Abstract
The ATRX gene encodes a chromatin remodeling protein that has two important domains, a helicase/ATPase domain and a domain composed of two zinc fingers called the ADD domain. The ADD domain binds to histone tails and has been proposed to mediate their binding to chromatin. The putative ATRX homolog in Drosophila (XNP/dATRX) has a conserved helicase/ATPase domain but lacks the ADD domain. In this study, we propose that XNP/dATRX interacts with other proteins with chromatin-binding domains to recognize specific regions of chromatin to regulate gene expression. We report a novel functional interaction between XNP/dATRX and the cell proliferation factor DREF in the expression of pannier (pnr). DREF binds to DNA-replication elements (DRE) at the pnr promoter to modulate pnr expression. XNP/dATRX interacts with DREF, and the contact between the two factors occurs at the DRE sites, resulting in transcriptional repression of pnr. The occupancy of XNP/dATRX at the DRE, depends on DNA binding of DREF at this site. Interestingly, XNP/dATRX regulates some, but not all of the genes modulated by DREF, suggesting a promoter-specific role of XNP/dATRX in gene regulation. This work establishes that XNP/dATRX directly contacts the transcriptional activator DREF in the chromatin to regulate gene expression.
The human Alpha-Thalassemia and mental Retardation X-linked syndrome (hATRX) gene encodes a chromatin remodeling protein that contains several conserved motifs. The ATRX protein has a helicase/ATPase domain of the SWI/SNF family at its carboxyl terminus, and this domain is characteristic of the catalytic subunits of chromatin remodeling complexes (1). At its amino terminus, the ATRX protein has a domain composed of a Plant Homeo Domain (PHD) and a GATA-like zinc-finger motif, known as the ATRX-DNMT3A-DNMT3L (ADD) domain because this configuration of Zinc fingers has been found in the DNA-methyl-transferases DNMT3A and DNMT3L (2). The ADD domain of the DNMT3A and DNMT3L proteins has been shown to bind histone tails (3). This interaction has been proposed to mediate the recruitment of these proteins to chromatin (4). The ADD domain of hATRX is very similar to the one present in the DNMTs and recently has been reported to bind H3 when its K4, and K9 residues are methylated (5–7).
Mutations in hATRX are associated with several syndromes linked to the X chromosome. For this reason, most of the patients affected are males. Patients with mutations in hATRX have developmental defects in the urogenital system, suffer severe dysfunctions in the central nervous system, suffer from α-thalassemia, have impaired motor abilities, and display a characteristic facial dysmorphism. About 50% of the mutations detected in patients afflicted with ATRX syndrome are located in the ADD region, and ∼30% are in the SNF2 domain (1).
Some of the phenotypes observed in patients carrying ATRX mutations may be due to the role of ATRX in the regulation of transcription. As a result, different molecular studies have focused on elucidating the role played by ATRX in transcription (8,9). A recent genome-wide analysis of human and mouse cells demonstrates that ATRX can bind G-rich tandem repeat sequences. Importantly, genes associated with these tandem repeat sequences are deregulated when ATRX is mutated (10). Furthermore, the same study shows that ATRX can directly bind to G-rich sequences that may form G quadruplex structures (10). In addition, mammalian ATRX is capable of interacting with other cellular factors including Heterochromatin Protein 1 (HP1), Methyl-CpG-binding protein (MeCP2), cohesin, CCCTC-binding factor (CTCF) and the Death Associated Protein-6 (DAXX) presumably acting together as a H3.3 chaperone (11–15). The interaction of ATRX with CTCF and MeCP2 results in the repression of imprinted gene expression in the postnatal mouse (15). In addition, evidence suggests that in mice, the PAR genes are positively regulated by ATRX (16). In fact, expression of the α-globin genes is affected when ATRX is mutated, and this effect is determined by the number of the tandem G-rich repeats sequences upstream of this gene, suggesting that the ATRX mechanisms that influence gene expression are complex (10). Indeed, it is not clear if all the cases involve a direct interaction with chromatin or if this activity is mediated by an interaction with other undescribed factors. Therefore, the mechanism by which ATRX regulates gene expression is currently unknown.
In Drosophila, XNP/ATRX is present as two isoforms (dATRXS and dATRXL) that are derived from the use of two alternative translational start codons (17). While some reports suggest that XNP/dATRXL interacts with HP1 in heterochromatic regions, including the chromocenter, other studies show evidence that dATRXL can also be located in euchromatin (18,19). The dATRXS isoform has also been shown to be distributed in different regions across polytene chromosomes (19). In Drosophila, XNP/dATRX mutants act as suppressors of position effect variegation (18,19). Therefore, it is likely that XNP/dATRX is a chromatin-associated protein in the fly. However, although both XNP/dATRX isoforms conserve the ATPase/helicase domain, they lack the human ADD domain, suggesting that these proteins participate in the control of gene expression through protein–protein interactions with other cell factors that may have DNA or chromatin binding domains.
To test this hypothesis and to find putative XNP/dATRX partners, we used XNP/dATRX as a bait to screen an embryonic cDNA library through a yeast two-hybrid analysis, We identified, among several factors, that the transcription factor DREF interacts in vitro and in vivo with dATRX. DREF recognizes the consensus DNA element 5′-TATCGATA-3, which is highly prevalent in core promoters and is known as the DNA replication-related element (DRE) (20). In Drosophila, DREF regulates the expression of several genes that are required during the S phase of the cell cycle, such as PCNA, E2F and DNApol-α (22–24). Physically, DREF interacts with factors involved in chromatin structure and dynamics (25,26), and the wide spectrum of genes regulated by DREF suggests that this transcription factor acts in conjunction with a diverse array of transcriptional regulators.
The interaction between XNP/dATRX and DREF results in the regulation of pannier (pnr) gene transcription. Mechanistically, we found that DREF binds to DRE elements present in the pnr promoter, where it works to maintain correct levels of pnr gene expression. The interaction of XNP/dATRX with DREF results in the repression of pnr transcription. These results suggest a dynamic interaction between XNP/dATRX and DREF to control the expression of a factor that is required for various biological processes during the development of Drosophila.
MATERIALS AND METHODS
Fly stocks
The wild-type flies used in this study were Oregon R(OreR), and fly stocks were maintained at 25°C with standard food. All stocks were obtained from the Bloomington, Indiana stock center. RNAi transgenic flies for DREF and XNP/dATRX on chromosome 3 were obtained from the Vienna Drosophila RNAi center (VDRC). Df(3R)Exel6202 is a deficiency that does not have XNP/dATRX (deletes polytene chromosome bands 96D1-96E2), In this work, we called this deficiency Df(ATRX).
Establishment of UAS-RNAi transgenic flies
The pSymp-UAST plasmid was kindly provided by Dr E. Giordano and used to make transgenic flies for expression of an inducible RNAi against XNP/dATRX. A fragment containing XNP/dATRX bases 3765–4087 was cloned into the pSymp-UAST. The resulting transgene is called UAS-dATRXi. Transgenic flies were made by Genetic Services Inc. Transgenic flies carrying a transgene used for the expression of an inducible RNAi for DREF (UAS-DREFi) were obtained from the Vienna Drosophila RNAi Center (VDRC).
Genetic crosses
All stocks were crossed first with w1118 or with OreR flies in order to homogenize the genetic background. The transgene called UAS-dATRX is P{Mae-UAS.6.11}XNPUY3132 (27). It carries an UAS sequence inserted at the 5′-UTR of the XNP/dATRX gene. Transgenic RNAi lines carrying the transgenes in chromosomes 2 or 3 were established and balanced with CyO for chromosome 2 and with TM2, Ubx, TM3, Ser or TM6B, Tb balancers for chromosome 3. The drivers used to express the UAS-XNP/dATRX, UAS-XNPi or UAS-DREFi transgenes were Act5C-Gal4, 455.2-Gal4 (28) or pnr-Gal4. These drivers direct transgene expression ectopically, in the scutellum, or in the pnr domains, respectively.
Antibodies
Antibodies were raised in rats or rabbits against XNP/dATRX using the peptide p4:CVVRLKRVSLPKTKPAQ which recognizes XNP/dATRXL. The polyclonal rabbit anti-DREF antibody has been previously reported (29). Polyclonal rabbit anti-Pnr antibody was kindly provided by Dr Ginés Morata.
Immunostaining of polytene chromosomes
Salivary glands from third instar larvae were fixed in solution I (PBS, 3.7% paraformaldehyde and 1% Triton X-100) and then in solution II (3.7% paraformaldehyde, 50% acetic acid). The chromosomes were spread on poly-l-Lysine coated microscope slides. Anti-DREF antibody was used at 1:200 and anti-dATRX antibody at 1:100. Secondary antibodies ALEXA fluor 488 or 568 (Invitrogen) were used at 1:500. Images were taken on a confocal laser scanning microscope (Zeiss LSM 510 META).
Electrophoretic mobility shift assays
Electrophoretic mobility shift assays (EMSAs) were performed as previously described (30). Nuclear extracts were obtained from adult flies or embryos using the protocol described in (31). Oligonucleotides sequences are indicated in Figure 4. Competitions were performed with a 100-fold molar excess of unlabeled oligonucleotides, and 100 000 cpm of γ-32P ATP-labeled paired oligonucleotides were used per lane. EMSA-WB was performed as described in (32).
Figure. 4.
