Summary
Carotid blowout is a devastating complication in patients with head and neck malignancy. Various treatments including high risk surgery, carotid stenting or carotid occlusion using either coils or detachable balloons have been described. The key for any treatment is the rapidity at which it can be performed. We describe treatment of common carotid blowout secondary to neoplastic infiltration using four Amplatzer vascular plugs deployed in less than ten minutes.
Key words: Amplatzer vascular plug, carotid blow-out, common carotid artery
Introduction
Carotid blowout (CB) refers to rupture of the carotid artery or its branches and is a devastating complication of head-and-neck cancers with very high morbidity and mortality. Surgical management of CB is usually technically difficult especially in those previously irradiated. Endovascular management of CB by occlusion with coils or detachable balloons has been described1,2. Detachable balloons, although fast, are being used less commonly and have a significant learning curve. Coil occlusion is time consuming and potentially risky for thromboembolism. Covered stents achieve immediate hemostasis in patients with head-and-neck cancers and CB, but long-term safety, stent patency, and permanency of hemostasis appeared unfavorable3. For active extravasation from a CB, it is necessary to use a device that achieves rapid occlusion.
Amplatzer vascular plug (AVP) has been used for transcatheter embolizations in peripheral vasculature; occlusion of abnormal vessel communications and other neurovascular conditions4,5. We describe endovascular AVP treatment of CB presenting with life-threatening hemoptysis.
Case Report
A 50-year-old man with longstanding oropharyngeal squamous cell carcinoma was referred for endovascular treatment of intractable epistaxis/hemoptysis. This patient had a history of childhood sarcoma treated by whole head and neck radiation. Many years later, he developed squamous cell carcinoma. Despite repeated surgery and radiotherapy he developed extensive local spread of carcinoma. The patient had two episodes of life-threatening hemoptysis at home and in the emergency room which was partially controlled by posterior pharyngeal packing as the patient had a tracheostomy.
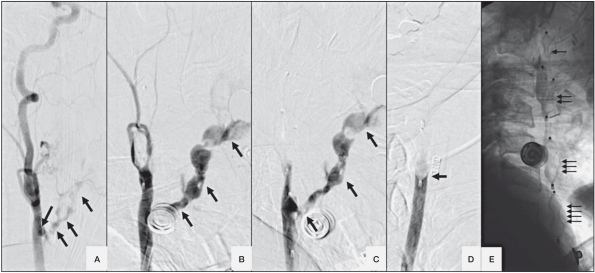
He was not initially bleeding in the angio-suite and CCA angiogram showed no tumor blush or extravasation. However he suddenly had a torrent of blood coming out of his mouth and nose requiring constant suctioning. He became tachycardic and hypotensive. An angiogram showed extravasation of contrast from distal left CCA out into the oropharynx (Figure 1A). We needed fast hemostasis. Unfortunately there was no time for a test occlusion. Detachable balloons and AVP were considered. The proximal location of the CB and the urgency favoured use of AVP. A 6F Envoy guiding catheter was placed in the proximal left ICA. With road map guidance, one 8 mm AVP (AGA medical corporation, MN, USA) was deployed in the left ICA and a second in the left ECA to prevent back bleeding. There was immediate occlusion of flow in left ICA (Figure 1B). The guiding catheter was withdrawn into left CCA and a 10 mm AVP was deployed at the point of CB. There was small residual extravasation (Figure 1C) and a second 10 mm AVP was deployed just proximal to the last one resulting in complete occlusion. The angiographic extravasation (Figure 1D) and bleeding through his mouth stopped immediately. The total time taken from the decision-making to the deployment of all four AVP was less than ten minutes which provided dramatically fast life-saving occlusion and hemostasis.
Figure 1.
Left CCA lateral angiogram. A) Extravasation of contrast from CCA (arrows). B) No filling of ICA after deployment of AVP in the left ICA and ECA with continued extravasation (arrows). C) No filling of left the ECA and ICA but continued extravasation after deployment of the third AVP (arrows). D) After deployment of the fourth AVP, no filling of ICA, ECA and no extravasation. E) 4 AVPs (numbered arrows).
Despite transfusion, the patient’s systolic blood pressure dropped to 60 mmHg, which later improved with further transfusion (7 units packed RBC total) and i.v. fluids. His oxygen saturation remained 100% throughout the procedure. The patient remained neurologically unchanged. Post-occlusion angiogram of right ICA showed very good collateral flow through the anterior communication artery. The patient was discharged home three days after the procedure with no further bleeding and no neurologic deficit.
Discussion
CB refers to rupture of the carotid artery and its branches. It is one of the most devastating complications associated with head-and-neck cancers. CB is associated with approximately 60% neurological morbidity and 40% mortality in patients with associated conditions such as pharyngocutaneous fistula, recurrent tumour, or radiation necrosis6. The history of radiation therapy adds a 7.6-fold increased risk of developing CB in these patients7.
Surgical treatment of extracranial carotid artery pseudoaneurysm or CB has been by arterial resection and reconstruction or carotid artery ligation. However, this can be associated with haemodynamic instablity and decreased cerebral perfusion. There is a high risk of perioperative rupture of a pseudoaneurysm8.
Endovascular treatments including permanent balloon or coil embolisation expanded the therapeutic options for CB patients1. Balloon occlusion can achieve immediate occlusion but as high as 15-20% have immediate or delayed cerebral ischaemia2. Another disadvantage of balloons is their tendency to slowly deflate. Detachable balloons are technically demanding; time consuming and are less and less available. Coil occlusion requires more time in these unstable patients.
