Summary
We report our experience with endovascular treatment of supra-aortic arteries and follow-up results in patients with Takayasu’s arteritis (TA) presenting with neurological symptoms. Of the 20 patients with TA who underwent cerebral angiography for neurological manifestations between May 2002 and May 2009, 12 (11 females, one male; mean age, 39 years; range 31-56 years) underwent endovascular treatment and evaluated outcome for 21 lesions, including nine common carotid arteries, four vertebral arteries, four subclavian arteries, two internal carotid arteries, and one brachiocephalic artery. Eight patients underwent multiple endovascular procedures for different lesions in single or multiple stages. Mean angiographic and clinical follow-up durations were 34 months (range, 11-79 months) and 39 months (range 11-91 months), respectively.
Technical success was achieved for 20 procedures in 11 patients. One procedure failed, with 50% residual stenosis after stenting due to dense calcification of vessel walls. There were no procedure-related complications. Restenosis occurred at two lesions in two patients were treated by re-stenting. Asymptomatic occlusion occurred at two lesions in one patient. Ten patients remained in 0-1 on the modified Rankin scale (mRs) during mean 39 months. One patient, however, had a score of 3 on mRs due to a traumatic contusion during follow-up. One patient died from cardiac failure 36 months after successful angioplasty.
Our data suggest that endovascular treatment of symptomatic supra-aortic lesions of TA is effective and durable in selected patients with neurologic symptoms.
Key words: Takayasu’s arteritis, angiography, stroke, stenosis, angioplasty
Introduction
Takayasu’s arteritis (TA) is a chronic inflammatory arteriopathy of unknown etiology that most commonly affects the aorta and its major branches as well as the pulmonary artery. Clinical manifestations vary depending on the sites and severity of the occlusive vascular lesions1-3. The neurological manifestations of TA result primarily from decreased blood flow caused by steno-occlusive lesions in the arch and cervical arteries to the brain and/or shifting of the blood flow (steal). Collateral vessel formation is common, due to characteristic proximal arterial involvement and the slowly progressive nature of the disease. Therefore, neurological manifestations are most often associated with obstructive lesions of multiple arch and cervical arteries, occurring during the latest stages of disease progression4. These neurological manifestations, which have been reported in 57% to 80% of patients, may include headache, dizziness, visual disturbance or loss, stroke, and transient ischemic attack (TIA)2-8 and may be major causes of morbid events and even premature death4,7,9,10. In one study, 6.8% of patients with TA developed hemiplegia and 4.5% had loss of vision at a mean follow-up of 33.2±37 months9. Moreover, cerebrovascular events contributed to the deaths of 20% of these patients.
Management strategies for TA include medical therapy with steroids or immunosuppressive agents and revascularization procedures2,3,7,11-13. During the active phase of the disease, steroids have been shown to improve systemic inflammatory symptoms within a few days to weeks. Cytotoxic drugs such as methotrexate or cyclophosphamide have been used when steroids could not induce remission2,11,12. In the chronic stage, the principle of treatment is revascularization of the affected organ, either by surgery or endovascular treatment13-19. Although surgical treatment has been used to bypass the stenosed segment, the diffuse, proximal, and multifocal involvement of the arch vessels may make surgical revascularization difficult. Surgical repair of steno-occlusive lesions at the origin of arch vessels is much more complex because it requires an intrathoracic approach, as opposed to endarterectomy of cervical carotid bifurcation stenosis in atherosclerosis, and is often complicated by graft reocclusion, anastomotic site aneurysm, and/or morbidity.
Recent advances in endovascular treatment have made percutaneous transluminal balloon angioplasty and stenting feasible for inactive stenotic/occlusive arterial or aortic lesions due to TA13-17,20. Endovascular treatment is less invasive than surgical repair, as well as being a cost-effective and safe therapeutic option for the relief of stenotic lesions in patients with TA. There have been few studies, however, of endovascular treatment for lesions in the supra-aortic arteries that cause neurological manifestations and the long-term impact of endovascular treatment remains still uncertain. We report our experience with endovascular treatment of supra-aortic arteries for neurological manifestations due to TA and the long-term follow-up results.