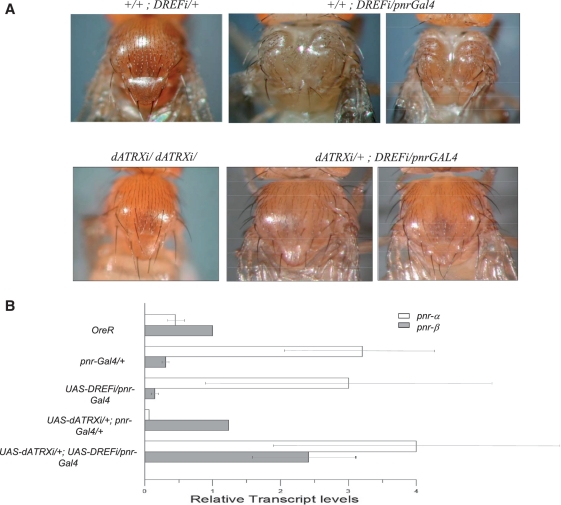
Suppression of the thoracic cleft induced by DREF knockdown in pnr-Gal4 heterozygous flies, by depletion of XNP/dATRX. (A) UAS-DREFi (top panel, left) or UAS-dATRXi (bottom panel, left) have a normal thorax, meanwhile flies with UAS-DREFi expression in the pnr domain driven by the pnr-Gal4 driver (middle and right top panels) show strong thoracic cleft defects. Depletion of XNP/dATRX in these flies, accomplished by the expression of the UAS-dATRXi transgene in the pnr domain, alleviates this defect (middle and right bottom panels). (B) pnr transcript levels are influenced by DREF and XNP/dATRX dosages. pnr-a andβ transcript levels in the thorax of flies depleted of DREF and DREF and XNP/dATRX in the pnr expression domain (genotypes as in Figure 4A). Note that in the thorax of DREF depleted flies there is a significant reduction of the pnr-β transcript levels. These levels are recovered when DREF and XNP/dATRX are simultaneously depleted in the pnr expression domain and partially recovered the ratio between pnr-α and pnr-β mRNAs. The quantification was obtained from three independent experiments and each cDNA was amplified three times by qRT–PCR. The Dmp8 transcript levels were used as reference control, which is not affected by the XNP/dATRX levels.
Pull-down assays
Pull-down assays were performed using the TNT-Quick-Coupled Transcription/Translation System (Promega) according to the manufacturer's instructions. A clone from the Drosophila Genomics Resource Center containing full length DREF cDNA was used for the in vitro translation of DREF.
Yeast two-hybrid screen
Yeast two hybrid assays were performed using the BD Matchmaker library construction and screening kit (Biosciences-Clontech) according to manufacturer's instructions. The cDNA library was made from 0 to 12 h embryos. The assay was performed using the amino-terminus (amino acids 1–221) or the carboxy-terminus (amino acids 1147–1308) of XNP/dATRX as bait. Screening for positive interactions was performed by co-transformation and/or yeast mating. Positive interactions were analyzed by growth in QDO medium and X-α-galactosidase or β-galactosidase assays.
Co-immunoprecipitation assays
Embryo or adult fly nuclear extracts were prepared as described in (33). This protocol allows us to maintain the ‘transcriptionally active’ nuclear fraction free of membranes and DNA. Co-IP was performed as described in (34).
qRT–PCR assays
RNA was obtained using the Trizol method or the protocol reported in (35) from 0 to 12 h embryos or adult flies expressing the RNAi against XNP/dATRX or from overexpressing dATRX. Equal amounts of RNA (1 µg) were used for first strand synthesis reactions. qRT–PCR reactions were performed using the light cycler DNA master SYBR green 1. All of the oligonucleotide sequences used in this study are available upon request. Relative quantification was performed by the ΔΔct-method. All reactions were performed in triplicate.
ChIP, ChIP–western and Re-ChIP assays in embryos
The 0- to 14-h-old embryos were collected, dechorionated and fixed (36). The fixation reaction was stopped by adding glycine (125 mM). Embryos were washed and resuspended in lysis buffer, and sonication was performed until the size of chromatin reached between 200 and 800 bp. Pre-clearing, antibody incubations, immunoprecipitation and phenol:chlorophorm extractions were performed as described in (30). For the ‘mock’ condition, a pre-immune sera against XNP/dATRX or an anti-GFP antibody were used. The following oligonucleotides were used for pnr-β: FWD 5′-CGGCCTGCTAAGTGAGTGTGA-3′, REV 5′-CCGGTTGGATGTGCGCTGAG-3′ and for pnr-a: FWD 5′-CCTTCAGTACCCACAATATCGCC-3′, REV 5′-GAACTAAGATTTAAACCACC-3′. qPCRs were then performed using the light cycler DNA master SYBR green 1 run in a Lyght cycler 1.5 (ROCHE), and the relative quantification was performed as follows. The percentage of INPUT was calculated using the formula:
where input dilution factor = (fraction of the input chromatin saved)−1 as described in Champion-ChIP TM qPCR Primers. Quantitative real-time PCR analysis of chromatin, SA. Biosciences 2008. All the assays were performed at least three independent times unless stated otherwise. All of the data were obtained from at least three independent experiments (37). For the ChIP–western experiments, the ChIP protocol was followed through the final wash of the agarose beads and protein complexes with TE buffer. The denatured complexes were migrated in an 8% polyacrylamide gel and transferred to a nitrocellulose membrane. WB was conducted using the anti-XNP/dATRX antibodies (described above) or the anti-DREF antibody.
For the Re-ChIP experiments, the ChIP protocol was followed through the last wash of the agarose beads and protein complexes with TE buffer, after which the agarose beads were resuspended in 100 µl of 10 mM DTT and incubated at 37°C for 1 h to dissociate the protein complexes from the beads. The supernatant was removed and diluted in IP buffer, and immunoprecipitation was performed using the second antibody. The standard ChIP protocol was followed to obtain the DNA that was then analyzed by qPCR as described above.
RESULTS
XNP/dATRX physically interacts with DREF
To find putative XNP/dATRX interacting proteins, we performed a yeast two-hybrid screen. To achieve this, we constructed a cDNA library from 0 to 12 h embryos in which cDNAs were linked to the sequence encoding the GAL4 activation domain. Meanwhile, the N-terminal (amino acids 1–221) and C-terminal (amino acids 1147–1308) regions of XNP/dATRX were used as baits. The SNF2/helicase–ATPase domain was not used in this screen because it is also conserved in other factors. Several bona fide candidates were identified, as well as some encoded proteins involved in gene transcription and chromatin dynamics (Supplementary Table S1). Among these candidates, we decided to analyze DREF (Supplementary Figure S1) in more detail because it is a transcription factor that participates in several cellular functions and has been shown to interact with proteins involved in chromatin dynamics, such as Mi-2 (29,38).
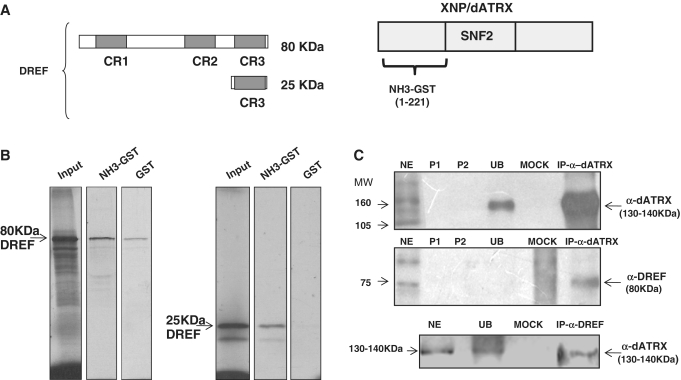
In the yeast two-hybrid screen, the activation domain of DREF called the CR3 was found to interact with the N-terminal portion of XNP/dATRX (Figure 1). To confirm this interaction, we performed in vitro GST-pull-down and co-immunoprecipitation (CoIP) experiments. In the pull-down experiments, a full length DREF or CR3 domain sequences were in vitro transcribed and translated to produce an S35-labeled DREF protein, or the S35-labeled CR3 domain, to monitor their binding with a XNP/dATRX-N-terminal-GST fusion protein (Figure 1A). In these pull-down assays we found that both, the S35-labeled DREF protein and the S35-labeled CR3 domain bound more strongly to XNP/dATRX-N-terminal-GST coupled to a glutathione sepharose column than to GST alone (indicated in the corresponding lanes as NH3-GST in Figure 1B).
Figure 1.
Physical interaction between XNP/dATRX and DREF. (A) Representation of the fragments assayed by pull down, the three conserved regions of DREF are shown (gray boxes), CR3 corresponds to the region that interacts with XNP/dATRX in the two-hybrid assay. On the right side, the 221 amino acids of XNP/dATRX used for the two-hybrid and the pull-down assays is shown. (B) Pull-down assay. For both panels: the first lane shows the amount of transcription/translation labeled protein (full-length DREF or the CR3 domain) used for each experiment (input), the second lane is the experimental interaction (GST-dATRX and dDREF) and the third lane is the control interaction (GST and dDREF) for each analyzed polypeptide. (C) CoIP of XNP/dATRX and DREF. Transcriptional active nuclear fraction from adult flies or 0–24 h embryos (NE); P1 is the pre-clearing 1; P2 is pre-clearing 2; UB correspond to the unbound protein fraction; Mock is the incubation with an unrelated antibody, the upper and middle panels correspond to an IP performed against XNP/dATRX. Upper panel was blotted with α-dATRX, middle panel with α-dDREF and the lower panel again with α-dATRX after an IP against DREF.
