Abstract
Two maneuvers known to stimulate electrogenic sodium bicarbonate cotransporter 1 (NBCe1) activity are 1) deletion from the cytosolic amino-terminus (Nt) of NBCe1-C of an 87-amino acid sequence that contains an autoinhibitory domain (AID); and 2) binding of the protein IRBIT to elements within the same 87-amino acid module in a different variant, NBCe1-B. Helpful to understanding the relationship between these two phenomena would be an appreciation of the relative magnitude of stimulation caused by each maneuver for the same NBCe1 variant. In the present study, we performed two-electrode voltage-clamp on Xenopus oocytes expressing human NBCe1-B constructs, with and without human IRBIT constructs. We find that removal of the AID stimulates NBCe1-B to the same extent as coexpression of wild-type IRBIT. The potency of wild-type IRBIT apparently is reduced by the action of endogenous oocyte protein phosphatases: a mutant IRBIT that cannot be influenced by the action of protein phosphatase-1 stimulates NBCe1-B to an extent 50% greater than can be achieved by removal of the NBCe1-B AID. Thus the stimulatory effect of IRBIT cannot be explained solely by masking of autoinhibitory determinants within the AID. Finally, we find that an NBCe1-B construct that lacks amino acid residues 2–16 of the Nt is fully autoinhibited, but cannot be stimulated by IRBIT, indicating that autoinhibitory and IRBIT-binding determinants within the cytosolic Nt are not identical.
Keywords: bicarbonate, acid-base, SLC4A4, NBC1
electrogenic sodium bicarbonate cotransport (NBCe) activity was first detected in the salamander renal proximal tubule (3), and the cDNA encoding the transporter NBCe1-A (a product of the slc4a4 gene), was cloned on the basis of mRNAs purified from kidney of the salamander (22). Mammalian orthologs of NBCe1-A were subsequently cloned from human and rat kidney cDNA libraries (5, 21). We now know that there are at least five mammalian NBCe1 gene products, NBCe1-A through NBCe1-E (4, 14). NBCe1-A is predominantly expressed in the kidney, NBCe1-B is widely expressed throughout the body, and NBCe1-C is thought to be mainly expressed in the brain. When expressed in Xenopus oocytes, all NBCe1 variants (12, 16, 23) mediate the coupled transport of 1 Na+ ion and 2 HCO3− ions or their thermodynamic equivalent (e.g., 1 CO32− ion replacing 2 HCO3− ions, see Refs. 10, 13). NBCe1-A includes at its extreme NH2-terminus (Nt), a 41-amino acid (AA) module that contains an autostimulatory domain (ASD).1 NBCe1-A expressed in Xenopus oocytes exhibits a twofold greater activity than a truncated construct that lacks the 41-AA module containing the ASD (16). NBCe1-B and NBCe1-C both exhibit lower activity than NBCe1-A (16). In these variants, the 41-AA module containing the ASD is replaced with an 85-AA module that includes an autoinhibitory domain (AID).2 Rat NBCe1-C expressed in Xenopus oocytes exhibits only one-third the activity of a truncated construct that lacks the first 87 AA of the Nt (16). NBCe1-C differs from NBCe1-B only in its COOH-terminus (Ct) (2), but the Ct of NBCe1-C, at least in the absence of the Nt AID, is relatively stimulatory to NBCe1 activity compared with the Ct of NBCe1-B (16). The relative activities of full-length NBCe1-B compared with an AID-less NBCe1-B have not been described.
Concurrent with the discovery that the first 87 AA of NBCe1-C contains an AID, another group reported that the equivalent sequence in human NBCe1-B included binding determinants for the protein binding partner IRBIT [inositol trisphosphate (IP3)-receptor (IP3R) binding protein released with IP3] (25). IRBIT is known to interact with many transmembrane proteins, including the IP3R, CFTR, and Na/H exchanger 3 (see Refs. 1, 11, 32). IRBIT enhances the activities of NBCe1-B and -C (25, 26), and also enhances the three electroneutral Na+-coupled HCO3− cotransporters NBCn1, NDCBE, and NBCn2 (20).
In secretory epithelia, such as those of the pancreatic and parotid-salivary ducts where NBCe1-B is abundant, IRBIT is responsible for the coordinated upregulation of transporters that contribute toward transepithelial ion and fluid secretion (31, 32). IRBIT must be phosphorylated to interact with its binding partners (1, 25), and the potency of IRBIT with respect to IP3R binding is reduced by action of protein phosphatases (1, 8). In the original study, coexpression of NBCe1-B with IRBIT in Xenopus oocytes resulted in a sevenfold increase in transporter activity compared with NBCe1-B alone (25). Considering these data, it seems reasonable to propose that the mechanism of action of IRBIT on NBCe1-B could involve IRBIT binding to and masking the AID, thereby relieving transporter autoinhibition (18, 24, 31).
Despite the preceding, straightforward hypothesis, the available data, taken at face value, in fact are consistent with the hypothesis that IRBIT stimulates the AID-containing NBCe1 variants (i.e., NBCe1-B, -C, or -E) to more than twice the extent that can be accounted for by relief of autoinhibition alone. However, these data were gathered by different groups studying different NBCe1 variants from different species. Moreover, they do not address the action of endogenous oocyte phosphatases that might render IRBIT less than maximally potent. Helpful to reconciling the hypothesis with the data would be experiments examining IRBIT coexpression vs. AID removal with the same NBCe1 variant, and with consideration of the potential effects of phosphatases. In the present study, we revisit the effect of IRBIT coexpression on NBCe1-B activity and for the first time study the effect of AID removal from human NBCe1-B. We find that NBCe1-B is equally stimulated by AID removal and coexpression of IRBIT with full-length NBCe1-B. Moreover, for the first time, we show that IRBIT cannot stimulate an AID-less NBCe1-B construct. A mutant IRBIT that lacks a protein phosphatase-1 (PP-1) binding site proves to be more potent than wild-type (WT) IRBIT with respect to NBCe1-B activation, stimulating NBCe1-B to a 50% greater extent than can be achieved by AID removal. Finally, we find that at least one determinant in the NBCe1-B Nt that is required for IRBIT stimulation of the transporter lies in the earliest part of the Nt (residues 2–16), a region that is unnecessary for the AID to exert its full autoinhibitory (AI) effect.
MATERIALS AND METHODS
NBCe1-B Constructs
Subcloning enhanced green fluorescent protein-tagged NBCe1-B.
Our starting point was human NBCe1-B that had been subcloned into pGH19 (7). We replaced the stop codon in the NBCe1-B cDNA with an AgeI restriction site using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), according to the manufacturer's recommendations. Taking advantage of a unique NotI restriction site located within the vector (downstream of the cDNA), we excised vector sequence between the AgeI and NotI sites. The excised sequence was replaced with a complementary fragment that had been excised from a modified version of NBCe1-A-EGFP.pGH19 (27).3 This fragment included (from 5′ to 3′) an AgeI site, enhanced green fluorescent protein (EGFP) cDNA, a termination codon, pGH19 vector sequence identical to that which had been excised from NBCe1-B.pGH19, and a NotI site. Ligating the two complementary DNAs, we created NBCe1-B-EGFP.pGH19. By virtue of its construction, this clone encodes human NBCe1-B fused to EGFP at its Ct via a 5-AA linker sequence “SPVAT”. We call this construct BWT. The sequence of all constructs used in this report was confirmed by automated sequencing performed by the Keck Sequencing Center (Yale University, New Haven, CT).
