Abstract
Epidemiological studies have revealed an inverse association between Helicobacter pylori infection and the incidence of allergic asthma. This association is consistent with the hygiene hypothesis, which posits that exposure to microbes early in life prevents the later development of allergic diseases, and has been reproduced in mouse models of asthma. In this issue of the JCI, Oertli and colleagues report that H. pylori infection in neonates elicits tolerogenic DCs that produce IL-18, which drive the generation of Tregs that subsequently protect the mice from allergic asthma. This finding strengthens the intriguing link between pathogen exposure and allergic disease.
Nearly half of the world’s population is infected with the Gram-negative bacterium Helicobacter pylori. Infection rates are higher in developing countries than in developed countries; in fact, the bacterium is gradually disappearing from many populations in developed countries (1). Infection seems to occur in early childhood in most cases, with the incidence of infection increasing with age. German scientists first observed the spiral-shaped bacterium in the lining of the human stomach in 1875 (2). However, Australian scientists, Marshall and Warren, were the first to culture this bacterium in 1984, and they went on to link the presence of H. pylori in the gut to inflammation in the stomach (gastritis) and ulceration of the stomach or duodenum (peptic ulcer disease) (3). Marshall and Warren were awarded the 2005 Nobel Prize in Physiology or Medicine for this finding. H. pylori infection has been subsequently associated with gastric cancer, mucosa-associated tissue lymphoma, gastroesophageal reflux disease, and iron deficiency anemia (4).
The flip side of the equation is that, in an epidemiological study, Chen and Blaser reported an inverse association between infection with H. pylori expressing the virulence factor cytotoxin-associated protein A (CagA) and the incidence of asthma and allergy (5). This observation is consistent with the hygiene hypothesis, which states that exposure to microbes (both pathogenic and commensal) early in life prevents the later development of allergic diseases (6). The basis for this phenomenon is unclear, but, recently, Müller and colleagues demonstrated that H. pylori infection prevents allergic asthma in mouse models of the condition through the induction of Tregs (7).
The logical question raised by this discovery was what is the mechanism underlying the induction of Tregs in mice infected with H. pylori? In this issue of the JCI, Oertli, Müller, and colleagues provide insight into this process (8). Specifically, they show that DCs exposed to H. pylori are programmed to become tolerogenic, driving Treg differentiation and thereby protection from asthma, through the production of IL-18. This study suggests novel cellular and molecular pathways regulating allergic diseases in individuals infected with H. pylori. However, there remain many unanswered questions regarding the underlying mechanisms of H. pylori infection–associated asthma protection.
H. pylori–conditioned tolerogenic DCs
DCs, originally discovered by Steinman and Cohn in 1973 (9), are now well established to be the critical antigen-presenting cells responsible for activating naive T cells. DCs link the innate and acquired immune systems by detecting and responding to danger signals, such as those on invading pathogens. DCs respond to danger signals by endocytosing local antigens, which they then degrade, generating antigenic peptides that form complexes with MHC molecules that are then expressed on the DC surface in order to initiate antigen-specific T cell responses. DCs also respond to danger signals by undergoing a process known as maturation, during which they begin to express the chemokine receptor CCR7, which enables them to migrate, in a chemokine-dependent manner, to the lymph nodes draining the site of antigen encounter.
In addition to initiating the adaptive immune response to invading pathogens, DCs also continuously present self antigens, in the context of MHC molecules, to promote self tolerance (the lack of immune responsiveness to self antigens). Interestingly, certain helminth and bacterial pathogens, including Fasciola hepatica, Candida albicans, Schistosoma japonicum, Schistosoma mansoni, Bordetella pertussis, and Vibrio cholerae, are known to promote DC tolerogenicity by producing TGF-β and IL-10 mimetics or by inducing host DCs to produce these cytokines and consequently drive Treg induction to modulate the host immune response (10).
The effects of H. pylori infection described by Oertli et al. (8) are mediated by pathogen-induced tolerogenic DCs. In vitro exposure of mouse bone marrow–derived DCs to H. pylori induced a population of DCs that were unable to activate T cells. Comparable populations of DCs were present in the mesenteric lymph nodes and lamina propria (the thin layer of loose connective tissue that lies beneath mucosal epithelia, which contains lymphoid tissues and subsets of leukocytes) of H. pylori–infected mice and patients, respectively.
