Summary
Posterior cerebral aneurysms are rare vascular lesions and usually present as non-saccular or dissecting in nature. We present a retrospective review of our experience in the deliberate parent artery occlusion of posterior cerebral artery (PCA) aneurysms.
From June 2006 to June 2010, 12 patients (seven men, five women) with posterior cerebral artery non-saccular aneurysms presented to our department and were treated by parent artery occlusion. There were eight (66.7%) aneurysms located at the P2 segment, two (16.7%) at the P2-3 junction, one (8.3%) at the P1-2 junction and one (8.3%) at the P3 segment. Ten of the 12 patients were treated by aneurysm together with parent artery occlusion and two were treated by proximal occlusion.
The procedure was technically successful in all cases. Angiography was performed immediately after the procedure in all patients and showed occlusion of the parent vessel with no filling of the aneurysm. Only one patient (8.3%) developed procedure-related transient hemianopsia and recovered within one month. The other 11 patients showed no additional neurological symptoms after procedure.
Deliberate parent artery occlusion by detachable coils appears to be well tolerated for P2 or distal segment of PCA in our limited case series. We propose that this technique could be a good treatment option in treating non-saccular aneurysms in this location.
Key words: posterior cerebral artery, occlusion, aneurysm, embolization
Introduction
Posterior cerebral aneurysms are rare vascular lesions and usually present as nonsaccular or dissecting nature1-3. Neurosurgical management of these aneurysms is challenging even in most experienced hands because of their deep locations and richness of perforating branches. Endovascular treatment for these aneurysms is technically feasible and has become a less invasive alternative to the neurosurgical approach during the last decade. However, in some situations, an endovascular reconstructive approach is impossible due to the irregular morphology or dissecting nature of the lesions, and therefore a deconstructive approach becomes inevitable. We retrospectively reviewed our experiences of deliberate parent artery occlusion for 12 patients with posterior cerebral artery aneurysms.
Methods
From June 2006 to June 2010, 12 patients (seven men, five women) with posterior cerebral artery aneurysms presented to our department and were treated by parent artery occlusion. All of the aneurysms were non-saccular (serpentine, fusiform) or dissecting aneurysms and could not be easily treated by simple coiling. Patients’ ages ranged from 16 to 73 (mean age, 44 years), five patients presented with SAH, six with headache, three with hemiparesis, two with visual disturbances and one with memory loss.
The involved area of the PCA was classified into four segments according to Zeal and Rhoton (28): there were eight (66.7%) aneurysms located at the P2 segment, two (16.7%) at the P2-3 junction, one (8.3%) at the P1-2 junction and one (8.3%) at the P3 segment. The clinical and angiographic details of the 12 patients are summarized in Table 1.
Table 1.
Summary of data in the 12 patients with PCA aeneurysms.
| Pat. No. |
Age/ Sex |
Site | Type/ Size* |
Symptoms | H-H grade |
Treatment | Angiographic- outcome |
F/u (m) |
Clinical-angiographic follow-up finding |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31/M | P2/L | Serpentine/ large |
SAH | 1 | PAO | Total | 21 | GOS=5, no symptoms, no recanalization on angiography |
| 2 | 49/M | P2/R | Serpentine/ large |
Headaches hemiparesis (L) |
0 | PAO | Total | 17 | GOS=4, mild hemiparesis, but return to work. |
| 3 | 24/F | P2/R | Serpentine/ large |
Headaches hemiparesis (L) |
0 | PAO | Total | 2 | GOS≈5, the hemiparesis recovered, no recanalization on angiography |
| 4 | 47/M | P2/R | Dissecting/ large |
Headaches | 0 | PAO | Total | 25 | GOS=5, no symptoms |
| 5 | 54/F | P2/R | Fusiform/ small |
SAH, memory loss |
1 | PAO | Total | 13 | GOS=5, no symptoms |
| 6 | 39/M | P3/L | Serpentine/ large |
Headaches | 0 | PAO | Total | 6 | GOS=5, no symptoms, no recanalization on angiography |
| 7 | 46/F | P2-3 junction/ L |
