Summary
This study evaluated the efficacy of intra-arterial nimodipine infusion for symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage (aSAH). Clinical data collected from 42 consecutive patients with symptomatic vasospasm after aSAH were retrospectively reviewed. Forty-two patients underwent 101 sessions of intra-arterial nimodipine infusion. Angiographic response, immediate clinical response, and clinical outcome were evaluated at discharge and six months later.
Angiographic improvement was achieved in 82.2% of patients. The immediate clinical improvement rate was 68.3%, while the deterioration rate was 5.0%. A favorable clinical outcome was achieved in 76.2% at discharge and 84.6% six months. Vasospasm-related infarction occurred in 21.4%. There was no drug-related complication. The nimodipine group showed satisfactory outcomes. Nimodipine can be recommended as an effective and safe intra-arterial agent for the treatment of symptomatic vasospasm after aSAH.
Key words: intracranial aneurysm, subarachnoid hemorrhage, vasospasm, intra-arterial infusion, nimodipine
Introduction
Symptomatic vasospasm is a major cause of disability and death after aneurysmal subarachnoid hemorrhage (aSAH). It occurs in about 30% of patients between the fourth and fourteenth day after initial bleeding and may last up to the fourth week1. Preventive therapies include oral medication of nimodipine2, lumbar drainage of bloody cerebrospinal fluid3, cisternal toilet4 and intra-venous infusion of magnesium sulfate5, while therapeutic measures against symptomatic vasospasm comprise ‘triple-H therapy’ (hypertension, hypervolemia, and hemodilution) as well as endovascular treatments such as balloon angioplasty and intra-arterial drug infusion6-26. Moreover, new drugs are under investigation27-29.
Balloon angioplasty was introduced in the early 1980s. It is effective in vasospasm of the proximal and large vessels, and its effect is known to be more sustainable than that of intra-arterial drug infusion. However, it has a limitation to reach the small and distal vessels, and it requires expert neurointerventionists due to the high risk of arterial dissection, rupture and occlusion9,30,31. Papaverine has been widely used as an intra-arterial drug for vasospasm therapy since 199215,16. It is effective in relieving vasospasm of the small and distal vessels as well as large and proximal ones, and the procedure is easier than that of balloon angioplasty. In addition, clinical results have been favorable8-10,12,19,22,24,26. However, the effect is frequently transient and some problems have been identified, including the discrepancy between angiographic response and clinical outcome. Neurological complications after papaverine infusion have also been reported, such as an increase in intracranial pressure (ICP), monocular blindness and brainstem depression 8,12,16,19,22,24,30,31. On the contrary, results of the recent preliminary studies with calcium channel blockers such as nimodipine, nicardipine and verapamil have been favorable, and the complication rates were low7,12-14,17,18.
At our institute, papaverine had been used until 2005. After experiencing some complications related to papaverine infusion, we substituted nimodipine for papaverine as an agent for intra-arterial delivery.
The purpose of this study was to evaluate the efficacy of intra-arterial nimodipine for the treatment of symptomatic vasospasm after aSAH.
Materials and Methods
Patient Population
A total of 451 patients were treated for ruptured intracranial aneurysms at our institute during the past three years between January 2006 and December 2008. Under the approval by the Institutional Review Board, we retrospectively reviewed medical records and imaging data of 42 patients (9.3%), who received intra-arterial nimodipine infusion for symptomatic cerebral vasospasm.
Management of Patients with Ruptured Intracranial Aneurysms
When a ruptured aneurysm was identified in a patient, immediate surgical or endovascular treatment was performed within hours after admission. Open surgery was performed in 14 patients (12 clipping and two clipping plus bypass), and endovascular treatment was performed in 28 patients (26 coil embolization and two trapping of parent vessels). Ventricular drainage was performed as necessary in 20 patients with poor clinical grades for the control of ICP and removal of intraventricular blood. Lumbar drainage was initiated in eight patients with acute symptomatic and unobstructed hydrocephalus. Cisternal lavage of hematoma was not routinely done during surgery. All the patients received intravenous infusion of nimodipine (2 mg/h) followed by the oral medication (60 mg every 4 h) for 21 days, with maintenance of normovolemic status.
Medication for seizure prevention was indicated in patients with cerebral parenchymal injury. Medical conditions (serum electrolyte, glucose, renal function, and cardiopulmonary function) as well as nutritional status were regularly checked and corrected if abnormal findings were noted.
Management of Symptomatic Vasospasm
We performed daily checks on flow velocity using transcranial Doppler (TCD). When the flow velocity tended to increase, we started ‘triple-H therapy’. Cerebral angiography was performed in patients with symptomatic vasospasm accompanied by a significant increase in flow velocity on TCD as well as clinical symptoms/signs refractory to medical treatment without other causes (rebleeding, hydrocephalus, seizure, brain swelling, electrolyte imbalance, and other medical problems).