Materials and Methods
Patients
Between May 2002 and May 2009, 20 patients with TA underwent digital subtraction cerebral angiography (DSA) for neurological manifestations were retrospectively reviewed from the prospective neurointerventional data registry. Of these, 12 consecutive patients (11 females, one male; mean age, 39 years; range, 31-56 years) underwent percutaneous transluminal balloon angioplasty (PTA) and/or stenting for stenotic lesions of supra-aortic arteries caused by TA and were included in this study.
TA was diagnosed according to the American College of Rheumatology Criteria for the Classification of TA21. The presence of at least three of the following six criteria was considered consistent with a diagnosis of TA: onset age <40 years, claudication of an extremity, decreased brachial artery pulse, >10 mmHg difference in systolic blood pressure between arms, a bruit over the subclavian arteries or the aorta, and arteriographic evidence of narrowing or occlusion of the entire aorta, its primary branches, or large arteries in the proximal upper and lower extremities.
Patients with active systemic disease, as manifested by symptoms such as fever, musculoskeletal pains, or increased erythrocyte sedimentation rate (ESR) received immunosuppressive therapy before endovascular treatment. ESR was measured by the Westergren method (Espette, Korea; reference range <15 mm/h in men and <20 mm/h in women). Prednisolone (1 mg/kg/d) was the first-line immunosuppressive drug, with the addition of methotrexate (7.5 mg/wk) in patients unresponsive to steroids. The doses of immunosuppressive agents were adjusted according to each patient’s ESR and clinical status. Endovascular treatment was performed after the ESR had been normalized in these patients.
Of the 12 included patients, five had ischemic stroke, three had TIA (transient hemiparesis, aphasia, or visual loss), three had dizziness and one had decreased vision. Twenty arteries were treated in these 12 patients including nine common carotid arteries (CCAs), four vertebral arteries, four subclavian arteries, two internal carotid arteries (ICAs), and one brachiocephalic artery. Our study protocol was approved by our Institutional Review Board.
Interventional Procedures
The patients in our study were premedicated daily with 100 mg aspirin and 75 mg clopidogrel for at least four days before endovascular treatment. Following the procedure, patients were continued on 100 mg/day aspirin indefinitely and 75 mg/day clopidogrel for at least three months. All procedures were performed with the patients under local anesthesia with sedatives, and electrocardiogram, arterial oxygen saturation, and blood pressure parameters were appropriately monitored. After a 6 to 9F sheath was introduced into the femoral artery, the baseline activated clotting time was obtained. A bolus injection of 50 to 80 IU/kg of heparin was followed by 500-1000 IU/hr as necessary to achieve an activated clotting time around 200 seconds. Using standard catheterization techniques, a 6F to 9F guiding catheter was introduced into the proximal portion of the stenotic artery. In all patients except those with occluded subclavian arteries, the stenosis was initially crossed by a 0.014-inch microguidewire; in some patients, a filter-type protection device was deployed in the cervical portion of the ICA and vertebral artery. If the lesion was too narrow and tortuous for a microguidewire, the latter was manipulated to pass the lesion using a microcatheter and the wire was then replaced by a 0.014-inch microguidewire of intermediate stiffness or with a tapered tip, followed by removal of the microcatheter22. The balloon ranged from 4 to 7 mm in diameter and 15 to 20 mm in length. The balloon was inflated two to three times at six to 12 atmospheres for ten to 20 seconds until its waist disappeared. If stenting was to be performed, a self-expandable stent delivery catheter was advanced over the immobilized microguidewire or filterwire after predilatation. After stent deployment, postdilatation was performed using a 5 mm or 8 mm diameter angioplasty balloon if residual stenosis was >30%. For occluded subclavian arteries, an additional radial approach was used. To obtain access, the ipsilateral radial artery was punctured, a 4F sheath was introduced proximally into the radial artery, and the occluded site was probed using a 4F catheter and a 0.035-inch guidewire through the radial route23. After this system passed the occluded site, the microguidewire located in the subclavian artery proximal to the occluded site could be advanced, using the femoral route, to the axillary artery, followed by stent delivery through this microguidewire and its successful deployment24.
Following angioplasty or stenting, selective angiography was performed to determine the degree of dilation and whether dissection had occurred and to evaluate the intracranial circulation for signs of distal embolization25.
