Abstract
Neuroblastoma (NB) is the second most common extracranial tumour of childhood. Angiogenesis plays a crucial role in the growth and development of NB and Vascular Endothelial Growth Factor (VEGF), one of the most potent angiogenic stimuli of angiogenesis, has been studied extensively in vitro. VEGF165 has been shown to be the predominant angiogenic isoform expressed in NB cell lines and tumours. In this study, we investigated the anti-angiogenic isoform of VEGF-A, generated from distal splice site selection in the terminal exon of VEGF (VEGF165b) and shown to be down-regulated in epithelial malignancies. The expression of both the pro- (VEGFxxx) and anti-angiogenic isoforms (VEGFxxxb) was compared in a range of NB tumours and ganglioneuromas (GN). Whereas VEGFxxxb and VEGFxxx were both expressed in GN, specific up-regulation of the VEGFxxx isoforms was seen in NB at RNA and protein levels. Highly tumorigenic NB cell lines also showed up-regulation of the angiogenic isoforms relative to VEGFxxxb compared to less tumorigenic cell lines, and the isoforms were differentially secreted. These results indicate that VEGF165 is up-regulated in NB and that there is a difference in balance of isoform expression from anti-angiogenic VEGF165b to angiogenic VEGF165. Treatment with recombinant human VEGF165b significantly reduced the growth rate of established xenografts of SK-NB-E(2)C cells (4.24-± 1.01 fold increase in volume) compared with those treated with saline (9.76± 3.58, p<0.01, ANOVA). Microvascular density (MVD) was significantly decreased in rhVEGF165b-treated tumours (19.4±1.9 vessels/mm3) in contrast to the saline-treated tumours (45.5±8.6 vessels/mm3). VEGF165b had no significant effect on the proliferative or apoptotic activity, viability or cytotoxicity of SK-NB-E(2)C cells after 48 hours. In conclusion, VEGF165b is an effective inhibitor of NB growth. These findings provide the rationale for further investigation of VEGF165b in NB and other paediatric malignancies.
Keywords: neuroblastoma, vascular endothelial growth factor, VEGF165b, VEGFxxx, balance of isoforms
INTRODUCTION
Neuroblastoma (NB) is the second most common extra-cranial paediatric cancer and is responsible for approximately 15% of all childhood cancer deaths [1]. The clinical hallmark of NB is heterogeneity, with the likelihood of tumour progression varying widely according to age at diagnosis and anatomic stage, pathologic and molecular biologic features [1]. Neuroblastic tumours display a wide range of differentiation. At one end of the spectrum are the undifferentiated NBs with a uniformly poor prognosis. At the other end are benign ganglioneuromas (GNs), which are composed of mature ganglion cells and Schwann cells. The most important biological feature in NBs is MYCN amplification. It occurs in up to 25% of primary untreated NBs and is highly correlated with advanced stage, rapid disease progression and a poor prognosis.
In contrast to the excellent 95% five-year survival rate in infants, it is only 40% in children more than one year of age (Janoueix-Lerosey 2010). Clearly, new treatment strategies are needed for children with disseminated, high-risk disease. Novel treatment approaches that are being evaluated include inhibitors of angiogenesis [2,3], a biologic process whereby new blood vessels are formed by tumours. Angiogenic factors, especially vascular endothelial growth factor, VEGF, and its receptors have been studied extensively in NB cell lines and tumours [4-12]. The studies have not however, discriminated between the two families of VEGF isoforms. The pro-angiogenic isoforms generated by use of a proximal slice site (PSS) in the terminal exon, exon 8, (VEGFxxx) and the anti-angiogenic isoforms, generated by use of a more distal splice site in exon 8 (VEGFxxxb). The first description of the anti-angiogenic family of VEGFxxxb isoforms involved VEGF165b, the predominant splice variant. It is a competitive inhibitor of VEGF165 and has been shown to inhibit VEGF165-induced endothelial proliferation, migration, vasodilatation and in vivo angiogenesis [13-16]. Furthermore, VEGF165b is down-regulated in various adult cancers [13,16] and its overexpression inhibits the growth of a variety of human tumour xenografts in mice [13,16-19].
The aim of this study was two-fold: firstly, to compare the expression levels of the pro- and anti-angiogenic isoforms of VEGF in neuroblastic tumour samples and in cell lines, and secondly, to study the biological properties of rhVEGF165b in NB cell lines in vitro and in NB xenografts.
