Summary
Faster recanalization correlates with better outcomes in acute ischemic stroke. We analyzed times from arrival in ER to end of treatment in patients undergoing endovascular treatment for acute ischemic stroke at our institution.
We retrospectively studied patients who underwent IA procedures for stroke from 2005 to 2009 noting the times of arrival to ER, CT scan, arrival to DSA, arterial puncture and recanalization from our endovascular database. A subgroup analysis was performed based on administration of GA, use of mechanical devices and whether the procedure was performed during regular hours or after hours.
Of 101 patients, 53 were male, with a median age of 66 years (range 18-87). There were 81 anterior circulation strokes. Median ER to CT time was 22 min (2-1025), CT to DSA arrival time 80 min (range 4-990), DSA arrival to puncture time 24 min (range 0-75) and puncture to recanalization time 84 min (range 11-206). 23.3% of patients had an ER to CT time interval of > 60 min and 71.3 % had a CT to DSA time interval of > 60 min contributing to significant in-hospital delays. For subgroup analysis the Mann-Whitney test was used. No significant differences in CT to DSA arrival (p=0.8), DSA arrival to puncture (p=0.1) and puncture to recanalization (p=0.59) times were noted between patients with and without GA. No significant difference was noted in puncture to recanalization times with or without device (p=0.78). 39 cases were done during regular (R) hours and 62 after (A) hours. Median ER to CT time (R=18 min, A = 27 min, p 0.02), CT to DSA arrival time (R=64 min, A=90 min, p 0.004) and DSA arrival to puncture time (R=18 min, A=25 min, p 0.003) was significantly higher after hours.
ER to CT and CT to DSA arrival times in patients undergoing endovascular stroke therapy show wide variability and therefore, considerable scope for reduction. Time differences during regular and after hours should serve as a reminder to make efforts to reduce overall ischemic times in spite of staffing patterns and resource availability.
Key words: stroke, thrombolysis, recanalization, tPA
Introduction
Thrombolysis for acute ischemic stroke has been validated by the NINDS trial1 and the importance of rapid treatment has been shown from analysis of pooled data which suggests that the chance of a favorable outcome after acute ischemic stroke decreases as the time interval from stroke onset to start of treatment increases2. Early recanalization has also been shown to salvage tissue at risk as defined by magnetic resonance imaging (MRI)3. Treatment algorithms for timely intervention in patients with acute ischemic stroke are complicated by various factors such as delay in stroke recognition at the community level4, late arrival to emergency rooms4 as well as heterogeneity of stroke mechanisms5.
Endovascular treatment methods to recanalize large vessel occlusions in acute ischemic stroke patients are being used increasingly where such expertise is available. Even in these patients considerable delays may occur before the patients are brought to the endovascular suite. Often, time is misperceived by the treating team and strategies to reduce time to recanalization are not easily recognized. We reviewed our data for patients undergoing endovascular treatment for acute ischemic strokes to identify areas of in-hospital delays that may potentially prolong time to recanalization and delay reperfusion of ischemic penumbra.
Materials and Methods
We retrospectively studied patients who underwent endovascular treatment for acute ischemic strokes at our institution from February 2005 to March 2009 with approval from the institutional review board. Data were collected from patient charts as well as from images for the procedure which are time-stamped in our picture archiving and communication system (PACS).
At our institution, patients for endovascular treatment are selected by a multidisciplinary approach involving the in-house stroke neurology fellow and the attending stroke neurologist as well as the neurointerventionalist. Patients who are ineligible for IV tPA and who demonstrate a proximal vessel occlusion on CT angiography with a baseline unenhanced CT scan showing no evidence of extensive ischemic changes based on ASPECTS scoring are chosen for endovascular therapy. Those patients who received iv tPA without improvement or worsening NIHSS scores are also included. Typical cutoff window for anterior circulation strokes is six hours from onset. For posterior circulation strokes, patients are chosen on an individual case-by-case basis.