Carotid stent placement combined with GDC has been reported to achieve immediate hemostasis but will continue to have a risk of immediate or delayed cerebral ischemia9. Covered stents have been used in the treatment of ICA pseudoaneurysm10. However, stent sizing and placement for the CB is not always straightforward in cases of malignancy involving both the larger lumen CCA or carotid bulb and the smaller lumen ICA. Also a 9F arterial sheath is required to accommodate the outer diameter of their delivery system. There is potential possibility of further pseudo-aneurysm formation at either end of the covered stent margin possibly due to surrounding soft tissue weakness or due to the rigid lower end of the covered stent structure.
The AVP is a self-expanding, cylindrical device consisting of 144 nitinol mesh wires and is available in sizes ranging from 4 to 16 mm in 2-mm increments. It is advanced from a loader through a 5F to 8F guiding catheter. The AVP has platinum markers on both ends (Figure 1E). A stainless steel micro-screw is welded to the proximal platinum marker band with screw attachment to the 135-cm-long delivery wire. It is delivered by retracting the guiding catheter while holding the wire steady resulting in vessel occlusion when it resumes the cylindrical shape. It is detached by rotating the delivery wire counter-clockwise.
Peripheral vascular applications of the AVP have been reported in the literature4. Selection of a device approximately 30–50% larger than the vessel diameter is recommended. Since the AVP is a flexible nitinol wire mesh, it adjusts to the shape of the vessel and, consequently, oversizing prevents device migration after deployment. The AVP can be used alone for vascular occlusion or combined with other embolizing agents5.
In our case, we used the AVP to rapidly occlude an artery as the patient was hemodynamically unstable. Test occlusion in this case could not be done due to the patient’s critical condition. Technically, AVP is the easiest and safest of all occluding devices. This was life saving for our patient. In addition the cost of AVP embolization can be significantly lower than for coil embolization.
A major limitation of the AVP in the neck may be the possible risk of distal thromboembolism due to the flow through the porous body of the device until the complete occlusion of the artery is achieved. This can be minimized by using more than one AVP in a vessel. However in our case we had an immediate occlusion of the ICA and ECA after deployment of one AVP. But we deployed two AVP in the CCA for adequate coverage of the CB. Another limitation of this device is the requirement of distal placement of a 5 to 8F guiding catheter or sheath depending on the diameter of the vessel to be occluded.
Conclusion
In conclusion, the AVP can be used as an alternative device for fast occlusion of extracranial carotids especially in hemodynamically unstable patients. The possibility of distal thromboembolism should be kept in mind, and can be prevented by use of AVP in combination with other occlusion material or using two tandem AVPs in the same arterial segment to shorten the time required to achieve total thrombosis.
References
- 1.Citardi MJ, Chaloupak JC, Son YH, et al. Management of carotid artery rupture by monitored endovascular therapeutic occlusion (1988–1994) Laryngoscope. 1995;105:1086–1092. doi: 10.1288/00005537-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Scavee WJ, Mormont E, Coulier B, et al. “Pseudoaneurysm of the internal carotid artery: treatment with a covered stent.”. Cardiovasc Intervent Radiol. 2001;24:283–285. doi: 10.1007/s00270-001-0012-z. [DOI] [PubMed] [Google Scholar]
- 3.Lesley WS, Chaloupka JC, Weigele JB, et al. Preliminary experience with endovascular reconstruction for the management of carotid blowout syndrome. Am J Neuroradiol. 2004;24:975–981. [PMC free article] [PubMed] [Google Scholar]
- 4.Cil BE, Canyigit M, Ozkan OS, et al. Bilateral multiple pulmonary arteriovenous malformations: endovascular treatment with the Amplatzer vascular plug. J Vasc Interv Radiol. 2006;17:141–145. doi: 10.1097/01.rvi.0000186954.74462.ce. [DOI] [PubMed] [Google Scholar]
- 5.Geyik S, Cil BE, Yavuz K, et al. Neuroapplication of Amplatzer vascular plug: a novel device for parent artery occlusion. Neuroradiology. 2008;50(2):179–183. doi: 10.1007/s00234-007-0307-0. [DOI] [PubMed] [Google Scholar]
- 6.Chaloupka JC, Roth TC, Putman CM, et al. Recurrent carotid blowout syndrome: diagnostic and therapeutic challenges in a newly recognized subgroup of patients. Am J Neuroradiol. 1999;20:1069–1077. [PMC free article] [PubMed] [Google Scholar]
- 7.Maran AG, Amin M, Wilson JA. Radical neck dissection: a 19-year experience. J Laryngol Otol. 1989;103:760–776. doi: 10.1017/s002221510011000x. [DOI] [PubMed] [Google Scholar]
- 8.Charbel FT, Gonzales-Portillo G, Hoffman W, et al. Distal internal carotid artery pseudoaneurysms: technique and pitfalls of surgical management: two technical case reports. Neurosurgery. 1999;45:643–648. doi: 10.1097/00006123-199909000-00043. [DOI] [PubMed] [Google Scholar]
- 9.Mericle RA, Lanzino G, Wakhioo AK, et al. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery. 1999;43:1229–1234. doi: 10.1097/00006123-199811000-00130. [DOI] [PubMed] [Google Scholar]
- 10.Saket RR, Razavi MK, Sze DY, et al. Stent-graft treatment of extracranial carotid and vertebral arterial lesions. J Vasc Interv Radiol. 2004;15:1151–1156. doi: 10.1097/01.rvi.0000134496.71252. [DOI] [PubMed] [Google Scholar]