METHODS
TISSUE SAMPLES
Thirty neuroblastic tumours were obtained from the archives of the Department of Histopathology, Bristol Royal Infirmary, Bristol, UK. These comprised 9 GN and 21 NB, of which 6 were MYCN amplified. All the snap-frozen samples showed at least 60% viable tumour. A representative formalin-fixed block was selected for immunohistochemical staining from each tumour. Full ethical approval was obtained from North Somerset and South Bristol Research Ethics Committee for the use of anonymised samples (REC reference number 07/Q2006/49).
CELL LINES
SK-N-BE(2)C, SH-IN, SHEP and SH-SY5Y cell lines, a kind gift from Dr. Barbara A. Spengler (Fordham University, New York, USA), were grown in DMEM-F12 1:1 Glutamax medium (Invitrogen). IMR-32 cell line, purchased from EACACC, was grown in MEM medium with 2mM L-glutamine (Sigma). Cell lines were grown at 37°C at 5% CO2 in media with 10% fetal bovine serum, 50 U/ml penicillin and 50 μg/ml streptomycin (Sigma), and 1% MEM non-essential amino acid solution (Sigma). The properties of the cell lines [20] are summarised in Table 1.
Table 1. Neuroblastoma cell lines.
The cell types are named N for neuroblastic, S for non-neuronal, substrate-adherent, and I for the cell type that is intermediate in morphology between N and S. S-cells form no tumours in nude mice. In contrast, N-type cells form tumours in athymic mice with a frequency of 12%, I-type cells have the highest malignant potential, forming tumours in 80% of mice. SH-IN, SH-SY5Y and SH-EP are subclones of the same cell line: SK-N-SH (20).
| CELL LINE | CELL TYPE | MYCN | TUMORIGENESIS |
|---|---|---|---|
| BE(2)-C | I-type | Amplified | Yes |
| IMR-32 | N-type | Amplified | Yes |
| SH-IN | I-type | Non amplified | Yes |
| SH-SY5Y | N-type | Non amplified | Yes |
| SH-EP | S-type | Non amplified | No |
RNA PURIFICATION, REVERSE TRANSCRIPTION, PCR and qPCR AMPLIFICATION
Total RNA was extracted from cell lines and tumour samples using TRI reagent (Sigma) according to manufacturer’s instructions. Two μg of RNA was treated with RNAse-free DNase (Promega) and reverse transcribed with M-MLV reverse transcriptase using oligo dT primers and random hexamers (Promega, UK).
For conventional PCR, 200 ng of cDNA was used with FastStart Universal SYBR Green Mastermix (Rox) 2x (Roche Diagnostics) and 0.8 μM of each primer. The forward primer was in exon 7 and a reverse primer in exon 8b, allowing the amplification of both isoforms in the same PCR reaction. All samples were run in parallel with negative controls, no cDNA or reverse transcriptase, and two positive controls, VEGF165 pcDNA and VEGF165b pcDNA. Products were run on a 3% agarose gel and visualised using ethidium bromide staining under an ultraviolet illuminator. The expected band sizes are 195 bp for VEGFxxx and 129 bp for VEGFxxxb.
For qPCR, 80 ng of cDNA was used, and the reactions were run in Opticon (BioRad). Three endogenous control genes, SDHA, HPRT1 and β-actin were used to normalize the data. Table 2 shows the primer sequences and cycling conditions. The comparative Ct method was used for relative quantification. All samples were run in duplicate.
Table 2. RT-PCR and qPCR primer sequences and cycling conditions.
Cycling conditions comprised an initial 10 min of polymerase activation at 95°C and a final extension of 10 min at 72°C.