To evaluate the procedural flow of patients through the hospital system we identified specific time points for various events such as time of arrival of the patient to the emergency room (ER time), the time of performance of the CT scan (CT time), time of arrival of patient to the angiography suite (DSA suite arrival time), the time of arterial puncture (puncture time) and in patients where recanalization occurred, the time of recanalization (recanalization time). Recanalization was defined as a thrombolysis in myocardial infarction (TIMI) score of 2 or 3 for the arterial occlusive lesion as defined by Khatri et al for the interventional management of stroke (IMS 1) investigators6. Interval times such as ER to CT, CT to DSA suite arrival, DSA suite arrival to puncture and puncture to recanalization were calculated in minutes for each patient. The aggregate data for the time interval from ER arrival to CT scan (ER to CT interval) excluded patients who had “in hospital” strokes and the time interval for arterial access to recanalization (puncture to recanalization interval) was restricted to those patients who demonstrated recanalization by the defined criteria. Average time intervals and variability for ER to CT (excluding in-hospital strokes), CT to DSA suite arrival, DSA suite arrival to puncture and puncture to recanalization (TIMI 2/3) were evaluated and described as summary statistics.
Further subcategorizing the dataset, we identified patients who had a procedure performed under general anesthesia (GA), those in whom an approved mechanical device was used for recanalization and those who had their procedure during regular hours (8 am to 5 pm on weekdays) or after hours. Median time intervals of each of the above categories were tabulated and analyzed using the Mann Whitney U non parametric test.
Results
Of 101 patients, 53 were male (52.4%), with a median age of 66 years (range 18-87). There was a predominance of anterior circulation strokes (80.2%). Fifty-one (50.5%) patients had some mechanical device used during the intra-arterial procedure. General anesthesia was administered in 30 (29.7%) patients and 39 (38.6%) procedures were done during regular hours (Table 1).
Table 1.
Patient demographics and distribution of stroke territory
| Instances (%) | ||
|---|---|---|
| Male patients | 53 | |
| Mean age (range) | 63.06 ± 14.05 (18-87) | |
| Stroke Territory | ||
| Anterior Circulation | ||
| ICA | 24 (23.8) | |
| M1 MCA | 45 (44.5) | |
| M2 MCA | 10 (9.9) | |
| M3 MCA | 1 (1) | |
| ACA | 1 (1) | |
| Posterior Circulation | ||
| Basilar | 19 (18.8) | |
| Vertebral Artery | 1 (1) | |
| Total Recanalization Rate | 65 (64.4) | |
| In-Hospital Strokes | 16 (15.8) | |
| Device Usage | 51 (50.5) | |
| General Anesthetic Administration | 30 (29.7) | |
| Regular Working Hours | 39 (38,6) | |
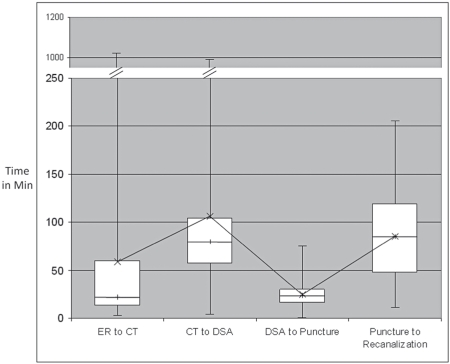
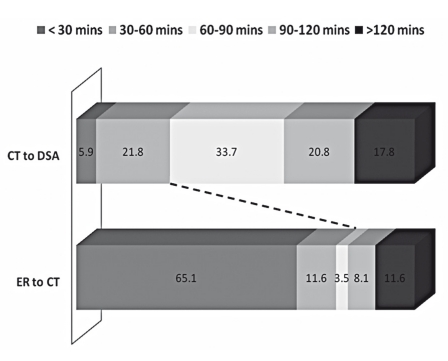
Median and range of interval times for ER to CT (excluding in-hospital strokes), CT to DSA suite, DSA suite to puncture and puncture to recanalization (TIMI 2/3) are depicted in Figure 1. Median ER to CT time was 22 min (range 2-1025), CT to DSA suite arrival time 80 min (range 4-990), DSA suite arrival to puncture time 24 min (range 0-75) and puncture to recanalization time 84 min (range 11-206). To understand time interval variability that contributes to in hospital delays specifically, we looked at two specific time intervals. Analysis of this ER to CT and CT to DSA suite intervals shows a wide range of variability. It is interesting to note that 20/86 (23.3%) have an ER to CT interval time > 60 mins and 72/101 (71.3%) have a CT to DSA interval time > 60 minutes. (Figure 2).
Figure 1.