Vasospasm was suspected when TCD values were mean flow velocity of ≥120 cm/s and peak flow velocity of ≥190 cm/s, or the flow velocities increased more than 50 cm/s above the initial value32.
Once symptomatic vasospasm was identified, intra-arterial drug infusion was performed immediately. Sometimes the procedures were repeated in cases of recurrent symptomatic vasospasm until clinical symptoms and signs did not deteriorate.
Nimodipine was diluted with normal saline (1: 3 dilution). After a microcatheter was placed proximal to the affected vessel, nimodipine was infused from 3 to 6 mg at a rate of 6 mg/h per vessel. When transient hypotension occurred during procedure, infusion was stopped temporarily until blood pressure became normalized.
Imaging Analysis
To evaluate the angiographic response to intra-arterial nimodipine infusion, diameters of the narrowest segment of affected vessels were measured on the pre and post-treatment angiogram. The degree of vasospasm at each period was rated as mild when narrowing was 0% to 24%, moderate when 25% to 49% and severe when 50% or more, with the diameter on initial angiography as a reference.
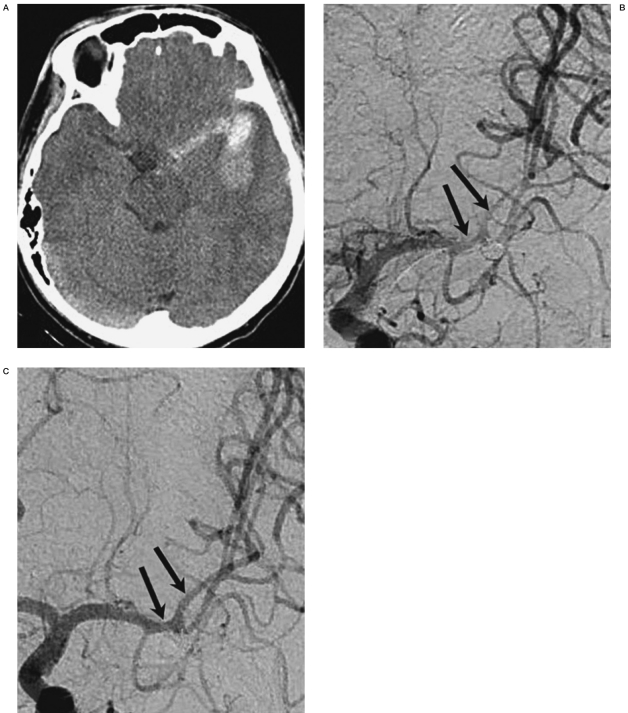
The reference diameter was obtained from the follow-up angiography when a patient showed angiographic vasospasm on the ictus day. Angiographic response was determined as poor when the degree was unchanged and good when improved, comparing the pre- and post-treatment angiograms (Figure 1).
Figure 1.
30-year-old male with Hunt and Hess grade III and Fisher’s group 3 were treated for the aneurysm at the left middle cerebral artery (MCA) with coil embolization on the day of initial bleeding (A). On the 6th day after the initial bleeding, vasospasm more than 50% at the left MCA was identified with altered mentality (arrows) (B). After 7 intra-arterial nimodipine infusions, MCA was almost normalized (grade 1) (C). There was no vasospasm-related infarction at discharge and the patient returned to his workplace.
In cases with multiple affected vessels, the result was considered good when there was at least one good response and poor when there were all poor responses. All these assessments were made blindly.
Vasospasm-related cerebral infarction was evaluated with the follow-up computed tomographic scan and/or magnetic resonance imaging (MRI) at least two weeks after completion of vasospasm therapy. Pre-existing infarction before aSAH, and infarction related to clipping or embolization were excluded. If the infarction gave rise to significant neurological deficits, it was considered major infarction. If it had trivial or non checkable deficit, it was considered minor infarction.
Clinical Evaluation
Short-term clinical response was defined as a change in clinical symptoms within 24 hours after intra-arterial drug infusion, and it was graded as improved, unchanged or deteriorated. Clinical outcome was assessed at discharge and six months later, using the modified Glasgow Outcome Scale (GOS) score (GOS score of 5, good recovery; 4, moderate disability; 3, severe disability; 2, vegetative state; 1, death)33. The clinical outcome was categorized into a favorable (GOS scores of 4) and 5) and unfavorable outcome (GOS scores of 1 to 3). Procedure and drug-related complications were evaluated. When abrupt changes in neurological signs and symptoms occurred during drug infusion or they recovered after stopping the drug, this was designated drug-related complications. Transient change in blood pressure was not included. Mortality was defined as a patient having passed away within one month after the initial bleeding.