| RT-PCR | SENSE AND ANTISENSE SEQUENCES | PCR CONDITIONS |
|---|---|---|
| VEGFxxx/VEGFxxxb | 35 Cycles: | |
| S: 5′-GTTTGTACAAGATCCGCAGACGT-3′ | 95°C for 15s | |
| A: 5′-ATGGATCCGTATCAGTCTTTCCTGG-3′ | 56°C for 30s | |
| 60°C for 30s | ||
|
| ||
| qPCR | SENSE AND ANTISENSE SEQUENCES | PCR CONDITIONS |
|
| ||
| VEGFxxx | S: 5′-GTTTGTACAAGATCCGCAGACGT-3′ | 45 Cycles: |
| A: 5′-GTTCCCGAAACCCTGAGGG-3′ | 95°C for 15s | |
| 60°C for 60s | ||
| SRPK1 | Validated primers (Qiagen, QT00059556) | 45 Cycles: |
| 95°C for 15s | ||
| 55°C for 30s | ||
| 72°C for 30s | ||
| β-Actin | S: 5′- AACGGGAAGCTCACTGGCATG-3′ | 45 Cycles: |
| A: 5′- TCCACCACCCTGTTGCTGTAG-3′ | 95°C for 15s | |
| 60°C for 60s | ||
| HRPT I | S: 5′-TGACACTGGCAAAACAATGCA-3′ | 45 Cycles: |
| A: 5′-GGTCCTTTTCACCAGCAAGCT-3′ | 95°C for 15s | |
| 60°C for 60s | ||
| SDHA | S: 5′-TGGGAACAAGAGGGCATCTG-3′ | 45 Cycles: |
| A: 5′-CCACCACTGCATCAAATTCATG-3′ | 95°C for 15s | |
| 60°C for 60s | ||
ELISA and cELISA
Tumour samples and cell lines were lysed using RIPA lysis buffer containing protease inhibitors and the protein concentration was measured using Precision Red (Cytoskeleton, Inc), according to the manufacturer’s guidelines. Pan-VEGF and VEGFxxxb ELISA was performed using the VEGF DuoSet ELISA kit DY293 and DY3045, respectively (R&D Systems, Abingdon, UK). Briefly, 1 μg/ml goat anti-human pan-VEGF or 2 μg/ml anti-human VEGxxxb (Clone 56/1) were used as a capture antibodies, overnight at room temperature (RT). After washing and blocking steps, serial dilutions of, rhVEGF165 or rhVEGF165b standards were added to each well in triplicate, at concentrations ranging from 15.625 pg /ml to 4 ng/ml. Sample lysates were typically diluted 1:5 in blocking solution and added in duplicate to each well. For cell ELISA, viable NB cells, were counted in a haemocytometer with trypan blue exclusion staining, and plated in triplicate at different concentrations, up to 105 cells per well in serum-free medium (100 μl/well) on the ELISA plates coated as above, and incubated at 37°C and 5% CO2 for 20 h. Either biotinylated goat anti-human VEGF (0.1 μg/ml), or mouse anti-human VEGFxxxb (0.25 μg/ml), was used as detection antibody. Absorbance was read in a plate reader Opsys MR 96-well plate reader (Dynex Technologies, Chantilly, VA, USA) at 450 nm, with the control reading at 570 nm. Revelation Quicklink 4.25 software was also used to construct a standard curve from mean absorbance values of standards enabling the estimation of VEGF concentration for each sample. The actual VEGF concentrations were calculated according to Varey et al [16].
IMMUNOHISTOCHEMISTRY
Pan-VEGF and VEGFxxxb immunostaining was performed on 3 μm sections of formalin-fixed, paraffin-embedded tumour. Sections were pretreated in sodium citrate buffer, 0.01M (pH 6.0) at 800 Watts for two cycles of 6 min followed by immersion in 3% hydrogen peroxide for 5 min. Sections were kept in ULTRA-V block (Thermoscientific, UK) for 2 h at RT. After washing, each slide was incubated with 10 μg/ml mouse anti-human VEGF IgG (clone VG1, DAKO, UK) or 12 μg/ml mouse monoclonal anti-human VEGF165b IgG (clone 56/1) antibody, diluted in PBS overnight at 4°C. Negative control slides were treated with isotype-specific mouse IgG (Sigma) at the same concentration as the primary antibody. After washing, sections were incubated with biotinylated horse anti-mouse IgG (Vector Lab) at 2 μg/ml diluted in 1.5% normal horse serum at 4°C overnight. Normal colon sections served as positive controls.
VEGFR2 immunohistochemistry was performed on 3 μm sections of 4% paraformaldehyde paraffin-embedded tissue from NB tumour mice xenografts. Sections were incubated with rabbit monoclonal anti-VEGFR2 (55B11, Cell Signalling) at 1:300 dilution or normal goat IgG overnight at 4°C. Following washing, sections were incubated with biotinylated goat anti-rabbit IgG at 1:750 dilution at RT for 1 h in a humid chamber.
The sections were incubated in Vectastain ABC solution (PK4000; Vector Lab) for 30 min at RT, followed by DAB substrate and counterstaining in haematoxylin. The images were captured using a Leica DM LB2 microscope and IM50 digital imaging system.
VEGFR2 staining of the endothelium was used to measure the MVD and the vessel area in the tumours. For that purpose, 5 to 10 random images were taken of each tumour section, and the blood vessels present within quantified. The average blood vessel number for each tumour section was calculated and divided by the image area in order to determine the MVD. For the area measurements, ImageJ was used.