Oertli et al. went on to show that direct contact between H. pylori and DCs was essential for the induction of tolerogenic DCs (8). In investigating the molecular basis for these events, they determined that neither the host molecules TLR2 and MyD88 (a key intracellular signal transduction molecule for TLRs) nor the H. pylori type IV secretion system (which delivers CagA into epithelium) were involved. As no evidence of a role for the TLR family of pathogen-sensing receptors in DC recognition of H. pylori was found by Oertli et al., it is possible that one or more members of the nod-like receptor (NLR) family of pathogen-sensing receptors is involved. In this context, Watanabe et al. have shown that γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP; a cell wall peptidoglycan derivative from H. pylori) activates DCs in a manner dependent on nucleotide-binding oligomerization–domain containing 1 (Nod1, also known as NLRC1) when added to culture medium and also induces type I IFN and, eventually, IL-10 through the Nod1-TBK1-IRF7 pathway in human epithelial cell lines (11). Future studies should examine whether this pathway is also involved in H. pylori induction of IL-18–producing tolerogenic DCs.
Treg induction by DC-derived IL-18
The role of IL-18 in allergic diseases is complicated. IL-18 is produced mainly by macrophages and epithelial cells in a caspase-1–dependent manner (12). IL-18 was first identified based on its ability, in combination with IL-12, to induce the production of IFN-γ by T cells and other leukocytes. Therefore, coadministration of IL-18 and IL-12 induces potent antiallergic activity. On the other hand, IL-18 in combination with IL-2 increases the expression of CD40L on CD4+ T cells and, in the absence of antigen, can also induce the production of Th2 cytokines such as IL-4 and IL-13, which are key contributors to the pathogenesis of asthma (13).
One of the most important observations made in the study by Oertli et al. (8) is that DC-derived IL-18 plays a central role in the conversion of naive T cells to Tregs that possess potent inhibitory activity against Th2 responses. Cytokine signals have been implicated in the generation of different classes of Tregs, just as they have been found to guide the generation of different subsets of helper T cells. For example, under Th1 inflammatory conditions, IFN-γ induces the expression of the transcription factor T-bet in FoxP3+ Tregs, optimizing these cells for the suppression of Th1 responses (14). On the other hand, Tregs in the periphery express high levels of IFN regulatory factor-4 (IRF4), which is required for these cells to be able to suppress Th2 responses (15). It is unclear at this stage whether IL-18 induces general Treg populations or specifically induces Tregs optimized for suppressing Th2 responses, which are key to the pathogenesis of asthma. Therefore, it will be very important to define how IL-18–mediated signals regulate the activation of FoxP3, the master regulator of Treg induction (16), and to define the effect of IL-18 on the signaling pathways that determine Treg heterogeneity.
Tolerogenic signals from the gut to the airways
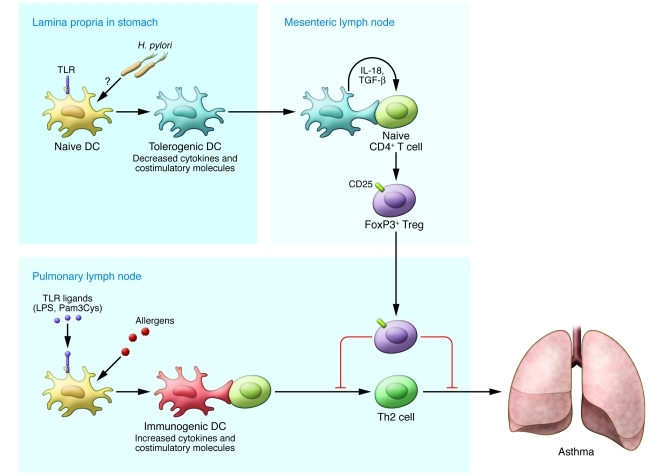
One of the biggest puzzles raised by the work of Oertli et al. is how H. pylori in the mucus of the stomach influences DCs in the lamina propria. It has been reported that H. pylori is able to sense the pH gradient within the mucus layer, moving away from the acidic lumen toward the more neutral pH environment of the epithelial cell surface, even invading the gastric mucosa and translocating to the gastric lymph nodes (17, 18). However, it remains unclear how efficiently live H. pylori migrate to the lamina propria and the draining lymph nodes, in which they may regulate the function of DCs. Ito et al. have shown that H. pylori are mainly phagocytosed in the lamina propria and translocated into the gastric lymph nodes by macrophages, but not by DCs, in the induction of gastritis (19). At the same time, there are a number of reports suggesting that Peyer’s patches (aggregations of lymphoid tissue in the small intestine) are the sites of T cell priming and effector T cell generation in H. pylori infection (20, 21). In a clinical setting, H. pylori infection only occurs in the stomach. It seems unlikely that DCs in the lamina propria of the stomach egress to the airways via the lymphatics and circulation. Although this fact highlights a shortcoming of the model used by Oertli et al. (8), in which H. pylori–conditioned DCs were administered intranasally, their study represents important proof of the concept that H. pylori–exposed DCs become tolerogenic and provide protection from allergic disease. However, important questions remain about how events in the gut affect the lungs. As illustrated in Figure 1, we believe that antigen-independent systemic surveillance by Tregs generated in the mesenteric lymph nodes draining the H. pylori–infected gut represents the most plausible mechanism for how this could occur.