Dissecting/ small |
Headaches | 0 | PAO | Total | 8 | GOS=5, no symptoms |
| 8 | 73/F | P2/L | Dissecting/ small |
SAH | 2 | PAO | Total | 7 | GOS=5, no symptoms |
| 9 | 56/M | P1-2 junction /L |
Dissecting/ small |
SAH, mild hemiparesis(R) |
2 | PAO | Total | 12 | GOS=5, no symptoms, the hemiparesis recovered. |
| 10 | 16/M | P2/R | Dissecting/ large |
SAH, hemianopsia |
1 | Proximal PAO |
Total | 6 | GOS=5, no symptoms, no recanalization on angiography |
| 11 | 44/M | P2-3/R | Dissecting/ small |
Blurred vision |
0 | PAO | Total | 4 | GOS≈5, transient hemianopsia but gradually recovered within 1 mo. |
| 12** | 50/F | P2/R | Dissecting/ large |
Headache | 1 | Proximal PAO |
Total | 6 | GOS=5, no symptoms, no recurrence after proximal PAO |
|
* Aneurysms were classified as large (> 10 mm) or small (<10 mm) according to the longitudinal diameter. ** This patient was treated by selective coiling for the first time, but 4 months later DSA showed enlargement of the aneurysm and compaction of the coil mass, then a proximal occlusion procedure was performed (Figure 3). Abbreviations: PAO parent artery occlusion; GOS Glasgow outcome scale; SAH subarachnoid hemorrhage; H-H grade Hunt-Hess grade; F/u follow-up. | |||||||||
All patients underwent PAO by coils. All of the procedures were performed under general anesthesia and systemic heparinization. In these cases, 5000 U of heparin were administered at the start of the procedure, followed by 1000 U every hour until completion. In all patients, a 6F femoral sheath was placed in the common femoral artery, and selective catheterization of the vertebral artery was performed. For patient 7, we performed an internal carotid artery approach instead because of her fetal PCA (case illustration). A microcatheter (Echelon 10/14, MTI-EV3, Irvine, CA, USA ) was introduced by a microguidewire (Silverspeed 10/14, MTI-EV3, Irvine, CA, USA) in a coaxial fashion through the guiding catheter, followed by selective catheterization of the artery with the aneurysm in each case. The tip of the microcatheter was placed in the aneurysm. We did not perform BOT in any of these patients before parent artery occlusion. Ten of the 12 patients were treated by aneurysmal lumen and parent artery occlusion, and the other two underwent proximal artery occlusion. Coils were selected based on the size of the aneurysms and the parent artery to be occluded. Angiography was performed after coil placement to confirm occlusion of the parent artery and the aneurysm. Heparinization was continued for 24 hours in all patients after the procedure. In this series, only one patient (case 11) developed procedure-related transient hemianopsia and gradually recovered within one month, the postoperative course of other patients was uneventful; these patients were discharged, on average, by postoperative day 4 (range, day 3 through day 6). Clinical follow-up ranged from two to 25 months (average, 10.3 months). Of the 12 patients, five had follow-up angiography.
Results
Immediate Results
Of the 12 aneurysms in our series, seven (59%) were dissecting, four (33%) were serpentine and one (8%) was fusiform. The aneurysms were classified as large ≥10 mm) in seven patients and small (<10 mm) in five patients according to the longitudinal diameter. The procedure was technically successful in all cases. Angiography was performed immediately after the procedure in all patients and showed occlusion of the parent vessel with no filling of the aneurysm. All patients had retrograde flow into the peripheral branches of the occluded artery via leptomeningeal collaterals, but in no case was there flow into the aneurysm itself. There were no device-related complications during the procedure.
Angiographic Results
Follow-up angiography was performed in five out of 12 patients from two to 25 months after embolization. In each of the five patients, the angiogram showed no residual filling of the aneurysm.
Clinical Results
The period of clinical follow-up by telephone was 10.3 months on average (range, two-25 months). During the follow-up period, no SAH occurred; one patient developed procedure-related hemianopsia and recovered within one month.