Definitions and Follow-up
Type of stenosis was classified, based on the length of the stenotic lesion, as focal (<2 cm) or diffuse (≥2 cm). An intervention was considered technically successful if the residual stenosis was <30%. Long-term success of the endovascular procedure was documented by digital subtraction angiography, CT angiography, MR angiography or Doppler ultrasound. Restenosis was defined by the presence of symptom recurrence and >50% restenosis on follow-up angiography.
Results
The demographic, clinical and angiographic findings and date, site, method and endovascular treatment device of individual patients are summarized in Table 1 and the ESR and type of lesion are summarized in Table 2. Five PTAs and 16 stentings were performed for 21 lesions of these 12 patients. Thirteen lesions were diffuse and eight were focal (Table 2). Five patients underwent multiple endovascular procedures for different lesions in a single session. Two patients underwent staged procedures at two sessions, one (patient 1) for a restenosis and the second (patient 7) for a progressed stenosis, and one patient underwent staged procedures during three sessions due to new neurological symptoms (patient 2) (Figure 1). The ESR was increased in six patients (50%) (Table 2).
Table 1.
Neurological manifestations, angiographic findings and endovascular treatments.
| Case No. |
Age/ Sex |
Symptom | Mechanism of Sx and rationale for recanalization |
Angiographic findings (% stenosis) | Procedure site | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBCA | RCCA | RICA | RVA | RSCA | LCCA | LICA | LVA | LSCA | |||||
| 1 | 33/F | R hemiplegia | Stroke (left MCA infarct) Restenosis |
N | 70% 70% |
N | 90% | O | O | O | O | O | RVA, RCCA RCCA |
| 2 | 38/F | TIA, Vis Dist | Repeated TIA, prior to AVR LSCA steal |
N | O open† |
O | 80% | 70% | D | 70% | O | O | RVA, LICA LSCA, RCCA |
| 3 | 32/F | Dizzi, Vis Dist | LCCA steal | N | N | N | N | N | 80% | N | N | N | LCCA |
| 4 | 35/F | L hemiplegia | Stroke (right PCA infarct) | N | O | O | N | 50% | O | O | 80% | 50% | LVA |
| 5 | 36/F | Dizzi, Vis Dis | Both p-com and RSCA steal | O | O | O | O | O | O | O | 80% | O | LSCA |
| 6 | 31/F | Dizzi | RCCA steal | N | 80% | N | N | N | N | N | N | N | RCCA |
| 7 | 56/M | R hemiplegia | Stroke (left BG infarct) Progressive stenosis |
99% good‡ |
N 80% |
N N |
N N |
N N |
O O |
N O |
80% good‡ |
50% 50% |
LVA, RBCA RCCA |
| 8 | 39/F | Dizzi, Vis Dist | RCCA & LSCA steal | N | 80% | N | N | N | N | N | N | O | RCCA |
| 9 | 49/F | R hemiplegia | Previous stroke (left brain) | N | 40% | N | N | N | 80% | N | N | O | LCCA |
| 10 | 34/F | TIA | TIA & prior to AVR | O | O | O | O | O | N | 80% | O | O | LICA |
| 11 | 34/F | R hemiplegia | Stroke (left pontine infarct) | N | N | N | N | 80% | N | N | N | 80% | LSCA, RSCA |
| 12 | 52/F | TIA | Progressive TIA | N | 80% | N | O | O | 80% | N | O | O | RCCA, LCCA |
|
† The occluded right CCA was recanalized by small vascular channels on follow-up angiogram. ‡ Good means a state of good preservation without restenosis of the stented artery. Numbers in columns of angiographic finding are percent stenosis of the arteries. Dizzi, dizziness; Vis Dist, visual disturbance; Sx, symptom; AVR, aortic valve replacement; p-com, posterior communicating arter; L, left; LCCA, left common carotid artery; LICA, left internal carotid artery; LVA, left vertebral artery; LSCA, left subclavian artery; N, normal; O, occlusion; RBCA, right brachiocephalic artery; RCCA, right CCA, RICA, right ICA; RVA, right VA; RSCA, right SCA. | |||||||||||||
Table 2.