Box plot showing time in minutes and variability of ER to CT time, CT to DSA suite arrival time, DSA to puncture time and puncture to recanalization time. The boxes represent the inter-quartile range, the line within each box represents the median and the interconnected x represents the mean. Note the difference between mean and median for the first two intervals, representing dispersion.
Figure 2.
Percentage-wise break up of ER to CT and CT to DSA intervals in 30 minute blocks (<30 min, 30-60 min, 60-90 min, 90-120 min and > 120 min).
For the various subgroup analyses, the time intervals were categorized based on administration of General anesthetic, use of an approved mechanical device during the procedure and performance of procedure during regular hours or after hours (Table 2). Interval time from ER arrival to CT scan showed a significant difference between regular hours and after hours (median 18 versus 27 minutes, p = 0.02) as did interval time from CT to DSA suite arrival (median 64 versus 90 minutes, p=0.004) and DSA suite arrival to puncture (median 18 versus 25 minutes, p = 0.003). There was also a significant difference between DSA suite arrival to puncture time between those patients in which a device was used versus those in which one wasn’t used (median 20 versus 27 minutes, p = 0.01). There was no significant difference in any interval times in patients in whom GA was used as against those in whom the procedure was performed using moderate sedation and local anesthesia. There was also no difference in puncture to recanalization time when a device was used (88 min) versus those when none was used (88 minutes versus 75 minutes, p = 0.78) (Table 2).
Table 2.
Median time interval in minutes and p values (Mann-Whitney U test) subcategorized based on administration of GA, intra-arterial use of device and occurrence during regular or after hours
| Recanalization | ER to CT | CT to DSA suite arrival |
DSA suite arrival to puncture |
Puncture to |
|---|---|---|---|---|
| GA versus no GA | ||||
| GA | 24 | 86 | 24 | 84 |
| No GA | 21 | 80 | 23 | 85 |
| p value | 0.31 | 0.8 | 0.1 | 0.59 |
| Device versus no device | ||||
| Device | 22 | 80 | 20 | 88 |
| No device | 23 | 80 | 27 | 75 |
| p value | 0.27 | 0.8 | 0.01 | 0.78 |
| Regular versus after hours | ||||
| Regular hours | 18 | 64 | 18 | 96 |
| After hours | 2 | 90 | 25 | 87 |
| p value | 0.02 | 0.004 | 0.003 | 0.34 |
Discussion
In the treatment of patients with acute ischemic strokes, the time interval from onset of symptoms to treatment is critical. The chance of a favorable outcome decreases with time as the ischemic penumbra proceeds to infarct and this was shown from the pooled analysis of data from the ATLANTIS, ECASS and NINDS tPA trials 2. In fact, delayed treatment in itself may be detrimental as suggested by animal data that late reperfusion results in higher rates of hemorrhagic conversion7. Secondary analysis of ECASS data for intravenous tPA administration showed that treatment three to six hours after onset has a higher rate of symptomatic hemorrhage than treatment within three hours8. Both the PROACT and PROACT II trials have shown benefit of intra-arterial thrombolysis in patients treated up to six hours after onset9,10 although a detailed time point analysis was not performed in those patients.
The most important conclusion from our study is that there is wide variability in time intervals from ER to CT and CT to DSA suite arrival for patients undergoing endovascular treatment procedures at our institution. These are the points in the time flow that contribute most to in-hospital delays offers an opportunity to shorten these interval times in the overall drive to reduce total ischemic times. Although there are some differences in DSA suite arrival to puncture time intervals between regular and after hours, the absolute values as well as variability is much less and therefore any gain made from shortening this time would be limited to a few minutes. The process of recanalization itself from the time of puncture is highly variable and maybe limited by factors such as vascular tortuosity, clot consistency and limitations of currently available technology for mechanical clot retrieval. With improvements in technology in the future, these time intervals may show some improvement in the overall effort to reduce recanalization times.
Our data show that 23.3% of patients with acute ischemic stroke who subsequently go on to have IA therapy take more than 60 minutes to have a CT scan after being triaged in ER. Delay in having a CT scan could be due to uncertainty regarding “stroke” as a diagnosis at triage, respiratory or hemodynamic compromise warranting intubation, airway support and overall stabilization before obtaining a CT scan. Sometimes, the CT scanner itself may not be immediately available due to it being used for other patients or technical faults. It is also interesting to note that forty percent (8/20) of patients who had ER to CT time more than 60 minutes had basilar artery strokes. Basilar artery strokes have a clinically variable presentation. Continuing medical education of triage and emergency medical staff in tertiary stroke centers by sensitizing them to the various presentations of basilar artery strokes is essential in reducing interval times in this potentially fatal disease.