Creation of truncated NBCe1-B-EGFP constructs.
We PCR-amplified above NBCe1-B-EGFP.pGH19 as a template to obtain a clone that lacks the initial 85 AA that includes the Nt-AID. We call this clone BΔN85. That is, the NH2-terminal 85 AA are replaced by a single initiator Met, using two primers: the forward primer 5′-CGAAGCCCGGGCCACCATGTCTCCTGCTGCAGAACGC-3′ (in which underlined sequence is an XmaI site, italicized sequence is a kozak sequence, and the bolded sequence is the initiator methionine) and the reverse primer 5′-AGCAGATACGAATGGCTACA-3′, the complementary sequence of which lies in the pGH19 vector downstream of the multiple cloning site. The PCR product was digested with XmaI and XbaI and then subcloned into pGH19 vector again. Note that, because NBCe1-B and NBCe1-A sequence converge at the point of this truncation, BΔN85 is the same as AΔN41 (i.e., NBCe1-A lacking the Nt ASD). To generate other truncations of NBCe1-B-EGFP.pGH19, we employed the same protocol with different forward primers: 1) to create BΔN4, we used GCAAGCCCGGGCCACCATGGCTGTCCTGGACAGAGGG; 2) to create BΔN16, we used GCAAGCCCGGGCCACCATGGTGTGTGATGAAGAAGAAG; 3) to create BΔN49, we used GCAAGCCCGGGCCACCATGGGGCACAAAGAAAAGAAGG; and 4) to create BΔN59, we used GCAAGCCCGGGCCACCATGATCTCTGAGAACTACTCTG.
IRBIT Constructs
Subcloning hemagglutinin-tagged IRBIT.
Our starting point was human IRBIT cDNA in a pCMV-SPORT6 vector (Open Biosystems, IMAGE ID: 6179838). This cDNA was used as template in a PCR reaction using two primers (forward primer 5′-CGAAGGGATCCGCCACCATGGGATACCCATACGACGTACCAGATTACGCTATGTCGATGCCTGACGCG-3′ and reverse primer 5′-CGAAGTCTAGATTAGTATCTGTAATAATTAGG-3′) to amplify a PCR product that included (from 5′ to 3′) a 5-bp spacer sequence, a BamHI site, a Kozak sequence, cDNA encoding Met-Gly followed by a hemagglutinin (HA) epitope-tag (“MG-YPYDVPDYA”), IRBIT cDNA, a termination codon, an XbaI site, and a 5-bp spacer sequence. The PCR product was digested with BamHI and XbaI and ligated into complementarily digested pGH19 to create HA-IRBIT.pGH19.
Site-directed mutagenesis of HA-IRBIT to create super-IRBIT and sub-IRBIT.
Mutations were introduced into HA-IRBIT.pGH19 using the QuickChange site-directed mutagenesis kit (Stratagene). The putative PP-1 binding motif “KQIQF” (residues 40–44 of IRBIT) was mutated to “KQAQA” (which should not bind PP-1; Ref. 8) and “RRVRF” (a strong consensus PP-1-binding motif; Refs. 8, 28). In this paper, we will refer to WT-IRBIT as “WT-IRBIT”, IRBIT that is mutated to include the KQAQA motif as “super-IRBIT”, and IRBIT that is mutated to include the RRVRF motif as “sub-IRBIT”.
Expression in Xenopus Oocytes
cDNA constructs in pGH19 was linearized with NotI and then purified with the QIAquick PCR purification kit (Qiagen, Valencia, CA). Capped mRNAs from the linearized cDNAs were transcribed with the T7 Message Machine kit (Ambion, Austin, TX), according to the manufacturer's instructions. The cRNAs were purified with the RNeasy MinElute RNA Cleanup kit (Qiagen). Oocytes for cRNA injection, about one-half obtained by NASCO (Fort Atkinson, WI) and about one-half obtained by dissecting frogs in house, were prepared as described previously (17, 19). The protocols for housing and handling of Xenopus laevis were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University. In brief, Xenopus were anesthetized by immersion in a solution of 0.2% Tricaine. After 20 min, when the animal is unresponsive to touch, ovaries are surgically extracted, and the animal is terminated by cardiac excision. One day after preparation, oocytes were injected with 25 nl of H2O or cRNA. Except where otherwise stated, the injected cRNA solution contained a final concentration of 1 ng/nl NBCe1 cRNA and/or 0.33 ng/nl IRBIT cRNA in sterile, RNase-free H2O. The difference in cRNA concentrations represented an attempt to account for the threefold difference in open-reading-frame length. Thus each oocyte was injected with 25 ng of NBCe1 and/or 8 ng IRBIT. All experiments were performed at room temperature.
Physiological Solutions
Nominally HCO3−-free ND96 solution contained (in mM) 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES at a pH of 7.50. HCO3−-containing solutions included 33 mM NaHCO3 in place of 33 mM NaCl, and the pH of the solution was restored to pH 7.50 by equilibration with 5% CO2/95% O2. The osmolality of all solutions was adjusted to ∼195 mosmol/kg H2O by adding H2O or mannitol as appropriate.
Electrophysiological Measurements
Two-electrode voltage clamp was used to measure whole cell ionic currents in oocytes. Voltages and currents were recorded with a model OC-725C oocyte clamp (Warner Instruments, Hamden, CT). Electrodes were pulled from thin-walled borosilicate glass and had resistances of 0.5–2.0 MΩ when filled with 3 M KCl. In all experiments, the oocyte was placed in the recording chamber in ND96 solution and sequentially impaled with the two microelectrodes. The cell was superfused with ND96 until the membrane potential (Vm) had reached a stable value, indicating that the cell membrane had resealed around the sites of impalement. The voltage clamp was applied to hold the Vm at its spontaneous value, and then the voltage-clamp protocol was initiated. The voltage-clamp protocol used to generate current-voltage (I-V) relationships stepped the Vm from its spontaneous value to a holding potential of −160 mV for 100 ms and then back to the spontaneous Vm for an additional 100 ms before the next step, which was 20 mV more positive than the last. This cycle was repeated until the final holding potential step was to +20 mV. After the first set of voltage-clamp recordings in ND96 solution, the HCO3− solution replaced the ND96 solution, and the second set of voltage-clamp recordings was gathered ∼30 s after the solution change.
