Summary
Dural arteriovenous fistula (DAVF) can be separated into two types: DAVF which drains through an affected sinus (sinus type) and DAVF with direct reflux to the cortical vein (non-sinus type). The present report attempted to clarify the mechanism of formation and development of DAVF focusing on the emissary vein (EV) hypothesis.
First, inflammation occurs at the penetrating point of the EV on the dura due to idiopathic or secondary causes. Local inflammatory reactions induce vessel dilatation and neovascularization, and subsequently create arteriovenous (AV) connections on the arteriole level. Although EV communicating with dural arteries might play a role as draining routes at first, they start to degrade due to compression of enlarged emissary arteries or to a hemodynamic shift to the drainage pathway of least resistance. Following the occlusion of drainage pathway through EV into the sinus or cortical veins may form, resulting in clinically detectable DAVF. The AV shunt then expands to the surrounding dura associated with recruitment of feeders from distant sites induced by expression of angiogenetic factors and a shift in the hemodynamic balance. In sinus type DAVF, the sinus is progressively compartmentalized and finally occludes due to thrombogenesis with activated coagulopathy or to hemodynamic hypertrophy of the sinus wall. This progression results in the mature, aggressive DAVF with drainage impairments. Previous mechanistic hypotheses focusing on sinus hypertension and sinus thromboses cannot explain the pathogenesis of non-sinus type of DAVF. Although the etiology of DAVF may be concerned by the thrombo-occlusive change of sinus, the unique theory presented in this report may enable an understanding of the common etiology of both types of DAVF.
Key words: angiogenesis, dural arteriovenous fistula, emissary vein, inflammation, pathogenesis, sinus thrombosis
Introduction
Dural arteriovenous fistula (DAVF) is the acquired and progressive arteriovenous (AV) shunt disease on or between the dura matter, and its etiology remain controversial1-4. This disorder occurs not in the whole dura but at very specific locations. DAVF can be divided into two types based on the intervention of the drainage route and affected sinus; sinus type and non-sinus type. The sinus type has the shunt at the sinus wall or dural vein, and includes DAVF at the cavernous sinus (CS), transverse-sigmoid sinus (TS-SS), anterior condylor confluence (ACC), and superior sagittal sinus (SSS). The non-sinus DAVF has the shunt on the dura and directly drains into the pial veins, and includes tentorial, ethmoidal, craniocervical and spinal DAVF. However, even the sinus type DAVF ultimately changes to the isolated sinus with cortical reflux due to progressive sinus occlusion, similar to the non-sinus type. Such seemingly separated and complex pathogeneses of DAVF remain elusive. The formation and development of DAVF based on the hypothesis focusing on the emissary vein are explored in the present report.
Hypothesis
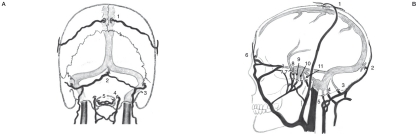
Etiologically, DAVF is revealed secondary to causes such as trauma, inflammation, or sinus thrombosis5-8. However, most causes are idiopathic and independent of the preceding hematological and immunological impairments. Therefore, comprehensive factors concerning the initiation of DAVF that covers all of the pathological features should be considered. From this perspective, we focused on the location of emissary veins (EV) and discovered that the distribution of EV definitely corresponds to those of the location of DAVF (Figure 1). According to this previous consensus and to new information, we propose following hypothesis concerning the development of DAVF which focuses on the emissary vein.
Figure 1.
Distribution of emissary veins. Note that their specific locations correspond well with those of the liable parts of DAVF (indicated in parentheses). A) Postero-anterior view. B) Lateral view 1. parietal (parasagittal ) emissary vein (EV), 2. occipital (torcular) EV, 3. mastoid EV, 4. condylar (condyloid) EV, 5. hypogroassal EV, 6. frontal (ethmoidal) emissary vein (EV), 7. petrosquamosal EV, 8. foramen ovale EV, 9. foramen spinosum EV, 10. foramen lacerum EV, 11. Basilar plexus EV and Trolard’s inferior petrooccipital vein (venous plexus of Raktzik).