Our data also show that 72.3% of patients have a CT to DSA suite arrival time more than 60 minutes. There could be various explanations for this phenomenon. These may include delays in coordinating the neurointerventional team including nursing and technical staff, especially after hours, availability of anesthesia personnel. More importantly there could be delays in the decision-making process to proceed with endovascular treatment including discussion with family members. Possible solutions to decrease this time interval may include contacting the treating team early in the time course, possibly having a member of the team evaluate the patient together with the acute stroke team. Having a critical care setup in the holding area of the angiography suite may prevent delays from having to take the patient to the ICU or stroke unit while awaiting the team to arrive.
The subgroup analysis, looking at differences in various time intervals with use of general anesthetic, mechanical device during procedures and occurrence during regular or after hours also showed a significant difference in ER to CT, CT to DSA suite arrival and DSA arrival to puncture time intervals between regular and after hours. This could be explained by issues such as after hours staffing patterns and resource availability. Our angiography team, including nursing and technical staff typically gets called in for emergent procedures after hours. There may be issues of availability of an angiography suite if another case is ongoing.
Another interesting finding is that there was no significant difference in interval times, especially DSA suite arrival to puncture time and puncture to recanalization times when procedures were performed under general anesthesia compared to those performed with moderate sedation and local anesthesia. This can be explained based on the fact that general anesthesia at our institution is more commonly employed for patients who are already intubated on arrival to the angiography suite and therefore the delays for typical anesthesia setup are minimized. We also note that there is no significant difference in puncture to recanalization times when a device was used compared to none being used. A possible explanation for this could be the nature and consistency of the thrombus. Soft non-adherent clots may be more amenable to local fibrinolytic therapy without the need for adjunctive device usage. With firmer clots that are adherent, it may require multiple attempts with a single device or use of a different device, which inherently involves a time penalty in the process. We cannot, however, explain the significant difference in DSA suite arrival to puncture times when a device was used compared to none being used.
There have been some reports of various aggregate times being studied for patients undergoing IA thrombolysis previously11,12. Kim et al.11 reported a median time interval of 303 minutes between symptom onset and start of procedure. Devlin et al.12 reported 150 min between symptom onset and hospital admission. Struffert et al.13 reported an average time interval of 151 minutes from last-known well to first angiography series. A few patients in the PROACT II trial have been treated with a door-to-drug time of less than one hour10. In the Interventional Management of Stroke trial (IMS-I), average time intervals for symptom onset to IV tPA administration, onset to angiogram and onset to IA tPA administration were 136 min, 183 min and 217 min respectively14. Analysis of time intervals are increasingly being incorporated into quality assurance programs. These could become important indicators of standards for certification of stroke programs.
In the coronary literature, shorter door to balloon times are associated with better survival in patients undergoing primary percutaneous coronary intervention and are used as hospital-level quality indicators for ST-elevation MI (STEMI)15,16. The importance of shortening total ischemic times in acute coronary syndromes has been emphasized with introduction of national level efforts to focus on systems improvement for achieving earlier recanalization17,18.
Our study has various limitations. It was performed in a retrospective manner with reliance on hospital charts and radiology imaging for accuracy of times. These may be subject to variations in documentation by staff members noting them. We did not factor in the time taken for the performance of CT angiography which theoretically could add a few more minutes to the overall time flow. While on the one hand it clearly adds a few minutes in acquiring and reconstructing the data, we think that information relating to aortic arch and vascular anatomy helps us anticipate access issues before the start of the procedure, helps us choose appropriate catheters and devices and may achieve faster overall times to recanalization.
Nevertheless, our results serve as a reminder to us that there is further room for improvement in our practice to reduce overall ischemic times and therefore improving patient outcome. This however needs to be convincingly proven in a prospective manner. Time goals for door to CT and door to needle times for IV thrombolysis do currently exist as best practice guidelines19. This needs to be broadened to include all patients with acute ischemic stroke, including those undergoing endovascular treatments.