Biotinylation
Biotinylation was performed using the Cell Surface Protein Isolation Kit (Pierce, Rockford, IL), according to the manufacturer's instructions. Briefly, groups of 10 oocytes were incubated for 1 h at 4°C in PBS (diluted to 200 mosmol/kgH2O) that contained 0.24 mg/ml of the biotinylation reagent Sulfo-NHS-SS-biotin. Following the incubation, nonreacted biotinylation reagent was quenched, and cells were disrupted by trituration in Tris-buffered saline that contained 1% Triton X-100 and protease inhibitors (Roche Applied Biosciences, Indianapolis, IN). At this point, a small aliquot of “total oocyte protein” was set aside for analysis by Western blot. The remainder of each homogenate was incubated for 1 h with neutravidin agarose in the mini-column provided in the kit, and nonbound protein (i.e., nonbiotinylated protein) was washed from the column. Finally, bound, biotinylated protein was eluted from the column with SDS-sample buffer that contained 50 mM DTT. Protein was resolved by SDS-PAGE on Novex 3–8% Tris-acetate gels (Invitrogen, Carlsbad, CA), transferred onto polyvinylidene difluoride membranes using the iBlot dry blotting system (Invitrogen), and immunoblotted using an anti-EGFP mouse-monoclonal primary antibody (JL-8, Clontech Laboratories, Mountain View, CA), followed by an horseradish peroxidase-conjugated goat-anti-mouse polyclonal antibody (MP Biomedicals, Solon, OH). Western blots were developed using ECL Plus reagents (GE Healthcare Biosciences Corp, Piscataway, NJ), and the signals were detected and imaged using a Typhoon Variable Mode Imager (GE Healthcare). Cells were processed in triplicate batches of 10, and each of the three resulting protein samples was resolved and analyzed in triplicate to reduce systematic error.
Data Analysis
Voltage-clamp data were collected and analyzed using pClamp and Clampfit software (version 10; Axon Instruments, Foster City, CA). Data were further analyzed with Microsoft Excel 2003. Values are given as means ± SE, and the number of replicate experiments is represented as n. Membrane conductance was calculated between −20 and +20 mV, where extracellular HCO3−-independent currents associated with NBCe1 expression are minimal (15). Statistical analyses (ANOVA with Tukey's post hoc analysis, and t-tests) were performed on data using Minitab 16 (Minitab, State College, PA).
RESULTS
The Effect of Deleting the Nt AID On NBCe1-B Activity
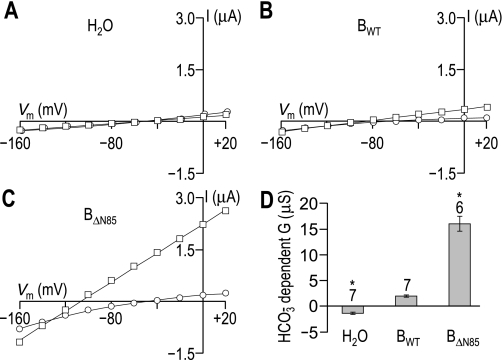
We injected control Xenopus oocytes with H2O and experimental oocytes with cRNA that encoded either BWT (i.e., full-length NBCe1-B) or BΔN85 (i.e., AID-less NBCe1-B). Three to four days after injection, we performed a voltage-clamp protocol that allowed us to determine the increase in membrane slope conductance between −20 and +20 mV that resulted from the application of CO2/HCO3−, the HCO3−-dependent slope conductance. As expected for cells expressing an electrogenic NBC, oocytes expressing either BWT or BΔN85 hyperpolarized immediately on exposure to CO2/HCO3−, whereas H2O-injected oocytes did not (data not shown). Figure 1, A–C, shows representative I-V relationships for oocytes injected with H2O or with the cRNA encoding either BWT or BΔN85. HCO3−-dependent slope conductances extracted from data such as these are summarized for a larger number of cells in Fig. 1D. We found that the HCO3−-dependent conductance was −1.4 ± 0.2 μS (n = 6) for H2O-injected cells, +2.0 ± 0.2 μS (n = 7) for oocytes expressing BWT, and 16 ± 1.5 μS (n = 7) for cells expressing BΔN85. Thus the deletion of the first 85 AA of WT NBCe1-B causes a substantial increase in the HCO3−-dependent slope conductance.
Fig. 1.
The stimulatory effect of truncating the amino terminus of the electrogenic sodium-HCO3 cotransporter NBCe1-B. The figure shows representative current-voltage (I-V) relationships obtained from Xenopus oocytes, first during superfusion with ND96 solution (○), and subsequently following a switch to our CO2/HCO3−-containing solution (□). A: I-V data from an oocyte that was injected with H2O. B: I-V data from an oocyte expressing full-length NBCe1-B (BWT). C: I-V data from an oocyte expressing NBCe1-B that is amino terminally truncated by 85 amino acids (AA) (BΔN85). D: bar chart summarizing the HCO3−-dependent slope conductances ± SE measured over the −20 to +20 mV range for a larger number of these cells. The number of replicates for each group is displayed above each bar, together with a note (*) of statistical difference from BWT (P < 0.05, ANOVA with Tukey's post hoc analysis). Note that the ND96 data points in A have been artificially shifted to the right by 2 mV to make them visible. Vm, membrane potential.
The Effect of WT-IRBIT Coexpression On NBCe1-B Activity
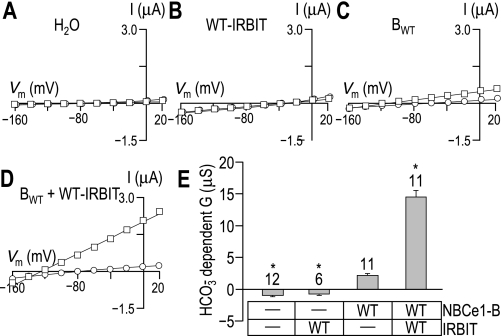
Into Xenopus oocytes, we injected a solution of BWT cRNA diluted in either H2O or a solution containing WT-IRBIT cRNA. As controls, we also injected some cells with either H2O alone or WT-IRBIT cRNA alone. Three to five days after injection, we performed a voltage-clamp protocol that allowed us to determine the increase in membrane slope conductance between −20 and +20 mV that resulted from the application of CO2/HCO3− for each set of oocytes. Figure 2, A–D, shows representative I-V relationships for oocytes injected with H2O, as well as for oocytes expressing WT-IRBIT, BWT, or BWT + WT-IRBIT. As expected for cells expressing an electrogenic NBC, oocytes expressing BWT or BWT + WT-IRBIT hyperpolarized immediately upon exposure to CO2/HCO3−, whereas H2O-injected and WT-IRBIT-expressing oocytes did not (data not shown). HCO3−-dependent slope conductances extracted from data such as those shown in Fig. 2, A–D, are summarized for a larger number of cells in Fig. 2E. We found that the HCO3−-dependent conductance was −0.9 ± 0.2 μS (n = 12) for H2O-injected cells and −0.7 ± 0.2 μS (n = 6) for cells expressing WT-IRBIT. In contrast, the HCO3−-dependent conductance was +2.2 ± 0.3 μS (n = 11) for oocytes expressing BWT alone and 14 ± 1.1 μS (n = 10) for oocytes expressing BWT + WT-IRBIT. Thus the coexpression of WT-IRBIT with WT NBCe1-B (see Fig. 2) causes an increase in the HCO3−-dependent slope conductance that is as substantial as that caused by the deletion of the first 85 AA (Fig. 1).