Illustrative Cases
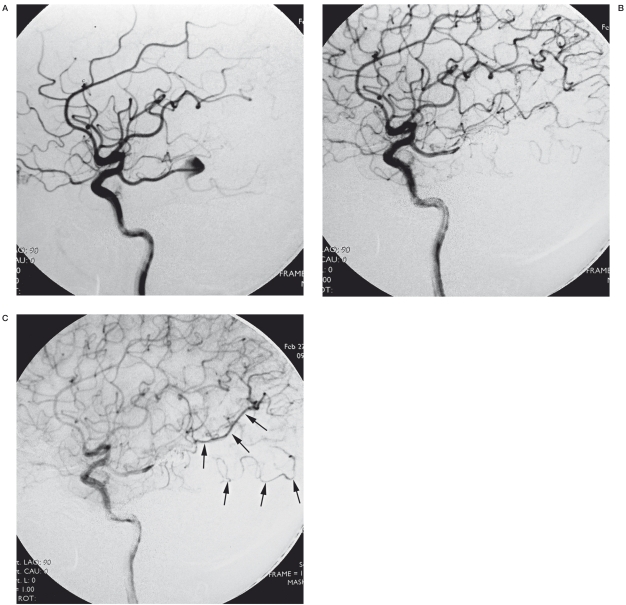
Case 7. A 46-year-old woman with chronic headache presented to our department (Figure 1). Computed tomographic scanning showed no special findings but the CTA revealed an aneurysm at the right PCA (not shown). Cerebral angiography confirmed the dissecting aneurysm involving the P2-3 junction and opacified from the right fetal posterior communicating artery. The patient was treated by coil occlusion of the aneurysm and parent PCA through an ICA-PcomA approach. From the postprocedural angiogram, we could find the retrograde flow through the leptomeningeal anastomosis to the PCA territory.
Figure 1.
Case 7. A) Right internal carotid artery injection angiogram showed the dissecting aneurysm located at the P2-3 junction of the fetal PCA. B) Angiogram after embolization of the aneurysm and the right PCA. C) Venous phase showed the retrograde flow of the leptomeningeal anastomosis (arrow).
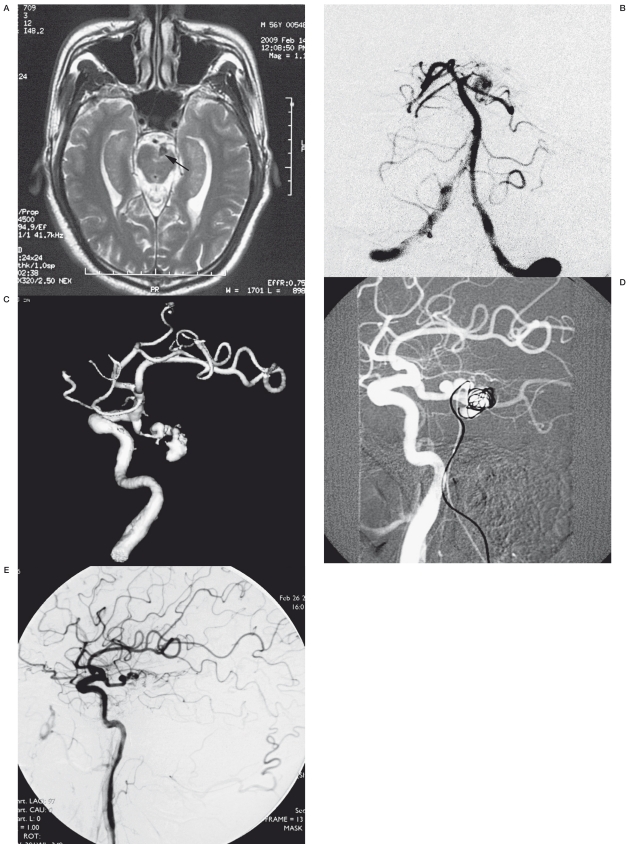
Case 9. A 56-year-old man presented with SAH and mild hemiparesis. MRI showed a flow void in front of the left peduncle. Cerebral angiogram showed the dissecting aneurysm at the junction of PcomA and the PCA, and the left P1 was aplastic. Because of the complex anatomical structure, we performed a bilateral femoral approach, a 6F guiding catheter was introduced into the left vertebral artery through the right femoral sheath for embolization and a 5F angiogram catheter was introduced into the left internal carotid artery through the left femoral sheath for road mapping and angiogram analysis. Post embolization angiogram showed complete occlusion of the aneurysm and the parent artery. The postprocedural cause was uneventful and the hemiparesis gradually recovered.