Results
Baseline Characteristics
Clinical information of the patients with aSAH is presented in Table 1. The male to female ratio was nearly 1:1 (20:22), and the mean age was 45.9±13.2 years old (range, 14-72). Hunt and Hess (HH) grades34 II and III were the most common (81.0%), and Fisher’s (F) group35 3 was the most common (76.2%). Most (92.9%) ruptured intracranial aneurysms were distributed in the anterior circulation system. Coil embolization was performed twice more than surgical clipping (28 versus 14).
Table 1.
Clinical information of the patients with aneurysmal subarachnoid hemorrhage.
| No. of patients | 42 |
|---|---|
| Sex (M : F) | 20:22 |
| Age, mean ± SD (years) | 45.9 ± 13.2 |
| HH grade, no. (%) I II III IV V |
3 (7.2) 24 (57.1) 10 (23.8) 4 (9.5) 1 (2.4) |
| F group, no. (%) 1 2 3 4 |
0 (0) 7 (16.7) 32 (76.2) 3 (7.1) |
| Location of aneurysm, no. (%) Internal carotid artery Anterior cerebral artery Middle cerebral artery Posterior circulation |
12 (28.6) 12 (28.6) 16 (38.1) 2 (4.7) |
| Treatment for aneurysm, no. (%) Clipping Coil embolization> |
14 (33.3) 28 (66.7) |
| F group: Fisher's group35, HH grade: Hunt and Hess grade34, SD: standard deviation. | |
Basal information of intra-arterial nimodipine infusion for the symptomatic vasospasm is shown in Table 2.
Table 2.
Treatment outcome of symptomatic vasospasm.
| Interval between bleeding and initial IA drug infusion, mean days ± SD (range) | 8.5 ± 5.0 (0-24) |
|---|---|
| Location of vasospasm, no. of sessions (%) Bilateral ACA alone MCA alone ACA + MCA ICA + ACA + MCA Others* |
28 (28.0) 8 (7.9) 23 (22.8) 53 (52.5) 14 (13.9) 2 (2.0) |
| Vasospasm-related symptoms, no. of sessions (%) Altered mentality Neurological deficits Both |
33 (32.7) 31 (30.7) 37 (36.6) |
| Sessions per patient (mean ± SD) | 101/42 (2.4 ± 1.9) |
| Angiographic response, no. of sessions (%) Good Poor |
83 (82.2) 18 (17.8) |
| Short-term clinical response, no. of sessions (%) Improved Unchanged Deteriorated |
69 (68.3) 27 (26.7) 5 (5.0) |
| Favorable GOS (4 and 5), no. of patients (%) At discharge At 6 months after discharge |
32 (76.2) 33 (84.6) |
| Mortality, no. of patients (%) | 1 (2.4) |
| Vasospasm-related infarction, no. of patients (%) Major infarction Minor infarction |
9 (21.4) 4 (9.5) 5 (11.9) |
| Complications, no. of sessions (%) Procedure-related Drug-related |
3 (3.0) 3 (3.0) 0 (0) |
|
ACA: anterior cerebral artery, GOS: Glasgow outcome scale score 33, IA: intra-arterial, ICA: internal carotid artery, MCA: middle cerebral
artery, ns: not significant, SD: standard deviation. * One was at the ICA and the other was at ICA + MCA. | |
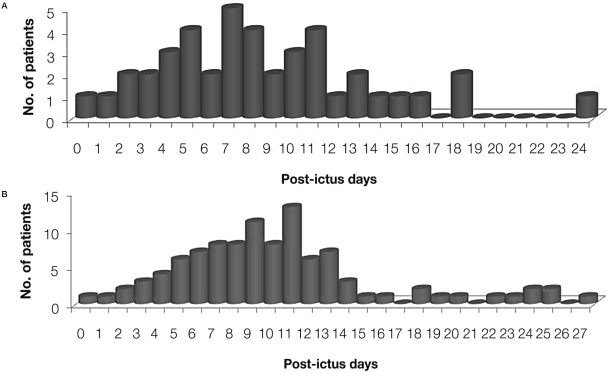
The mean interval between initial bleeding and intra-arterial nimodipine infusion for the symptomatic vasospasm was 8.5±5.0 days (range, 0-24) (Figure 2A). Four patients showed symptomatic vasospasm within 3 days. Vasospasm therapy was frequently repeated (Figure 2B).
Figure 2.
Distribution of the interval between bleeding and intra-arterial nimodipine infusion for the symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. Mean interval between initial bleeding and initial treatment for the symptomatic vasospasm was 8.5±5.0 days (range, 0-24 d) (A). Mean interval between initial bleeding and total vasospasm therapy (including repeated procedures) was 10.1±5.4 days (range, 0-27 d) (B).