Fig. 2.
The stimulatory effect of coexpressing wild-type inositol trisphosphate-receptor binding protein released with inositol trisphosphate (WT-IRBIT) with NBCe1-B. The figure shows representative I-V relationships obtained from oocytes, first during superfusion with ND96 solution (○), and subsequently following a switch to our CO2/HCO3−-containing solution (□). A: I-V data from an oocyte that was injected with H2O. B: data from an oocyte expressing only WT-IRBIT. C: data from an oocyte expressing only NBCe1-B (BWT). D: data from an oocyte expressing NBCe1-B plus WT-IRBIT (BWT + WT-IRBIT). E: bar chart summarizing the HCO3−-dependent slope conductances ± SE measured over the −20 to +20 mV range for a larger number of these cells. The number of replicates in each group is displayed above each bar, together with a note (*) of statistical difference from BWT (P < 0.05, ANOVA with Tukey's post hoc analysis). Note that the ND96 data points in A and B have been artificially shifted to the right by ∼2 mV to make them visible.
Increasing the Relative Dose of WT-IRBIT
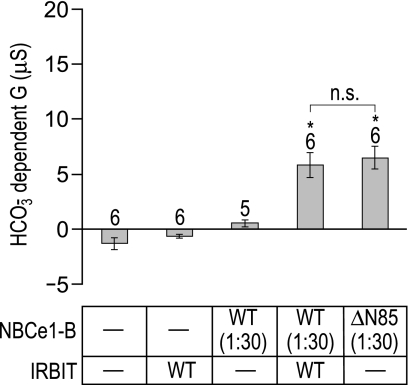
Our next goal was to determine whether the stimulatory effects of AID removal in Fig. 1 or WT-IRBIT coexpression in Fig. 2 may have been underestimated, either because of saturation of the assay, or because of an insufficient WT-IRBIT-to-NBCe1-B ratio. Therefore, we repeated the protocols in Figs. 1 and 2 after diluting 30-fold the cRNA for BWT and BΔN85, while maintaining the regular dose of WT-IRBIT. Thus the IRBIT-to-NBCe1 cRNA ratio was increased 30-fold. We injected Xenopus oocytes with H2O, WT-IRBIT, diluted BWT(1:30), diluted BΔN85(1:30), and diluted BWT(1:30) cRNA + regular dose WT-IRBIT cRNA. Figure 3 shows the averaged HCO3−-dependent slope conductances for these cells. Note that the HCO3−-dependent conductances with this protocol were about one-half those for BΔN85 in Fig. 1D or for BWT + WT-IRBIT in Fig. 2E. In this set of experiments (i.e., Fig. 3), we find that the HCO3−-dependent conductance was not significantly different for oocytes expressing BWT + WT-IRBIT or for oocytes expressing BΔN85 alone. Thus, in terms of fractional stimulation, WT-IRBIT was no more effective when paired with a reduced, nonsaturating dose of WT NBCe1-B cRNA (Fig. 3) than a 30-fold higher dose of cRNA (Fig. 2).
Fig. 3.
Comparison of the stimulatory effects of amino terminal truncation and WT-IRBIT coexpression on NBCe1-B action. The bar chart summarizes the HCO3−-dependent slope conductances ± SE measured over the −20 to +20 mV range for a number of oocytes that were injected either with a normal dose of H2O, a normal dose of WT-IRBIT cRNA (IRBIT/WT), a 1:30 dilution of NBCe1-B cRNA [NBCe1-B/WT(1:30)], a 1:30 dilution of NBCe1-B cRNA plus a normal dose of IRBIT cRNA [NBCe1-B/WT(1:30) + IRBIT/WT], or a 1:30 dose of AID-less NBCe1-B cRNA [ΔN85(1:30)]. The number of replicates in each group is displayed above each bar, together with a note (*) of statistical difference from BWT (P < 0.05, ANOVA with Tukey's post hoc analysis) or a note of nonsignificance (n.s.) between specific pairs of interest (indicated by a horizontal bracket), as assessed by ANOVA post hoc analysis.
The Effect of AID Removal or WT-IRBIT Coexpression on the Abundance of NBCe1-B Constructs in the Plasma Membrane
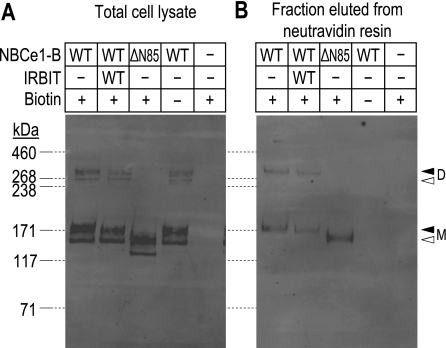
To confirm, as reported by others (16, 25), that the observed increase in HCO3−-dependent conductance in Xenopus oocytes was not due to an increased abundance of the cotransporters to the plasma membrane, we labeled proteins exposed to the extracellular fluid with a biotinylation reagent. Oocytes expressing BWT, BWT + WT-IRBIT, or BΔN85 (Fig. 4, lanes 1–3) were exposed to a biotinylation reagent, isolated, and resolved by SDS-PAGE. In one set of control experiments, we subjected BWT-expressing oocytes to the biotinylation protocol, but omitted the biotinylation reagent (Fig. 4, lane 4). In a second set of control experiments, we subjected H2O-injected oocytes to the biotinylation protocol (Fig. 4, lane 5). Total and plasma membrane-resident (i.e., biotinylated) protein extracts were resolved by SDS-PAGE and visualized by Western blotting. Figure 4A shows a representative Western blot of total oocyte protein extract (one-fifth of an oocyte equivalent per lane). EGFP immunoreactivity was detected only in extracts from oocytes that were expressing BWT or BΔN85. In the case of BWT, with or without WT-IRBIT, monomer and dimer were consistently detected as doublets, likely representing nonglycosylated (tight lower band in each doublet) and glycosylated versions (more diffuse upper band of each doublet; see Ref. 6). BΔN85 monomer was also detected as a doublet, but no dimer was evident in any of the samples analyzed. This observation is consistent with the hypothesis that the initial 85 AA normally can stabilize the dimeric state of the transporter, even under the strong reducing and denaturing conditions of SDS-PAGE. If we normalize the total abundance of BWT in oocytes that were expressing only BWT to 100%, the abundance of BWT in cells that were expressing BWT + WT-IRBIT was 112 ± 24% (n = 4 sets of oocytes), whereas the abundance of BΔN85 was 97 ± 15% (n = 3 sets of oocytes). Thus all three populations expressed similar amounts of NBCe1-B construct.
Fig. 4.