ESR at procedure, type of lesion and immediate and follow-up results.
| Case No |
ESR (mm/h) |
Date | Site | Type of lesion |
Method | Device (mm) |
Technical Success |
Cx | Angiographic F/U | Clinical F/U | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | Tool | Results | Period | Results (mRs) | |||||||||
| 1 | 40 | 2002-07-11 2002-07-11 2003-04-03 |
RVA RCCA RCCA |
Focal Diffuse Diffuse |
PTA PTA Stent |
Balloon 4×15 Balloon 4×15 Precise 7×40 |
Success Success Success |
No No No |
71 9 62 |
DUS DSA DUS |
Good distal flow 60% restenosis No restenosis |
83 | Improve (1) |
| 2 | 73 | 2002-10-21 2003-01-22 2009-05-28 2009-05-28 |
RVA LICA LSCA RCCA |
Focal Diffuse Diffuse Diffuse |
PTA Stent Stent Stent |
Balloon 4×20 Smart 8×60 Precise 8×30 Wallstent7×50 |
Success Success Success Success |
No No No No |
79 79 11 11 |
DSA DSA CTA CTA |
No restenosis No restenosis No restenosis No restenosis |
91 | Improve (1) |
| 3 | 19 | 2003-06-19 | LCCA | Diffuse | Stent | Smart 7×80 | Success | No | 11 | CTA | No restenosis | 13 | Improve (1) |
| 4 | 44 | 2004-02-16 | LVA | Focal | PTA | Balloon 5×20 | Success | No | 36 | Death (6) | |||
| 5 | 4 | 2004-11-29 | LSCA | Diffuse | PTA | Balloon 7×20 | Success | No | 19 | MRA | No restenosis | 32 | Improve (0) |
| 6 | 30 | 2005-10-14 | RCCA | Diffuse | Stent | Zilver 8×50 | Success | No | 44 | DUS | 50% restenosis | 44 | Improve (0) |
| 7 | 7 | 2005-11-10 2005-11-10 2006-04-25 |
LVA RBCA RCCA |
Focal Diffuse Diffuse |
Stent Stent Stent |
Precise 5×30 Precise 10×40 Precise 8×20 |
Success Success Success |
No No No |
46 46 39 |
CTA CTA CTA |
No restenosis Occlusion Occlusion |
55 | SAH (3) |
| 8 | 25 | 2005-12-14 | RCCA | Diffuse | Stent | Precise 9×30 | 50% residual |
No | 42 | DUS | No change | 42 | Improve (1) |
| 9 | 21 | 2006-10-16 | LCCA | Diffuse | Stent | Precise 9×40 | Success | No | 32 | DUS | No restenosis | 32 | Improve (0) |
| 10 | 16 | 2007-12-31 | LICA | Focal | Stent | Precise 9×30 | Success | No | 12 | DUS | No restenosis | 16 | Improve (1) |
| 11 | 7 | 2008-05-15 2008-05-15 |
LSCA RSCA |
Diffuse Focal |
Stent Stent |
Precise 8×30 Precise 8×30 |
Success Success |
No No |
12 12 |
CTA CTA |
No restenosis No restenosis |
13 | Improve (0) |
| 12 | 10 | 2009-02-16 2009-02-16 |
RCCA LCCA |
Focal Focal |
Stent Stent |
Wallstent 7×40 Wallstent 7×40 |
Success Success |
No No |
11 11 |
DUS DUS |
No restenosis No restenosis |
11 |
Improve (0) |
|
CTA, CT angiography; DUS, Doppler ultrasound; MRA, MR angiography; LCCA, left CCA; LICA, left ICA; LVA, left vertebral artery; LSCA, left subclavian artery; RBCA, right brachiocephalic artery; RCCA, right CCA, RICA, right ICA; RVA, right vertebral artery; RSCA, right subclavian artery; SAH, subarachnoid hemorrhage | |||||||||||||
Figure 1.
