grade. Arrestin 2-enhanced green fluorescent protein (eGFP) and arrestin 3-eGFP (bovine) were kindly supplied by J. Benovic (Thomas Jefferson University, Philadelphia, PA) and M. Caron (Duke University, Durham, NC).
Peptides.
CCK peptides were synthesized in our laboratory, as described elsewhere (41), and were purified to homogeneity by reverse-phase, high-performance liquid chromatography (41). Identities of all peptides were confirmed by mass spectrometry. Peptides previously prepared and characterized include d-Trp-OPE, rhodamine- and Alexa 488-conjugated d-Trp-OPE (rhodamine-d-Trp-OPE and Alexa-d-Trp-OPE), and Alexa 488-conjugated Gly-[(Nle28,31)CCK-26–33] (Alexa-CCK) (20, 44).
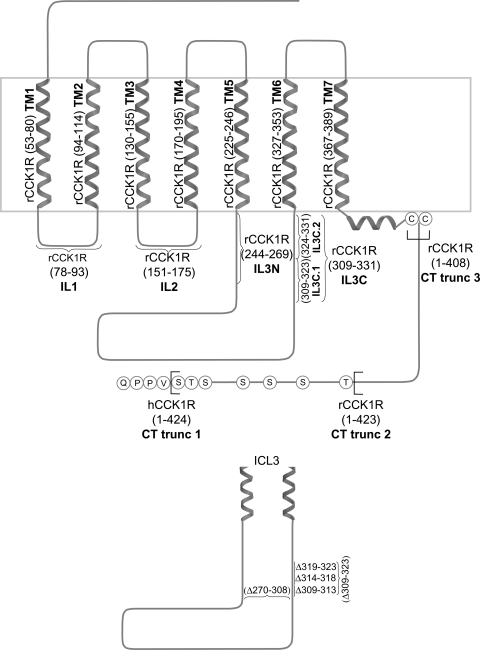
Peptides corresponding to predicted transmembrane (TM) segments of the rat CCK1R were synthesized and purified to homogeneity. Peptides corresponding to TM1, TM2, TM5, TM6, and TM7 were previously prepared and utilized (19). Additional peptides corresponding to TM3 and TM4 were synthesized and purified, following the same techniques previously reported (19). The sequences for these peptides are shown in Table 1 and illustrated schematically in Fig. 5.
Table 1.
Amino acid sequences of transmembrane segment peptides from rat CCK1R used in competitive inhibition experiments
| Receptor Segment | Sequence |
|---|---|
| TM1 | EWQSALQILLYSIIFLLSVLGNTLVITV |
| TM2 | FLLSLAVSDLMLCLFCMPFNL |
| TM3 | KTTTYFMGTSVSVSTFNLVAISLERY |
| TM4 | KSHALKVIAATWCLSFTIMTPYPIYS |
| TM5 | QTFLLLILFLLPGIVMVVAYGL |
| TM6 | VIRMLIVIVVLFFLCWMPIFSANAWRA |
| TM7 | PISFILLLSYTSSCVNPIIYCFM |
CCK1R, type 1 cholecystokinin receptor; TM1–TM7, transmembrane segments 1–7.
Fig. 5.
Regions of the CCK receptor. Schematic diagram illustrates predicted CCK1R topology and highlights synthetic transmembrane (TM) peptides used in competitive inhibition studies, regions of intracellular domains used in competitive coexpression studies, and sites of carboxyl-terminal truncations and 3rd intracellular loop deletions used in attempts to elucidate the mechanism of receptor internalization stimulated by d-Trp-OPE. Residues relevant for each construct are shown in parentheses. TM1–TM7, transmembrane segments 1–7; IL1 and IL2, 1st and 2nd intracellular loops; IL3N and IL3C, amino- and carboxyl-terminal portions of 3rd intracellular loop; CT trunc 3, CHO cell line expressing the rat CCK1R truncated at residue 408; CT trunc 2, CHO cell line expressing the rat CCK1R truncated at residue 423 (16); CT trunc 1, CHO cell line expressing hCCK1R truncated at residue 424.
Cell lines.
Several different cell lines were utilized: many of these were previously characterized by our research group (relevant regions illustrated in Fig. 5): CHO-rCCK1R, a Chinese hamster ovary (CHO) cell line expressing the rat CCK1R (17); CHO-rCCK1R-YFP, a CHO cell line expressing the rat CCK1R tagged with yellow fluorescent protein (YFP) (19); CHO-hCCK1R, a CHO cell line expressing the human CCK1R (7); IL1, CHO-rCCK1R coexpressing the region corresponding to the receptor first intracellular loop residues 78–93; IL2, CHO-rCCK1R coexpressing the region corresponding to the receptor second intracellular loop residues 151–175; IL3N, CHO-rCCK1R coexpressing the amino-terminal portion of the receptor third intracellular loop residues 244–269; IL3C, CHO-rCCK1R coexpressing the carboxyl-terminal portion of the receptor third intracellular loop residues 309–331 (11); CT trunc 3, a CHO cell line expressing the rat CCK1R truncated at residue 408; and CT trunc 2, a CHO cell line expressing the rat CCK1R truncated at residue 423 (16).
Cell lines stably expressing receptor constructs were prepared for the study to complement these previously published cell lines (see Fig. 5). This included CHO-rCCK1R cells coexpressing the carboxyl-terminal portion of the third intracellular loop carboxyl-terminal peptide (residues 324–331, IL3C.2) and CHO-rCCK1R cells coexpressing the amino-terminal portion of the third intracellular loop carboxyl-terminal peptide (residues 309–323, IL3C.1). These were prepared by transfection of CHO cells or CHO-rCCK1R cells using Lipofectamine LTX and Plus reagent (Invitrogen Life Sciences) as directed by the manufacturer. Cells were seeded at 1 × 106 cells in a 56-cm2 dish 24 h prior to transfection. Opti-MEM (Invitrogen Life Sciences) was incubated with 14 μg of plasmid DNA and 14 μl of Plus reagent and incubated at room temperature for 5 min. Lipofectamine LTX (21 μl) was added, and the cells were incubated for another 30 min at room temperature. Media in 56-cm2 dishes were replaced with Opti-MEM, and after the 30-min incubation period the DNA-Plus reagent-Lipofectamine LTX mixture was added to cells and the cells were incubated for 6 h at 37°C. After 6 h, the transfection mixture was aspirated, and Ham's F-12 medium containing 5% fetal clone II was added to the cells and incubated for an additional 24 or 48 h at 37°C. General selection was achieved with 500 μg/ml hygromycin for 2 wk, with clonal populations of cells then selected by limiting dilution.