Comparison of the effects of amino terminal truncation and WT-IRBIT coexpression on NBCe1-B protein expression. A: representative Western blot of total oocyte lysates (samples are equivalent to 1/5th cell per lane) prepared from cells that were expressing full-length NBCe1-B (NBCe1-B/WT), full-length NBCe1-B + WT-IRBIT (NBCe1-B/WT + IRBIT/WT), truncated NBCe1-B (NBCe1-B/ΔN85), and cells that were injected with H2O following subjection to our biotinylation procedure (samples marked “+ biotin”). Samples marked “− biotin” were processed in the absence of biotinylation reagent. B: representative Western blot of biotinylated protein (equivalent to 1 cell per lane) that were isolated, using a neutravidin resin, from the same oocyte lysates that were sampled in A. Molecular mass markers are displayed to the left of A and are extended to B by dashed lines. The open triangles to the right of B indicate the migration position of the putatively non- or core-glycosylated BWT monomer (M) and dimer (D) visible in A, but not in B. The solid triangles indicate the migration position of putatively complex-glycosylated BWT M and D visible in both A and B.
Figure 4B shows a representative Western blot of biotinylated oocyte protein extract (one oocyte equivalent per lane). EGFP immunoreactivity was detected only in extracts from oocytes that were expressing BWT or BΔN85 and then only from cells that had been treated with biotinylation reagent. In biotinylated extracts, neither BWT nor BΔN85 was evident as doublets, possibly because these NBCe1-B constructs are present in the plasma membrane only in their mature glycosylated forms. Again, as with total protein extracts, SDS-stable dimers of BΔN85 were not evident. If we normalize the plasma membrane abundance of BWT in oocytes that were expressing only BWT to 100%, the abundance of BWT in the plasma membrane of cells that were expressing BWT + WT-IRBIT was 80 ± 15% (n = 4 sets of oocytes), whereas the abundance of BΔN85 was 71 ± 2% (n = 4 sets of oocytes). Thus we confirm that the greater functional expression (i.e., the combination of surface expression and intrinsic/per-molecule cotransporter activity) of BWT + WT-IRBIT vs. BWT or of BΔN85 vs. BWT cannot be explained by increased plasma membrane abundance. Moreover, because both functional expression and plasma membrane abundance are indistinguishable for BWT + WT-IRBIT vs. BΔN85, we conclude that average per-molecule activity is virtually identical in the two cases. Because the plasma membrane abundance of both BWT + WT-IRBIT and BΔN85 may be less than that for BWT, our electrophysiological data may underestimate the per-molecule activation of BWT + WT-IRBIT and BΔN85.
Comparing the abundance of total protein to the abundance of surface protein, we estimate that 11 ± 1% of total BWT is in the plasma membrane in cells expressing BWT alone (n = 4 sets of cells), 9 ± 1% of BWT is in the plasma membrane of cells expressing BWT + WT-IRBIT (n = 4 sets of cells), and 9 ± 1% of total BΔN85 is in the plasma membrane of cells expressing BΔN85 alone (n = 3 sets of cells). Thus the net plasma membrane accumulation of NBCe1-B constructs is similar in all three cases.
The Effect of Mutant-IRBIT Coexpression on the Activity of NBCe1-B Constructs
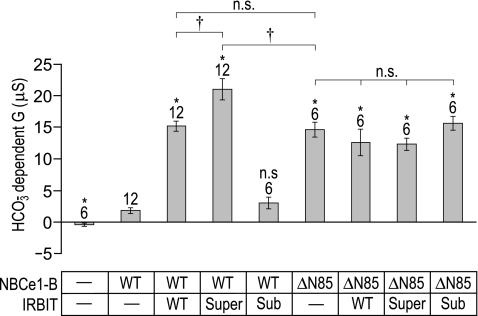
To determine whether the action of endogenous Xenopus oocyte PP-1 was reducing the potency of the heterologously expressed WT-IRBIT in our oocyte expression system, we mutagenized the putative PP-1 binding motif in IRBIT (“KQIQF”, see Ref. 8) to either 1) the strong consensus PP-1 binding site “RRVRF”, expected to yield a lower stimulatory capacity (“sub-IRBIT”); or to 2) the extremely weak PP-1 binding site “KQAQA”, expected to yield a high stimulatory capacity (“super-IRBIT”). We injected Xenopus oocytes with our usual doses of cRNA encoding BWT or BΔN85 and one of three IRBIT constructs: WT-, sub-, or super-IRBIT. In this set of experiments (Fig. 5), we found that the HCO3−-dependent conductance for H2O-injected cells was −0.4 ± 0.3 μS (n = 6). BWT coexpressed with sub-IRBIT exhibits a HCO3−-dependent conductance that is indistinguishable from that of BWT expressed alone (3.0 ± 0.9 vs. 1.8 ± 0.4 μS, n = 6). However, BWT coexpressed with super-IRBIT exhibits a HCO3−-dependent conductance that is significantly greater than that exhibited by BWT coexpressed with WT-IRBIT (21 ± 1.6 vs. 15 ± 0.8 μS) or than that exhibited by BΔN85 that is expressed alone (15 ± 1.2 μS).
Fig. 5.
The effect of coexpressing WT or mutant IRBITs with WT or truncated NBCe1-B. The bar chart summarizes the HCO3−-dependent slope conductances ± SE of oocytes expressing NBCe1-B/WT or truncated NBCe1-B (NBCe1-B/ΔN85), with or without one of three IRBIT constructs (WT-, super-, or sub-IRBIT). The number of replicates in each group is displayed above each bar, together with a note of statistical difference (*) from WT NBCe1-B (P < 0.05, ANOVA with Tukey's post hoc analysis). Also provided is a note of significance (†) or of nonsignificance (n.s.) between specific pairs of interest (indicated by a horizontal bracket), as assessed by ANOVA post hoc analysis, as well as t-test.
Note that none of the three IRBIT constructs had a significant effect on the already stimulated BΔN85. The data in Fig. 5, taken together with the data from the previous figures, indicate that an IRBIT construct (in this case super-IRBIT) can enhance the activity of NBCe1-B more than the truncation of the NH2-terminal AID.
The Functional Expression of NBCe1-A vs. Fully Stimulated NBCe1-B
Even BWT + super-IRBIT cannot match the functional expression of NBCe1-A. In day-matched experiments (not shown), we found that BWT + super-IRBIT mediated a conductance of 17 ± 1.2 μS (n = 6), whereas NBCe1-A mediated a conductance of 35 ± 2.7 μS (n = 6). McAlear et al. (16) report that the surface expression levels of NBCe1-A and BΔN87 are indistinguishable. Inasmuch as our data in Fig. 4 indicate that the plasma-membrane expression levels of BWT and BΔN85 are indistinguishable, we conclude that the extra activity of NBCe1-A vs. fully stimulated NBCe1-B reflects the presence of the ASD of NBCe1-A.