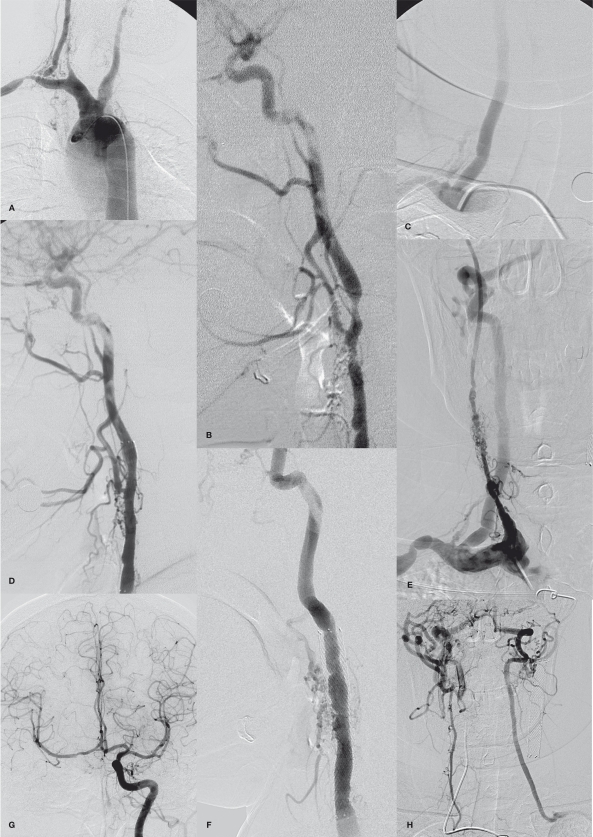
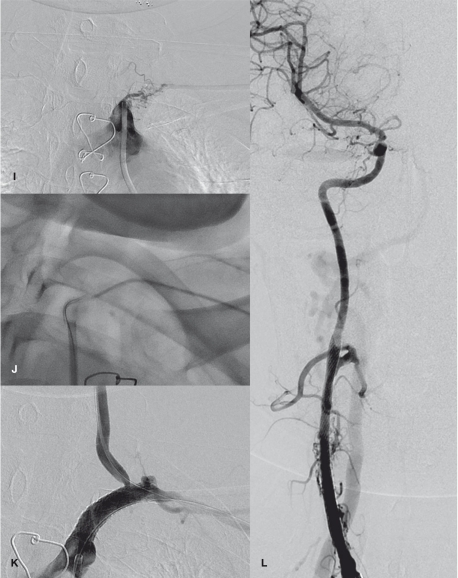
A 38-year-old woman with TA presenting with recurrent loss of consciousness and dizziness due to multiple steno-occlusion in neck vessels and both subclavian and both common carotid artery (CCA) steal phenomena. Initial aortogram (A) and the left CCA angiogram (B) revealed severe stenoses in the right vertebral artery (VA) ostium and in the left internal carotid artery (ICA) bifurcation. The right CCA and the left subclavian arteries are occluded. (C) Angioplasty of the right VA ostium reveals good patency resulting in improved consciousness. (D) One year later, the left ICA lesion was stented to increase perfusion of the cerebrum to improve her residual dizziness caused by the subclavian steal phenomenon. Brachiocephalic arteriogram (E), the left CCA-gram (F, G), the left subclavian arteriogram (H), and (I) right VA grams obtained 6 years later shows good preservation of the ostium of the right VA (E) and the stented lumen of the left ICA (F). There was a spontaneous recanalization of the right CCA with fine vascular channels (E). The right cerebral hemisphere was supplied by collaterals via the anterior communicating artery from the left ICA and also via the external carotid artery from the right VA (G, H). Also note the left subclavian steal due to the left subclavian artery occlusion (H). Using dual access routes via the femoral and radial arteries (I, J), the occluded left subclavian artery was recanalized by angioplasty followed by stenting (K). Good patency of the right CCA was obtained after stenting and resulted improvement of her visual disturbance (L). She was clinically stable during next 11 months without further symptom presentation.
The immediate and follow-up angiographic and clinical results are summarized in Table 2. Technical success was achieved in 20 procedures in 11 patients. One procedure failed with 50% residual stenosis due to dense calcification of the vessel walls (patient 8). There were no procedure-related complications. Angiographic follow-up was obtained for 19 lesions of 11 patients at a mean follow-up of 34 months (range 11-79 months). Restenosis occurred at two lesions in two patients (10%) without symptom recurrence; in the CCA of patient 1 nine months after PTA, treated by stenting, and in the CCA of patient 6, 44 months after stenting. Occlusion occurred at two lesions in one patient (patient 7). Mean clinical follow-up duration was 39 months (range 11-91 months). Neurological symptoms of ten patients were improved, as shown by a mRs score of 0-1. One patient died from cardiac failure 36 months after PTA (patient 4). One patient (patient 7) had a minor stroke three months later and returned to normal status; 41 months after stenting, this patient presented with a traumatic subarachnoid hemorrhage caused by fainting, CTA at the time of hemorrhage revealed a patent left vertebral artery ostial stent but occlusion of the right ICA and CCA stents. This patient’s final neurologic status was mRs=3. Before the hemorrhage, he had been followed due to diverse clinical problems but was not followed for the stent luminal patency. He had been taking aspirin 100 mg/day and clopidogrel 75 mg/day without developing clopidogrel resistance.