Some constructs, such as receptor constructs having deletions of residues 309–323 and 270–308 in the third intracellular loop (rCCK1R-Δ309–323 and rCCK1R-Δ270–308), were also studied as expressed transiently after transfection of CHO cells. For these studies, cells were transfected as described above but were then studied without clonal selection of stable expressing cell lines. At 24 h after transfection, cells were trypsinized and plated on glass coverslips in six-well dishes and incubated for an additional 48 h, at which time they were used in ligand-stimulated receptor internalization assays. Constructs not previously characterized were subjected to CCK receptor binding and intracellular calcium stimulation studies, as we previously reported (11, 20).
Receptor internalization assays.
The synthesis and characterization of Alexa-CCK or Alexa-d-Trp-OPE utilized in internalization experiments have been described by our laboratory (20). Immediately before labeling, cells plated on coverslips in six-well plates were incubated on ice for 10 min, then rinsed three times at 4°C in PBS, pH 7.4, containing magnesium and calcium (1.5 mM NaH2PO4, 8 mM Na2HPO4, 0.145 M NaCl, 0.1 mM MgCl2, and 0.08 mM CaCl2) and incubated on ice for an additional 10 min. Alexa-CCK and Alexa-d-Trp-OPE were diluted in 4°C PBS to 100 nM and 2 nM, respectively, and added to the coverslips to saturate surface receptors over a 90-min period. Coverslips were then rinsed three times with 4°C PBS, and internalization was started by placement of the cells in 37°C PBS and incubation for 5, 10, and 30 min. Coverslips were then placed in 2% paraformaldehyde for 15–30 min and washed with room temperature PBS and then mounted using Vectashield. For the 0-min time point, the cells were fixed prior to warming at 37°C. Images of cell fluorescence were captured as described previously (18). Fluorescence was observed using an inverted epifluorescence microscope (Axiovert 200M, Carl Zeiss, Thornwood, NY) with a fixed YFP filter set of excitation at 500/20 nm, dichroic mirror at Q515 long pass, and emission at 535/30 nm (Chroma Technology, Brattleboro, VT). Images were collected using an ORCA-12ER charge-coupled device camera (Hamamatsu, Bridgewater, NJ) with QED-InVivo 2.03 image acquisition software (Media Cybernetics, Silver Spring, MD). The final morphological images were assembled using Photoshop 7.0 (Adobe Systems, Mountain View, CA). Nonspecific, nonsaturable binding of the Alexa-conjugated peptides was assessed in each experiment using saturating amounts of unconjugated peptide (data not shown).
Arrestin translocation assays.
For these assays, media from CHO-hCCK1R cells that had been transfected with arrestin 2-eGFP or arrestin 3-eGFP and plated on coverslips 24 h previously were replaced with complete medium containing 0.1 μM CCK, 0.1 μM d-Trp-OPE, or medium alone. Cells were then stimulated for 0, 5, 30, or 60 min, washed twice with PBS, and then fixed in 2% paraformaldehyde for 15–30 min. Coverslips were then washed with room temperature PBS and mounted on slides, and fluorescence was observed using epifluorescence microscopy, as described above.
Morphological localization of internalized fluorescent peptides-Alexa 488.
CCK and rhodamine-d-Trp-OPE were used in nonsaturating concentrations (30 nM Alexa-CCK and 0.8 nM rhodamine-d-Trp-OPE) to simultaneously occupy random subsets of cell surface receptors at 4°C. Receptor internalization was allowed to proceed upon warming, as described above. Fluorescence was observed at 0 and 30 min using two selective settings with the inverted epifluorescence microscope (Axiovert 200M) described above (YFP filter set: excitation at 500/20 nm, dichroic mirror at Q515 long pass, and emission at 535/30 nm; tetramethylrhodamine isothiocyanate filter set: excitation at 535/50 nm, dichroic mirror at Q565 long pass, and emission at 610/75 nm). Pseudocolor images were collected with the rhodamine assigned red and the Alexa 488 assigned green. Background fluorescence was subtracted using a rolling ball radius of 50 pixels in ImageJ version 1.44 (1). Color images were then merged to reveal overlaid patterns of fluorescence.
Quantitation of plasma membrane fluorescence.
Fluorescent 18-bit images collected as previously described were analyzed in ImageJ version 1.44. Briefly, the background fluorescence was subtracted from all images using a rolling ball radius of 15 pixels. The region of interest manager application of ImageJ was then used to analyze the pixel intensity of the entire cell and its intracellular region. Plasma membrane fluorescence was calculated by subtracting the intracellular fluorescence from whole cell fluorescence and expressing this as a percentage of whole cell fluorescence. Five cells from each of three independent experiments (n = 15) were used for each sample group. Statistical analysis was carried out using a two-tailed Student's t-test, and data were plotted using Prism (version 4.0, GraphPad, San Diego, CA).