To confirm the yeast two-hybrid analysis and the results of the GST pull-downs, we performed a Co-IP experiment using protein extracts from the transcriptionally active chromatin fraction from adult flies and embryos (see ‘Material and Methods’ section) and an anti-XNP/dATRX antibody that only recognizes the large XNP/dATRX isoform (dATRXL). The presence of DREF in the proteins precipitated by the dATRXL antibody was determined using an anti-DREF antibody. Figure 1C shows that XNP/dATRX could be efficiently immunoprecipitated with the antibody and that DREF co-immunoprecipitated with it. We also performed a reciprocal CoIP using the DREF antibody to immunoprecipitate proteins from the adult flies or embryo extracts and found that XNP/dATRX also co-immunoprecipitates (Figure 1C). Intriguingly, the detection of DREF by western blot analysis after the CoIP using the XNP/dATRX antibody suggests that most of the DREF protein interacts with XNP/dATRX because no signal can be detected in the unbound fraction. However, we cannot discard the possibility that under our experimental conditions, the unbound DREF is unstable and degraded during the clearing procedure. Nevertheless, taken together, the results presented in this section strongly suggest that dATRX and DREF interact physically in Drosophila.
XNP/dATRX behaves genetically as a repressor of pnr function
Because pnr expression is likely regulated by XNP/dATRX (39), we studied the role of XNP/dATRX and DREF (see below) on gene expression. To do this, we used as a test system the modification of adult phenotypes shown by pnr mutant flies in conditions where the levels of ATRX or DREF are manipulated through the expression of transgenes carrying UAS-dATRX or sequences encoding RNA is for XNP/dATRX (UAS-dATRXi) or DREF (UAS-DREFi). This allowed us to reduce the levels of these proteins in particular domains, according to the Gal4-driver used.
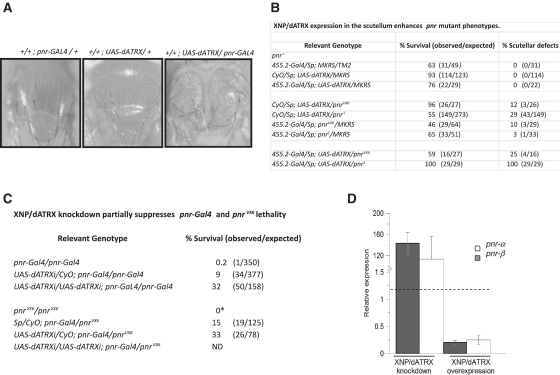
pnr encodes two isoforms, Pnr-α and Pnr-β, transcribed from two different promoters (40). Peña-Rangel et al. (39) showed that overexpression of XNP/dATRX enhances the thorax and scutellar abnormalities found in heterozygote individuals carrying the pnr-Gal4 mutation. In pnr-Gal4, a transposable element carrying the Gal4 gene is inserted just upstream of the first pnr exon transcribed from the pnr-β promoter (40). Consequently, in pnr-Gal4 individuals, Gal4 expression closely mimics normal pnr-β expression in wing imaginal discs and in adult flies (40). In homozygote pnr-Gal4 wing discs, the levels of pnr-β transcripts are reduced, while those of pnr-α are increased (41). pnr-Gal4 flies die as homozygotes, and only 1–3% develop into adults. pnr-Gal4 heterozygote adult flies have a very slight dorsal thoracic cleft and disorganization of bristles, sometimes missing a few inner and post-vertical bristles (39). For this set of experiments, we also used the pnr1 and pnrVX6 alleles (40). pnr1 presents a stop codon at position 180 (W180@), interrupting the first zinc finger found in Pnr-β, and it is considered to retain some activity (42). pnrVX6 is a small deficiency that deletes the complete pnr locus (40). pnr1 and pnrVX6 are recessive lethal, and their adult phenotypes are normal in heterozygosis.
We found that individuals expressing the transgene UAS-dATRX in the pnr adult territory, using the pnr-Gal4 line as a GAL4 source, showed a strong thoracic cleft and scutellar defects (Figure 2A) similar to the enhancement of the pnr defects found by Peña-Rangel et al. (39) with the overexpression of XNP/dATRX.
Figure 2.
Modification of pnr mutant phenotypes by XNP/dATRX dosage. (A) Enhancement of the thoracic cleft phenotype by the expression of the UAS-dATRX transgene in the pnr-expression domain. The genotypes of each organism are indicated at the top. Note the enhancement of the thorax cleft defect in the UAS-dATRX/pnr-Gal4 fly. This result is in agreement with Peña-Rangel et al. (39) where they used a different P-UAS construct that overexpresses XNP/dATRX in the pnr expression domain (27). (B) Enhancement of pnrVX6 and pnr1 scutellar defects by the expression of theUAS-dATRX transgene in the scutellum. Relevant genotypes are indicated. The scutellar defects consists of a reduction in the scutellum size and deformations in the scutellar border (data not shown). No defects in the macrochaetes were observed in these flies. (C) XNP/dATRX depletion rescues the lethality of pnr mutant flies. *Homozygous pnrVX6 flies die as embryos (40). Homozygous pnr-Gal4 or pnr-Gal4/pnrVX6 adult flies survive in the presence of one or two copies of the UAS-dATRXi transgene. (D) Modification of pnr transcript levels by XNP/dATRX. Expression of transgenes was driven by the Act5C-Gal4 driver. Depletion of XNP/dATRX by ectopic expression of the UAS-dATRXi transgene increases the transcript levels of the two pnr isoforms in Drosophila embryos. On the contrary, ectopic expression of XNP/dATRX by the UAS-dATRX transgene, reduced bothpnr mRNA levels. The quantification of the mRNA of mutant embryos was performed by RT–qPCR compared with the relative mRNA levels of wild-type embryos of the same age. The relative expression levels are adjusted to one, which is the level of each pnr isoform transcript in wild-type flies. Data are from three different experiments.
As pnr-Gal4 is by itself a pnr allele and a pnr GAL4 driver, interpretation of these results could be difficult. Thus, we drove the expression of the UAS-dATRX transgene with two other drivers, the ectopic Act5C-Gal4 and the 455.2-Gal4 drivers. The latter one was used to direct the UAS-dATRX transgene expression to the scutellum in wild-type pnr or in pnr1 or pnrVX6 backgrounds (Figure 2). Importantly, these experiments validate pnr-Gal4 as a suitable tool for these kinds of experiments because equivalent results can be obtained with different drivers and with different pnr alleles.
We found that ectopic expression of UAS-dATRX with the Act5C-Gal4 driver in the presence of wild-type pnr or with pnrVX6 is semilethal or lethal, respectively, and surviving adult flies (when wild-type pnr is present) do not show any morphological defects (data not shown). With the 455.2-Gal4 driver, we found lethality that could not be attributed to the effect of UAS-dATRX expression (Figure 2B); however, expression of UAS-dATRX in the scutellum enhances the scutellar defects shown by the flies of the control genotypes (flies with the 455.2-Gal4 driver and with pnr1 or pnrVX6 but without the UAS-dATRX transgene). These defects are not observed when the UAS-dATRX transgene is expressed in a wild-type pnr background (Figure 2B).
Based on these genetic results, we hypothesized that XNP/dATRX may act as a repressor of pnr expression. Therefore, we evaluated whether a reduction in XNP/dATRX levels (using RNA interference) could suppress the phenotypes found in pnr-Gal4 flies. Flies expressing the UAS-dATRXi transgene with the Act5C-Gal4 driver did not exhibit any visible defect, although there was a reduction of XNP/dATRX mRNA levels (data not shown). In contrast, the expression of UAS-dATRXi with the pnr-Gal4 driver suppressed the lethal phenotype of homozygous pnr-Gal4 flies (Supplementary Figure S2 and Figure 2Chttp://nar.oxfordjournals.org/cgi/content/full/gkr865/DC1). The suppression of lethality was greater when two copies of the UAS-dATRXi transgene were present (Figure 2C). Similar results were obtained with a different UAS-dATRXi RNAi line obtained from the Vienna Drosophila RNAi stock collection (data not shown). In addition, pnr-Gal4/pnrVX6 heteroallelic flies do not survive well (40); however, this is alleviated with the expression of one copy of the UAS-dATRXi transgene (Figure 2C). Furthermore, embryonic transcript levels of pnr isoforms respond to XNP/dATRX levels. Through the action of the Act5C-Gal4 driver during embryogenesis, XNP/dATRX depletion (by UAS-dATRXi expression) or overexpression (by UAS-dATRX expression) increases or decreases, respectively, the transcript levels of both pnr isoforms (Figure 2D). Thus in summary, overexpression of XNP/dATRX causes or enhances phenotypes that can be interpreted as a reduction of pnr function; meanwhile, reduction of XNP/dATRX levels rescues these phenotypes, suggesting that dATRX negatively regulates pnr expression.