CELL VIABILITY, LDH RELEASE, CASPASE 3/7 ACTIVITY AND BrdU INCORPORATION
104 SK-N-BE(2)C cells were seeded in triplicate in 96-well plates. After 24 h, the cells were treated with VEGF165b (0.5, 1, 2.5 and 5nM final concentration). As controls for the assays, cells were treated with 10 ng/ml of basic fibroblast growth factor, bFGF (Invitrogen) or 10 mM sodium butyrate (Sigma). Treatments were compared with serum-free media control. Cells were incubated at 37°C and 5% CO2. After appropriate incubation, the specific assays were performed: Alamar Blue Assay (Biosource, UK); cell proliferation ELISA BrdU colorimetric Kit (Roche Diagnostics); Caspase-Glo 3/7 Assay (Promega, UK) and CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, UK). The assays and calculations were performed as stated in the manufacturer’s protocol. All experiments were repeated a minimum of two times.
IN VIVO TUMOUR GROWTH EXPERIMENTS
Thirteen female nude mice (BALB/c nu/nu, University of Bristol) were given subcutaneous injections (sc) into the dorsal lumbar area with 107 SK-NB-E(2)C cells. Tumour growth was followed with twice-weekly caliper measurements of the length and width, beginning when the tumours became palpable. Twice-weekly sc administration of either 100 μg of VEGf165b (Philogene Inc, New York) or saline was initiated in each group when the tumour was 816.2±453.1 mm3 and 1001.8±631.1 mm3, respectively. Tumours were allowed to reach 15 mm in diameter before sacrifice, when they were excised and fixed in 4% paraformaldehyde. Tumour volumes were calculated according to the formula (length × width × (length + width)/2.
STATISTICAL ANALYSES
If not indicated otherwise, data are shown as mean ± SEM. All statistical analyses were calculated with GraphPad Prism (GraphPad Software Inc, San Diego, California, USA). All results were considered statistically significant at p<0.05.
RESULTS
PRO-ANGIOGENIC VEGFxxx IS UP-REGULATED IN NEUROBLASTOMAS
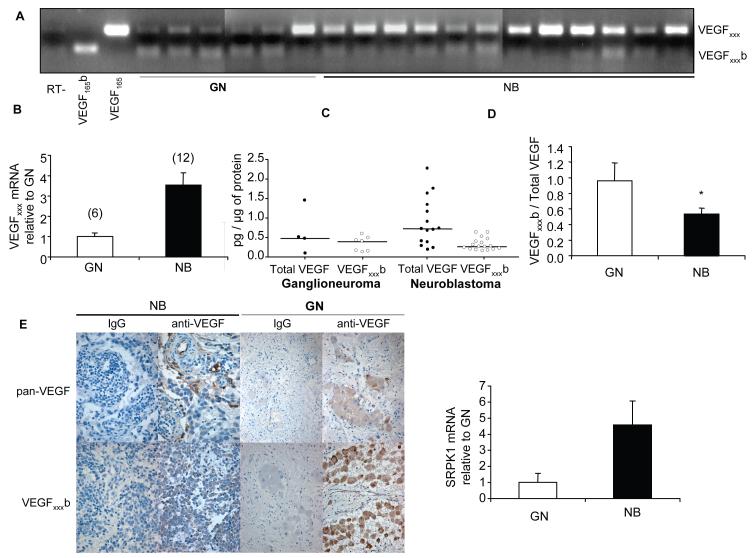
Neuroblastoma and ganglioneuroma samples were assessed for VEGFxxx and VEGFxxxb isoform expression at mRNA and protein levels. Using a forward primer in exon 7 and a reverse primer in exon 8b we amplified both isoforms in the same PCR reaction. The size of the amplicon for VEGFxxx and VEGFxxxb was 195 bp and 129 bp, respectively. VEGFxxx but not VEGFxxxb was up-regulated in NB compared to GN at the mRNA level (Figure 2A). These results were confirmed by qPCR for VEGFxxx, which showed a 3.54 fold increase in NB compared to GNs (Figure 2B). The relative quantities of VEGFxxx transcript in each sample were normalized to the expression of three house-keeping genes previously shown to be stable in NB [21]. Subsequently we quantified VEGFxxx and VEGFxxxb cellular protein in tissue homogenates by ELISA. NB samples showed a significantly lower ratio between VEGFxxxb and total VEGF (0.53±0.08) than GNs (0.96±0.23) (Figures 2C, D).
Figure 2. VEGFxxx is up-regulated in neuroblastoma but not ganglioneuroma at mRNA and protein levels.