Discussion
We have shown here that PTA and/or stenting of supra-aortic arteries provided good results in patients with TA presenting with neurological manifestations. Lesions dilated by endovascular treatment were well preserved angiographically during a follow up period of 11 months to six years, and these patients led lives stable for 11 months to eight years after endovascular treatment without developing new or recurrent neurological manifestations. There- fore, one- or multi-staged PTA and/or stenting of supra-aortic arteries in patients with TA presenting with neurological manifestations seems to be feasible and durable.
Endovascular treatment has emerged as the initial mode of treatment for stenosis of the aorta, renal, and subclavian arteries caused by TA17,26-28. Less is known, however, about the role of endovascular treatment for lesions in the carotid, brachiocephalic, and vertebral arteries7,10,29-31. One case report showed an excellent ten-year outcome after PTA for lesions of the brachiocephalic artery and CCA29, and PTA and stenting of 12 lesions of arch arteries for severe cerebral ischemia gave good results during follow-up periods of three months to 49 months7. Symptomatic restenosis of two patients with diffuse-type stenosis at stenting was successfully retreated by cutting balloon angioplasty7. We also observed restenosis in two lesions with diffuse stenosis; one, initially treated with PTA, was retreated by stenting, and the second, initially treated by stenting, was treated by medication because it was asymptomatic. These restenotic lesions were tolerable clinically during follow-up of 44-83 months. The results of these two studies showed that diffuse lesions of arch vessels are associated with a higher rate of restenosis than focal lesions and that PTA of these restenotic lesions, using cutting balloon and stenting, results in good long-term outcomes. Previous studies have shown good results in patients undergoing stenting of multiple vessels in a single session7,10,29,32,33. The multiplicity of progressive lesions is challenging in the management of TA, with endovascular treatment taking priority over surgery. In our study, five out of 12 patients underwent multiple endovascular procedures in a single session, whereas three patients underwent multiple procedures during two or three sessions due to progressive stenosis, new neurological symptoms and/or restenosis. None of these procedures showed evidence of technical failure, and the results of long-term clinical follow-up were good. We observed a significant residual stenosis after stenting due to dense calcification of the vessel walls. Another study reported a technical failure in stenting of an occluded subclavian artery due to an inability to cross the occluded segment33. In TA, unlike atherosclerotic lesions, the vessels are firm, scarred, nonulcerated and fibrotic. Thus, arch vessels require higher balloon inflation pressure or a cutting balloon during angioplasty. Revasculariz- ation of the occluded vessels is often crucial in patients with TA. The hard nature of the occluded segment is a main cause of technical failure. If the subclavian artery is occluded, an approach in which the occluded segment is crossed by dual access routes from the leg and arm may increase technical success23.
Because ESR at the time of admission may affect the results of endovascular treatment, it is important to strictly control active disease by administering immunosuppressive agents before and after endovascular treatment. On an emergency basis, patients should be administered steroids prior to endovascular procedures and steroids should be continued for prolonged periods thereafter31. The intensity and duration of the immunosuppressive regimen should be determined according to each patient’s clinical condition and disease activity. We therefore administered prednisolone to patients with increased ESR before endovascular treatment, adding methotrexate if a patient was unresponsive to steroids. We found that this protocol was successful in lowering the ESR before intervention. Although our study is limited by the small number of subjects, we assessed the long-term results of endovascular treatment for supra-aortic lesions in patients with TA.
Conclusions
Single- or multi-staged procedures for different lesions of TA are often needed in patients with neurologic manifestations which are dependent on each patient’s hemodynamic status. In conjunction with medical treatment, PTA and/or stenting of supra-aortic arteries in TA is feasible and durable, and provides good symptomatic relief in patients with multiple steno-occlusive lesions of supra-aortic arteries.