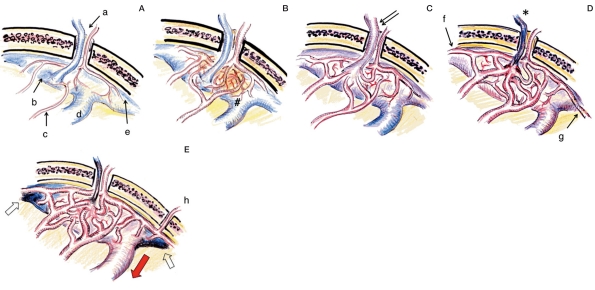
Emissary veins connecting the intracranial and extracranial venous system through the bone are distributed in specific parts of the vault or base of the skull9. They are usually accompanied by and penetrate together through the same foramen with emissary arteries (transosseous perforating arteries) (Figure 2A). Figure 2 shows the scheme of this process in the DAVF at SSS (a representative of sinus type DAVF). First, some inflammatory reaction occurs at the penetrating site of EV. It may be reasonable to consider the cause of inflammation to the infection of adjacent tissue such as sinusitis and mechanical inflammation after trauma (including catheter intervention). Sinus thrombosis is occasionally observed before the occurrence of DAVF7, however, such thrombosis might be the result of focal inflammation. In most cases with DAVF, inflammation will develop undetected or as an autoimmune allergic reaction. Local inflammation may expand with expression of various cytokines, cause vessel dilatation, and open the physiological AV shunt at the level of capillary vessels (Figure 2B).
Figure 2.
Mechanism of development of the DAVF at the superior sagittal sinus (SSS) as a representative of the sinus type DAVF. A) Normal site. Emissary vein (EV) and artery (a) is penetrating through a foramen of the parasagittal skull. EV is connected with the venous lacunae (b). Meningeal arteries (c) have no connection with SSS (d) and cortical vein (e). B) Neovascularization (arrow) and vessel dilatation induced by dural inflammation (#). C) AV shunt formation at the level of dural arteriole and penetration into the sinus (initial stage of DAVF). Note the shunt flow draining into the sinus as well as EV (double arrow). D) Shunt development with thrombosis of an emissary vein (asterisk) and recruitment of distal arteries from anterior falx artery (f) and posterior meningeal arteries (g). E) Maturation of DAVF with the reflux to cortical veins (red arrow) due to sinus occlusion (white arrow). Note the further recruitment of feeders from the other side or transosseous branches (h).
In sinus type DAVF, a micro AV shunt between the emissary artery and vein will enlarge to the adjacent sinus wall. The increase in shunt flow triggers drainage into the sinus, and subsequently changes the main drainage route from the EV to the sinus. As a result, the sinus will be occupied by the shunt flow more than the normal intracranial venous outflow pathway (Figure 2C). While this shift in shunt flow direction decreases the role of EV as the drainage pathway, the developed and swollen emissary artery compresses the accompanied EV and impairs the drainage flow. This results in occlusion of the EV (Figure 2D). This degeneration of the EV is an important process in the formation of a well-known style of sinus type of DAVF. However, in some cases of DAVF at CS and SSS the EV may remain patent and serve as a drainage route to the pterygoid plexus or parietal vault. Also an EV that connects with diploic veins can form an enlarged intraosseous venous lake, and appear as a new channel of sinus or duplication. The shunt recruits other dural feeders from the distant and contralateral parts due to angiogenetic and hemodynamic factors, and forms the extended vascular network as a new DAVF10. Extracranial arteries on the skull or under the skull base are often mobilized through the bone. Such active recruitment of feeders is considered to be due to angiogenesis enhanced by the expression of vasculogenetic factors at the affected dura (including vascular endothelial growth factor (VEGF))11-16.
The next key process in maturation of the DAVF is the occlusive change of the draining system. Although draining pathway may finally occlude due to intrasinus thrombosis associated with hypercoagulopathy, the essence of the occlusive mechanism should be hypertrophy of the sinus wall12. This occlusive process is typical in the DAVF at CS17. Its drainage route gradually occludes from the inferior petrosal sinus and superior ophthalmic vein11, and occasionally causes a paradoxical worsening of visual acuity and chemosis with ocular hypertension following occlusion of the anterior drainage route. During this progression, the thrombophilic abnormalities characteristic of DAVF are also reported18-22. In some cases such thrombotic change of drainage route may occur in the initial stage preceding the development of the shunt.