Case 10. A 16-year-old boy presented SAH and hemianopsia. The angiogram showed a large dissecting aneurysm at the left P2 segment. In the capillary phase, we could see poor opacification of the left PCA territory.
We performed proximal occlusion to treat this aneurysm.
The postprocedural angiogram showed excellent leptomeningeal anastomosis to the PCA territory without aneurysmal opacification. Follow-up angiogram showed no recurrence, and the hemianopsia recovered.
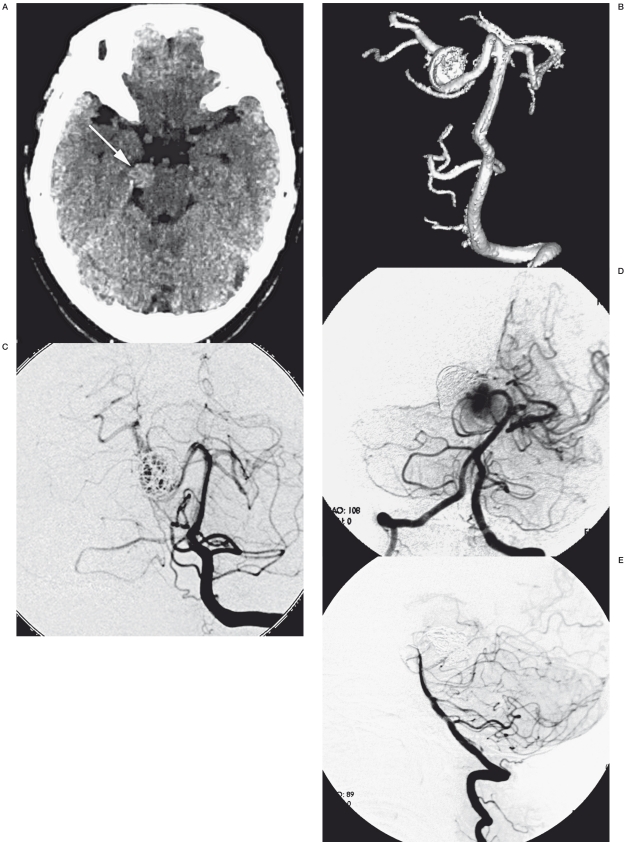
Case 12. A 50-year-old woman presenting migrainous headache came to our hospital for diagnosis and treatment. An enhanced CT showed a high intensity in the right ambient cistern. DSA was performed and showed a large dissecting aneurysm in the P2 segment. For the first time, we just performed endosaccular occlusion of the aneurysm with preservation of the right PCA. However, four months later the patient suffered a sudden onset of headache and vomiting. Although no SAH presented in the CT scan, the angiogram revealed the enlarged aneurysm and compact coil mass. We performed proximal occlusion for the second treatment. The patient tolerated the treatment well and was discharged three days after the procedure without any additional clinical symptoms.
Discussion
Anatomical Characteristics of PCA
According to Zeal and Rhoton4, the PCA is divided into four anatomical segments. Multiple collaterals exist at different segments of the PCA, which is the theoretical basis for parent artery occlusion treatment in our series. The medial posterior choroidal artery (MPChA) main trunk proceeds to the interventricular foramen and produces a lateral branch that joins the arterial system of the choroid plexus of the lateral ventricle and anastomoses to branches of the anterior choroidal artery (AChA) and lateral posterior choroidal arteries, and a medial branch has a recurrent course on the roof of the third ventricle, where it anastomoses with its counterpart of the opposite side; the long circumflex arteries of P1 segment and the superior cerebellar artery territory at quadrigeminal plate level; splenial branches of ACA and PCA (at P3-4 segments) and leptomeningeal collaterals from ACA and MCA4-7.
Characteristics of PCA Aneurysms
Posterior cerebral artery aneurysms are uncommon with an incidence less than 1%, but they are an important cause of SAH4,8,9. PCA aneurysms have some of their own specific clinical and morphologic features. They occur more frequently in young individuals with an average age of 38 years, as opposed to the 50-60 years for aneurysms at other sites of the intracranial circulation8. The mean age of this series was 44 years, a little older than previously reported. Morphologically, aneurysms in this location tend to be large or giant in size and exhibit a relatively high proportion of non-saccular types2,10-12. In addition, there was a predilection of dissecting aneurysms formed in this location2, which was explained by the hemodynamic shearing force of blood flow from the PcomAs or traumatic injury of the tentorial edge13. Of the 12 aneurysms in our series, seven (59%) were dissecting, four (33%) were serpentine and one (8%) was fusiform.