(A) Extraction of mRNA from neuroblastic tumour samples followed by RT-PCR using primers to detect both families of isoforms. (B) Relative amounts of VEGFxxx mRNA in neuroblastic tumour samples, NB and GN, represented as mean ± SEM. qPCR values were calculated in relation to expression of three reference genes (SDHA, HPRT I and β-actin). VEGFxxx was 3.54 times greater in malignant tumours than in benign tumours. (C) ELISA results on total VEGF and VEGFxxxb protein expression by different neuroblastic tumour samples showed as data points and median. Results normalized by protein. (D) VEGFxxxb relative expression decreases significantly with the malignancy of the tumour (unpaired t test, p<0.05). Mean ± SEM. (E) Immunohistochemical staining of VEGFxxxb and total VEGF in neuroblastic tumours. (F) Relative amounts of SRPK1 mRNA in NB and GN, represented as mean ± SEM. qPCR values were calculated in relation to expression of three reference genes (SDHA, HPRT I and β-actin). SRPK1 was 4.58 times greater in NB than in GN.
These results were confirmed by immunohistochemistry. GNs showed a higher intensity of VEGFxxxb staining than NBs, in which the total VEGF was more abundant. Cytoplasmic staining for total VEGF was identified in the neuroblasts at different stages of differentiation including ganglion cells. On the other hand, weak staining for VEGFxxxb was detected in the neuroblasts while the ganglion cells displayed abundant cytoplasmic reactivity (Figure 2E). These findings suggest that VEGF acts in a paracrine manner in NB. VEGF it is produced by the tumour cells, and binds to its two receptors on the surface of the tumour endothelium, stimulating tumour angiogenesis. Furthermore, VEGFR1 and VEGFR2 mRNA in NB specimens suggests an autocrine effect of VEGF in NB (Langer et al. 2000).
Neuroblastic tumour samples were also assessed for the expression of SRPK1 (Serine/arginine protein kinase 1) at mRNA level by qPCR. SRPK1 showed a 4.58 fold increase in NB compared to GN indicating an up-regulation of this splice factor kinase in NB.
VEGFxxx IS UP-REGULATED IN HIGHLY TUMOURIGENIC, MYCN AMPLIFIED NB CELL LINES
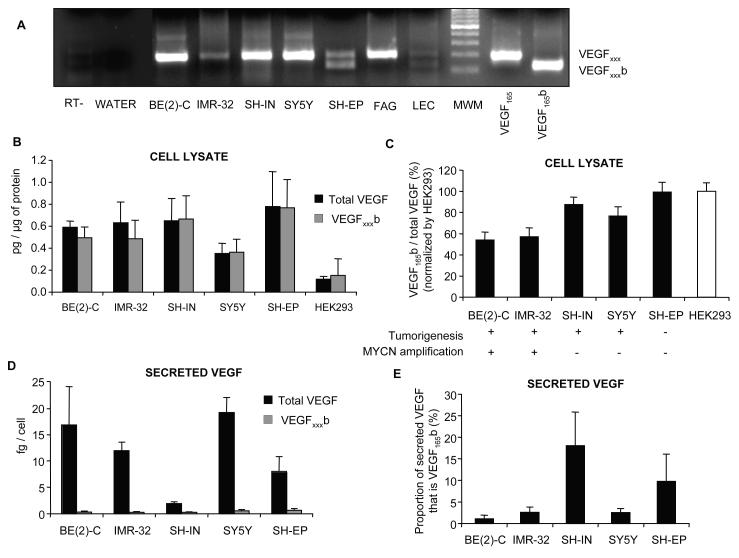
Five NB cell lines with different tumorigenic potential and MYCN amplification status (Table 1) were also evaluated for the expression of VEGFxxx and VEGFxxxb isoforms at mRNA and protein levels. VEGFxxx mRNA was detected reliably in all NB cell lines. However, only SH-EP, a non-tumorigenic cell line, consistently expressed VEGFxxxb isoforms (Figure 3A). The total VEGF and VEGFxxxb protein in the NB cell lysates was measured by ELISA. A modified cell ELISA, (cELISA) was also used to measure the levels of secreted VEGF and VEGFxxxb. Human Embryonic Kidney 293 cells (HEK293) were used as a positive control for the expression of VEGFxxxb. Protein extracted from all NB cell lines contained anti-angiogenic VEGFxxxb isoforms (Figure 3B). Pan-VEGF ELISA showed similar levels of expression of total VEGF for all cell lines (Figure 3B). SK-N-BE(2)C and IMR-32, MYCN amplified cell lines, showed the lowest ratio of VEGFxxxb/total VEGF (Figure 3C). On the other hand, and keeping with mRNA data, the non-tumorigenic cell line SH-EP showed the highest levels of VEGFxxxb (Figure 3C). Of note however, the relative amount of VEGFxxxb to total VEGF in the cells was very high, in contrast with the mRNA levels in which VEGFxxxb was difficult to detect. To determine whether this difference could be accounted for by differential secretion of VEGF isoforms from the cells, cELISA was carried out. After 20 h incubation, all NB cell lines secreted VEGF at levels between 1 and 20 fg/cell (Figure 3D). In contrast, VEGFxxxb secretion was 1-2 orders of magnitude lower, varying between 0.2 and 0.6 fg/cell (Figure 3E). Of all NB cells only SH-IN and SH-EP secreted significant proportion of its VEGF as VEGFxxxb (Figure 3F).