Overall, 101 sessions of vasospasm therapy were performed in 42 patients, and the mean session per patient was 2.4±1.9. In detail, the numbers of sessions were one in 21 patients (50%), two in six, three in six and four or more in nine.
Treatment Results
Short-term therapeutic response is summarized in Table 2. Angiographic response after intra-arterial nimodipine infusion was satisfactory in 82.2% of the sessions. With regard to the short-term clinical response, the improvement rate was 68.3% of the sessions.
Overall, 39 out of 42 patients (one patient died and two were lost) were followed up at six months. Favorable clinical outcome was achieved in 76.2% of 42 patients at discharge, and 84.6% of 39 patients at six months after discharge (Table 2).
The mortality rate was 2.4% (n=1), and the cause of death was brain swelling after the vasospasm-related infarction. Vasospasm-related infarction occurred in 21.4% of the patients and the rate of major infarction was 9.5% (Table 2).
The procedure-related complication rate was 3.0%. Complications included two asymptomatic thromboses in the middle cerebral artery (MCA) branches, one of which was resolved using intra-arterial tirofiban injection, and one transient catheter-related MCA spasm. On the other hand, there was no drug-related complication (Table 2).
Transient decrease in blood pressure below 90/60 mmHg during the drug infusion occurred during 14 sessions in nine patients (14%).
Discussion
Short-Term Radiological and Clinical Responses
Papaverine was a prototype drug for intra-arterial infusion in cerebral vasospasm. In the literature, radiological and clinical responses after papaverine infusion ranged from 43% to 100% and 0% to 100%, respectively (Table 3)8-10,12,19,22,24,26. However, the effect of intra-arterial papaverine infusion was measured with different methods in each study, and clinical response was generally worse than radiological one. In a review article, cerebral blood flow was improved in 60% of patients after papaverine infusion, while clinical improvement was achieved in only 43%30. Recently, the results in preliminary studies with nimodipine have been reported (Table 3)7,13,14,18. In the current study, short-term angiographic and clinical responses were satisfactory (82.2% and 68.3%, respectively), and clinical deterioration rate was low (5%). There was no discrepancy between clinical and angiographic responses. Clinical improvement was compatible with or sometimes better than angiographic one. Therefore, we consider the intra-arterial nimodipine therapy as an effective treatment modality for cerebral vasospasm.
Table 3.
Literature review.
| Study [ref. no.] | Drug | No. of patients |
Dose (mg) |
Radiological improvement (%) |
Short-term clinical improvement (%) |
Final favorable clinical outcome (%) |
Complications (%) |
Infarction (%) |
Mortality (%) |
|---|---|---|---|---|---|---|---|---|---|
| Clouston et al. (1995)8 | PPV | 14 | 510-600 | 95 | 50 | 50 | 21.4 | na | 7.1 |
| Elliott et al. (1998)9 | PPV | 13 | 300 | 100 | 69 | 62 | 0 | na | 0 |
| Fandino et al. (1998)10 | PPV | 10 | 300-360 | 100 | 100 | 70 | Na | na | na |
| Firlik et al. (1999)12 | PPV | 15 | 300-600 | 78 | 26 | Na | 20 | na | na |
| Kaku et al. (1992)15 | PPV | 10 | 6-20 | 92 | 80 | 80 | 0 | na | 0 |
| Kassell et al. (1992)16 | PPV | 12 | 60-300 | 66.7 | 33.3 | Na | 16.7 | na | 0 |
| Liu et al. (2004)19 | PPV | 17 | 60-165 | 36 | 71 | 59 | 23.5 | 11.8 | 17.6 |
| McAuliffe et al. (1995)22 | PPV | 21 | 300-500 | 76 | 52 | Na | 14.3 | na | 9.5 |
| Sawada et al. (1997)24 | PPV | 14 | NA | 47 | 21 | Na | 64.3 | na | na |
| Vajkoczy et al. (2001)26 | PPV | 8 | 300 | 51 | 0 | 0 | Na | na | 50 |
| Biondi et al. (2004)7 | NMP | 25 | 1-5 | 43 | 76 | 72 | 0 | na | 8 |
| Hänggi et al. (2008)13 | NMP | 26 | 0.8-3.2 | 69.2 | 18.2 | 61.1 | 23.1 | 61.1 | 5.6 |
| Hui and Lau (2005)14 | NMP | 9 | mean 3.3 | 66.6 | 89 | 77.8 | 11.1 | na | 0 |
| Kim et al. (2009)18 | NMP | 19 | 3-5 | 79.3 | 68.4 | 79.0 | Na | na | 0 |
| Badjatia et al. (2004)6 | NCP | 18 | 2.5-5 | 100 | 42.1 | Na | 33.3 | na | na |
| Tejada et al. (2007)25 | NCP | 11 | 10-40 | 100 | 91 | 90 | 36.4 | 30 | 9.1 |
| Nogueira et al. (2009)23 | NCP | 6 | 2-10 | 41±43 | na | Na | Na | na | na |
| Feng et al. (2002)11 | VPM | 29 | 3.1±0.3 | 44±9 | 29.4 | Na | 0 | na | na |
| Keuskamp et al. (2008)17 | VPM | 10 | 41±29 | 83.3 | 66.7 | Na | 0 | na | na |
| Mazumdar et al. (2006)21 | VPM | 15 | 2.5-10 | 0 | na | Na | Na | na | na |
| Current study | NMP | 42 | 3.3±1.0 | 82.2 | 68.3 | 84.6 | 3.0 | 21.4 | 2.4 |
| Na: not available, NCP: nicardipine, NMP: nimodipine, PPV: papaverine, VPM: verapamil. | |||||||||
Clinical Outcome