RESULTS
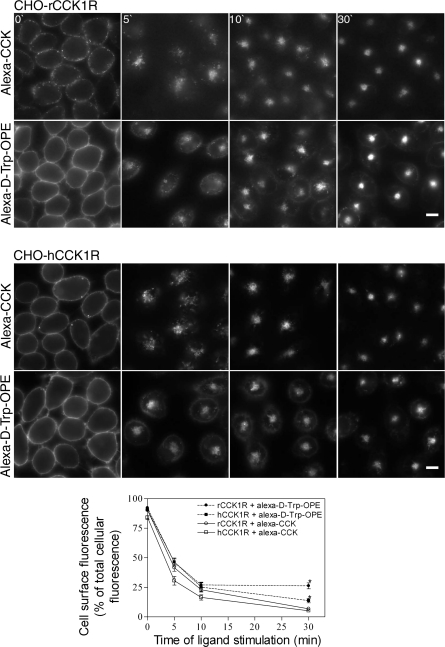
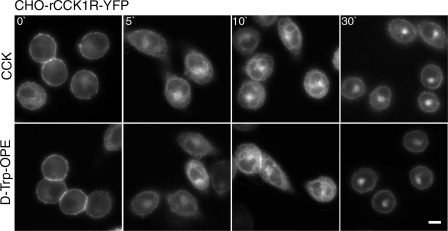
As previously reported (44), occupation of the CCK1R with the d-Trp-OPE analog of CCK is known to not stabilize association of the receptor with its G protein (44) and does not elicit intracellular calcium (44), cAMP (44), or phosphorylation (44) responses. However, it did result in prompt internalization of the ligand-occupied rat and human CCK1R in receptor-bearing CHO cells (Fig. 1). The time course of this was similar to that stimulated by receptor occupation with the natural full agonist CCK, although cell surface labeling was more extensive and intense with the fluorescent d-Trp-OPE ligand, and some of this fluorescence appeared to remain at the cell surface, unlike the fluorescent CCK ligand, in which all surface labeling internalized over time (44). Quantitation of membrane fluorescence (Fig. 1, bottom) demonstrated significantly more plasma membrane fluorescence after 30 min with Alexa-d-Trp-OPE than with Alexa-CCK in CHO-rCCK1R (P < 0.0001) and CHO-hCCK1R (P < 0.0003). This observation was similar to a previous report of the kinetics of internalization of this receptor (44). A possible reason for the residual plasma membrane fluorescence at later time points is that the antagonist Alexa-d-Trp-OPE binds not only to functional, fully folded CCK1Rs, but it may also bind to receptors that are not normally folded or fully functional. The rat CCK1R construct tagged with YFP was used to validate this observation, demonstrating that nonfluorescent d-Trp-OPE was also able to stimulate receptor internalization (Fig. 2). Indeed, similar kinetics of internalization were observed using tagged and untagged peptide.
Fig. 1.
Ligand-stimulated cholecystokinin (CCK) receptor internalization. Representative fluorescence images show internalization of Alexa-CCK (100 nM) and Alexa-d-Tyr-Gly-[(Nle28,31,d-Trp30)CCK-26–32]-2-phenylethyl ester (d-Trp-OPE, 2 nM) occupying the CCK receptor over time into receptor-expressing Chinese hamster ovary (CHO) cells. Top and middle: cells expressing rat and human type 1 CCK receptor (rCCK1R and hCCK1R). Images are typical of data observed in 3 independent experiments. Scale bars, 20 μm. Bottom: quantitation of fluorescence on plasma membrane at 0, 5, 10, and 30 min for rat and human receptors occupied with Alexa-CCK (100 nM) and Alexa-d-Trp-OPE (2 nM). Results are representative of analysis of 5 individual cells from each of 3 independent experiments.
Fig. 2.
Fluorescently tagged CCK receptor internalization stimulated by nonfluorescent ligands. Representative fluorescence images show internalization of the yellow fluorescent protein (YFP)-tagged CCK receptor over time after occupation with ligands. Top and bottom: cells expressing the rCCK1R-YFP occupied with 0.1 μM CCK and 0.1 μM d-Trp-OPE. Images are typical of data observed in 3 independent experiments. Scale bar, 20 μm.
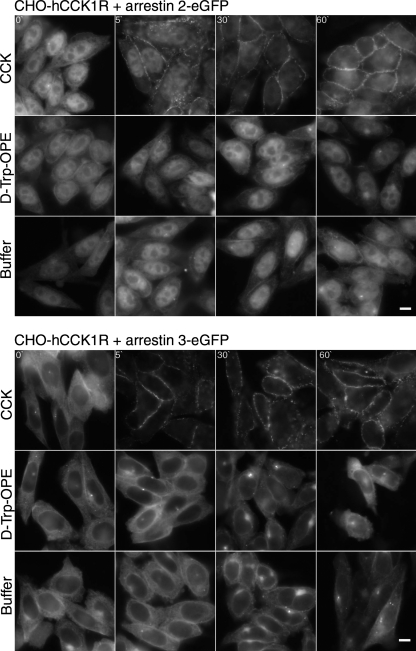
The dominant pathway of internalization of the CCK1R occupied by CCK or d-Trp-OPE in these cells has previously been defined as clathrin-dependent endocytosis (44, 46). Arrestin proteins have been shown to play a major role in the internalization of many GPCRs utilizing this pathway (10, 13, 35). For this reason, we studied the translocation of fluorescent analogs of arrestin 2 and arrestin 3 (4, 34) (Fig. 3). As expected, stimulation with the full agonist CCK that is known to stimulate receptor phosphorylation (44) resulted in time-dependent translocation of arrestin 2 and arrestin 3 to the plasma membrane. In contrast, stimulation with a saturating concentration of d-Trp-OPE did not result in a change in the cytosolic distribution of either of the arrestins, and this distribution was not different from control cells treated with buffer alone.
Fig. 3.
Translocation of arrestins after stimulation with CCK and d-Trp-OPE. Representative fluorescence images show arrestin 2-enhanced green fluorescent protein (eGFP, top) and arrestin 3-eGFP (bottom) translocation stimulated by CCK receptor ligands (0.1 μM) and buffer control in CHO cells expressing the hCCK1R. Images are typical of data from 5 independent experiments. Scale bars, 20 μm.
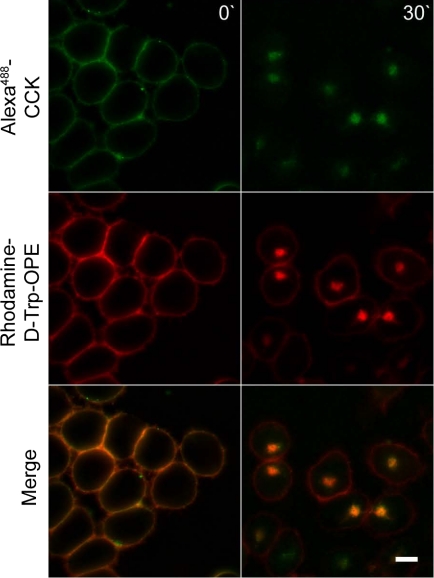
Since arrestins appear to play a role in internalization of CCK1R stimulated by the natural agonist ligand, but not by d-Trp-OPE, it was important to evaluate whether both internalization events resulted in similar or different intracellular trafficking. To explore this, receptors on the same cell occupied with rhodamine-d-Trp-OPE and Alexa-CCK were studied and were shown to traffic similarly and to be colocalized to the same intracellular compartment (Fig. 4).