Figure 3. Differential expression and secretion of VEGF isoforms by neuroblastoma cell lines.
(A) Extraction of mRNA from NB cell lines followed by RT-PCR using primers to detect both families of isoforms. FAG is fetal adrenal gland and LEC, lymphatic endothelial cells. (B) ELISA for VEGFxxxb and total VEGF on cell lysates for different NB cell lines where MYCN oncogene amplification is associated with poor prognosis. Results normalized to protein. HEK293 cells were used as positive control for the expression of VEGF xxxb. (C) VEGFxxxb relative expression decreases significantly in highly tumorigenic, MYCN amplified cell lines (One-way ANOVA, post test for linear trend, p<0,01). Results normalized to HEK293 cells. (D) Cell ELISA for VEGFxxxb and total VEGF showing a significant secretion of VEGF (1-20fg/cell) in all neuroblastoma cell lines. VEGFxxxb secretion was 1-2 orders of magnitude lower (0.2-0.6fg/cell). Results normalized by cell number. (E) Proportion of secreted VEGF that is VEGFxxxb. Only SH-IN and SH-EP cells secreted more than 5% of its VEGF as VEGFxxxb.
rhVEGF165b SIGNIFICANTLY REDUCES HUMAN NEUROBLASTOMA GROWTH RATE IN VIVO
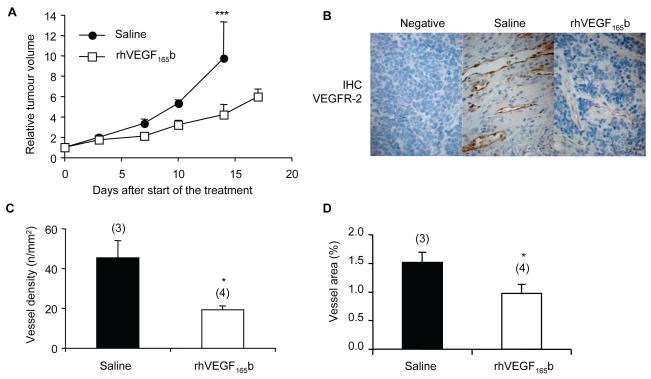
Treatment with rhVEGF165b reduced NB tumour growth rate of established xenografts (4.24-± 1.01 fold increase in volume) compared with those treated with saline (9.76± 3.58, p<0.01, ANOVA). The difference between the groups became statistically significant at treatment day 14 (Figure 4A). The excised tumours were sectioned and immunostained for VEGFR2 using an antibody, which shows species cross-reactivity against human and mouse cells. VEGFR2 staining was evident in vascular endothelium of the stroma (Figure 4B). There was no significant staining of the NB tumour cells, suggesting a direct effect of rhVEGF165b on the tumour vessels rather than an anti-proliferative effect on the tumour cells in vivo. MVD and vessel area analyses in 5 to 10 random fields of each tumour (Figure 4C, D) revealed a decrease in the number of vessels in rhVEGF165b-treated tumours (19.4±1.9 vessels/mm2) in contrast to the saline-treated tumours (45.5±8.6 vessels/mm2, p<0.05, unpaired t-test), as well as a reduction in the vessel area (0.98±0.16% mm2/tumour area in rhVEGF165b-treated tumours compared to 1.52±0.18% in saline-treated xenografts).
Figure 4. rhVEGF165b reduces tumour growth rate when administered subcutaneously twice a week.