Clinical outcome in the current study was satisfactory in about 80% of the patients. Studies with papaverine have reported that favorable clinical outcome was variably achieved in 0% to 80%, and those with nimodipine showed favorable outcome in 61% to 79% (Table 3). Papaverine studies reported mortality rates ranging from 0% to 17.6%, and nimodipine studies from 0% to 5.6%. In the current study, mortality was 2.4%, the cause of which was related to vasospasm. In our opinion, nimodipine seemed to show comparable clinical outcome and lower mortality rate. Clinical outcome has seldom been the focus of previous studies on vasospasm therapy because vasospasm is one of the factors influencing clinical outcome and it is difficult to identify and compare each factor. A series of factors may influence clinical outcome, such as patient factors (severity of initial hemorrhage, age, sex, medical conditions), aneurysm factors (size, location, morphology), and institutional factors (availability of endovascular treatment, patient volume, type of facility)36. Therefore, it would be appropriate to understand that the clinical outcome is related to intra-arterial drug infusion to a degree.
Vasospasm-Related Infarction
The incidence of infarction in patients with aSAH is not widely known in spite of its clinical impact (Table 3). According to Heros et al.37, one fourth of patients with aSAH demonstrated symptomatic vasospasm, and half of those patients later died of infarction. In a papaverine study, eight out of ten vascular territories in eight patients succumbed to infarction26. In another study19, 12% (2/17) of patients died of cerebral infarction after receiving papaverine infusion. In the current study, vasospasm-related infarction was identified in 21.4% (major infarction in 9.5% and minor infarction in 11.9%). In a nicardipine study, 30% of patients experienced infarction25. On the basis of the previous reports and the current study, nimodipine seemed to be at least as effective as papaverine in the prevention of vasospasm-related infarction.
Complications Related to Intra-Arterial Drug Infusion
The estimated complication rate of intra-arterial papaverine infusion has been reported to be about 10%30,31. Complications included increase in ICP, mydriasis, monocular blindness, brainstem depression, seizure, thrombocytopenia, precipitation of crystal and paradoxical vasospasm (Table 3). At our institute, the papaverine-related complication rate was 19.3% of the patients and most of them resulted from the increase in ICP (unpublished data). In contrast, we observed no nimodipine-related complications except transient hypotension. Transient decrease in blood pressure normally recovered within a few minutes.
No neurological complications have been reported from the use of nimodipine in the literature7,13,14. In other studies with verapamil and nicardipine, complication rates ranged from 0% to 36.4%, and most of them were procedure-related complications and transient hypotension6,11,17,21,23,25. A nicardipine study reported transiently increased ICP6. Thus, calcium channel blocker including nimodipine seemed to be superior in terms of complications.
Differences in the Drug Pathomechanisms
As shown in Table 3, the papaverine studies show a discrepancy between the angiographic and clinical response, and a higher complication rate than nimodipine and other kinds of calcium channel blockers. One of the causes might be the direct neurotoxic effect of papaverine.
One study demonstrated high signal intensity on diffusion-weighted and fluid-attenuated inversion recovery MRI at the gray matter within the vascular territories treated with papaverine, and concordant neuronal injury upon pathological examination38. In animal studies, opening of the blood brain barrier and endothelial cell injury after papaverine infusion were reported39,40.
On the contrary, nimodipine is known to have neuroprotective properties as well as a vasodilating effect, including inhibition of free radical, reduction of cellular damage induced by calcium influx at reperfusion and an increase in cerebral oxygen metabolism41-43.
An alternative explanation could be that they exert a differential effect on regional microcirculation.
One study with papaverine demonstrated that it reduced cerebral blood flow in ischemic regions44, while a study with nimodipine revealed that the magnitude and duration of vasodilatation of the penetrating arterioles were greater and longer than those of superficial pial arterioles after nimodipine infusion in rat brain45.
The other explanations include the paradoxical vasospasm occurring after papaverine infusion12,46, embolism caused by precipitation of papaverine28, and anaphylactoid reaction by papaverine itself or preservatives.