Fig. 4.
Colocalization of internalized Alexa-CCK and rhodamine-d-Trp-OPE. Representative fluorescence images show CCK receptors on the same cell occupied with Alexa-CCK (30 nM) and rhodamine-d-Trp-OPE (0.8 nM). Top: images taken with the YFP filter set. Middle: images taken with the tetramethylrhodamine isothiocyanate filter set. Bottom: overlay of the 2 channels, with yellow identifying areas of colocalization. Images are typical of data observed in 3 independent experiments. Scale bars, 20 μm.
The next series of studies was designed to explore possible regions of the CCK1R that were responsible for the d-Trp-OPE-induced internalization of this receptor. Figure 5 shows the regions that were studied.
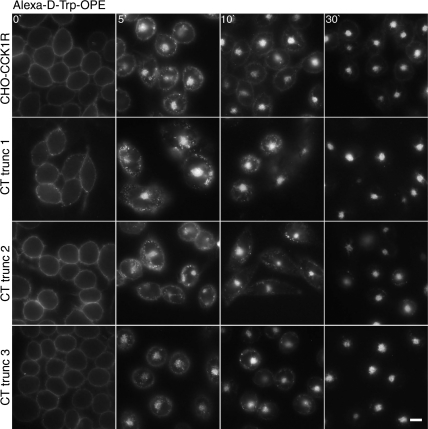
The carboxyl-terminal tail region of many GPCRs is known to also play a role in receptor internalization, and PDZ-domain protein association with this region has been linked to regulation of receptor trafficking (33, 43). Indeed, a proline-rich region that could interact with PDZ-domain proteins is present in the distal carboxyl-terminal tail of the CCK1R (last 4 residues APPP in rCCK1R and VPPQ in hCCK1R). We, therefore, truncated this region of the receptor in hCCK1R residues 1–424 (CT trunc 1) and examined the ability of the fluorescent d-Trp-OPE to stimulate its internalization after binding (Fig. 6). There was no significant effect of removal of the distal carboxyl-terminal tail region of the CCK receptor on this process.
Fig. 6.
Effects of receptor carboxyl-terminal truncation on d-Trp-OPE-stimulated CCK receptor internalization. Representative fluorescent images show Alexa-d-Trp-OPE (2 nM) internalization after occupying CCK receptor constructs expressed on CHO cells. Cells express wild-type receptor and 3 constructs with different degrees of truncation of its carboxyl-terminal tail region. Images are typical of data observed in 3 independent experiments. Scale bar, 20 μm.
The carboxyl-terminal tail region of many GPCRs also includes potential sites of phosphorylation and sites of fatty acid acylation (palmitoylation) that are known to be utilized in the CCK1R (15, 24, 31, 37, 38). Therefore, receptor constructs with more severe degrees of truncation were also employed. CHO cell lines that express these carboxyl-terminal truncations in the rat CCK1R have previously been prepared and studied (16). The first truncation eliminated the carboxyl-terminal tail sites of phosphorylation, residues 1–423 (CT trunc 2), and was found to have no effect on d-Trp-OPE-stimulated internalization (Fig. 6). The next truncation also eliminated the region of the tail between the phosphorylation domain and the two vicinal cysteine residues known to be palmitoylated, residues 1–408 (CT trunc 3), and also had no effect on d-Trp-OPE-stimulated internalization (Fig. 6).
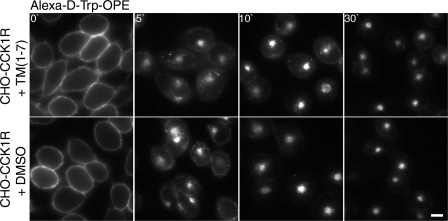
In an effort to determine which receptor domain might be involved in protein-protein interactions mediating the d-Trp-OPE-induced CCK receptor internalization, we performed a series of competitive inhibition experiments. We previously utilized this approach to demonstrate which region of GPCRs might be involved in receptor oligomerization (19) and receptor trafficking (11). As illustrated in Fig. 7, we utilized a combination of all seven predicted transmembrane segment peptides of the rat CCK1R, TM1–TM7 (Table 1), in an analogous competition assay. Indeed, this had no effect on d-Trp-OPE-induced internalization.
Fig. 7.
Competitive inhibition of d-Trp-OPE-stimulated CCK receptor internalization by synthetic TM segment peptides. Representative fluorescent images show Alexa-d-Trp-OPE (2 nM) internalization after occupation of rCCK1R expressed on CHO-CCK1R cells in the presence of a cocktail containing synthetic peptides corresponding to TM1–TM7 of rat CCK1R or the carrier control for these peptides (DMSO only). Images are typical of data observed in 3 independent experiments. Scale bar, 20 μm.