In a separate set of day-matched experiments (not shown), we find that oocytes expressing NBCe1-A exhibit a HCO3−-dependent conductance of 33 ± 2.1 μS (n = 9) and confirm the finding of Shirakabe and coworkers (25) that the conductance of NBCe1-A is not stimulated by coexpression with WT-IRBIT (34 ± 2.9 μS, n = 4). Furthermore, we find that NBCe1-A is not stimulated by coexpression with super-IRBIT (29 ± 1.0 μS, n = 6) or sub-IRBIT (27 ± 1.4 μS, n = 4).
Defining Elements Within NBCe1-B Required for Autoinhibition and for Stimulation by IRBIT
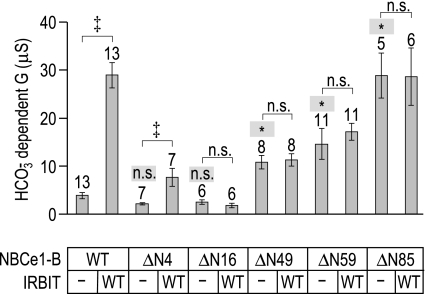
We created four novel Nt truncations of NBCe1-B that lacked, not considering the initiator Met, the first 3 AA (BΔN4), the first 15 AA (BΔN16), the first 48 AA (BΔN49), or the first 58 AA (BΔN59). We expressed these four truncated mutants, as well as BΔN85, in Xenopus oocytes with and without WT-IRBIT and measured their activities by two-electrode voltage clamp. The results of these experiments are shown in Fig. 6. BΔN4 and BΔN16 have a fully intact AID (i.e., they are fully inhibited and their activity is not statistically different from that of BWT; ANOVA analysis of full-length and truncated constructs without WT-IRBIT, P > 0.05). Further truncations gradually erode the AID (i.e., their activities are statistically greater than that of BWT; ANOVA analysis of full-length and truncated constructs without WT-IRBIT, P < 0.05). Thus the removal of a further 33 residues from BΔN16 (i.e., BΔN49) removes ∼40% of the inhibition of the AID. Truncating yet another 10 residues (BΔN59) removes another 10% of the autoinhibition, resulting in a total loss of ∼50% of the AID. Finally, the removal of an additional 26 residues (BΔN85) removes a further 50% of the autoinhibition, fully abrogating the effect of the AID. Thus auto inhibitory determinants are located throughout a region of the NBCe1-B Nt that is delimited by AA residues 17 and 85.
Fig. 6.
Localizing autoinhibitory and IRBIT-binding determinants within the NBCe1-B amino terminus. Bar chart summarizes the HCO3−-dependent slope conductances ± SE of oocytes expressing NBCe1-B/WT or truncated NBCe1-B constructs (NBCe1-B/ΔN4 to NBCe1-B/ΔN85), with or without WT-IRBIT. The number of replicates in each group is displayed above each bar, together with a note of statistical difference (* on a shaded background) or nonsignificance (n.s. on a shaded background) from WT NBCe1-B (P < 0.05, ANOVA on samples without IRBIT with Tukey's post hoc analysis). Also provided is a note of significance (‡) or of nonsignificance (n.s.) between specific pairs of interest (indicated by a horizontal bracket), as assessed by t-test.
When paired with BWT and BΔN4, WT-IRBIT increases the mean HCO3−-dependent conductance (t-tests of NBCe1-B constructs with and without WT-IRBIT, P < 0.05). However, WT-IRBIT only stimulated BΔN4 mildly compared with its large stimulatory effect on BWT. None of our other constructs, that is to say, BΔN16, BΔN49, BΔN59, or BΔN85, was significantly stimulated by WT-IRBIT. Thus even the first 3 AA are critical for WT-IRBIT stimulation of NBCe1-B, and the first 15 are essential.
In data not shown, we found that BΔN8 exhibits a severe defect in functional expression. Oocytes expressing this construct hyperpolarized on average by only 13 ± 7 mV (n = 5) following the application of our CO2/HCO3− solution, but did not exhibit sufficient NBCe1-like activity to cause a detectable increase in membrane conductance. In two oocytes coexpressing BΔN8 and WT-IRBIT, the application of CO2/HCO3− elicited a hyperpolarization of 28 and 29 mV. However, we detected no increase in membrane conductance.
DISCUSSION
The present study, which is the first in which it has been possible to compare directly the effect of Nt truncation vs. the effect of IRBIT coexpression, produced several novel observations. 1) AID removal stimulates NBCe1-B activity to an extent that is similar to that caused by WT-IRBIT coexpression (Figs. 1–4). 2) WT-IRBIT cannot stimulate an NBCe1-B that lacks the first 84 AA, or even the first 15 AA, of the Nt (Figs. 5 and 6). 3) A heterologously expressed sub-IRBIT that is engineered to interact strongly with endogenous PP-1 cannot stimulate NBCe1-B (Fig. 5). 4) The mutant super-IRBIT that is engineered to be impervious to the effects of endogenous PP-1 is more potent than WT-IRBIT in regard to NBCe1-B activation (Fig. 5). 5) The stimulatory effect of super-IRBIT can exceed the stimulatory effect of AID removal (Fig. 5). 6) The stimulatory effect of IRBIT is not as great as the stimulatory effect of the ASD. 7) At least one IRBIT binding determinant is distinct from any necessary component of the AID (Fig. 6).
Stimulation of NBCe1-B by AID Removal and IRBIT Coexpression
In Xenopus oocytes, AID-less NBCe1-B (i.e., BΔN85) exhibits a greater functional expression than full-length NBCe1-B (Fig. 1), but the same functional expression as full-length NBCe1-B in the presence of WT-IRBIT (Fig. 2). Thus both AID removal and WT-IRBIT coexpression appear to stimulate NBCe1-B to the same extent. These results hold true when we reduce the injection dose of BWT and BΔN85 cRNA by a factor of 30, a maneuver that appears to reduce the functional expression of NBCe1-B by one-half and thus should increase the relative abundance of WT-IRBIT over NBCe1-B (Fig. 3). Thus our measurements are unlikely to be confounded by insufficient WT-IRBIT abundance. Furthermore, in a set of experiments performed 5–6 days after injection of cRNA (vs. only 4 days for all other protocols in the paper), we find that it is possible to measure HCO3−-dependent conductances of up to 30 μS (Fig. 6), double the conductance measured in the experiments shown in Figs. 1 and 2. Thus our measurements are unlikely to be confounded by saturation of signal.
The extent of NBCe1-B stimulation effected either by amino terminal truncation or WT-IRBIT coexpression is difficult to quantify precisely due to the small magnitude of the current mediated by unstimulated, full-length NBCe1-B. However, we estimate the increase to be in the range of five- to eightfold. We find no evidence that either amino terminal truncation or WT-IRBIT coexpression causes a substantial increase in the abundance of NBCe1-B in the plasma membrane of oocytes (Fig. 4). Others have made similar observations for the amino terminal truncation of NBCe1-C (16) and the effect of WT-IRBIT coexpression on NBCe1-B (25). Therefore, the stimulatory effect of these two maneuvers likely represents a per-molecule activation of NBCe1-B.