Acknowledgements and Funding
We acknowledge the assistance of Sun Moon Whang, B.S. and Eun Hye Kim, R.N. in the collection of patient data as well as that of Yun Gyeong Jeong in preparing the manuscript. This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A080201).
References
- 1.Arnaud L, Kahn JE, Girszyn N, et al. Takayasu’s arteritis: An update on physiopathology. Eur J Intern Med. 2006;17(4):241–246. doi: 10.1016/j.ejim.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Ogino H, Matsuda H, Minatoya K, et al. Overview of late outcome of medical and surgical treatment for Takayasu arteritis. Circulation. 2008;118(25):2738, 2727–2747. doi: 10.1161/CIRCULATIONAHA.107.759589. [DOI] [PubMed] [Google Scholar]
- 3.Kerr G. Takayasu’s arteritis. Curr Opin Rheumatol. 1994;6(1):32–38. doi: 10.1097/00002281-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Suh DC, Kim JK, et al. Correlation of neurological manifestations of Takayasu’s arteritis with cerebral angiographic findings. Clin Imaging. 2005;29(2):79–85. doi: 10.1016/j.clinimag.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa K. Natural history and classification of occlusive thromboaortopathy (Takayasu’s disease) Circulation. 1978;57(1):27–35. doi: 10.1161/01.cir.57.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa K. Survival and morbidity after diagnosis of occlusive thromboaortopathy (Takayasu’s disease) Am J Cardiol. 1981;47(5):1026–1032. doi: 10.1016/0002-9149(81)90208-3. [DOI] [PubMed] [Google Scholar]
- 7.Tyagi S, Gupta MD, Singh P, et al. Percutaneous revascularization of sole arch artery for severe cerebral ischemia resulting from Takayasu arteritis. J Vasc Interv Radiol. 2008;19(12):1699–1703. doi: 10.1016/j.jvir.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Churg J. Large vessel vasculitis. Clin Exp Immunol. 1993;93(Suppl 1):11–12. doi: 10.1111/j.1365-2249.1993.tb06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, et al. Takayasu’s arteritis. Clinical study of 107 cases. Am Heart J. 1977;93(1):94–103. doi: 10.1016/s0002-8703(77)80178-6. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi JC, Sakai N, Manaka H, et al. Multiple supra-aortic stenting for Takayasu arteritis: extensive revascularization and two-year follow-up. Am J Neuroradiol. 2002;23(5):790–793. [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman GS. Treatment of resistant Takayasu’s arteritis. Rheum Dis Clin North Am. 1995;21(1):73–80. [PubMed] [Google Scholar]
- 12.Ishikawa K. Effects of prednisolone therapy on arterial angiographic features in Takayasu’s disease. Am J Cardiol. 1991;68(4):410–413. doi: 10.1016/0002-9149(91)90845-c. [DOI] [PubMed] [Google Scholar]
- 13.Martin EC, Diamond NG, Casarella WJ. Percutaneous transluminal angioplasty in non-atherosclerotic disease. Radiology. 1980;135(1):27–33. doi: 10.1148/radiology.135.1.6127750. [DOI] [PubMed] [Google Scholar]
- 14.Yagura M, Sano I, Akioka H, et al. Usefulness of percutaneous transluminal angioplasty for aortitis syndrome. Arch Intern Med. 1984;144(7):1465–1468. [PubMed] [Google Scholar]
- 15.Khalilullah M, Tyagi S, Lochan R, et al. Percutaneous transluminal balloon angioplasty of the aorta in patients with aortitis. Circulation. 1987;76(3):597–600. doi: 10.1161/01.cir.76.3.597. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Han MC, Kim SH, et al. Takayasu arteritis: angiographic findings and results of angioplasty. Am J Roentgenol. 1989;153(5):1069–1074. doi: 10.2214/ajr.153.5.1069. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Gupta H, Saxena A, et al. Results of renal angioplasty in nonspecific aortoarteritis (Takayasu disease) J Vasc Interv Radiol. 1998;9(3):429–435. doi: 10.1016/s1051-0443(98)70294-5. [DOI] [PubMed] [Google Scholar]