Duration of the Effect of Intra-Arterial Drug Infusion
Recurrent vasospasm after intra-arterial drug infusion has long been a problem. Many drugs directly act on smooth muscle cells of the cerebral vasculature46,47, and prompt vasodilatation after drug infusion is commonly observed. However, the effect sometimes does not last long enough to maintain normal cerebral perfusion and the exact duration is unknown. There are only some animal studies on the duration of action after papaverine48-50 and nimodipine infusion47,51, respectively.
In the current study, nimodipine tended to show short duration of action.
Twenty-one (50%) out of 42 patients underwent intra-arterial drug infusion more than once, with a mean session number of 2.4±1.9. Regarding the session numbers of drug infusion in the clinical studies, it was hard to find a difference.
Elliott et al.9 and Liu et al.19 demonstrated that repeated infusions of papaverine were performed in 54% and 68% within 24 hours, respectively.
Mean sessions per person in the papaverine study were 1.5 to 1.79,12,30. On the other hand, about 11% to 90% of patients received repeated infusion within various time interval (range, 1-8 days), and 1.1 to 2.8 sessions per person were performed in the nimodipine studies7,13,14,18. To overcome the short duration, there has been an attempt to infuse a nimodipine continuously via a catheter placed in the internal carotid artery or vertebral artery for several days20. However, the risk of thromboembolism can be a problem with this procedure. Therefore, further studies on the pharmacological characteristics of existing drugs and development of a new drug with high efficacy, long duration, and low complication are needed.
Study Limitations
This study collected data retrospectively and the number of patients was small. It was difficult to rule out the cumulative or exponential effect of oral/ intravenous and intra-arterial administration of nimodipine.
Conclusions
In the current study, nimodipine demonstrated favorable results in angiographic response and clinical outcome, and low complication rate. Intra-arterial nimodipine infusion is an effective and safe treatment for symptomatic vasospasm. In the future, development of new drugs for the prevention and treatment of cerebral vasospasm with greater efficacy and longer duration is anticipated.
Acknowledgments
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (grant no: A06-0171-B51004-06N1-00040B).
References
- 1.Kassell NF, Sasaki T, Colohan AR, et al. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 2.Pickard JD, Murray GD, Illingworth R, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. Br Med J. 1989;298:636–642. doi: 10.1136/bmj.298.6674.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klimo P, Jr, Kestle JR, MacDonald JD, et al. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 2004;100:215–224. doi: 10.3171/jns.2004.100.2.0215. [DOI] [PubMed] [Google Scholar]
- 4.Hamada J, Kai Y, Morioka M, et al. Effect on cerebral vasospasm of coil embolization followed by microcatheter intrathecal urokinase infusion into the cisterna magna: a prospective randomized study. Stroke. 2003;34:2549–2554. doi: 10.1161/01.STR.0000094731.63690.FF. [DOI] [PubMed] [Google Scholar]
- 5.van den Bergh WM, Algra A, van Kooten F, et al. Magnesium sulfate in aneurysmal subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2005;36:1011–1015. doi: 10.1161/01.STR.0000160801.96998.57. [DOI] [PubMed] [Google Scholar]
- 6.Badjatia N, Topcuoglu MA, Pryor JC, et al. Preliminary experience with intra-arterial nicardipine as a treatment for cerebral vasospasm. Am J Neuroradiol. 2004;25:819–826. [PMC free article] [PubMed] [Google Scholar]
- 7.Biondi A, Ricciardi GK, Puybasset L, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. Am J Neuroradiol. 2004;25:1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 8.Clouston JE, Numaguchi Y, Zoarski GH, et al. Intraarterial papaverine infusion for cerebral vasospasm after subarachnoid hemorrhage. Am J Neuroradiol. 1995;16:27–38. [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott JP, Newell DW, Lam DJ, et al. Comparison of balloon angioplasty and papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 1998;88:277–284. doi: 10.3171/jns.1998.88.2.0277. [DOI] [PubMed] [Google Scholar]
- 10.Fandino J, Kaku Y, Schuknecht B, et al. Improvement of cerebral oxygenation patterns and metabolic validation of superselective intraarterial infusion of papaverine for the treatment of cerebral vasospasm. J Neurosurg. 1998;89:93–100. doi: 10.3171/jns.1998.89.1.0093. [DOI] [PubMed] [Google Scholar]