The most common presentation of PCA aneurysms described before is a subarachnoid hemorrhage (SAH)1,8,14. In our case series, five (5/12, 41.7%) of the 12 patients presented with aneurysmal rupture. Previous researchers2,5,13,15,16 found that the P2 segment was the most common location of an aneurysm, and this was the case in our series (8/12, 66.7%). In addition, three (25%) dissecting aneurysms were at the P1-2 junction or P2-3 junction, which we thought related to the hemodynamic shearing force. Large aneurysms in this location can present with symptoms of mass effect on the adjacent brain structure and result in neurological deficits. Three patients in our series without SAH presented hemiparesis. We assume that was because of the mass effect and surrounding brain parenchymal edema. Memory deficit in our studies may be caused by involvement of hypothalamic pathways entering and exiting from the mamillary bodies or compression of the parahippocampal gyrus. However, this might also be the sequelae of SAH or ischemic complications caused by perforators’ occlusion. Pia and Fontana3 reported a 27% incidence of visual disturbances in patients harboring PCA aneurysms, with a prevalence of oculomotor palsy and hemianopsia. In our series, two patients (17%) had visual disturbances: one with a large P2 dissecting aneurysm and the other with a P2-3 junction dissecting aneurysm. The mechanisms of visual disturbances were postulated as ischemic events resulting from arterial dissection or compression of the adjacent optic tract.
Interventional Treatment of PCA Aneurysms
Neurosurgical treatment of PCA aneurysms is an extremely challenging task even in the most experienced hands. Surgical complications associated with management of PCA aneurysms include retraction injury to the temporal, occipital, or parietal lobes and oculomotor nerve, occlusion of thalamoperforating arteries, and cerebral infarction secondary to prolonged temporary occlusion of vessels8,17-19. In contrast, the endovascular approach is relatively easier and not associated with manipulation of the surrounding tissues. In addition, the development of interventional materials and the accumulated experiences make it far safer than before. There were no device-related complications in this series.
Endovascular treatment varies depending on the nature of the aneurysm (saccular, fusiform or dissecting) and its location along the different anatomical segments of the PCA. The primary goal of endovascular treatment of PCA aneurysms is still isolation of the aneurysms and preservation of the parent artery. Several techniques have been described for achieving this purpose, including endosaccular coiling, balloon remodeling technique and stent assisted coiling3,20. Adjunctive techniques make it possible to occlude wide neck or fusiform aneurysms with patency of the parent artery. However, for certain kinds of aneurysms as illustrated in our series, preservation of the parent artery seemed impossible, and the combined occlusion of the sac and parent artery appears to be the only viable option by endovascular means. Patient 12 in our series was treated by endosaccular coiling with the parent artery preserved for the first time, but the four-month follow-up DSA showed the aneurysm became obviously larger than before. The dissecting nature of this aneurysm conveys that the entire vessel is diseased. Without parent vessel sacrifice, this portion of the vessel may represent a nidus for recurrence2,13,21. Therefore, for large non-saccular or dissecting aneurysms, a reconstructive approach might not be a judicious option, exposing the patient to the risk of SAH.
In recent years, the so-called “flow-diverting devices” such as the Pipeline Embolization Device (PED, Chestnut Medical Technologies, Inc., Menlo Park, CA, USA) or several kinds of covered stents have emerged. These devices provide a technique that could occlude the aneurysm while maintaining the patency of the parent artery. Several fundamental clinical studies6,22,23 have been published to confirm the effectiveness of these devices. However, the essence of all these devices is stenting. To deliver a stent into a target vessel, a microguidewire must be advanced across the aneurysm into the distal segment of the parent artery at first. If the guidewire could not go through the aneurysm or the target vessel was too tortuous, then the stent could not be deployed to cover the aneurysm neck. In our case series, the dissecting or serpentine nature of the aneurysm limited the application of these devices. Furthermore, the experience of using these kinds of devices in peripheral arteries is limited.