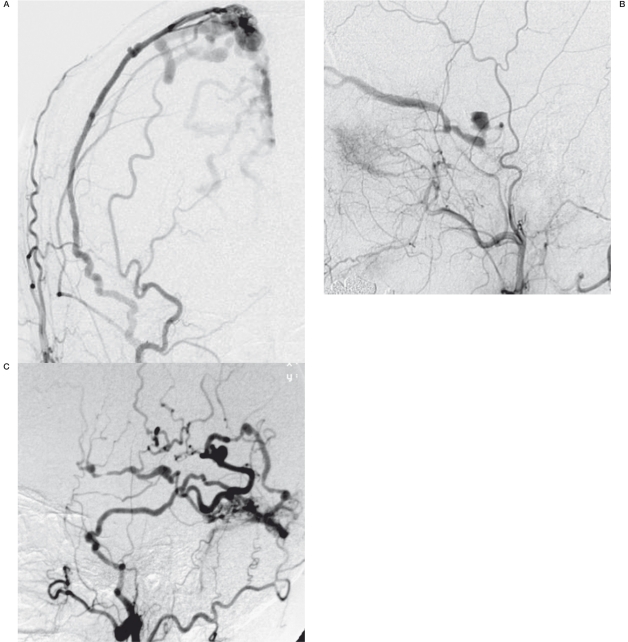
Next, the same process progresses in the upstream side because the remaining upstream drainage with more hemodynamic stress may yield the hypertrophic change of sinus wall12,23. As a result, the meeting point of the shunt flow will be isolated, and shunt flow without exit to the sinus may reflux into cortical veins (Figure 2E). Such a matured and aggressive type of DAVF with an affected isolated sinus or dural vein24 (Figure 3), may be the final expression of this process. However, this final isolated part of the DAVF will not be always consistent with the first trigger point of a micro AV shunt, because the inflammatory extension and recruitment of many dural arteries will easily cause the movement of the main shunt point.
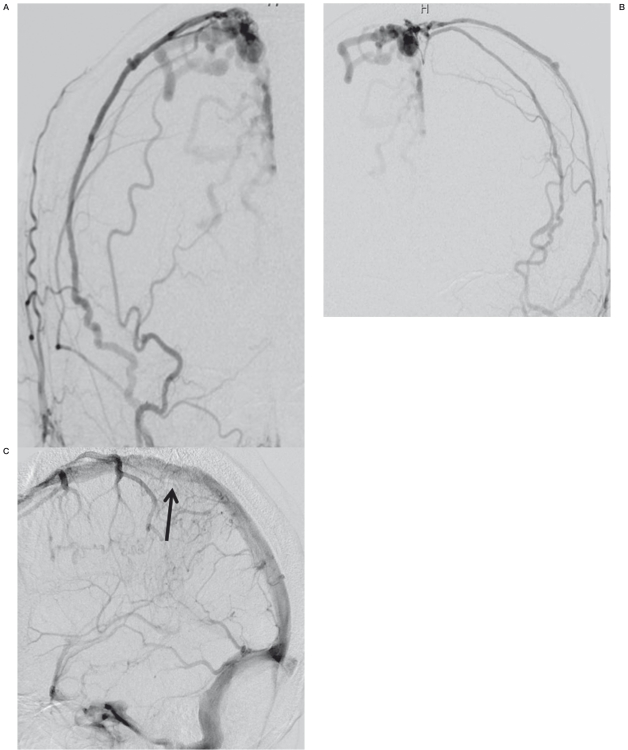
Figure 3.
Examples of matured sinus type DAVF A) DAVF at superior sagittal sinus. B) DAVF at cavernous sinus. C) DAVF at transverse-sigmoid sinus.