(A) Treatment of established murine NB tumours with either saline or rhVEGF165b (100μg subcutaneously twice a week). Values of tumour fold increase in volume were reduced in the VEGF165b-treated group in comparison with saline-treated controls (from 9.76±3.58 to 4.24±1.01 on day 14, ANOVA, p<0.001). (B) VEGFR2 immunohistochemical staining of mice tumours. The staining was mostly detected in the vascular endothelium (in brown). (C, D) Quantification of the blood vessel number and blood vessel area present in the saline-treated tumours versus the VEGF165b-treated tumours. Treatment with rhVEGF165b decreased the vessel density and the area of the blood vessels in the tumours (unpaired t test, p<0,05).
rhVEGF165b IS NOT CYTOTOXIC FOR SK-NB-E(2)C NEUROBLASTOMA CELLS
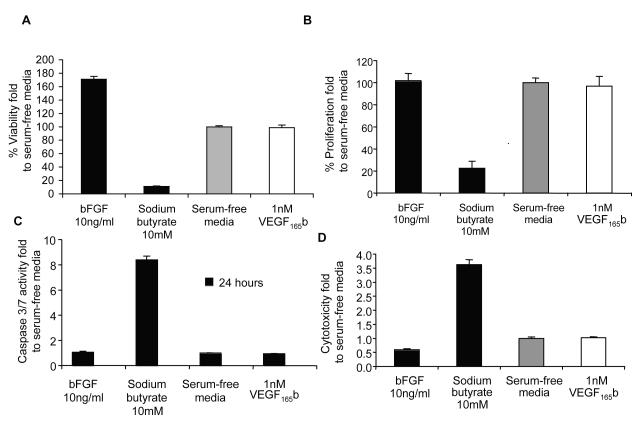
To determine whether this decrease could be due to a direct effect of rhVEGF165b on SK-NB-E(2)C cells, in vitro proliferation and cytoxicity assays were performed. Twenty-four or 48-hours-treatment of SK-NB-E(2)C cells with different rhVEGF165b concentrations (0.5, 1, 2.5 and 5 nM) showed no effect on cell viability (Figure 5A), proliferation (Figure 5B), apoptosis (Figure 5C) or cytoxicity (Figure 5D) implying a distinctive anti-angiogenic role of VEGF165b in reducing tumour growth rate.
Figure 5. rhVEGF165b has no effect on SK-N-BE(2)C neuroblastoma cells.
Results normalized by serum-free media control. Data represent mean ± SEM. After 24 hours or 48 hours, SK-N-BE(2)C cells treated with 0.5, 1, 2.5 and 5 nM did not show any significant difference at (A) viability, (B) proliferation, (C) apoptosis or (D) cytotoxicity levels (Student’s t test, p>0.05). Representative data for one concentration of rhVEGF165b (1nM) and one time point is shown.
DISCUSSION
A characteristic feature of NB is increased vascularity, which correlates with increasing stage [7]. Overexpression of VEGF, a key player in angiogenesis, has been reported in NB cell lines and tumour samples [4-12,22]. However, these studies have not distinguished between pro- and anti-angiogenic VEGF isoforms. Studies by Bates’ group have shown that there are two families of isoforms generated by proximal or distal site selection of exon 8 resulting in pro-angiogenic (VEGFxxx) and anti-angiogenic (VEGFxxxb) isoforms [13,19,23,24]. VEGF165 and VEGF165b bind to VEGFR2 with equal affinity but VEGF165b fails to stimulate angiogenesis in vivo.
VEGF165b inhibits several VEGF165-mediated processes and all data indicate a cancer-associated switch from anti- to pro-angiogenic VEGF expression by an alteration in splicing [25,26]. This switch to the pro-angiogenic isoforms has been shown at mRNA and protein levels. The anti-tumour effect of VEGF165b, has been confirmed in a variety of xenograft tumour models of adult epithelial cancers [13,16-18]. Heterotopic xenograft colon carcinomas treated with VEGF165b are less haemorrhagic and less vascularised with decreased MVD [18]. In addition, a low level of VEGF165b is correlated with poor prognosis in colon carcinoma [27].
This is the first study showing a relative down-regulation of the anti-angiogenic isoform of VEGF in NB, an embryonal tumour of childhood. These findings support our hypothesis that the angiogenic VEGF family of isoforms, VEGFxxx, is up-regulated in NB cell lines and tumours compared to its anti-angiogenic VEGFxxxb. The comparison of expression, at both mRNA and protein levels, between NB and GN, a benign tumour, extend the previous studies conducted in adult epithelial cancers to a childhood cancer, and provide further evidence that the balance of VEGF switches from the anti- to the pro-angiogenic isoforms in majority of tumours. SRPK1 is a kinase involved in the activation of ASF/SF2, a splice factor that has previously been shown to promote the VEGF-A PSS selection (Nowak et al 2008, Nowak et al 2010). Recently there have been several reports showing how the alteration of splicing can be used as a target in therapy. Both the use of isoform-specific antisense oligonucleotides, or small molecules that affect splicing factors and kinases involved in splicing, such as SRPK1, are promising candidates for new drugs. High expression of SRPK1 has been detected in several tumours and correlates with tumour grade (Hayes et al 2007, Krishnakumar et al 2008). Up-regulation of SRPK1 mRNA in NB suggests that it is a potential target for cancer treatment.