Figure 1. H. pylori–exposed tolerogenic DCs drive naive CD4+ T cells to become Tregs.
DCs in the airways capture allergens, process antigens, and migrate into pulmonary lymph nodes, in which they initiate antigen-specific Th2-skewed immune responses, which ultimately cause asthma. When H. pylori infection occurs in neonates, H. pylori–exposed DCs in the lamina propria of the gut display impaired maturation, resulting in tolerogenic DCs. IL-18 derived from these DCs acts directly on naive CD4+ T cells in gut regional lymph nodes (mesenteric lymph nodes), causing them to become Tregs that subsequently suppress asthmatic immune responses in the airways.
Why is neonatal infection so critical?
As demonstrated in a paper from Müller and colleagues (7), asthma is strongly reduced in mice infected with H. pylori neonatally but not in mice infected during adulthood. In neonatally infected mice, the reduction in asthma was accompanied by an increase in Tregs; in mice infected during adulthood, numbers of Tregs remained unchanged. These observations raise the question, why do Tregs dominate in H. pylori infection in neonates? Given the data from Oertli et al. (8), this phenomenon could be due to a difference in IL-18 production between H. pylori–programmed DCs in neonates and adults. However, it could also be due to a barrier difference between the guts of neonates and adults. Although the finding that asthma is strongly reduced in mice infected with H. pylori neonatally but not in those infected during adulthood is consistent with the epidemiologic data indicating that H. pylori infection occurs early in life, its mechanistic basis remains unclear.
DC-derived IL-18 as a potential therapeutic target
In summary, the study by Oertli, Müller, and colleagues (8) has extended our understanding of the molecular and cellular basis of the hygiene hypothesis in the context of H. pylori infection. They have revealed that DCs exposed to H. pylori are destined to become tolerogenic, driving Treg differentiation through IL-18 production and thereby inducing protection from allergic asthma. If DC-derived IL-18 is found to be generally involved in Treg induction in vivo, targeting IL-18 as a means of Treg regulation could present an attractive strategy for the prevention and treatment of the various human diseases in which Treg function is dysregulated, which include autoimmune diseases and cancer as well as asthma.
Acknowledgments
We are very grateful to Francis H.W. Shand for reviewing the manuscript and providing helpful comments.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(3):801–804. doi:10.1172/JCI61466
See the related article beginning on page 1082.
References
- 1.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284(5418):1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ. An endangered species in the stomach. Sci Am. 2005;292(2):38–45. doi: 10.1038/scientificamerican0205-38. [DOI] [PubMed] [Google Scholar]
- 3.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 4.Superbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167(8):821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 6.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold IC, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. . J Clin Invest. 2011;121(8):3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oertli M, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori–specific immune tolerance, and asthma protection. . J Clin Invest. 2012;122(3):1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. . J Exp Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe T, et al. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest. 2010;120(5):1645–1662. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y, et al. Activation of Interferon-gamma inducing factor mediated by Interleukin-1beta converting enzyme. Science. 1997;275(5297):206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto T, et al. Nonredundant roles for CD1d-restricted natural killer T cells and conventional CD4+ T cells in the induction of immunoglobulin E antibodies in response to interleukin 18 treatment of mice. J Exp Med. 2003;197(8):997–1005. doi: 10.1084/jem.20021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF-4 to control Th2 responses. Nature. 2009;458(7236):351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber S, et al. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc Natl Acad Sci U S A. 2004;101(14):5024–5029. doi: 10.1073/pnas.0308386101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen AM, Krogfelt KA. Helicobacter pylori: an invading microorganism? A review. FEMS Immunol Med Microbiol. 2003;36(3):117–126. doi: 10.1016/S0928-8244(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, et al. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab Invest. 2008;88(6):664–681. doi: 10.1038/labinvest.2008.33. [DOI] [PubMed] [Google Scholar]
- 20.Nagai S, et al. Role of Peyer’s patches in the induction of Helicobacter pylori-induced gastritis. Proc Natl Acad Sci U S A. 2007;104(21):8971–8976. doi: 10.1073/pnas.0609014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiriya K, et al. Essential role of Peyer’s patches in the development of Helicobacter-induced gastritis. Int Immunol. 2007;19(4):435–446. doi: 10.1093/intimm/dxm008. [DOI] [PubMed] [Google Scholar]