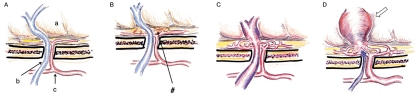
It is quite easy to adopt this mechanistic hypothesis to non-sinus type DAVF. Figure 4 demonstrates the scheme of the development of ethmoidal (anterior skull base) DAVF as a representative of non-sinus type DAVF. EV at the anterior skull base (Figure 4A) connecting with the cortical vein will create, secondary to the ethmoidal inflammation, a micro AV shunt at the skull base dura (Figure 4B). Subsequently the EV will occlude according to the same process as described above (Figure 4C,D). As a result, all the shunt flow supplied from ethmoidal arteries drains into the cortical veins, which is the common style encountered in the clinical setting. The pathological process at the spine or craniocervical junction DAVF can be explained by the same mechanism.
Figure 4.
Mechanism of development of the DAVF: an ethmoidal (anterior skull base) DAVF as a representative of the non-sinus type DAVF. A) Normal state. A, frontal cortex; b, frontal emissary vein; c, dural branches from anterior ethmoidal artery. B) Neovascularization and dilatation induced with dural inflammation (#). C) AV shunt formation at the level of dural arteriole and reflux into an EV (initial stage of DAVF). D) Shunt development and increased reflux into the varicose cortical vein (white arrow) following the occlusion of an emissary vein.
Atypical DAVF
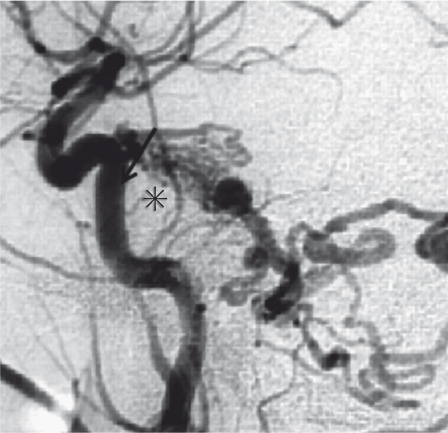
1) Tentorial DAVF. It is true that there is no EV on the tent. The name of this type of DAVF may come from the participation of tentorial artery, however the main shunt point is not on the tent but at the clival dura (Figure 5). The tentorial artery as the feeder usually creates the AV shunt just posterior to its origin, and the shunt flow immediately drains into the subtentorial venous complex. Although there are some classifications of various type of tentorial DAVF25, the most common type of tentorial DAVF at the clivus may be caused by the pathological process of a concerned EV, petrosal bridging vein26, that connects between the ventral venous system in the posterior fossa and the basilar plexus. The exceptional tentorial DAVFs affecting the posterior tentorial sinus or the confluence are considered to be variants of TS-DAVF with the influence of EV at the petrous and occipital skull.
Figure 5.
Tentorial DAVF. The lateral view of the left carotid angiogram showing the dilated feeder from the tentorial artery (arrow) and AV shunt at the clival portion (asterisk) immediately draining into the prepontine venous network.
2) DAVF at the anterior condylor confluence. DAVF at the anterior condylor confluence has been newly defined as the generic clinical entity, and includes DAVF of the inferior petrosal sinus, DAVF of the marginal sinus, hypoglossal DAVF, DAVF of the anterior condylor vein within the hypoglossal canal, and jugular foramen DAVF27. The anterior condylor confluence (ACC) is extracranially located, and is a major venous crossroad at the posterior base of the skull. Tributaries from the hypoglossal canal, petroclival fissure, and the vertebral venous plexus meet together at the ACC and drain into the jugular bulb through multiple channels28. As seen in previous nomenclature, one of the important drainage routes in the anterior condylor vein is the EV passing though the hypoglossal canal. However, in most cases, the anterior condylor vein has been already occluded, and other venous systems (including the lateral condylor vein, inferior petroclival vein, and inferior petrosal sinus) may function as a drainage route via ACC. Specific characteristics of this type of DAVF include patients suffering from strong tinnitus just when the DAVF is initially formed. Hypoglossal palsy develops in some cases. DAVF at the ACC tends to be diagnosed in the early stages. Therefore, as the original drainage route, the anterior condylor vein occasionally remains one of the draining veins.
3) Vault DAVF. This rare type of DAVF is located at the temporal or occipital convexity, and is a non-sinus type DAVF. An aggressive feature of this type of DAVF is that it directly drains into cerebral cortical veins. According to our theory, it may be caused by the focal inflammation around the atypically located EV, or due to the congenital focal connection between pial and dural veins.