DREF binds to DRE elements in the pnr control region
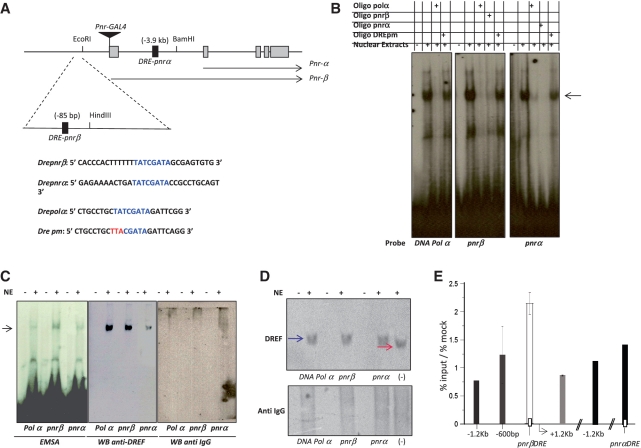
In silico analyses have revealed more than 250 DRE elements in the promoter regions of various genes, including genes involved in DNA metabolism, transcriptional regulation, protein synthesis and signal transduction mechanisms. Therefore, because DREF interacts with XNP/dATRX, we decided to search for DRE elements in the pnr regulatory region. The pnr locus encodes two isoforms expressed from two different promoters, named pnr-α and pnr-β (41). Two DNA elements containing the DRE consensus sequence were found in the vicinity of the pnr regulatory region: one −85 bp from the putative transcriptional start site of the β-isoform and the other 3.9 kb upstream of the putative pnr-α transcription start site (Figure 3A). We named these two elements Dre-pnrα and Dre-pnrβ. Next, to determine whether oligonucleotides containing the putative DRE elements were shifted by proteins in adult fly nuclear extracts, we performed an EMSA analysis. As a positive control, we used the DNA polymerase-α DRE element (Dre-polα), a known DREF binding site. As a non-competing element, we used the same Dre-polα sequence with a three-base mutation in the consensus element, as reported by Ida et al. (21; Figure 3A). Figure 4B shows that proteins in the nuclear extracts formed a complex with either Dre-pnrα, Dre-pnrβ or Dre-polα; in all three cases the radioactive binding signal was inhibited by a 100-fold molar excess of the Dre-polα non-radioactive oligonucleotide but not by the mutated Dre-pm oligonucleotide at the same molar excess (Figure 3B). These results suggested that the two DRE elements present in the pnr control region could be recognized by a nuclear factor and that this interaction could be inhibited with the DRE-polα oligonucleotide but not by the mutated Dre-pm oligonucleotide. Next, we assessed whether DREF was part of the shifted complexes. Initially, we used the DREF antibody to identify super-shifted complexes; however, this antibody was not able to produce any super-shift, even with the positive control Dre-polα oligonucleotide. After DREF antibody incubation with the nuclear extracts and with each oligonucleotide, the formation of the complex was reduced, suggesting that the DREF antibody sequestration inhibits the putative DREF–DNA interaction (data not shown). Therefore, to assess the presence of DREF in these complexes, we performed an EMSA–western experiment. Indeed, we found that the DREF antibody recognizes the complexes bound at the Dre-pnrα and Dre-pnrβ oligonucleotides (Figure 3C). No signal was detected in a similar western blot analysis performed with an antibody which does not recognize DREF. To confirm that DREF binds to the Dre-pnr elements, we incubated nuclear extracts with or without the different oligonucleotides to allow the formation of the complexes. The presence of DREF in the formed complexes was determined by western blot analyses with the anti-DREF antibody in the nuclear protein extracts separated electrophoretically under native conditions (Figure 3D). These results show that the migration of DREF is shifted in the presence of the Dre-pnrα, Dre-pnr-β and Dre-polα oligonucleotides suggesting, as in the EMSA analysis, that DREF found in the nuclear extracts interacts with Dre-pnra, Dre-pnr-β and Dre-polα elements. To complement these data, we performed ChIP experiments using chromatin obtained from 0 to14 h developed embryos. DREF was detected at the Dre-pnrβ elements and at the DRE-PCNA element, which was used as a positive control, indicating that DREF binds the Dre-pnrβ element in vivo (Supplementary Figure S3). In addition, we performed ChIP experiments with several PCR primer sets over the pnrα and pnrβ promoter region and found that the DREF signals peaks at the two putative DRE elements but it is enriched at the DRE-pnrβ element (Figure 3E). The detection of DREF at the DRE-pnrα element was marginal (Figure 3E and Supplementary Figure S3). However, we cannot exclude the possibility that this element could be recognized by DREF at other developmental stages. We also performed a bioinformatics search for DRE elements in the putative regulatory regions of the pnr homologs in other Drosophila species. Assuming that pnr also encodes two isoforms in these other species, we found that a putative Dre-pnrβ element is conserved in other Drosophila species (Supplementary Figure S4). A putative Dre-pnr-α element was found to be conserved only to a minor extent. Taken together, these results suggest that DREF recognizes at least one DRE element located upstream of the pnr-β promoter.
Figure 3.
DREF binds to two DRE elements in the pnr control region. (A) Localization of the two DRE (Dre-pnrα and Dre-pnrβ) consensus sequences near the transcription initiation site of the pnr-β (−85 bp) and pnr-α (−3.9 kb) transcripts. The sequence of the two Dre pnr elements, the Dre-polα and a mutant form (DRE pm) used as positive and negative controls respectively are also shown. The insertion site of the pnr-Gal4 driver is also indicated. (B) EMSA analysis of the DRE elements found in the pnr-β and α control region. P32-labeled double-strand oligonucleotides with the sequences indicated in the panel A were incubated with Drosophila nuclear extracts with or without competing non-labeled oligonucleotides (100-fold molar excess of the same and the mutant non-radioactive oligonucleotides). The arrow shows the shifted complex. (C) EMSA-western blot used to identify the presence of DREF in the shifted complexes. In the left panel a typical EMSA is observed after the incubation of nuclear extracts with the Dre-Polα, Dre-pnrα and Dre-pnrβ oligonucleotides. The central panel shows a western blot using an anti-DREF antibody after the transfer of the EMSA to a nitrocellulose membrane. The right panel shows a western blot after the transfer of the DRE-protein complexes, but using a not related antibody as a control. (D) Native DREF is shifted in the presence of the DRE oligonucleotides. Western blots are shown after the transfer of total nuclear proteins previously incubated with the DRE oligonucleotides and separated in a native polyacrilamide gel. Note that in the presence of the different DRE oligonucleotides the band recognized by the DREF antibody is shifted when compared to the one without DNA indicated as (−). A control western blot using an unrelated antibody is also shown. (E) ChIP assays using a specific antibody against DREF to determine the distribution DREF factor around and on the putative pnr-α and pnr-βDRE in chromatin derived from embryos. Graphs show results from three independent immunoprecipitation reactions (n = 3). ChIP signals quantified by means of quantitative polymerase chain reaction, are presented as percentage of the input/percentage of the mock antibody. As it can be observed in the figure, the pnr-β chromatin region shows the maximum enrichment when the precipitation was carried out with the DREF antibody.
Genetic interaction between DREF and XNP/dATRX in pnr gene regulation
In the previous sections, we showed that DREF binds directly to XNP/dATRX and to the DRE sites at the pnr promoter, suggesting a possible role of DREF in the control of pnr gene expression. Therefore, we investigated the effect of knocking down DREF using the pnr-Gal4 driver. We used the transgenic UAS-DREFi line from the Vienna Drosophila RNAi stock collection (43). A reduction in the DREF protein level was detected in the thorax of adult flies expressing the UAS-DREFi RNAi transgene (Supplementary Figure S5). Interestingly, individuals expressing the UAS-DREFi transgene with the pnr-Gal4 driver showed a dramatic enhancement of thoracic cleft defects (Figure 4A and Table 1). We also noticed a reduction in macrochaete number in the pnr expression domain, as well as the presence of thinner bristles when compared to the ones in pnr-Gal4/+ flies without the transgene (Figure 4A and Table 1).
Table 1.
DREF and XNP/dATRX knockdowns modify pnr thorax cleft and machrochaete defects
| Relevant genotype | Thoracic cleft defects (%) | Macrocheate (%) |
|---|---|---|
| +/+;UAS-DREFi/TM3 | 0 (0/177)a | 0 (0/177) |
| +/+; pnr-Gal4/MKRS | 0 (0/160) | 0 (0/160) |
| +/+; pnr-Gal4/UAS-DREFi | 100 (86/86) | 100 (86/86) |
| UAS-dATRXi/+; pnr-Gal4/ UAS-DREFi | 0 (0/73)b | 65 (48/73) |
aFlies with thoracic cleft and absence of macrochaetes from the total number of flies counted for each genotype.
bThe reduction of the thoracic cleft phenotype and the absence of machrochaetes are shown in Figure 4A.
Depletion of DREF in flies expressing the UAS-DREFi transgene with the 455.2-Gal4 driver, causes the disappearance of machrochaetes in the scutellum (Supplementary Figure S6, middle panel). Interestingly, when a mutant pnr allele such as pnr1 (Supplementary Figure S6, right panel) or pnrVX6 (data not shown) is added, the scutellum is smaller, with irregular borders. These results, together with the binding of DREF in the promoter region of pnr-β, suggest that DREF is a positive regulator of pnr expression.
Because our data show that XNP/dATRX and DREF may be antagonistic pnr transcriptional regulators, we tested the effect of XNP/dATRX knockdown in the UAS-DREFi/pnr-Gal4 flies, expecting that the enhancement of the thoracic cleft presented by DREF depleted pnr-Gal4 flies (Figure 4A, upper panel) would be suppressed by the depletion of XNP/dATRX. Indeed, we found that this was the case. Figure 4A (lower panel) shows a reduction of the thoracic cleft defect of UAS-DREFi/pnr-Gal4 flies when the levels of XNP/dATRX were diminished (Table 1).
We determined the levels of pnr-a and pnr-β transcripts in the thorax of these flies (Figure 4B). We found that in heterozygous pnr-Gal4 flies, the pnr-β transcript level is reduced and the pnr-α transcript level is increased, in accordance with previous studies in which the transcript levels of the different isoforms were studied (41). When DREF is depleted in the pnr domain, pnr-β transcript levels are further diminished, supporting the positive role of DREF in pnr-β expression. On the contrary, when XNP/dATRX is depleted, the pnr-β transcript level is increased in comparison to the level found in wild-type flies, supporting the negative role of XNP/dATRX in pnr expression. In this latter condition, we found a decrease in the pnr-α transcript level compared to the wild-type level probably due to indirect effects of Pnr-β on pnr-α transcription (41). The differences between the transcript levels of pnrα in embryos versus the wing disc when XNP/dATRX is depleted could be attributed to differences in pnr regulation during development. Interestingly, depletion of both DREF and XNP/dATRX in the pnr-Gal4 domain recovers the ratio between pnr-α and pnr-β transcript levels (Figure 4B). These results support the idea that besides their physical interaction, DREF and XNP/dATRX also interact genetically and have antagonistic roles in pnr transcription. In addition, the depletion of XNP/dATRX does not affect DREF protein levels, suggesting that dATRX does not regulate DREF gene expression (Supplementary Figure S7).