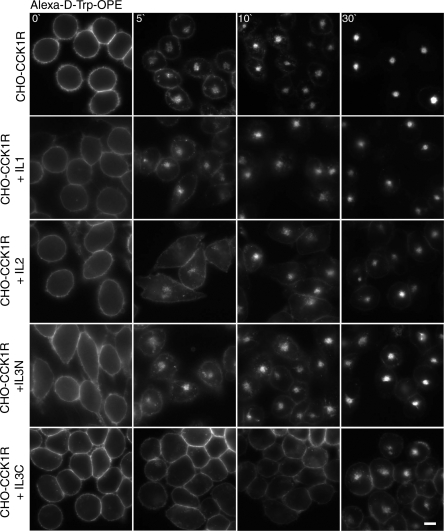
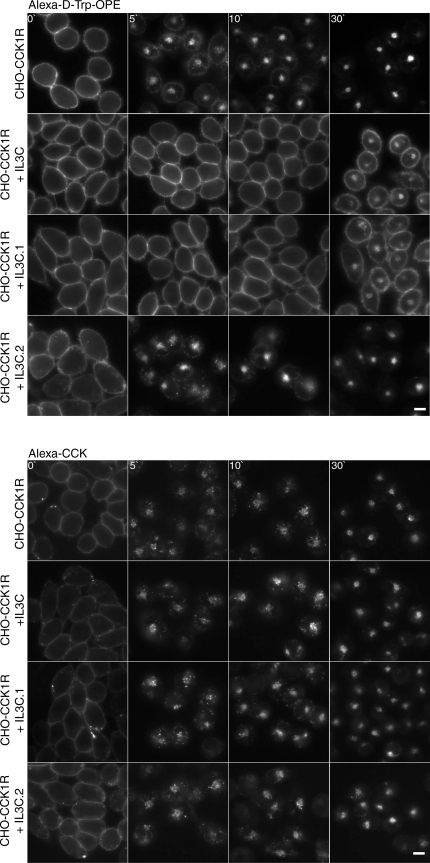
As illustrated in Fig. 8, we also utilized peptides corresponding to the perimembranous region of each of the intracellular loops of the rat CCK1R in analogous assays. In a previous study, stable cell lines expressing the CHO-rCCK1R and these peptides were utilized in a similar strategy for examining full agonist CCK-induced trafficking, and the second intracellular loop peptide was shown to affect CCK receptor recycling, but none of the peptides had an inhibitory effect on internalization (11). Here, in response to d-Trp-OPE occupation, the same peptides were utilized, and the peptide corresponding to the carboxyl end of the third intracellular loop (IL3C) resulted in markedly slowed and reduced receptor internalization but normal CCK-stimulated internalization. Since this peptide is predicted to span the lower portion of TM6 and the top of the loop, we also prepared constructs encoding each of these regions separately (Fig. 9). Of particular interest, the peptide at the top of the loop and extending into TM6, including residues 324–331 (IL3C.2), had no effect on the d-Trp-OPE-stimulated receptor internalization in this assay. Instead, the peptide representing residues 309–323 (IL3C.1) had the same inhibitory effect on receptor internalization in response to d-Trp-OPE occupation as the longer peptide but had no effect on CCK-stimulated internalization. This established that the inhibition by this peptide of d-Trp-OPE-stimulated CCK receptor internalization did not result from disruption of the internalization machinery.
Fig. 8.
Competitive inhibition of d-Trp-OPE-stimulated CCK receptor internalization by coexpressing intracellular domain peptides. Representative fluorescence images show Alexa-d-Trp-OPE internalization after occupation of rCCK1R expressed on CHO-CCK1R cells coexpressing IL1, IL2, IL3N, and IL3C. Images are typical of data observed in 3 independent experiments. Scale bar, 20 μm.
Fig. 9.
Competitive inhibition of d-Trp-OPE- and CCK-stimulated CCK receptor internalization by coexpressing peptides representing portions of IL3C. Representative fluorescence images show Alexa-d-Trp-OPE (2 nM; top) and Alexa-CCK (100 nM; bottom) internalization after occupation of the rCCK1R expressed on CHO-CCK1R cells coexpressing IL3C, as well as fragments of this peptide (IL3C.1 and IL3C.2). Images are typical of data observed in 3 independent experiments. Scale bars, 20 μm.
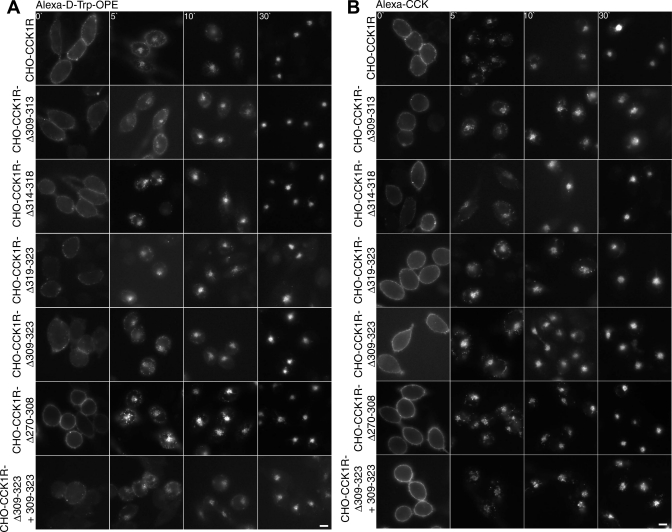
Deletion mutants of the CCK1R were created to investigate further the role of residues 309–323 of the CCK1R in the internalization of the Alexa-d-Trp-OPE occupied receptor. Four deletion mutants were characterized using Alexa-d-Trp-OPE and Alexa-CCK. These consisted of rat CCK1R with deletion of residues 309–313 (rCCK1R-Δ309–313), deletion of residues 314–318 (rCCK1R-Δ314–318), deletion of residues 319–323 (rCCK1R-Δ319–323), and deletion of all 15 residues (rCCK1R-Δ309–323). Figure 10 shows the internalization of these four deletion mutants after occupation with Alexa-d-Trp-OPE and Alexa-CCK. The results demonstrate that all these constructs were internalized in response to both peptides, such as wild-type receptor (Fig. 10). However, neither peptide stimulated an intracellular calcium response, and both bound differently from their binding characteristics to wild-type receptor {IC50 values (nM) for peptide competition for 125I-d-Tyr-Gly-[(Nle28,31)CCK-26–33] binding to intact receptor-bearing cells: 2.2 ± 0.7 (CCK) and 5.9 ± 0.9 (d-Trp-OPE) for wild-type rCCK1R and 0.27 ± 0.02 (CCK) and 0.77 ± 0.08 (d-Trp-OPE) for rCCK1R-Δ309–323}. This suggested impact on receptor conformation and function, making the internalization difficult to interpret. It is possible that the antagonist-stimulated internalization of this construct follows a mechanism distinct from that in the wild-type receptor. Coexpression of the 309–323 peptide failed to inhibit the internalization of this construct stimulated by CCK or d-Trp-OPE (Fig. 10).