- 18.Tada Y, Sato O, Ohshima A, et al. Surgical treatment of Takayasu arteritis. Heart Vessels Suppl. 1992;7:159–167. doi: 10.1007/BF01744563. [DOI] [PubMed] [Google Scholar]
- 19.Gu YQ, Wang ZG. Surgical treatment of cerebral ischaemia caused by cervical arterial lesions due to Takayasu’s arteritis: preliminary results of 49 cases. ANZ J Surg. 2001;71(2):89–92. doi: 10.1046/j.1440-1622.2001.01998.x. [DOI] [PubMed] [Google Scholar]
- 20.Dong ZJ, Li SH, Lu XC. Percutaneous transluminal angioplasty for renovascular hypertension in arteritis: experience in China. Radiology. 1987;162(2):477–479. doi: 10.1148/radiology.162.2.2879316. [DOI] [PubMed] [Google Scholar]
- 21.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Choi BS, Choi JW, et al. Stent implantation of multichanneled pseudoocclusion of the internal carotid artery. J Vasc Interv Radiol. 2009;20(3):391–395. doi: 10.1016/j.jvir.2008.12.413. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Jung JH, Kwon H-J, et al. Landmark–wire technique of symptomatic subclavian artery occlusion. Interventional Neuroradiology. 2009;15(4):401–405. doi: 10.1177/159101990901500404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang KQ, Wang ZG, Yang BZ, et al. Long-term results of endovascular therapy for proximal subclavian arterial obstructive lesions. Chin Med J (Engl) 2010;123(1):45–50. [PubMed] [Google Scholar]
- 25.Suh DC, Kim JK, Choi JW, et al. Intracranial stenting of severe symptomatic intracranial stenosis: results of 100 consecutive patients. Am J Neuroradiol. 2008;29(4):781–785. doi: 10.3174/ajnr.A0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyagi S, Kaul UA, Nair M, et al. Balloon angioplasty of the aorta in Takayasu’s arteritis: initial and long-term results. Am Heart J. 1992;124(4):876–882. doi: 10.1016/0002-8703(92)90967-z. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi S, Singh B, Kaul UA, et al. Balloon angioplasty for renovascular hypertension in Takayasu’s arteritis. Am Heart J. 1993;125(5):1386–1393. doi: 10.1016/0002-8703(93)91012-4. Pt 1. [DOI] [PubMed] [Google Scholar]
- 28.Tyagi S, Verma PK, Gambhir D, et al. Early and long-term results of subclavian angioplasty in aortoarteritis (Takayasu disease): comparison with atherosclerosis. Cardiovasc Intervent Radiol. 1998;21(3):219–224. doi: 10.1007/s002709900248. [DOI] [PubMed] [Google Scholar]
- 29.Murakami R, Korogi Y, Matsuno Y, et al. Percutaneous transluminal angioplasty for carotid artery stenosis in Takayasu arteritis: persistent benefit over 10 years. Cardiovasc Intervent Radiol. 1997;20(3):219–221. doi: 10.1007/s002709900141. [DOI] [PubMed] [Google Scholar]
- 30.Staller BJ, Maleki M. Percutaneous transluminal angioplasty for innominate artery stenosis and total occlusion of subclavian artery in Takayasu’s-type arteritis. Cathet Cardiovasc Diagn. 1989;16(2):91–94. doi: 10.1002/ccd.1810160204. [DOI] [PubMed] [Google Scholar]
- 31.Sharma BK, Jain S, Bali HK, et al. A follow-up study of balloon angioplasty and de-novo stenting in Takayasu arteritis. Int J Cardiol. 2000;75(Suppl 1):S147–S152. doi: 10.1016/s0167-5273(00)00192-3. [DOI] [PubMed] [Google Scholar]
- 32.Pyun HW, Suh DC, Kim JK, et al. Concomitant multiple revascularizations in supra-aortic arteries: short-term results in 50 patients. Am J Neuroradiol. 2007;28(10):1895–1901. doi: 10.3174/ajnr.A0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaida H, Sakai N, Nagata I, et al. [Stenting for the occlusive carotid and subclavian arteries in Takayasu arteritis] No Shinkei Geka. 2001;29(11):1033–1041. [PubMed] [Google Scholar]