4) Multiple, de novo, recurrent DAVF. These clinical features cannot be explained with the single inflammation theory, and spreading or multifocal inflammation should be considered (Figure 6). Although recurrence of the same lesion can be due to incomplete occlusion of the shunt29, de novo creation of DAVF independent from the previous ones may follow the newly developing process, possibly be promoted with constitutional factors.
Figure 6.
Multiple DAVF at superior sagittal, transverse and sigmoid sinus (arrows).
Discussion
Previous theories on DAVF etiology
Previous recognition of the etiology of DAVF has been directed to sinus hypertension1,4 and thrombosis5,8. It is true that such abnormal situations may create the experimental AV shunt1-4. However, if one considers that the sinus hypertension is the initial trigger, it should be caused secondary to the thrombosis or outlet stenosis. It is unreasonable to adopt this theory into non-sinus type, because this type has no correspondence with the sinus. In other hand AV shunt formation can easily create the condition of sinus hypertension. Thus, the conventional discussion over the etiology of AV shunt formation between sinus occlusion and sinus hypertension is just a chicken-or-egg question. We consider that sinus hypertension concerning sinus wall hypertrophy may not be the cause, but rather one factor in the development of DAVF. Our theory based on inflammatory initiation affecting EV can explain both types of DAVF and subsequent development with pathological changes of the drainage route is not contradictory to the previous sinus-oriented theory.
Supporting clinical situation
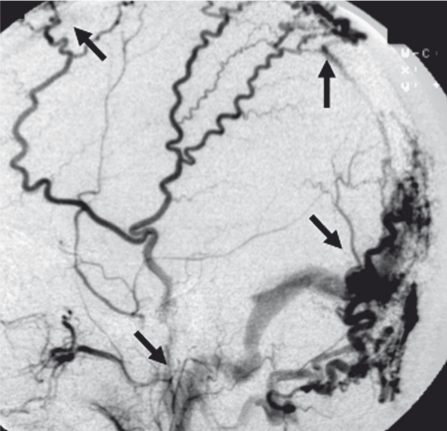
This hypothesis is supported by some familiar features encountered in clinical cases. First, in the case of mature SSS-DAVF, shunt flow usually drains into the cortical vein through an isolated sinus with the particular congestion of pial veins. However, in spite of such an aggressive type with reflux to the cortical vein, SSS is still patent in some particular cases. (Figure 7) This unusual situation suggests the influence of EV at the initial location of the micro AV shunt. As seen in Figure 7, the parasagittal (parietal) EV has no direct connection with SSS itself and drains from venous lacunae. The abnormal state mentioned above can be interpreted as follows; the occlusive change of drainage site might occur at the channel between venous lacunae and SSS after the formation of AV shunt, therefore SSS as the normal cortical drainage route is independent from DAVF and can be preserved. It may suggest that the shunt point is located not the sinus wall of SSS but venous lacunae, exit of EV. In the early stage of CS-DAVF without ocular symptoms there are various drainage routes into the pterygoid plexus as well as the superior ophthalmic vein and inferior petrosal sinus. Similarly, the anterior condylor vein is patent in the initial stage of ACC-DAVF. In such young DAVF, EV of the foramen ovale or foramen lacerum and hypoglossal EV still remain as the original drainage pathway. This fact suggests the possibility that the EV plays an important role in the initial stages of newly developed DAVF.
Figure 7.
A) Isolated venous lacunae B) External carotid angiogram showing a parasagittal DAVF fed by both meningeal feeders draining into the cortical veins via isolated venous lacunae. C) Internal carotid angiogram shows no involvement of the SSS (arrow).
One often encounters a multiplicity about the location or TS-SS-DAVF. This fact is also explainable using the present hypothesis. At the confluence, the lateral side and the sigmoid junction, the initially affected EVs: may be torcular, petrosquamosal and mastoid EVs, respectively.
Unfortunately, our hypothesis has not yet been proven in a pathological specimen of clinical cases as well as from the animal experiments. Further, it is difficult to explain the etiology of DAVF in locations without emissary veins or those without arterial supply coming from emissary arteries with the exception of osteodural AV shunts. Although our theory was not based on the anatomical, physiopathological or clinical observational convincing background, if the very early stage of DAVF is incidentally found, inflammatory investigation into the inflammation and meticulous observation of the flow change will be helpful to predict the development of DAVF and also to support this theory.