Hallacq et al.15 have shown their preferable results of proximal P2 aneurysms treated safely with parent vessel sacrifice. In their series, endovascular occlusion of the parent artery at the level of the aneurysmal neck was the aim. Lazinski et al.13 reported six PCA dissecting aneurysms, three of them were treated by occlusion of the parent PCA and received favorable clinical and angiographic outcome. According to our experience, for some large dissecting aneurysms, only proximal occlusion might also be effective, without the risk of device-related aneurysmal perforation. Our cases 10 and 12 with large dissecting aneurysms were treated mainly by proximal artery occlusion; the results were excellent without residual opacification from collateral anastomosis. We postulated that was because there was already blood flow stagnation in large aneurysm lumen10,21, simply prevention of the antegrade flow could result in intraaneurysmal thrombosis.
Some researchers8 consider that surgical revascularization is necessary. Based on our studies, we believe surgical revascularization is not always warranted in distal PCA aneurysms, although it may be combined with parent artery occlusion. The morbidity rate of homonymous hemianopsia developing after proximal P2 occlusion in a patient is 4.5% to 14%1,14,15,18. Distal bypass followed by endovascular coiling or parent vessel sacrifice is not an ideal treatment because it carries the dual risk of thromboembolic events related to endovascular navigation and risks associated with the bypass itself25. Because anticoagulation is used to minimize endovascular risks, the risks of epidural hematoma and other bleeding events related to the bypass procedure increase. However, ischemic complications do exist. Accordingly, preservation of the parent artery is still one of the primary goals for the endovascular treatment of PCA saccular aneurysms.
Several investigators have reported that proximal ligation or trapping of a PCA aneurysm can be performed without revascularization7. Parent artery occlusion resulted in 100% occlusion of all aneurysms in the absence of a revascularization process in our small series; the initial clinical status was the only factor that influenced the prognosis. Only one patient (1/12, 8%) developed procedure-related transient hemianopsia after the PAO procedure and gradually recovered within one month. This result compares favorably with previously published surgical and endovascular series12,18. Analysis of the angiograms showed that after the point of occlusion, the distal temporal branches are still opacified because of sump aspirators for flow, allowing revascularization of the distal PCA via a leptomeningeal supply11,15,18.
We did not consider test occlusion before permanent occlusion in these patients, because occlusion of the PCA at the level of the P2 is generally well-tolerated by virtue of a rich collateral arterial network (case illustration, Figures 1 and 3). Moreover, it is technically impossible for our 12 patients using the endovascular reconstructive approach due to the irregular morphology of the aneurysms.
Figure 2.
Case 9. A) A T2-weighted MRI showed a flow void at the basil cistern with compression of the left peduncle. B) Left vertebral injection showed the dissecting aneurysm and aplastic P1 segment. C) Left carotid artery injection showed the aneurysm was opacified better from anterior circulation. D) Trans-vertebral embolization under the roadmap from LICA injection. E) Postprocedural angiogram showed the aneurysm were completely occluded
Figure 3.
Case 12. Av CT scan showed relative high-intensity at the right basal cistern. B) A 3D angiogram showed the large aneurysm at the right PCA. C) Endosaccular palliative embolization of the aneurysm with the patency of the parent PCA. D) 4 months later, another angiogram showed the enlarged aneurysm and compaction of the coil mass. E) Angiogram showed the proximal embolization of the parent artery and no residual flow to the aneurysm.
Therefore, we suggest that deliberate parent artery occlusion become the first treatment option of non-saccular or dissecting aneurysms of the PCA.
Even for the wide-necked saccular aneurysm at the distal segment of the PCA, the strategy could also be a viable alternative to the adjunctive techniques. This strategy is based on our experience and the following reasons: the high risk of alternative reconstructive surgery in distal PCA7,11,15,23-29, the relative lack of data on the use of balloon or stent assistance in distal vessels, and finally and most critically, the data in the surgical literature suggesting that there is no difference in outcome whether the parent artery is preserved during the treatment of aneurysms in this arterial territory19,20. However, theoretically, this strategy could not be applied for proximal P1 segment aneurysms due to the glomerate perforators to the mesencephalon and diencephalon.