In a mammalian cell line that is overexpressing NBCe1-B, IRBIT coexpression stimulates NBCe1-B activity without significantly altering the abundance of NBCe1-B in the plasma membrane (31). However, in mammalian cells that are also overexpressing WNK/SPAK (with no lysine/Ste20-related proline-alanine-rich kinase) proteins, IRBIT not only stimulates the per-molecule activity of NBCe1-B, but also antagonizes the effects of the WNK/SPAK pathway, which normally acts to reduces NBCe1-B abundance in the plasma membrane. Thus, in this situation, IRBIT increases both per-molecule activity and the abundance of plasma membrane resident NBCe1-B (31). Because 1) IRBIT coexpression does not result in increased abundance of NBCe1-B in the oocyte plasma membrane; and 2) oocytes do not appear to express substantial endogenous WNK/SPAK activity (9, 29, 30), we presume that the stimulation of NBCe1-B that we and others observe in oocytes is due solely to the per-molecule activation of NBCe1-B by IRBIT.
Exaggerated Stimulation of NBCe1-B by Super-IRBIT
Because IRBIT activation of NBCe1-B and autoinhibition of NBCe1-C both require the initial 85 AA that are common to the two variants, it is attractive to hypothesize that IRBIT binds to and masks the AID, thereby relieving inhibition and appearing to stimulate NBCe1. Indeed, at first glance, our data appear to suggest that truncating the initial 85 residues from NBCe1-B results in the same gain of function as coexpression of WT-IRBIT (Figs. 1–3). However, under these assay conditions, WT-IRBIT is not exerting its maximal stimulatory effect on NBCe1-B. When we replace the putative PP-1 binding site in IRBIT (“KQIQF”) with a strong consensus PP-1 binding site (“RRVRF”), we find that this “sub-IRBIT” is no longer able to stimulate NBCe1-B, consistent with the hypothesis that an endogenous oocyte PP-1 inactivates sub-IRBIT (Fig. 5). Others have demonstrated the decreased potency of this mutant IRBIT with respect to IP3R binding (8).
When we replace the putative PP-1 binding site in IRBIT with a sequence (“KQAQA”) that does not interact with PP-1 (8), a greater potency is exhibited by this “super-IRBIT” with respect to NBCe1-B activation. In this case, the stimulatory effect of super-IRBIT on NBCe1-B exceeds the effect of AID removal by 50%. Because we have no reason to think that WT-IRBIT and super-IRBIT stimulate NBCe1-B by different mechanisms, we consider that the action of super-IRBIT better represents the true maximal per-molecule stimulatory effect of IRBIT on NBCe1-B. Thus numerically we can account for two-thirds of the stimulatory effect of IRBIT with a model in which IRBIT neutralizes the AID, but a further one-third of the stimulatory effect must be explained by another mechanism. How might we explain this extra enhancement? Four scenarios might be imagined:
1) AI determinants other than those in the initial 85 residues of NBCe1-B might be neutralized by IRBIT. However, as super-IRBIT cannot stimulate AID-less NBCe1-B, this effect presumably would still require IRBIT binding to the Nt.
2) IRBIT binding results in a conformation change in NBCe1-B, independently of the masking of the AID, that enhances transporter activity.
3) IRBIT contains a sequence that otherwise enhances NBCe1-B activity (e.g., by raising local concentrations of substrates).
4) The initial 85 residues of NBCe1-B not only include AI determinants, but also autostimulatory (AS) determinants. IRBIT might sequester the AI determinants, leaving just the AS determinants to exert their effects. Truncation would remove both AI and AS determinants, leading to an underestimation of the effect of truncation on AI relief.4
Existing data do not allow us to distinguish among these four scenarios.
Evidence That Essential IRBIT Binding Determinants Include Elements Unnecessary for the AID
Functional studies indicate that residues 2–87 of NBCe1-C, and therefore the same residues of NBCe1-B, include an AID (16). Binding studies demonstrate that one necessary (although not sufficient) IRBIT-binding determinant is located in the first 18 AA of NBCe1-B (25). Thus it is not unreasonable to suggest that IRBIT binds to determinants in and/or near the AID, thereby relieving the effects of the AID. In the present study, we find that WT-IRBIT stimulates BΔN4 poorly and does not stimulate BΔN16 at all, even though both NBCe1-B constructs retain an intact AID. We can, therefore, define the necessary determinants of the AID more precisely as including no more than residues 17–85 of NBCe1-B. Although defining the minimal extent to the AID (or the AI elements therein) will require further refinement, it is clear that at least some IRBIT-binding and AI determinants are not identical.
Comparison of Present and Previous Data
AID removal.
The original 2006 study of NBCe1 autoinhibition by McAlear and coworkers (16) reported that, as expressed in Xenopus oocytes, the activities of rat NBCe1-C and rat NBCe1-B are nearly identical, whereas the activity of the CΔN87 truncation of CWT is twofold greater than that of BΔN87 (which is identical to AΔN43). The enhanced activity of CΔN87 over BΔN87 is proposed to be due to a stimulatory effect of the unique Ct of NBCe1-C in the absence of the amino terminal AID (16). The McAlear study did not directly address the effect of AID removal from NBCe1-B. However, they did find that the activity of CΔN87 is threefold greater than that of CWT. We find that human BΔN85 has at least a fivefold greater activity than BWT. However, the apparent difference between the threefold and fivefold stimulations may not be meaningful, given the numerous systematic differences between the 2006 study and the present study (e.g., rat vs. human, retention of an extra 2 AA, non-EGFP-tagged vs. EGFP-tagged, method of data analysis, different laboratories).
IRBIT coexpression.
The 2006 study by Shirakabe and coworkers (25) reported that, as expressed in Xenopus oocytes, IRBIT stimulates BWT by approximately sevenfold and that the activity of WT-IRBIT-stimulated BWT is comparable to that of NBCe1-A (AWT). Because McAlear et al. find that AWT has twice the activity of BΔN87 (i.e., AΔN43), the Shirakabe and McAlear data, taken together, are consistent with the hypothesis that IRBIT can stimulate BWT by twice the amount that could be accounted for solely by relief of transporter autoinhibition. This inference is consistent with our observation that super-IRBIT stimulates BWT substantially more than truncating BWT to BΔN85. However, in contrast to the observation by Shirakabe, we find that the activity of BWT + super-IRBIT is substantially less than that of AWT. This apparent disparity might to be explained by a large sample variation (see Fig. 4 of the 2006 study), which could mask a significant difference between AWT and IRBIT-stimulated BWT.
The relationship between NBCe1-B, IRBIT, and PP-1.