Conclusion
According to previous theories concerning the mechanism of DAVF, it is very important to consider the occlusive pathway and hypertension of the affected sinus. However, previous theories did not indicate an initiation of this pathological situation which can explain both sinus and non-sinus type DAVF and the promoting factors to develop the extension of DAVF. We propose a new theory: the inflammatory vascular network at the penetration site of emissary veins may induce local shunt formation. Subsequent occlusion of the affected EV completes the usual figure of DAVF, and, the sinus (venous) occlusive pathway and arterial recruitment are important steps in the maturation of the DAVF. Although our theory is only a speculation without the base of experimental study, it may enable to understand the common etiology of the two (sinus and non-sinus) types of DAVF, and is not contradictory to the previous sinus-oriented theory. Pathological proof of the initial stage of DAVF will be mandatory.
References
- 1.Hamada Y, Goto K, Inoue T, et al. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery. 1997;40:452–458. doi: 10.1097/00006123-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Herman JM, Spetzler RF, Bederson JB, et al. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83:539–545. doi: 10.3171/jns.1995.83.3.0539. [DOI] [PubMed] [Google Scholar]
- 3.Nishijima M, Takaku A, Endo S, et al. Etiological evaluation of dural arteriovenous malformations of the lateral and sigmoid sinuses based on histopathological examinations. J Neurosurg. 1992;76:600–606. doi: 10.3171/jns.1992.76.4.0600. [DOI] [PubMed] [Google Scholar]
- 4.Terada T, Higashida RT, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884–889. doi: 10.3171/jns.1994.80.5.0884. [DOI] [PubMed] [Google Scholar]
- 5.Houser OW, Baker HL, Jr, Rhoton AL, Jr, et al. Intracranial dural arteriovenous malformations. Radiology. 1972;105:55–64. doi: 10.1148/105.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Kusaka N, Sugiu K, Katsumata A, et al. The impsortance of venous hypertension in the formation of dural arteriovenous fistulas: a case report of multiple fistulas remote from sinus thrombosis. Neuroradiology. 2001;43:980–984. doi: 10.1007/s002340100596. [DOI] [PubMed] [Google Scholar]
- 7.Vilela P, Willinsky R, terBrugge K. Dural arteriovenous fistula associated with neoplastic dural sinus thrombosis: two cases. Neuroradiology. 2001;43:816–820. doi: 10.1007/s002340100570. [DOI] [PubMed] [Google Scholar]
- 8.Witt O, Pereira PL, Tillmann W. Severe cerebral venous sinus thrombosis and dural arteriovenous fistula in an infant with protein S deficiency. Childs Nerv Syst. 1999;15:128–130. doi: 10.1007/s003810050349. [DOI] [PubMed] [Google Scholar]
- 9.Lasjaunias P, Berenstein A. Surgical neuroangiography I. Berlin: Springer-Verlag; 1987. pp. 675–678. [Google Scholar]
- 10.Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1987;87:267–274. doi: 10.3171/jns.1997.87.2.0267. [DOI] [PubMed] [Google Scholar]
- 11.Klisch J, Kubalek R, Scheufler KM, et al. Plasma vascular endothelial growth factor and serum soluble angiopoietin receptor sTIE-2 in patients with dural arteriovenous fistulas: a pilot study. Neuroradiology. 2005;47:10–17. doi: 10.1007/s00234-004-1310-3. [DOI] [PubMed] [Google Scholar]
- 12.Kojima T, Miyachi S, Sahara Y, et al. The relationship between venous hypertension and expression of vascular endothelial growth factor: Hemodynamic and immunohistochemical examinations in a rat venous hypertension model. Surgical Neurol. 2007;68:277–284. doi: 10.1016/j.surneu.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 13.Tirakotai W, Bertalanffy H, Liu-Guan B, et al. Immunohistochemical study in dural arteriovenous fistulas and possible role of local hypoxia for the de novo formation of dural arteriovenous fistulas. Clin Neurol Neurosurg. 2005;107:455–460. doi: 10.1016/j.clineuro.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Terada T, Tsuura M, Komai N, et al. The role of angiogenic factor bFGF in the development of dural AVFs. Acta Neurochir (Wien) 1996;138:877–883. doi: 10.1007/BF01411267. [DOI] [PubMed] [Google Scholar]