Conclusion
Parent artery occlusion by detachable coils was well-tolerated for P2 or the distal segment of the PCA in our limited case series. This technique has clinical significance especially for the non-saccular aneurysms at the distal segment of the PCA. For the wide-necked saccular aneurysms, this technique could be a viable alternative to the adjunctive techniques. Further investigation is required in future clinical practice.
References
- 1.Ciceri EF, Klueznik RP, Grossman RG, et al. Aneurysms of the posterior cerebral artery: classification and endovascular treatment. Am J Neuroradiol. 2001;22:27–34. [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan LR, Estol CJ, Massaro AR. Dissection of the posterior cerebral arteries. Arch Neurol. 2005 Jul;62(7):1138–1143. doi: 10.1001/archneur.62.7.1138. [DOI] [PubMed] [Google Scholar]
- 3.Pia HW, Fontana H. Aneurysms of the posterior cerebral artery. Locations and clinical pictures. Acta Neurochir (Wien) 1977;38(1-2):13–35. doi: 10.1007/BF01401541. [DOI] [PubMed] [Google Scholar]
- 4.Zeal AA, Rhoton AL., Jr. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg. 1978;48:534–559. doi: 10.3171/jns.1978.48.4.0534. [DOI] [PubMed] [Google Scholar]
- 5.Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;(87) 2:141–162. doi: 10.3171/jns.1997.87.2.0141. [DOI] [PubMed] [Google Scholar]
- 6.Lasjaunias P, Berenstein A. In: Clinical vascular anatomy and variations. Vol 1. Berlin: Springer Verlag; 2001. Surgical neuroangiography; pp. 548–577. [Google Scholar]
- 7.Sherman P, Oka M, Aldrich E, et al. Isolated posterior cerebral artery dissection: report of three cases. Am J Neuroradiol. 2006;27(3):648–652. [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrante L, Acqui M, Trillò G, et al. Aneurysms of the posterior cerebral artery: do they present specific characteristics? Acta Neurochir (Wien) 1996;138(7):840–852. doi: 10.1007/BF01411263. [DOI] [PubMed] [Google Scholar]
- 9.Roh HG, Kim SS, Han H, et al. Endovascular treatment of posterior cerebral artery aneurysms using detachable coils. Neuroradiology. 2008 Mar;50(3):237–242. doi: 10.1007/s00234-007-0321-2. Epub 2007 Nov 13. [DOI] [PubMed] [Google Scholar]
- 10.Fanning AN, Kelleher MO, Ryder DQ. The Pretzeler sign: angiographic pattern of tortuous intraaneurysmal blood flow in a giant serpentine aneurysm. Br J Neurosurg. 2003;17(1):67–71. [PubMed] [Google Scholar]
- 11.Lv X, Li Y, Liu A, et al. Endovascular treatment of intracranial giant serpentine aneurysms. Neuroradiol J. 2007;20(2):237–241. doi: 10.1177/197140090702000220. [DOI] [PubMed] [Google Scholar]
- 12.Taylor CL, Kopitnik TA, Jr, Samson DS, et al. Treatment and outcome in 30 patients with posterior cerebral artery aneurysms. J Neurosurg. 2003; 99(1):15–22. doi: 10.3171/jns.2003.99.1.0015. [DOI] [PubMed] [Google Scholar]
- 13.Lazinski D, Willinsky R, terBrugge KG, et al. Dissecting aneurysms of the posterior cerebral artery: angioarchitecture and a review of the literature. Neuroradiology. 2000;42:128–133. doi: 10.1007/s002340050031. [DOI] [PubMed] [Google Scholar]
- 14.Cloft HJ, Kallmes DF, Jensen ME, et al. Endovascular treatment of ruptured, peripheral cerebral aneurysms: parent artery occlusion with short Guglielmi detachable coils. Am J Neuroradiol. 1999;20(2):308–310. [PMC free article] [PubMed] [Google Scholar]
- 15.Hallacq P, Poitin M, Moret J. Endovascular occlusion of the posterior cerebral artery for the treatment of P2 segment aneurysms: retrospective review of a 10-year series. Am J Neuroradiol. 2002;23:1128–1136. [PMC free article] [PubMed] [Google Scholar]