On the other hand, the simultaneous overexpression of XNP/dATRX and knockdown of DREF cause lethality and the organisms die during the embryo development. We also analyzed the effect in Drosophila of the simultaneous overexpression of both XNP/dATRX and DREF using the pnr-GAL4 driver. Intriguingly, we also found that this combination is lethal. Different possibilities may explain these two results, since both XNP/dATRX and DREF independently may regulate different set of genes (as it is suggested polytene chromosomes immunostainings, see ahead). Therefore it is possible that in either of these two conditions creates a dramatic de-regulation in gene expression during the fly development leading to organism lethality.
A recent publication (44) has shown that DREF depletion in the notum and in other subdomains of the wing disc reduces or eliminates bristles. This phenotype is due in part to the influence of DREF on PCNA gene expression and affects the endoreplication process of bristle development, which results in a lack of bristles. The reduction in the number and size of machrocheates in UAS-DREFi/pnr-Gal4 flies found in our study (Supplementary Figure S6) may be related to a cell proliferation defect independent of pnr. Nevertheless, the disruption of the thorax is only observed in UAS-DREFi/pnr-Gal4 flies (Figure 4, upper panel). Our genetic analyses indicate that we can separate these two phenotypes. We think that DREF depletion on the PCNA gene regulatory sequences would lead to the bristle defects, meanwhile depletion of this molecule on the pnr regulatory sequences would lead to the notum defects.
XNP/dATRX represses transcription of some, but not all, of the genes regulated by DREF
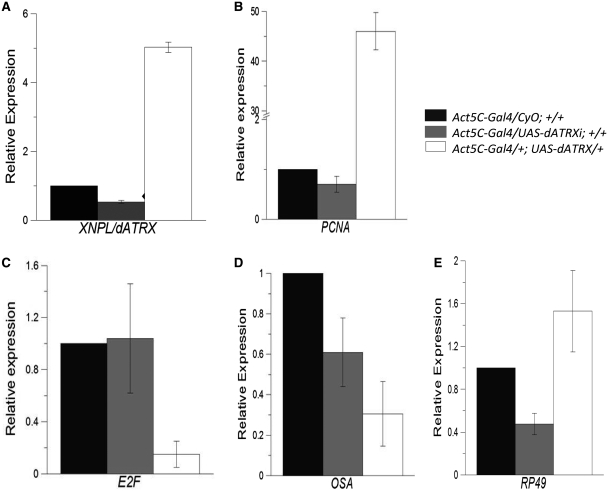
The genetic results suggest that XNP/dATRX negatively regulates the expression of pnr, which requires DREF to be properly expressed. Therefore, we studied the transcript levels of other genes that are positively regulated by DREF in conditions where XNP/dATRX levels were altered. To perform this analysis, we used the Act5C-Gal4 driver to overexpress or reduce XNP/dATRX levels ectopically. We measured the mRNA levels of E2F, osa and PCNA from embryonic total RNA through qRT–PCR. These three genes are positively regulated by DREF. As a control, we used the p8/TTDA transcript (31), which we found to be unaffected by XNP/dATRX. We also analyzed the transcript of the ribosomal protein rp49, which is generally used as a reference control to measure mRNA levels in Drosophila. However, it should be noted that genes encoding ribosomal proteins in mammals have been reported to be regulated by DREF (45).
In flies that ectopically express XNP/dATRX, we detected a reduction on the E2F and osa transcript levels (Figure 5C and D). However, the transcript levels of PCNA and rp49, which are also positively regulated by DREF, were enhanced (Figure 5B and E) This result is intriguing because E2F acts as an activator of PCNA transcription. At the same time, it has also been reported that E2F may act as a repressor of PCNA expression in some fly tissues (46). Thus, it is possible that the effect on PCNA mRNA levels may be indirect and not a direct con sequence of XNP/dATRX ectopic expression. To determine whether dATRX is found at the PCNA-DRE element in embryonic chromatin, we performed a ChIP experiment using specific dATRX antibodies. We found that dATRX is at the PCNA-DRE element (data not shown), suggesting that dATRX may have a direct impact on PCNA transcript levels; however, its interaction with DREF in this region is not clear because Re-ChIP experiments using DREF and dATRX antibodies pulled down the PCNA-DRE only marginally (Figure 6).
Figure 5.
Effect on the mRNA levels of different genes regulated by DREF by the overexpression and depletion of XNP/dATRX. Total RNA from flies either overexpressing XNP/dATRX or an XNP/dATRX RNAi construct under the control of the Act5C-Gal4 driver (ectopic expression) or without it, was purified from embryos and used in quantitative RT–PCR analysis for different gene transcripts. The genotypes are indicated for each bar color. Black bars indicate the quantification of the specific mRNA in flies with only the Act5C-Gal4driver; gray bars indicate flies expressing the XNP/dATRX RNAi and white bars correspond to flies overexpressing XNP/dATRX. As an internal control we used the mRNA levels of the Dmp8 transcript, which is not affected by the XNP/dATRX levels. The relative expression values are adjusted to 1, which correspond to Act5C-Gal4/CyO; +/+ organisms. (A) Quantification of mRNA levels of XNP/dATRX. Note that the levels of the specific mRNA is reduced when the RNAi against XNP/dATRX is expressed (RNAi-dATRX/Act5C-Gal4) and increased by the overexpression of XNP/dATRX in the UAS-XNP/dATRX (UY3132 line). (B–E) Quantification of the mRNA levels of E2F; osa; PCNA; rp49 in flies overexpressing XNP/dATRX or a RNAi against XNP/dATRX. In all cases the analyzed RNA was prepared from three independent experiments and each cDNA preparation amplified three times for posterior qRT–PCR.
Figure 6.
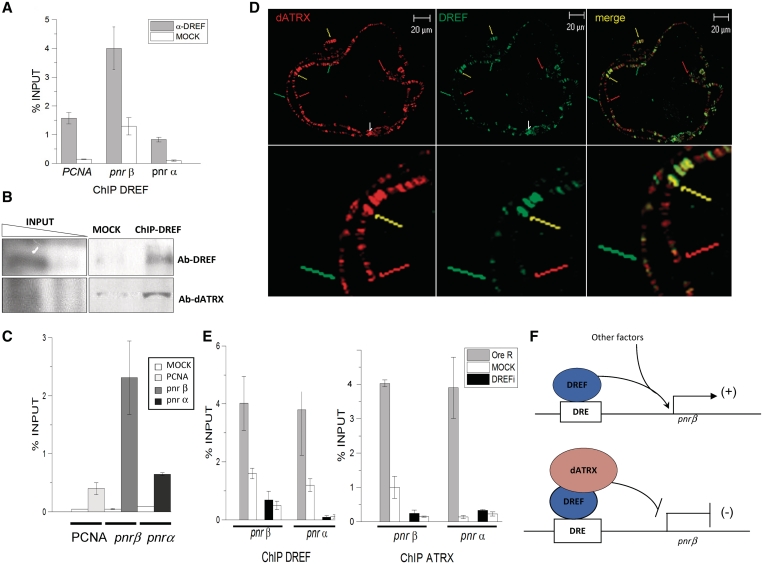
A XNP/dATRX–DREF complex binds the DRE-pnrβ element. (A) DREF-ChIP assay from embryos overexpressing XNP/dATRX using an Act5C-Gal4 driver. Note that even when XNP/dATRX overexpression reduces pnr mRNA levels (Figure 2), DREF is still located at the pnr-β and pnr-α-DRE elements. The ChIP experiment was performed by triplicate (P = 0.001). (B) ChIP-western assay. ChIP was performed with the anti-DREF antibody and the precipitated chromatin analyzed in a typical western blot to search for the presence of DREF and dATRX. Note that both proteins can be detected. (C) Re-ChIP experiment using the DREF antibody for the first ChIP and then the XNP/dATRX antibody for the Re-ChIP. An unrelated antibody was used as negative control for the first ChIP and then immunoprecipitated with the dATRX antibody (white bars, MOCK). The Re-ChIP shows the average of three independent experiments (P = 0.01). (D) Polytene chromosomes prepared from wild-type larvae were simultaneously stained with the anti-DREF (green signal) and anti-XNP/dATRX antibodies (red signal). Several merge bands containing both factors can be visualized (yellow arrows), regions where only XNP/dATRX is present (red arrows) and regions where only DREF is present (green arrows). (E) ChIP experiments using the DREF and XNP/dATRX antibodies in wild-type and DREFi knocked-down embryos. The genotype of the organisms tested is Act5C-Gal4/+; UAS-DREFi/+. OreR corresponds to immunoprecipitated chromatin from wild-type organism (gray bars). DREFi corresponds to immunoprecipitated chromatin from embryos expressing the anti DREF RNAi (black bars). Mock is the incubation with unrelated antibody (white bars). Antibodies used for each IP are indicated at the bottom of the figure. (F) Model on the role of DREF and XNP/dATRX in the transcriptional regulation of pnr-β. Among other factors that regulate pnr gene expression during development, the interaction between DREF and dATRX may participate in the establishment of an active or negative effect in gene regulation.