- 15.Uranishi R, Nakase H, Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999;91:781–786. doi: 10.3171/jns.1999.91.5.0781. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Lawton MT, Du R, et al. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery. 2006;59:687–696. doi: 10.1227/01.NEU.0000228962.68204.CF. [DOI] [PubMed] [Google Scholar]
- 17.Satomi J, Satoh K, Matsubara S, et al. Angiographic changes in venous drainage of cavernous sinus dural arteriovenous fistulae after palliative transarterial embolization or observational management: A proposed stage classification. Neurosurgery. 2005;56:494–502. doi: 10.1227/01.neu.0000153750.95524.62. [DOI] [PubMed] [Google Scholar]
- 18.Gerlach R, Boehm-Weigert M, Berkefeld J, et al. Thrombophilic risk factors in patients with cranial and spinal dural arteriovenous fistulae. Neurosurgery. 2008;63:693–698. doi: 10.1227/01.NEU.0000325730.77263.7E. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach R, Yahya H, Rohde S, et al. Increased incidence of thrombophilic abnormalities in patients with cranial dural arteriovenous fistulae. Neurol Res. 2003;25:745–748. doi: 10.1179/016164103101202101. [DOI] [PubMed] [Google Scholar]
- 20.Izumi T, Miyachi S, Hattori K, et al. Thrombophilic abnormalities among patients with cranial dural arteriovenous fistulas. Neurosurgery. 2007;61:262–269. doi: 10.1227/01.NEU.0000255529.46092.7C. [DOI] [PubMed] [Google Scholar]
- 21.Kraus JA, Stuper BK, Muller J, et al. Molecular analysis of thrombophilic risk factors in patients with dural arteriovenous fistulas. J Neurol. 2002;249:680–682. doi: 10.1007/s00415-002-0690-8. [DOI] [PubMed] [Google Scholar]
- 22.Safavi-Abbasi S, Di Rocco F, Nakaji P, et al. Thrombophilia due to Factor V and Factor II mutations and formation of a dural arteriovenous fistula: case report and review of a rare entity. Skull Base. 2008;18:135–143. doi: 10.1055/s-2007-1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahara Y, Miyachi S, Nagasaka T, et al. Radiological and pathological changes in the sinus of an experimental arteriovenous fistula of the rat. Interventional Neuroradiology. 2003;9(Suppl 1):101–105. doi: 10.1177/15910199030090S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyachi S. Endovascular treatment for dural arteriovenous fistula. No to Shinkei. 2008;60:907–914. [in Japanese] [PubMed] [Google Scholar]
- 25.Lawton MT, Sanchez-Mejia RO, Pham D, et al. Tentorial dural arteriovenous fistulae: operative strategies and microsurgical results for six types. Neurosurgery. 2008;62(3) Suppl 1:110–124. doi: 10.1227/01.neu.0000317381.68561.b0. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuhashi Y, Aurboomyawat T, Pereira VM, et al. Dural arteriovenous fistulas draining into the petrosal vein or bridging vein of the medulla: possible homologs of spinal dural arteriovenous fistulas. Clinical article. J Neurosurg. 2009;111:889–899. doi: 10.3171/2009.1.JNS08840. [DOI] [PubMed] [Google Scholar]
- 27.Miyachi S, Kojima T, Ohshima T, et al. Dural arteriovenous fistula at the anterior condylor confluence. Interventional Neuroradiology. 2008;14:303–311. doi: 10.1177/159101990801400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.San Millán Ruíz D, Gailloud P, Rüfenacht DA, et al. The craniocervical venous system in relation to cerebral venous drainage. Am J Neuroradiol. 2002;23:1500–1508. [PMC free article] [PubMed] [Google Scholar]
- 29.Ushikoshi S, Kikuchi Y, Houkin K, et al. Multiple dural arteriovenous fistulas. Neurol Med Chir (Tokyo) 1998;38:478–484. doi: 10.2176/nmc.38.478. [DOI] [PubMed] [Google Scholar]