- 16.Kocaeli H, Chaalala C, Abruzzo TA, et al. Results of surgical management for posterior cerebral artery aneurysms: 7-year experience in the endovascular era. Acta Neurochir (Wien) 2009;151(12):1583–1591. doi: 10.1007/s00701-009-0405-3. [DOI] [PubMed] [Google Scholar]
- 17.Eckard DA, O’Boynick PL, McPherson CM, et al. Coil occlusion of the parent artery for treatment of symptomatic peripheral intracranial aneurysms. Am J Neuroradiol. 2000;21:137–142. [PMC free article] [PubMed] [Google Scholar]
- 18.Hamada J, Morioka M, Yano S, et al. Clinical features of aneurysms of the posterior cerebral artery: a 15-year experience with 21 cases. Neurosurgery. 2005;56(4):662–670. doi: 10.1227/01.neu.0000156199.53041.32. [DOI] [PubMed] [Google Scholar]
- 19.Parkinson RJ, Eddleman CS, Batjer HH, et al. Giant intracranial aneurysms: endovascular challenges. Neurosurgery. 2008;62(6) Suppl 3:1336–1345. doi: 10.1227/01.neu.0000333798.67209.1f. [DOI] [PubMed] [Google Scholar]
- 20.Rooij WJ, Sluzewski M, Beute GN. Endovascular treatment of posterior cerebral artery aneurysms. Am J Neuroradiol. 2006;27(2):300–305. [PMC free article] [PubMed] [Google Scholar]
- 21.Sari A, Kandemir S, Kuzeyli K, et al. Giant serpentine aneurysm with acute spontaneous complete thrombosis. Am J Neuroradiol. 2006;27(4):766–768. [PMC free article] [PubMed] [Google Scholar]
- 22.Rasskazoff S, Silvaggio J, Brouwer PA, et al. Endovascular treatment of a ruptured blood blister-like aneurysm with a flow-diverting stent. Interventional Neuroradiology. 2010;16(3):255–258. doi: 10.1177/159101991001600304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zicherman J, Roychowdhury S, Demarco JK, et al. Endovascular treatment of a ruptured giant serpentine aneurysm of the superior cerebellar artery in a patient with a Chiari II malformation. Am J Neuroradiol. 2004;25:1077–1079. [PMC free article] [PubMed] [Google Scholar]
- 24.Arat A, Islak C, Saatci I, et al. Endovascular parent artery occlusion in large-giant or fusiform distal posterior cerebral artery aneurysms. Neuroradiology. 2002;4:700–705. doi: 10.1007/s00234-002-0747-5. [DOI] [PubMed] [Google Scholar]
- 25.Chang SW, Abla AA, Kakarla UK, et al. Treatment of distal posterior cerebral artery aneurysms: a critical appraisal of the occipital artery-to-posterior cerebral artery bypass. Neurosurgery. 2010;67(1):16–25. doi: 10.1227/01.NEU.0000370008.04869.BF. [DOI] [PubMed] [Google Scholar]
- 26.Isla A, Alvarez F, Roda JM, et al. Serpentine aneurysm: regrowth after a superficial temporal artery-middle cerebral artery by-pass and internal carotid ligation: case report. Neurosurgery. 1994;34(6):1072–1074. doi: 10.1227/00006123-199406000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Aletich VA, Debrun GM, Monsein LH, et al. Giant serpentine aneurysms: a review and presentation of five cases. Am J Neuroradiol. 1995;16:1061–1072. [PMC free article] [PubMed] [Google Scholar]
- 28.Coley SC, Hodgson JJ, Jakubowski J. Coil embolization of giant serpentine aneurysms: report of two cases arising from the posterior cerebral artery. Br J Neurosurg. 2002;16(1):43–47. doi: 10.1080/02688690120114219. [DOI] [PubMed] [Google Scholar]
- 29.Saito H, Ogasaware K, Kubo Y, et al. Treatment of ruptured fusiform aneurysm in the posterior cerebral artery with posterior cerebral artery–superior cerebellar artery anastomosis combined with parent artery occlusion: case report. Surg Neurol. 2006;65:621–624. doi: 10.1016/j.surneu.2005.09.009. [DOI] [PubMed] [Google Scholar]