Interestingly, in XNP/dATRX-depleted flies, the E2F, osa and PCNA transcript levels do not respond significantly to the reduction of XNP/dATRX dosage (Figure 5). This result contrasts with the pnr transcriptional deregulation that we have found as a consequence of XNP/dATRX depletion (Figure 2C), suggesting that pnr gene expression is particularly sensitive to XNP/dATRX levels. We also found that rp49 transcript levels are reduced under XNP/dATRX depletion, suggesting that XNP/dATRX may be a positive factor for rp49 expression. Taken together, these data show that XNP/dATRX can act as a repressor for some, but not all, DREF regulated genes, such as pnr, and suggest that XNP/dATRX exerts a promoter-specific effect on gene expression.
XNP/dATRX regulates pnr gene expression by binding DREF in chromatin
We have shown that both, DREF and XNP/dATRX are pnr transcriptional regulators. Thus, we investigated whether the negative effect of XNP/dATRX on pnr occurred via a direct interaction with DREF or through an independent mechanism. The fact that XNP/dATRX genetically and physically interacts with DREF supports the hypothesis that the repression of pnr by XNP/dATRX occurs via a physical contact with DREF. Overexpression of XNP/dATRX in the pnr expression domain enhanced the thoracic cleft phenotype, as was observed during DREF knockdown (Figures 2 and 4). In this situation, XNP/dATRX may be sequestering DREF and preventing it from interacting with DRE elements, thereby inhibiting transcription.
ChIP assays of pnr chromatin from XNP/dATRX overexpressing flies, show that in spite of XNP/dATRX increased levels, DREF still occupies the DRE-pnrβ element, suggesting that XNP/dATRX did not prevent the association of DREF with this DRE site (Figure 6A). To determine whether XNP/dATRX interacts with DREF at the DRE-pnrβ element, we performed ChIP-western and Re-ChIP experiments. The DREF antibody was used in a typical ChIP assay with wild-type-embryo chromatin, and the precipitated proteins were analyzed by western blot analysis to identify DREF and XNP/dATRX. We found that both DREF and XNP/dATRX can be identified in the precipitated chromatin by the specific antibodies (Figure 6B), indicating that XNP/dATRX can be co-localized together with DREF in the chromatin. In Re-Chip assays, in which we first precipitated the chromatin with the DREF antibody and then with the XNP/dATRX antibody and then quantified the precipitated DRE-pnrβ element region by qPCR, we found that both XNP/dATRX and DREF are bound to this element in vivo (Figure 6C). Interestingly, in this Re-Chip assay, there was no XNP/dATRX enrichment at the DRE-PCNA element, contrary to the XNP/dATRX enrichment found at the DRE-pnr-β element, suggesting that the XNP/dATRX transcriptional effect on PCNA (Figure 5), may be an indirect effect. It is important to note that Re-ChIP does not necessarily mean that XNP/dATRX is binding through DREF. The chromatin in these experiments is fragmented to 200- to 800-bp range, and therefore it is equally possible that XNP/dATRX is binding at neighboring DREF sites and is brought down by intervening DNA. However, the depletion of DREF reduces the occupancy of XNP/dATRX at the pnr DRE sites (see ahead).
To support the idea that DREF and XNP/dATRX can be located at the same chromosomal positions, we performed co-immunostaining experiments in polytene chromosomes to identify DREF and XNP/dATRX location sites. XNP/dATRX and DREF co-localize in around half of the sites (Figure 6D). In fact, we even observed co-localization of XNP/dATRX and DREF in the so-called pericentric focus (Figure 6D, white arrow, 18). However, some regions showed no co-localization of the two factors (Figure 6D), suggesting a highly dynamic behavior of both factors in the chromatin and that XNP/dATRX may interact with other factors besides DREF to bind specific chromatin regions (see below).
As previously mentioned, XNP/dATRX lacks the ADD motif. Therefore, we hypothesized that XNP/dATRX requires an interaction with other factors like DREF to bind to specific regions of the chromatin. Thus, we depleted DREF using RNAi and the Act5C-Gal4 driver in embryos and performed ChIP assays to determine the occupancy of DREF and ATRX in the DRE-pnr elements. We found that when DREF was depleted, the occupancy of both dATRX and DREF in the DRE-pnr elements was reduced (Figure 6E). This result indicates that dATRX requires the presence of DREF to bind to the chromatin at the DRE element in the pnr-β promoter.
DISCUSSION
XNP/dATRX interacts physically with DREF to regulate pnr gene expression
In Drosophila, little is known about the genes regulated by XNP/dATRX and how it is recruited to chromatin sites. In this work, we demonstrated that XNP/dATRX interacts with the trans-activation domain of the transcription factor DREF and that this interaction mediates XNP/dATRX recruitment to chromatin at the pnr promoter. Furthermore, depletion of DREF significantly reduces the occupancy of dATRX in these chromatin regions and suggests that XNP/dATRX occupancy at the DRE sites depends on the presence of DREF. This observation also suggests that the interaction between the two proteins at the pnr-β promoter results in the repression of pnr transcription.
Mammalian ATRX has two important domains: the helicase/ATPase SNF2 domain and the ADD domain. As mentioned before, only three proteins share the ADD domain: ATRX, DNMT3a and DNMT3L. The ADD domain of the DNMTs and hATRX are able to bind to histone tails, thus providing a model for how these proteins bind to chromatin (5–7). XNP/dATRX, as well as its counterpart in Caenorhabditis elegans, lacks the ADD domain present in the mammalian protein. Several studies have shown that XNP/dATRX is a chromatin-associated protein (17–19), but there is no evidence of direct binding to DNA sequences or to histones. For this reason, the identification of target sequences or promoters of XNP/dATRX has been elusive. We hypothesized that XNP/dATRX requires an interaction with other proteins to reach its target sites/promoters and to achieve its functions in chromatin. We found that one of these factors is DREF. DREF is a transcriptional activator that was initially found to regulate the expression of several genes required for DNA replication and cell proliferation (47,48). Recently, it has been demonstrated that DREF also activates the expression of many other genes in Drosophila and in human cells and that putative DRE motifs are present at many promoter regions in the fly genome (20).
The region of DREF that interacted with the amino terminus of XNP/dATRX corresponded to the transactivation domain of the protein (CR3 region). XNP/dATRXL bound to HP1α through a motif that covers amino acids 245–249 (17). Therefore, the DREF interaction with XNP/dATRX did not overlap with the HP1α interaction site. Moreover, it is important to mention that this interaction only occurs between DREF and the long isoform of XNP/dATRX because the short isoform does not contain this region. Further experiments with both isoforms of XNP/dATRX will be necessary to address the biological importance of both isoforms. CoIP experiments also confirmed the association between the two proteins, suggesting that this interaction took place in vivo in the cell nucleus (Figure 1C). Additionally, immunofluorescence analysis of polytene chromosomes indicated that both proteins co-localized at several, but not all, chromatin regions, suggesting that there may be other factors in addition to DREF that mediate XNP/dATRX recruitment to chromatin (Figure 6D). In fact, we identified several putative candidates using our two-hybrid screen (Supplementary Table S1).
It was initially reported that the longer isoform of XNP/dATRX is preferentially located in the chromocenter and less abundantly in other chromosomal regions (17). The short isoform apparently has a different distribution and is located in many regions on polytene chromosomes. In addition, it has recently been reported that XNP/dATRX is required for HP1α deposition in pericentric β-heterochromatin of the X chromosome (19). However, a second recent report challenged those results (18) because the authors found that XNP/dATRX is preferentially found in euchromatic regions. Furthermore, XNP/dATRX seems to co-localize with the H3.3 histone variant (which is a histone enriched in euchromatic regions) in polytene chromosomes (18). Moreover, it has recently been shown that hATRX interacts with the H3.3 histone in embryonic stem cell telomeres, and together with DAXX, it is important for the incorporation of H3.3 at the telomeres (49,50). Our experiments provide further support for the euchromatic localization of XNP/dATRX. Thus, it could be possible that XNP/dATRX represses the expression of genes located in euchromatin. However, a positive impact on gene expression cannot be discarded, and it would be interesting if XNP/dATRX also interacts with the H3.3 histone at the DRE sites. Nevertheless, our results in Drosophila suggest that DREF is one of the factors that interacts with XNP/dATRX to regulate gene expression and that it localizes to euchromatic regions.
The DREF-XNP/dATRX interaction has a functional relevance in the regulation of pnr
A previous report from another group (39) is in accordance with our own data which demonstrate that the ectopic expression of dATRX in the pnr expression domain enhances pnr loss-of-function phenotypes, suggesting that XNP/dATRX may act as a pnr negative transcriptional regulator. Specifically, reduction of the two XNP/dATRX isoforms with RNAi suppressed mutant phenotypes caused by different pnr alleles. This repressive role of XNP/dATRX in pnr expression is also supported by the fact that ectopic expression of XNP/dATRX in the embryo causes a reduction in the mRNA levels of the pnr isoforms and that the XNP/dATRX depletion by RNAi increases the pnr transcripts level. However, the DREF knockdown in the pnr-Gal4 expression domain enhances thoracic cleft defects caused by a reduction in pnr-β levels, and these defects are suppressed by the simultaneous XNP/dATRX knockdown. Thus, these factors interact genetically, and they have antagonistic roles in pnr regulation. Moreover, we also found that DREF can bind to the DRE element that is located in the pnr-β control region. Based on these results, we suggest that DREF activates pnr-β gene expression because its depletion enhances the loss of function phenotypes found in pnr-Gal4 flies. XNP/dATRX is targeted to the DRE element at the pnr-β promoter through its direct interaction with DREF and this interaction negatively regulates pnr (Figure 6F). However, this model opens the question of why XNP/dATRX depletion restores the pnr-β transcript levels in DREF-depleted thoraxes (Figure 4B) given the assumption that lowering DREF dosages would reduce consequently the amount of XNP/dATRX at the DRE regulatory sequence. A possible explanation is that the DRE element controlling pnr-β is very sensitive to changes in the DREF-XNP/dATRX ratio (Figure 4B). We also think that other factors binding to the regulatory regions of pnr, may influence DREF and XNP/dATRX location at the DRE and moreover DREF and/or XNP/dATRX, could be regulating the expression of upstream genes, therefore indirectly influencing pnr gene expression.