Fig. 10.
d-Trp-OPE- and CCK-stimulated internalization of deletion mutants of the CCK receptor. Representative fluorescence images show Alexa-d-Trp-OPE (2 nM; A) and Alexa-CCK (100 nM; B) internalization after occupation of CCK receptor constructs expressed on CHO cells. From top to bottom: cells expressing wild-type receptor, cells expressing 4 CCK receptor deletion constructs in the carboxyl-terminal region and 1 in the midregion of the 3rd intracellular loop, and data from the key rCCK1R-Δ309–323 construct coexpressing peptide 309–323. Images are typical of data observed in 3 independent experiments. Scale bars, 20 μm.
An additional CCK1R mutant representing deletion of residues 270–308 (rCCK1R-Δ270–308) in the midregion of the third intracellular loop was prepared and studied. This was the last remaining receptor region facing the cytosol that had not been evaluated in other studies. This, too, had no effect on the CCK and d-Trp-OPE ligand-stimulated internalization of this receptor (Fig. 10).
DISCUSSION
Bimolecular interactions are a common and critical mechanism for cell signaling and receptor regulation. Ligands bind to their receptors, representing the first such interaction in the vectorial cascade of events that often result in a cellular response. Binding of an agonist ligand to its GPCR elicits another key and common molecular interaction, the association of the receptor with a heterotrimeric G protein. This proximal mediator, in turn, initiates another cycle of events that activate various well-established signaling cascades within the cell, dependent on the particular G protein involved (36). These represent the classical signaling events stimulated by GPCRs. In addition to stimulating such signaling events, agonist occupation of this superfamily of receptors is known to commonly result in other bimolecular interactions involved in receptor regulation and desensitization, such as kinase-mediated phosphorylation (signaling kinases and GPCR kinases), arrestin association to block further G protein association, and molecular events that result in entry into clathrin-coated pits for endocytosis (25, 26, 28).
It is noteworthy that many partial agonists acting at GPCRs also stabilize G protein association and stimulate at least a subset of the molecular interactions described for full agonists (2, 14). There is also the recently recognized group of biased agonists that act predominantly through arrestin association, leading to different signaling pathways within the cell (27, 28, 30, 47).
In contrast, the ligands normally thought of as antagonists acting at these receptors do not stabilize G protein association and do not stimulate any of the signaling events typical of occupation of the same receptor with its natural agonist. Indeed, d-Trp-OPE is a peptide analog of CCK that has previously been identified as a antagonist (29) that is able to competitively eliminate CCK binding and CCK-stimulated signaling events (29, 44). Consistent with its identification as an antagonist, studies have demonstrated that it does not stabilize G protein association (44), that there is no intracellular calcium response stimulated by its binding to the CCK receptor (29, 44), and that there is no receptor phosphorylation stimulated by its binding to the CCK receptor (44).
Perhaps the most surprising observation for d-Trp-OPE action has been its ability to stimulate CCK receptor internalization (44). This was again confirmed in the present studies. There is some precedent for molecules identified as antagonists to stimulate the internalization of their receptors, although the vast majority of such ligands do not modify receptor trafficking. Peptide antagonists of the vasopressin, angiotensin, and bradykinin receptors have been reported to stimulate the internalization of their receptors (21, 22, 40); however, the mechanism for this effect has been unclear. As in the earlier observation (44), d-Trp-OPE stimulated the internalization of the rat CCK1R expressed on the CHO-CCK1R cell line to the same endocytic compartment observed after occupation with natural agonist hormone. In the present study, we also examined the effect of these ligands on trafficking of the human CCK1R in an analogous CHO cell line and found it to be identical. This provides a clue to mechanism, suggesting that d-Trp-OPE binding resulted in a conformational change in the CCK receptor that exposed an epitope that might mediate a bimolecular interaction that would lead to entry into the clathrin-dependent endocytic pathway.
Arrestin molecules are well known to play a role in directing GPCRs into clathrin-coated pits (10, 13). We, therefore, studied arrestin 2 and arrestin 3 translocation in relation to CCK receptor internalization stimulated by the natural hormonal full agonist peptide CCK and the presumed antagonist peptide analog d-Trp-OPE. Arrestin 2 and arrestin 3 were observed to translocate to the plasma membrane after stimulation with CCK, representing previously unreported observations. In contrast, neither of these arrestin molecules was translocated in response to d-Trp-OPE. The structurally related type 2 CCK receptor has also been described to recruit and bind arrestin after agonist stimulation (5, 32), although no analogous studies have been described using presumed antagonists of that receptor.
In the present study, in the apparent absence of CCK receptor association with known mediators of internalization after stimulation with d-Trp-OPE, we proceeded to systematically examine regions of the receptor that could potentially mediate a bimolecular interaction that could result in directing this receptor into the endocytic pathway. This was achieved using strategies of systematic deletional mutagenesis of this receptor, as well as competitive inhibition, which has been very useful to compete for a critical bimolecular interaction (11, 16, 19).
The carboxyl-terminal tail region of several GPCRs has been shown to bind numerous adaptor proteins involved in internalization. A series of truncations of residues from the carboxyl terminus of CCK1Rs were studied. These were performed with rat and human receptors. The shortest truncation eliminated the terminal four residues that include the proline-rich region, which has played a role in trafficking of other receptors in this superfamily (33). The next-longer truncation eliminated the serine- and threonine-rich region of the CCK receptor, which has been shown to be phosphorylated in response to full agonist (15, 24, 31, 37, 38), but likely not in response to d-Trp-OPE. The longest carboxyl-terminal tail truncation eliminated the entire tail region distal to the palmitoylated vicinal cysteines. For each of these constructs, there was no difference between Alexa-d-Trp-OPE- and Alexa-CCK-stimulated receptor internalization.
The possibility of an intramembranous interaction resulting in the receptor internalization was explored using the competing transmembrane peptide approach we previously utilized in examining CCK receptor oligomerization (19). Use of all seven transmembrane peptides in competitive mode did not disrupt the d-Trp-OPE-stimulated internalization process. This approach was then extended to the cytosolic loop regions adjacent to the predicted transmembrane segments. Again, this approach had been utilized previously (11) to evaluate molecular mechanisms of CCK-stimulated receptor trafficking. None of these regions were shown to be involved in CCK-stimulated internalization, while the second intracellular loop region was found to affect the recycling of this receptor (11). In the present study, the peptide corresponding to the carboxyl-terminal end of the third intracellular loop, indeed, had a prominent disruptive effect on d-Trp-OPE-stimulated CCK receptor internalization. This was clearly distinct from its lack of effect on CCK-stimulated internalization of the same receptor in the same cells. The differential effect of these two ligands acting at the same receptor was able to ensure that the internalization machinery was intact and not negatively affected by the competing peptide.