- 11.Feng L, Fitzsimmons BF, Young WL, et al. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. Am J Neuroradiol. 2002;23:1284–1290. [PMC free article] [PubMed] [Google Scholar]
- 12.Firlik KS, Kaufmann AM, Firlik AD, et al. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1999;51:66–74. doi: 10.1016/s0090-3019(97)00370-4. [DOI] [PubMed] [Google Scholar]
- 13.Hänggi D, Turowski B, Beseoglu K, et al. Intra-arterial nimodipine for severe cerebral vasospasm after aneurysmal subarachnoid hemorrhage: influence on clinical course and cerebral perfusion. Am J Neuroradiol. 2008;29:1053–1060. doi: 10.3174/ajnr.A1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui C, Lau KP. Efficacy of intra-arterial nimodipine in the treatment of cerebral vasospasm complicating subarachnoid haemorrhage. Clin Radiol. 2005;60:1030–1036. doi: 10.1016/j.crad.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Kaku Y, Yonekawa Y, Tsukahara T, et al. Superselective intra-arterial infusion of papaverine for the treatment of cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 1992;77:842–847. doi: 10.3171/jns.1992.77.6.0842. [DOI] [PubMed] [Google Scholar]
- 16.Kassell NF, Helm G, Simmons N, et al. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg. 1992;77:848–82. doi: 10.3171/jns.1992.77.6.0848. [DOI] [PubMed] [Google Scholar]
- 17.Keuskamp J, Murali R, Chao KH. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;108:458–463. doi: 10.3171/JNS/2008/108/3/0458. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Park IS, Park KB, et al. Intraarterial nimodipine infusion to treat symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2009;46:239–244. doi: 10.3340/jkns.2009.46.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JK, Tenner MS, Gottfried ON, et al. Efficacy of multiple intraarterial papaverine infusions for improvement in cerebral circulation time in patients with recurrent cerebral vasospasm. J Neurosurg. 2004;100:414–421. doi: 10.3171/jns.2004.100.3.0414. [DOI] [PubMed] [Google Scholar]
- 20.Mayer TE, Dichgans M, Straube A, et al. Continuous intra-arterial nimodipine for the treatment of cerebral vasospasm. Cardiovasc Intervent Radiol. 2008;31:1200–1204. doi: 10.1007/s00270-008-9346-0. [DOI] [PubMed] [Google Scholar]
- 21.Mazumdar A, Rivet DJ, Derdeyn CP, et al. Effect of intraarterial verapamil on the diameter of vasospastic intracranial arteries in patients with cerebral vasospasm. Neurosurg Focus. 2006;21:E15. doi: 10.3171/foc.2006.21.3.15. [DOI] [PubMed] [Google Scholar]
- 22.McAuliffe W, Townsend M, Eskridge JM, et al. Intracranial pressure changes induced during papaverine infusion for treatment of vasospasm. J Neurosurg. 1995;83:430–434. doi: 10.3171/jns.1995.83.3.0430. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira RG, Lev MH, Roccatagliata L, et al. Intra-arterial nicardipine infusion improves CT perfusion-measured cerebral blood flow in patients with subarachnoid hemorrhage-induced vasospasm. Am J Neuroradiol. 2009;30:160–164. doi: 10.3174/ajnr.A1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawada M, Hashimoto N, Tsukahara T, et al. Effectiveness of intra-arterially infused papaverine solutions of various concentrations for the treatment of cerebral vasospasm. Acta Neurochir (Wien) 1997;139:706–711. doi: 10.1007/BF01420042. [DOI] [PubMed] [Google Scholar]
- 25.Tejada JG, Taylor RA, Ugurel MS, et al. Safety and feasibility of intra-arterial nicardipine for the treatment of subarachnoid hemorrhage-associated vasospasm: initial clinical experience with high-dose infusions. Am J Neuroradiol. 2007;28:844–848. [PMC free article] [PubMed] [Google Scholar]
- 26.Vajkoczy P, Horn P, Bauhuf C, et al. Effect of intra-arterial papaverine on regional cerebral blood flow in hemodynamically relevant cerebral vasospasm. Stroke. 2001;32:498–505. doi: 10.1161/01.str.32.2.498. [DOI] [PubMed] [Google Scholar]
- 27.Pluta RM, Hansen-Schwartz J, Dreier J, et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31:151–158. doi: 10.1179/174313209X393564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayama CM, Liu JK, Couldwell WT. Update on endovascular therapies for cerebral vasospasm induced by aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2006;21:E12. doi: 10.3171/foc.2006.21.3.12. [DOI] [PubMed] [Google Scholar]
- 29.Vajkoczy P, Meyer B, Weidauer S, et al. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005;103:9–17. doi: 10.3171/jns.2005.103.1.0009. [DOI] [PubMed] [Google Scholar]