Interestingly, it has been reported that DREF associates with TRF2 and can recognize the PCNA core promoter (24). Therefore, it is possible that DREF may act as a co-activator that is necessary for the expression of genes required for cell proliferation, which are also regulated by TRF2. The DRE element, we identified at the pnr-β promoter overlaps with an identified sequence that is occupied by TRF2 (51). These data and our molecular and genetic assays suggest that DREF likely activates pnr-β expression through its interaction with TRF2. Therefore, it is possible that the mechanism of pnr gene inactivation by XNP/dATRX occurs via the interference of the interaction between DREF and TRF2.
XNP/dATRX has a possible dual role in transcriptional regulation
In this work, we present data indicating that in addition to pnr, the ectopic expression of XNP/dATRX also represses E2F and osa transcription. However, this was not true for other genes, such as PCNA, which requires DREF for its expression. In fact, in some examples, we found an increase in the level of specific mRNAs when XNP/dATRX was overexpressed. Contrary to the results observed with pnr, XNP/dATRX depletion did not affect the mRNA levels of PCNA, E2F and osa; however, rp49 mRNA levels were decreased in this condition (Figure 5). These results suggest that there is a selective effect of dATRX in gene regulation and that XNP/dATRX may be required for both gene activation and gene repression. A recent microarray analysis of a mouse model that lacks ATRX in the forebrain identified several overexpressed genes, as well as some genes that were downregulated. The functions of both sets of genes were diverse, but these results suggest that in mice, ATRX is required for gene activation and repression (15,16). In addition, the forebrain transcript analysis in the ATRX mutant mice indicated that ATRX is not a global regulator of gene expression, as it seems that ATRX acts through other factors like MeCP2, cohesin and CTCF in the regulation of imprinted genes in the postnatal mouse brain (15,16). A global analysis of changes in transcript levels caused by the XNP/dATRX overexpression and depletion in Drosophila will be required to achieve a comprehensive view of the influence of XNP/dATRX on gene regulation.
Pnr belongs to the GATA family of transcription factors, which has been conserved over the course of evolution. For example, there are six GATA family members in mammals. Therefore, it is important to assess whether ATRX regulates some of these factors in vertebrates. This is of particular interest because some patients afflicted with ATRX syndrome suffer from α-thalassemia, and it is known that the GATA 1 and GATA 2 factors are involved in the regulation of the globin genes (52). Moreover, it has recently been reported that human ATRX is recruited to G-rich tandem repeat sequences that have the potential to form G-quadruples, and these may have a negative impact on genes in the vicinity, such as the α-globin genes (10). It will be interesting in future studies to determine if XNP/dATRX is also recruited to G-rich sequences.
In conclusion, our results show that DREF is required for the proper expression of pnr and that XNP/dATRX binds to DREF at the DRE sites, resulting in the repression of pnr gene expression. This establishes a connection between a chromatin remodeling factor (XNP/dATRX) and a transcriptional activator (DREF) in the regulation of an important gene (pnr). In addition, these studies in Drosophila open new avenues for the study of human pathologies generated by mutations in hATRX. For example, human cells code for a DREF homolog, and as hATRX interacts with multiple factors to achieve its functions, it will be important to determine if hATRX functionally interacts with DREF in humans. These types of studies will allow us to assess whether genes regulated by DREF are affected in cells derived from patients afflicted with the ATRX syndrome.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table S1, Supplementary Figures S1–S7.
FUNDING
PAPIIT/UNAM IN 20109-3, CONACyT grant 48550, IXTLI/UNAM; Fundación Miguel Alemán, Howard Hughes Medical Institute (PAPIIT/UNAM IN-208808) (to M.Z.); CONACyT 99654 grants to M.V; L'Oreal-UNESCO –AMC scholarship to V.V.G. Funding for open access charge: Mexican Council of Research.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Virginia Barajas, Lucia Perezgasga, Shaday Michan and Javier Aguilar-Fuentes for help at the beginning of this work. The authors also thank Andrés Saralegui for his advice in confocal microscopy. Polyclonal rabbit anti-Pnr antibody was kindly provided by Dr Ginés Morata.
REFERENCES
- 1.Argentaro A, Yang JC, Chapman L, Kowalczyk MS, Gibbons RJ, Higgs DR, Neuhaus D, Rhodes D. Structural consequences of disease- causing mutations in the ATRX-DNMT3-DNMT3L (ADD) domain of the chromatin-associated protein ATRX. Proc. Natl Acad. Sci. USA. 2007;104:11939–11944. doi: 10.1073/pnas.0704057104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibbons RJ, Bachoo S, Picketts DJ, Aftimos S, Asenbauer B, Bergoffen J, Berry SA, Dahl N, Fryer A, Keppler K, et al. Mutations in transcriptional regulator ATRX establish the functional significance of a PHD-like domain. Nat. Genet. 1997;17:146–148. doi: 10.1038/ng1097-146. [DOI] [PubMed] [Google Scholar]
- 3.Cheng X, Blumenthal RM. Mammalian DNA methyltransferases: a structural perspective. Structure. 2008;16:341–350. doi: 10.1016/j.str.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Jurkowska R, Soeroes S, Rajavelu A, Dhayalan A, Bock I, Rathert P, Brandt O, Reinhardt R, Fischle W, et al. Chromatin methylation activity of Dnmt3a and Dnmt3a/3L is guided by interaction of the ADD domain with the histone H3 tail. Nucleic Acids Res. 2010;38:4246–4253. doi: 10.1093/nar/gkq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhayalan A, Tamas R, Bock I, Tattermusch A, Dimitrova E, Kudithipudi S, Ragozin S, Jeltsch A. The ATRX-ADD domain binds to H3 tail peptides and reads the combined methylation state of K4 and K9. Hum. Mol. Genet. 2011;20:2195–2203. doi: 10.1093/hmg/ddr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwase S, Xiang B, Ghosh S, Ren T, Lewis PW, Cochrane JC, Allis CD, Picketts DJ, Patel DJ, Li H, et al. ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat. Struct. Mol. Biol. 2011;18:769–776. doi: 10.1038/nsmb.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eustermann S, Yang JC, Law MJ, Amos R, Chapman LM, Jelinska C, Garrick D, Clynes D, Gibbons RJ, Rhodes D, et al. Combinatorial readout of histone H3 modifications specifies localization of ATRX to heterochromatin. Nat. Struct. Mol. Biol. 2011;18:777–782. doi: 10.1038/nsmb.2070. [DOI] [PubMed] [Google Scholar]
- 8.Bérubé NG, Healy J, Medina CF, Wu S, Hodgson T, Jagla M, Picketts DJ. Patient mutations alter ATRX targeting to PML nuclear bodies. Eur. J. Hum. Genet. 2008;16:192–201. doi: 10.1038/sj.ejhg.5201943. [DOI] [PubMed] [Google Scholar]
- 9.Baumann C, Schmidtmann A, Muegge K, De La Fuente R. Association of ATRX with pericentric heterochromatin and the Y chromosome of neonatal mouse spermatogonia. BMC Mol. Biol. 2008;13:9–29. doi: 10.1186/1471-2199-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law MJ, Coger KM, Voon HP, Hughes JR, Garrick D, Viprakasit V, Mitson M, De Gobbi M, Marra M, Morris A, et al. ATR-X syndrome protein targets tandem repeats and influences allele-specific expression in a size-dependent manner. Cell. 2010;143:367–378. doi: 10.1016/j.cell.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Xue Y, Gibbons R, Yan Z, Yang D, McDowell TL, Sechi S, Qin J, Zhou S, Higgs D, Wang W. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl Acad. Sci. USA. 2003;100:10635–10640. doi: 10.1073/pnas.1937626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De La Fuente R, Viveiros MM, Wigglesworth K, Eppig JJ. ATRX, a member of the SNF2 family of helicase/ATPases, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocytes. Dev. Biol. 2004;272:1–14. doi: 10.1016/j.ydbio.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Ishov AM, Vladimirova OV, Maul GG. Heterochromatin and ND10 are cell-cycle regulated and phosphorylation-dependent alternate nuclear sites of the transcription repressor Daxx and SWI/SNF protein ATRX. J. Cell Sci. 2004;117:3807–3820. doi: 10.1242/jcs.01230. [DOI] [PubMed] [Google Scholar]