The relationship between NBCe1-B, IRBIT, and PP-1 can be conceptualized in two ways. 1) Extrapolating from the model of Devogelaere and coworkers (8), PP-1 could bind to and dephosphorylate IRBIT, whereupon IRBIT would lose the ability to bind to and activate NBCe1-B. 2) According to the model of Yang and coworkers (31), PP-1 could bind to IRBIT, whereupon the PP-1/IRBIT complex could bind to NBCe1-B. PP-1 would then dephosphorylate NBCe1-B, stabilizing NBCe1-B in the plasma membrane. Independently, the IRBIT would enhance the per-molecule activity of NBCe1-B. In one respect, these two models appear to conflict. In model 1, a PP-1/IRBIT complex would be a less potent activator of NBCe1-B than would be IRBIT alone. In model 2, a PP-1/IRBIT complex would be a more potent activator of NBCe1-B. The in vivo reality is likely to be complex: PP-1 presumably dephosphorylates both NBCe1 and IRBIT, as well as the IRBIT antagonist SPAK (31). Our data are consistent with model 1, but do not speak to model 2, inasmuch as our experiments were performed in the nominal absence of an active WNK/SPAK pathway. However, in light of the data of Devogelaere et al. (8) and the present study, it seems unlikely that a PP-1/IRBIT complex would be the species that migrates to and activates NBCe1-B.
Conclusion
Our data are consistent with a model in which IRBIT activates NBCe1-B in the plasma membrane chiefly, but not exclusively, by relieving amino terminal autoinhibition of NBCe1-B. PP-1 binding to IRBIT would render IRBIT unable to activate NBCe1-B.
GRANTS
This work was supported by National Institutes of Health Grants NS18400 and DK30344 awarded to W. F. Boron.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.-K.L., W.F.B., and M.D.P. conceived and designed of research; S.-K.L. and M.D.P. performed experiments; S.-K.L. and M.D.P. analyzed data; S.-K.L., W.F.B., and M.D.P. interpreted results of experiments; S.-K.L., W.F.B., and M.D.P. prepared figures; S.-K.L., W.F.B., and M.D.P. drafted manuscript; S.-K.L., W.F.B., and M.D.P. edited and revised manuscript; S.-K.L., W.F.B., and M.D.P. approved final version of manuscript.
ACKNOWLEDGEMENTS
We thank Dale Huffman for computer support. We thank Benoit Devogelaere, Eva Sammels, Humbert De Smedt, and Jan Parys at Katholeike Universiteit Leuven; Mark Bevensee and Ian Thornell at the University of Alabama Birmingham; as well as Nicholas Courtney at Case Western Reserve University for helpful discussions.
Footnotes
It has not been determined whether 1) the 41-AA module in NBCe1-A is a single domain that has autostimulatory function due to the presence of one or more autostimulatory sequences, in which case the entire module could be considered to be an ASD; or 2) the 41-AA module is composed of multiple domains, one or more of which is an ASD. Similar considerations apply to the relationship between the 85-AA module in NBCe1-B/C and the AID.
See footnote 1.
In the original clone, EGFP cDNA is flanked by AgeI sites. In the modified clone, the downstream AgeI site has been destroyed by Quickchange mutagenesis.
Both BΔN4 and BΔN16 tended to exhibit HCO3−-dependent currents that were less than those mediated by full-length NBCe1-B, as though residues 2–16 might be mildly autostimulatory. However, these small differences did not reach statistical significance in this study and may be due to subtle differences in NBCe1-B-construct abundance in the plasma membrane.
REFERENCES
- 1. Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem 278: 10602–10612, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na/HCO3− cotransporter (NBC) with a novel C terminus, cloned from rat brain. Am J Physiol Cell Physiol 278: C1200–C1211, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO3− transport. J Gen Physiol 81: 53–94, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol 212: 1697–1706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3− cotransporter. J Biol Chem 272: 19111–19114, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+-HCO3− cotransporter (NBCe1). Am J Physiol Renal Physiol 284: F1199–F1206, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC). Am J Physiol Cell Physiol 276: C576–C584, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Devogelaere B, Beullens M, Sammels E, Derua R, Waelkens E, van Lint J, Parys JB, Missiaen L, Bollen M, De Smedt H. Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1,4,5-trisphosphate receptor. Biochem J 407: 303–311, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Grichtchenko II, Boron WF. Surface-pH measurements in voltage-clamped Xenopus oocytes co-expressing NBCe1 and CAIV: evidence for CO3− transport (Abstract). FASEB J 16: A795, 2002 [Google Scholar]
- 11. He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem 283: 33544–33553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heyer M, Muller-Berger S, Romero MF, Boron WF, Frömter E. Stoichiometry of the rat kidney Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes. Pflügers Arch 438: 322–329, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Lee S-K, Grichtchenko II, Boron WF. Distinguishing HCO3− from CO32− transport by NBCe1-A (Abstract). FASEB J 25: 656.–9., 2011 [Google Scholar]
- 14. Liu Y, Xu JY, Wang DK, Wang L, Chen LM. Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: A comparative study of NCBT genes. Genomics 98: 112–119, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3− cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol 292: C1787–C1798, 2007 [DOI] [PubMed] [Google Scholar]
- 16. McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3− cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol 127: 639–658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musa-Aziz R, Boron WF, Parker MD. Using fluorometry and ion-sensitive microelectrodes to study the functional expression of heterologously-expressed ion channels and transporters in Xenopus oocytes. Methods 51: 134–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parker MD, Daly CM, Skelton LA, Boron WF. IRBIT functionally enhances the electroneutral Na+-coupled bicarbonate transporter NCBE by sequestering an N-terminal autoinhibitory domain (Abstract). FASEB J 21: A1285, 2007 [Google Scholar]
- 19. Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3− cotransporter (NBCn2) with Cl− self-exchange activity. J Biol Chem 283: 12777–12788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parker MD, Skelton LA, Daly CM, Boron WF. IRBIT binds to and functionally enhances the electroneutral Na+-coupled bicarbonate transporters NBCn1, NDCBE and NCBE (Abstract). FASEB J 21: A1285, 2007 [Google Scholar]
- 21. Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 387: 409–413, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+-HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol 277: F611–F623, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Seki G, Yamada H, Horita S, Suzuki M, Sekine T, Igarashi T, Fujita T. Activation and inactivation mechanisms of Na-HCO3− cotransporter NBC1. J Epithel Biol Pharmacol 1: 35–39, 2008 [Google Scholar]
- 25. Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc Natl Acad Sci U S A 103: 9542–9547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thornell IM, Wu J, Bevensee MO. The IP3 receptor-binding protein IRBIT reduces phosphatidylinositol 4,5-bisphosphate (PIP2) stimulation of Na/bicarbonate cotransporter NBCe1 variants expressed in Xenopus laevis oocytes (Abstract). FASEB J 24: 815.–6., 2010 [Google Scholar]
- 27. Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol 291: C788–C801, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Wakula P, Beullens M, Ceulemans H, Stalmans W, Bollen M. Degeneracy and function of the ubiquitous RVXF motif that mediates binding to protein phosphatase-1. J Biol Chem 278: 18817–18823, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang CL, Liu X, Paliege A, Zhu X, Bachmann S, Dawson DC, Ellison DH. WNK1 and WNK4 modulate CFTR activity. Biochem Biophys Res Commun 353: 535–540, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 121: 956–965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest 119: 193–202, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]