- 30.Hoh BL, Ogilvy CS. Endovascular treatment of cerebral vasospasm: transluminal balloon angioplasty, intra-arterial papaverine, and intra-arterial nicardipine. Neurosurg Clin N Am. 2005;16:501–516. doi: 10.1016/j.nec.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Liu JK, Couldwell WT. Intra-arterial papaverine infusions for the treatment of cerebral vasospasm induced by aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2005;2:124–132. doi: 10.1385/NCC:2:2:124. [DOI] [PubMed] [Google Scholar]
- 32.Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl (Wien) 1988;42:81–84. doi: 10.1007/978-3-7091-8975-7_16. [DOI] [PubMed] [Google Scholar]
- 33.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 34.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 35.Fisher C, Kistler J, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Bederson JB, Connolly ES, Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 37.Heros RC, Zervas NT, Varsos V. Cerebral vasospasm after subarachnoid hemorrhage: an update. Ann Neurol. 1983;14:599–608. doi: 10.1002/ana.410140602. [DOI] [PubMed] [Google Scholar]
- 38.Smith WS, Dowd CF, Johnston SC, et al. Neurotoxicity of intra-arterial papaverine preserved with chlorobutanol used for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:2518–2522. doi: 10.1161/01.STR.0000144682.00822.83. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharjee AK, Kondoh T, Ikeda M, et al. MMP-9 and EBA immunoreactivity after papaverine mediated opening of the blood-brain barrier. Neuroreport. 2002;13:2217–2221. doi: 10.1097/00001756-200212030-00011. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura S, Hashimoto N, Goto Y, et al. Intraarterial infusion of high-concentration papaverine damages cerebral arteries in rats. Am J Neuroradiol. 1996;17:1891–1894. [PMC free article] [PubMed] [Google Scholar]
- 41.Aslan A, Gurelik M, Cemek M, et al. Nimodipine can improve cerebral metabolism and outcome in patients with severe head trauma. Pharmacol Res. 2009;59:120–124. doi: 10.1016/j.phrs.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Ercan M, Inci S, Kilinc K, et al. Nimodipine attenuates lipid peroxidation during the acute phase of head trauma in rats. Neurosurg Rev. 2001;24:127–130. doi: 10.1007/pl00012396. [DOI] [PubMed] [Google Scholar]
- 43.Roda JM, Carceller F, Díez-Tejedor E, et al. Reduction of infarct size by intra-arterial nimodipine administered at reperfusion in a rat model of partially reversible brain focal ischemia. Stroke. 1995;26:1888–1892. doi: 10.1161/01.str.26.10.1888. [DOI] [PubMed] [Google Scholar]
- 44.Heilbrun MP, Olesen J, Lassen NA. Regional cerebral blood flow studies in subarachnoid hemorrhage. J Neurosurg. 1972;37:36–44. doi: 10.3171/jns.1972.37.1.0036. [DOI] [PubMed] [Google Scholar]
- 45.Takayasu M, Bassett JE, Dacey RG., Jr Effects of calcium antagonists on intracerebral penetrating arterioles in rats. J Neurosurg. 1988;69:104–109. doi: 10.3171/jns.1988.69.1.0104. [DOI] [PubMed] [Google Scholar]
- 46.Clyde BL, Firlik AD, Kaufmann AM, et al. Paradoxical aggravation of vasospasm with papaverine infusion following aneurysmal subarachnoid hemorrhage. Case report. J Neurosurg. 1996;84:690–695. doi: 10.3171/jns.1996.84.4.0690. [DOI] [PubMed] [Google Scholar]
- 47.Gelebek V, Orer HS, Firat MM, et al. Selective intraarterial nimodipine treatment in an experimental subarachnoid hemorrhage model. Am J Neuroradiol. 2005;26:1357–1362. [PMC free article] [PubMed] [Google Scholar]
- 48.Nagai H, Noda S, Mabe H. Experimental cerebral vasospasm. Part 2: effects of vasoactive drugs and sympathectomy on early and late spasm. J Neurosurg. 1975;42:420–428. doi: 10.3171/jns.1975.42.4.0420. [DOI] [PubMed] [Google Scholar]
- 49.Ogata M, Marshall BM, Lougheed WM. Observations on the effects of intrathecal papaverine in experimental vasospasm. J Neurosurg. 1973;38:20–25. doi: 10.3171/jns.1973.38.1.0020. [DOI] [PubMed] [Google Scholar]
- 50.Varsos VG, Liszczak TM, Han DH, et al. Delayed cerebral vasospasm is not reversible by aminophylline, nifedipine, or papaverine in a “two-hemorrhage” canine model. J Neurosurg. 1983;58:11–17. doi: 10.3171/jns.1983.58.1.0011. [DOI] [PubMed] [Google Scholar]
- 51.Yin YH, Wang F, Pan YH, et al. Effects of dose-response of topical administration of nimodipine on cerebral vasospasm after subarachnoid hemorrhage in rabbits. Am J Med Sci. 2009;337:123–125. doi: 10.1097/MAJ.0b013e31817d1ca1. [DOI] [PubMed] [Google Scholar]