- 14.Baumann C, Viveiros MM, De La Fuente R. Loss of maternal ATRX results in centromere instability and aneuploidy in the mammalian oocyte and pre-implantation embryo. PLoS Genet. 2010;6:pii: e1001137. doi: 10.1371/journal.pgen.1001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kernohan YD, Jiang Y, Trembly DC, Bonvissuto AC, Eubanks JH, Mann MRW, Bérube NG. ATRX partners with Cohesin and MeCP2 and contributes to developmental silencing of imprinted genes in the brain. Dev. Cell. 2010;18:191–202. doi: 10.1016/j.devcel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Levy MA, Fernandes AD, Tremblay DC, Seah C, Bérubé NG. The SWI/SNF protein ATRX co-regulates pseudoautosomal genes that have translocated to autosomes in the mouse genome. BMC Genomics. 2008;9:468–472. doi: 10.1186/1471-2164-9-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassett AR, Cooper SE, Ragab A, Travers AA. The chromatin remodelling factor dATRX is involved in heterochromatin formation. PLoS One. 2008;3:e2099. doi: 10.1371/journal.pone.0002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneiderman JI, Sakai A, Goldstein S, Ahmad K. The XNP remodeler targets dynamic chromatin in Drosophila. Proc. Natl Acad. Sci. USA. 2009;106:14472–14477. doi: 10.1073/pnas.0905816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emelyanov AD, Konev YA, Vershilova E, Fyodorov DV. Protein complex of Drosophila ATRX/XNP and HP1A is required for the formation of pericentric beta-heterochromatin in vivo. J. Biol. Chem. 2010;285:15027–15037. doi: 10.1074/jbc.M109.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsukage A, Hirose F, Yoo MA, Yamaguchi M. The DRE/DREF transcriptional regulatory system: a master key for cell proliferation. Biochim. Biophys. Acta. 2008;1779:81–89. doi: 10.1016/j.bbagrm.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Ida H, Yoshida H, Nakamura K, Yamaguchi M. Identification of the Drosophila eIF4A gene as a target of the DREF transcription factor. Exp. Cell Res. 2007;313:4208–4220. doi: 10.1016/j.yexcr.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, Shin MJ, Yang DJ, Yamaguchi M, Park SY, Yoo MA. Transcriptional regulation of the Drosophila ANT gene by the DRE/DREF system. Genes Cells. 2007;12:569–579. doi: 10.1111/j.1365-2443.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- 23.Suyari O, Ida H, Yoshioka Y, Kato Y, Hashimoto R, Yamaguchi M. Identification of the Drosophila Mes4 gene as a novel target of the transcription factor DREF. Exp. Cell Res. 2009;315:1403–1414. doi: 10.1016/j.yexcr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Hochheimer A, Zhou S, Zheng S, Holmes MC, Tjian R. TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila. Nature. 2002;420:439–445. doi: 10.1038/nature01167. [DOI] [PubMed] [Google Scholar]
- 25.Hirose F, Ohshima N, Shiraki M, Inoue YH, Taguchi O, Nishi Y, Matsukage A, Yamaguchi M. Ectopic expression of DREF induces DNA synthesis, apoptosis, and unusual morphogenesis in the Drosophila eye imaginal disc: possible interaction with Polycomb and trithorax group proteins. Mol. Cell Biol. 2001;21:7231–7242. doi: 10.1128/MCB.21.21.7231-7242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart CM, Cuvier O, Laemmli UK. Evidence for an antagonistic relationship between the boundary element-associated factor BEAF and the transcription factor DREF. Chromosoma. 1999;108:375–383. doi: 10.1007/s004120050389. [DOI] [PubMed] [Google Scholar]
- 27.Nicolai M, Lasbleiz C, Dura JM. Gain-of-function screen identifies a role of the Src64 oncogene in Drosophila mushroom body development. J. Neurobiol. 2003;57:291–302. doi: 10.1002/neu.10277. [DOI] [PubMed] [Google Scholar]
- 28.Hinz U, Giebel B, Campos-Ortega JA. The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell. 1994;76:77–87. doi: 10.1016/0092-8674(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 29.Hirose F, Yamaguchi M, Handa H, Inomata Y, Matsukage A. Novel 8-base pair sequence (Drosophila DNA replication-related element) and specific binding factor involved in the expression of Drosophila genes for DNA polymerase alpha and proliferating cell nuclear antigen. J. Biol. Chem. 1993;268:2092–2099. [PubMed] [Google Scholar]
- 30.Valadez-Graham V, Razin SV, Recillas-Targa F. CTCF-dependent enhancer blockers at the upstream region of the chicken alpha-globin gene domain. Nucleic Acids Res. 2004;32:1354–1362. doi: 10.1093/nar/gkh301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguilar-Fuentes J, Fregoso M, Herrera M, Reynaud E, Braun C, Egly JM, Zurita M. p8/TTDA overexpression enhances UV-irradiation resistance and suppresses TFIIH mutations in a Drosophila trichothiodystrophy model. PLoS Genet. 2008;4:e1000253. doi: 10.1371/journal.pgen.1000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demczuk S, Harbers M, Vennström B. Identification and analysis of all components of a gel retardation assay by combination with immunoblotting. Proc. Natl Acad. Sci. USA. 1993;90:2574–2578. doi: 10.1073/pnas.90.7.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamakaka RT, Kadonaga JT. The soluble nuclear fraction, a highly efficient transcription extract from Drosophila embryos. Methods Cell Biol. 1994;44:225–235. doi: 10.1016/s0091-679x(08)60916-4. [DOI] [PubMed] [Google Scholar]
- 34.Leclerc V, Tassan JP, O'Farrell PH, Nigg EA, Leopold P. Drosophila Cdk8, a kinase partner of cyclin C that interacts with large subunit of RNA polymerase II. Mol. Biol. Cell. 1996;7:505–513. doi: 10.1091/mbc.7.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iltzsch MH, Bieber D, Vijayasarathy S, Webster P, Zurita M, Ding JZ, Mansour TE. Cloning and characterization of the cDNA coding for the alpha subunit of a stimulatory G-protein from Schistosoma mansoni, J. Biol. Chem. 1992;267:14504–14508. [PubMed] [Google Scholar]
- 36.Cross DP, Sang JH. Cell culture of individual Drosophila embryos. I. Development of wild type cultures. J. Embryol. Exp. Morphol. 1978;45:161–172. [PubMed] [Google Scholar]
- 37.Jiménez G, Ish-Horowicz D. A chimeric enhancer-of-split transcriptional activator drives neural development and achaete-scute expression. Mol. Cell. Biol. 1997;17:4355–4362. doi: 10.1128/mcb.17.8.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura K, Ida H, Yamaguchi M. Transcriptional regulation of the Drosophila moira and osa genes by the DREF pathway. Nucleic Acids Res. 2008;36:3905–3915. doi: 10.1093/nar/gkn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peña-Rangel MT, Rodríguez I, Riesgo-Escovar JR. A misexpression study examining dorsal thorax formation in Drosophila melanogaster. Genetics. 2002;160:1035–1050. doi: 10.1093/genetics/160.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heitzler P, Haenlin M, Ramain P, Calleja M, Simpson P. A genetic analysis of pannier, a gene necessary for viability of dorsal tissues and bristle positioning in Drosophila. Genetics. 1996;143:1271–1286. doi: 10.1093/genetics/143.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fromental-Ramain C, Vanolst L, Delaporte C, Ramain P. pannier encodes two structurally related isoforms that are differentially expressed during Drosophila development and display distinct functions during thorax patterning. Mech. Dev. 2008;125:43–57. doi: 10.1016/j.mod.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez AD, Shi W, Wilson BA, Skeath JB. pannier and pointed P2 act sequentially to regulate Drosophila heart development. Development. 2003;130:3015–3026. doi: 10.1242/dev.00488. [DOI] [PubMed] [Google Scholar]
- 43.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 44.Kawamori A, Yamaguchi M. DREF is critical for Drosophila bristle development by regulating endoreplication in shaft cells. Cell Struct. Funct. 2011;36:103–119. doi: 10.1247/csf.11004. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita D, Sano Y, Adachi Y, Okamoto Y, Osada H, Takahashi T, Yamaguchi M, Osumi T, Hirose F. hDREF regulates cell proliferation and expression of ribosomal protein genes. Mol. Cell Biol. 2007;27:2003–2013. doi: 10.1128/MCB.01462-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thacker SA, Bonnette PC, Duronio RJ. The contribution of E2F-regulated transcription to Drosophila PCNA gene function. Curr. Biol. 2003;13:53–58. doi: 10.1016/s0960-9822(02)01400-8. [DOI] [PubMed] [Google Scholar]
- 47.Hyun J, Jasper H, Bohmann D. DREF is required for efficient growth and cell cycle progression in Drosophila imaginal discs. Mol. Cell Biol. 2005;25:5590–5598. doi: 10.1128/MCB.25.13.5590-5598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohshima N, Takahashi M, Hirose F. Identification of a human homologue of the DREF transcription factor with a potential role in regulation of the histone H1 gene. J. Biol. Chem. 2003;278:22928–22938. doi: 10.1074/jbc.M303109200. [DOI] [PubMed] [Google Scholar]
- 49.Wong LH, McGhie JD, Sim M, Anderson MA, Ahn S, Hannan RD, George AJ, Morgan KA, Mann JR, Choo KH. ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 2010;20:351–360. doi: 10.1101/gr.101477.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication- independent chromatin assembly at telomeres. Proc. Natl Acad. Sci. USA. 2010;107:14075–14080. doi: 10.1073/pnas.1008850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isogai Y, Keles S, Prestel M, Hochheimer A, Tjian R. Transcription of histone gene cluster by differential core-promoter factors. Genes Dev. 2007;21:2936–2949. doi: 10.1101/gad.1608807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Escamilla-Del-Arenal M, Recillas-Targa F. GATA-1 modulates the chromatin structure and activity of the chicken alpha-globin 3' enhancer. Mol. Cell. Biol. 2008;28:575–586. doi: 10.1128/MCB.00943-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.