The peptide with the positive effect on d-Trp-OPE-stimulated CCK receptor internalization was 23 residues in length (residues 309–331), spanning the region of the carboxyl-terminal portion of the third intracellular loop and the bottom of the TM6 segment. Indeed, the latter contains residues that have been implicated in G protein coupling (BBxxB motif with 3 basic residues and spacers, KRVIR), and this region undergoes a conformational change in response to full agonist that theoretically exposes this sequence (8, 39). The two portions of this peptide were then studied individually. It was particularly interesting that the portion corresponding to the lower end of TM6 (residues 324–331), which included this consensus G protein-coupling motif (KRVIR), did not mimic the effect of the entire peptide to inhibit internalization. Instead, the portion limited to the loop domain (residues 309–323, with sequence LNRIRSSSSAANLIA) was able to reproduce the effect of the entire peptide on disrupting d-Trp-OPE-stimulated CCK receptor internalization.
Accessibility to different regions of the intracellular loop domains of GPCRs for bimolecular interactions is poorly understood. Clearly, there are conformational changes that can be induced by any number of allosteric events (23). Such a classical event is agonist binding to the ectodomain of the receptor, which results in exposure of the cytosolic face that interacts with a heterotrimeric G protein (9). It is interesting that the region of focus in the current project is quite near such a region, but the peptide competition studies have nicely established that this does not mediate the d-Trp-OPE-stimulated event. It is likely that the conformational change stimulated by d-Trp-OPE binding does not fully expose the G protein-coupling epitope, whereas CCK binding does. Given the relatively large size of the heterotrimeric G protein coupling with the receptor stimulated by full agonist, it is likely that such an interaction would block the bimolecular interaction that seems to mediate the d-Trp-OPE-stimulated event.
In an effort to determine whether this peptide segment was directly or indirectly involved in the d-Trp-OPE-stimulated CCK receptor internalization, deletion mutants were also prepared and studied. Deletion of this entire peptide (rCCK1R-Δ309–323) had no effect on the CCK- or d-Trp-OPE-stimulated receptor internalization. The inability to replicate the peptide competition experiment might be evidence against a required direct interaction between this peptide and a cellular protein directing the endocytosis, pointing toward an indirect effect. However, careful characterization of this deletion construct established that it has different ligand binding and no signaling activity in response to CCK or d-Trp-OPE peptides, suggesting that its conformation is different from that of the wild-type CCK receptor and may be regulated via a different mechanism. In support of the latter was the new observation that coexpression of the 309–323 peptide with this deletion construct failed to inhibit the internalization in response to CCK and d-Trp-OPE.
We have demonstrated that a CCK receptor ligand that does not stabilize G protein coupling or stimulate G protein-mediated signaling events and does not stimulate translocation or receptor association with arrestin proteins does stimulate the internalization of the CCK receptor. This seems to be achieved via a conformational change that includes exposure of a short region of the carboxyl-terminal end of the third intracellular loop. While it is not clear what molecule might be interacting with the receptor to direct its endocytosis, this provides still another type of regulatory event that can be stimulated by a ligand that seems to be otherwise devoid of biological activity. Such a regulatory event could lead to downregulation or desensitization of the receptor and could be useful in selected clinical settings.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-32878 and by the Mayo Clinic.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.E.C. and L.J.M. are responsible for conception and design of the research; E.E.C. performed the experiments; E.E.C. analyzed the data; E.E.C. and L.J.M. interpreted the results of the experiments; E.E.C. prepared the figures; E.E.C. and L.J.M. drafted the manuscript; E.E.C., K.G.H., and L.J.M. edited and revised the manuscript; E.E.C., K.G.H., and L.J.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank A. M. Ball, D. I. Pinon, and M. L. Augustine for excellent technical assistance.
REFERENCES
- 1.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004 [Google Scholar]
- 2.Amblard M, Lignon MF, Bernad N, Noel-Artis AM, Hauad L, Laur J, Rodriguez M, Galas MC, Fourmy D, Martinez J. Biological evaluation of JMV180 cholecystokinin analogs. Ann NY Acad Sci 713: 79–87, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Andresen BT. A pharmacological primer of biased agonism. Endocr Immune Disord Drug Targets 11: 92–98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barak LS, Ferguson SS, Zhang J, Martenson C, Meyer T, Caron MG. Internal trafficking and surface mobility of a functionally intact β2-adrenergic receptor-green fluorescent protein conjugate. Mol Pharmacol 51: 177–184, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Barak LS, Oakley RH, Shetzline MA. G protein-coupled receptor desensitization as a measure of signaling: modeling of arrestin recruitment to activated CCK-B receptors. Assay Drug Dev Technol 1: 409–424, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Carman CV, Benovic JL. G-protein-coupled receptors: turn-ons and turn-offs. Curr Opin Neurobiol 8: 335–344, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem 278: 52972–52979, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cheung AH, Huang RR, Graziano MP, Strader CD. Specific activation of Gs by synthetic peptides corresponding to an intracellular loop of the β-adrenergic receptor. FEBS Lett 279: 277–280, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Deupi X, Kobilka B. Activation of G protein-coupled receptors. Adv Protein Chem 74: 137–166, 2007 [DOI] [PubMed] [Google Scholar]