Acknowledgments
Funding for the study was internally available through the Department of Diagnostic Imaging, Foothills Medical Center, University of Calgary
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 3.Jansen O, Schellinger P, Fiebach J, et al. Early recanalisation in acute ischaemic stroke saves tissue at risk defined by MRI. Lancet. 1999;353(9169):2036–2037. doi: 10.1016/S0140-6736(99)01146-0. [DOI] [PubMed] [Google Scholar]
- 4.Wester P, Rådberg J, Lundgren B, et al. Factors associated with delayed admission to hospital and in-hospital delays in acute stroke and TIA: a prospective, multicenter study. Seek-Medical-Attention-in-Time Study Group. Stroke. 1999;30(1):40–48. doi: 10.1161/01.str.30.1.40. [DOI] [PubMed] [Google Scholar]
- 5.Muir KW, Grosset DG. Neuroprotection for acute stroke: making clinical trials work. Stroke. 1999;30(1):180–182. doi: 10.1161/01.str.30.1.180. [DOI] [PubMed] [Google Scholar]
- 6.Khatri P, Neff J, Broderick JP, et al. IMS-I Investigators. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36(11):2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 7.Fagan SC, Garcia JH. Hemorrhagic transformation in focal cerebral ischemia: influence of time to artery reopening and tissue plasminogen activator. Pharmacotherapy. 1999;19(2):139–142. doi: 10.1592/phco.19.3.139.30932. [DOI] [PubMed] [Google Scholar]
- 8.Steiner T, Bluhmki E, Kaste M, et al. The ECASS 3-hour cohort. Secondary analysis of ECASS data by time stratification. ECASS Study Group. European Cooperative Acute Stroke Study. Cerebrovasc Dis. 1998;8(4):198–203. doi: 10.1159/000015851. [DOI] [PubMed] [Google Scholar]
- 9.del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998;29(1):4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. J Am Med Asso. 1999;282(21):2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 11.Kim D, Jahan R, Starkman S, et al. Endovascular mechanical clot retrieval in a broad ischemic stroke cohort. Am J Neuroradiol. 2006;27(10):2048–2052. [PMC free article] [PubMed] [Google Scholar]
- 12.Devlin TG, Baxter BW, Feintuch TA, et al. The Merci Retrieval System for acute stroke: the Southeast Regional Stroke Center experience. Neurocrit Care. 2007;6(1):11–21. doi: 10.1385/NCC:6:1:11. [DOI] [PubMed] [Google Scholar]
- 13.Struffert T, Köhrmann M, Engelhorn T, et al. Penumbra Stroke System as an “add-on” for the treatment of large vessel occlusive disease following thrombolysis: first results. Eur Radiol. 2009;19(9):2286–2293. doi: 10.1007/s00330-009-1390-x. Epub 2009 Apr 7. [DOI] [PubMed] [Google Scholar]
- 14.IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35(4):904–911. doi: 10.1161/01.STR.0000121641.77121.98. Epub 2004 Mar 11. [DOI] [PubMed] [Google Scholar]
- 15.Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N Engl J Med. 2007;357(16):1631–1638. doi: 10.1056/NEJMra065985. [DOI] [PubMed] [Google Scholar]
- 16.McNamara RL, Wang Y, Herrin J, et al. NRMI Investigators. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47(11):2180–2186. doi: 10.1016/j.jacc.2005.12.072. Epub 2006 May 15. [DOI] [PubMed] [Google Scholar]
- 17.Antman EM. Time is Muscle: Translation into practice. J Am Coll Cardiol. 2008;52:1216–1221. doi: 10.1016/j.jacc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Nallamothu BK, Krumholz HM, Peterson ED, et al. D2B Alliance and the American Heart Association Get-With-The-Guidelines Investigators. Door-to-balloon times in hospitals within the get-with-the-guidelines registry after initiation of the door-to-balloon (D2B) Alliance. Am J Cardiol. 2009;103(8):1051–1055. doi: 10.1016/j.amjcard.2008.12.030. Epub 2009 Feb 28. [DOI] [PubMed] [Google Scholar]
- 19.Practice advisory: thrombolytic therapy for acute ischemic stroke--summary statement. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1996;47(3):835–839. doi: 10.1212/wnl.47.3.835. [DOI] [PubMed] [Google Scholar]