- 10.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-Arrestins and cell signaling. Annu Rev Physiol 69: 483–510, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Ding XQ, Rao RV, Kuntz SM, Holicky EL, Miller LJ. Impaired resensitization and recycling of the cholecystokinin receptor by co-expression of its second intracellular loop. Mol Pharmacol 58: 1424–1433, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SS, Caron MG. G protein-coupled receptor adaptation mechanisms. Semin Cell Dev Biol 9: 119–127, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271: 363–366, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Gaisano HY, Klueppelberg UG, Pinon DI, Pfenning MA, Powers SP, Miller LJ. Novel tool for the study of cholecystokinin-stimulated pancreatic enzyme secretion. J Clin Invest 83: 321–325, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gates LK, Ulrich CD, Miller LJ. Multiple kinases phosphorylate the pancreatic cholecystokinin receptor in an agonist-dependent manner. Am J Physiol Gastrointest Liver Physiol 264: G840–G847, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Go WY, Holicky EL, Hadac EM, Rao RV, Miller LJ. Identification of a domain in the carboxy terminus of CCK receptor that affects its intracellular trafficking. Am J Physiol Gastrointest Liver Physiol 275: G56–G62, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hadac EM, Ghanekar DV, Holicky EL, Pinon DI, Dougherty RW, Miller LJ. Relationship between native and recombinant cholecystokinin receptors: role of differential glycosylation. Pancreas 13: 130–139, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Harikumar KG, Akgun E, Portoghese PS, Miller LJ. Modulation of cell surface expression of nonactivated cholecystokinin receptors using bivalent ligand-induced internalization. J Med Chem 53: 2836–2842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harikumar KG, Dong M, Cheng Z, Pinon DI, Lybrand TP, Miller LJ. Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry 45: 14706–14716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harikumar KG, Pinon DI, Wessels WS, Prendergast FG, Miller LJ. Environment and mobility of a series of fluorescent reporters at the amino terminus of structurally related peptide agonists and antagonists bound to the cholecystokinin receptor. J Biol Chem 277: 18552–18560, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Houle S, Larrivee JF, Bachvarova M, Bouthillier J, Bachvarov DR, Marceau F. Antagonist-induced intracellular sequestration of rabbit bradykinin B2 receptor. Hypertension 35: 1319–1325, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Hunyady L. Molecular mechanisms of angiotensin II receptor internalization. J Am Soc Nephrol 10 Suppl 11: S47–S56, 1999 [PubMed] [Google Scholar]
- 23.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev 62: 265–304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klueppelberg UG, Gates LK, Gorelick FS, Miller LJ. Agonist-regulated phosphorylation of the pancreatic cholecystokinin receptor. J Biol Chem 266: 2403–2408, 1991 [PubMed] [Google Scholar]
- 25.Lefkowitz RJ. G protein-coupled receptor kinases. Cell 74: 409–412, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem 273: 18677–18680, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for β-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 24: 643–652, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science 308: 512–517, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Lignon MF, Galas MC, Rodriguez M, Laur J, Aumelas A, Martinez J. A synthetic peptide derivative that is a cholecystokinin receptor antagonist. J Biol Chem 262: 7226–7231, 1987 [PubMed] [Google Scholar]
- 30.Luttrell LM. Composition and function of G protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J Mol Neurosci 26: 253–264, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Lutz MP, Pinon DI, Gates LK, Shenolikar S, Miller LJ. Control of cholecystokinin receptor dephosphorylation in pancreatic acinar cells. J Biol Chem 268: 12136–12142, 1993 [PubMed] [Google Scholar]
- 32.Magnan R, Masri B, Escrieut C, Foucaud M, Cordelier P, Fourmy D. Regulation of membrane cholecystokinin-2 receptor by agonists enables classification of partial agonists as biased agonists. J Biol Chem 286: 6707–6719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol 48: 601–629, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matharu AL, Mundell SJ, Benovic JL, Kelly E. Rapid agonist-induced desensitization and internalization of the A2B adenosine receptor is mediated by a serine residue close to the COOH terminus. J Biol Chem 276: 30199–30207, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69: 451–482, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Neves SR, Ram PT, Iyengar R. G protein pathways. Science 296: 1636–1639, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Ozcelebi F, Miller LJ. Phosphopeptide mapping of cholecystokinin receptors on agonist-stimulated native pancreatic acinar cells. J Biol Chem 270: 3435–3441, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Ozcelebi F, Rao RV, Holicky E, Madden BJ, McCormick DJ, Miller LJ. Phosphorylation of cholecystokinin receptors expressed on Chinese hamster ovary cells. Similarities and differences relative to native pancreatic acinar cell receptors. J Biol Chem 271: 3750–3755, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Pauwels PJ, Gouble A, Wurch T. Activation of constitutive 5-hydroxytryptamine1B receptor by a series of mutations in the BBXXB motif: positioning of the third intracellular loop distal junction and its Goα protein interactions. Biochem J 343: 435–442, 1999 [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeiffer R, Kirsch J, Fahrenholz F. Agonist- and antagonist-dependent internalization of the human vasopressin V2 receptor. Exp Cell Res 244: 327–339, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Powers SP, Pinon DI, Miller LJ. Use of N,O-bis-Fmoc-d-Tyr-ONSu for introduction of an oxidative iodination site into cholecystokinin family peptides. Int J Pept Protein Res 31: 429–434, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Rao RV, Roettger BF, Hadac EM, Miller LJ. Roles of cholecystokinin receptor phosphorylation in agonist-stimulated desensitization of pancreatic acinar cells and receptor-bearing Chinese hamster ovary cholecystokinin receptor cells. Mol Pharmacol 51: 185–192, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci 4: 794–802, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Roettger BF, Ghanekar D, Rao R, Toledo C, Yingling J, Pinon D, Miller LJ. Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol Pharmacol 51: 357–362, 1997 [PubMed] [Google Scholar]
- 45.Roettger BF, Rentsch RU, Hadac EM, Hellen EH, Burghardt TP, Miller LJ. Insulation of a G protein-coupled receptor on the plasmalemmal surface of the pancreatic acinar cell. J Cell Biol 130: 579–590, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roettger BF, Rentsch RU, Pinon D, Holicky E, Hadac E, Larkin JM, Miller LJ. Dual pathways of internalization of the cholecystokinin receptor. J Cell Biol 128: 1029–1041, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terrillon S, Bouvier M. Receptor activity-independent recruitment of β-arrestin 2 reveals specific signalling modes. EMBO J 23: 3950–3961, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Violin JD, Lefkowitz RJ. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28: 416–422, 2007 [DOI] [PubMed] [Google Scholar]