VEGFxxx was also up-regulated in MYCN amplified NB cell lines. It has been shown in previous reports that MYCN siRNA inhibition of IMR-32 cells blocks VEGF secretion and that MYCN overexpression in SH-EP cells up-regulates VEGF-A (Kang et al. Oncogene 2008, Becker et al. Clin Canc Research 2010). Relative down-regulation of VEGFxxxb may also be enhanced by MYCN expression, supporting the crucial effect of this oncogene on neuroblastoma progression. Moreover, we show for the first time, using a novel assay for secreted VEGF that the VEGFxxxb isoforms are differentially secreted from the VEGFxxx isoforms. This adds a further level of complexity to the regulation of VEGF isoforms, as it is possible that switching the secreted levels of the two isoforms could also switch the angiogenic potential. There is nothing as yet known about the regulation of secretion of the different isoforms. It has been shown that N-glycolysation of VEGF is required for efficient secretion of VEGF but very little else is known about requisites for VEGF secretion.
Two phase I clinical trials with the anti-angiogenic agent Bevacizumab (Avastin) are in progress for children with NB. Anti-VEGF signalling drugs, either alone or in combination with other agents have been reported to inhibit NB growth. Bevacizumab has been shown to inhibit the growth of NB in vivo [28] and to suppress NB progression in the setting of minimal disease [29]. Targeted therapies against VEGF receptors, sunitinib (SU011248) [30,31], sorafenib [32] and AZD2171 [33] have shown variable cytotoxic effects in vitro associated with growth inhibition in murine models. Furthermore, sunitib and conventional cytotoxic drugs, such as cyclophosphamide, have been shown to have a synergistic effect in vitro and in vivo [31].
VEGF165b demonstrated significant growth inhibition of established NB xenografts in vivo, with no evidence of an in vitro effect on viability, apoptosis or cytotoxicity. There were fewer vessels in tumours treated with VEGF165b compared with control tumours and vessels were smaller in size. Furthermore, there was no expression of VEGFR2 in the tumorigenic cell line used for xenograft study, indicating that VEGF165b affects tumour growth through its inhibitory effect on angiogenesis rather than by a direct effect on tumour cells.
In conclusion, VEGF165b is a promising anti-angiogenesis agent in neuroblastoma. The endogenous nature and the lack of side effects such as hypertension and proteinuria that are associated with the inhibition of VEGFxxx comprise additional advantages of VEGF165b over a number of existing anti-angiogenic therapies. As inhibition of VEGF signalling may cause normalization of tumour vasculature leading to re-oxygenation of hypoxic regions and to enhanced delivery of anticancer reagent, there is potential for synergism [34] when agents such as VEGF165b are combined with chemotherapy drugs that target the tumour itself.
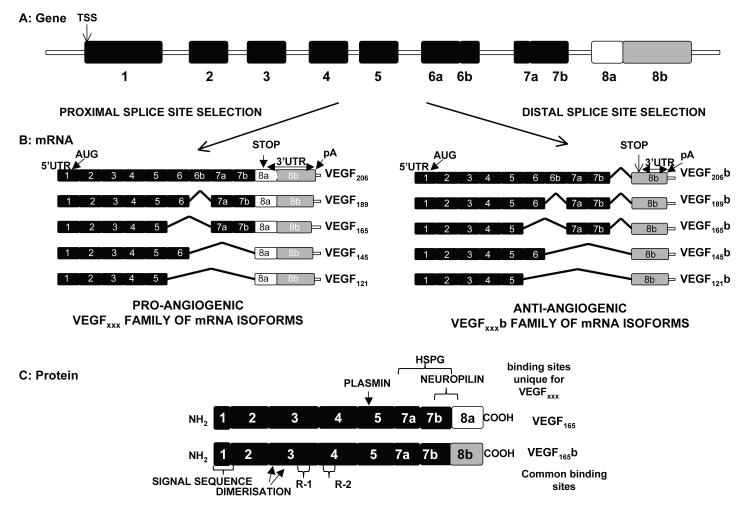
Figure 1. Organisation of the VEGF-A gene.
A. Gene structure. TSS=transcriptional start site. B. mRNA species. Alternative splicing of VEGF-A gene in the terminal exon results in two families of isoforms, the pro-angiogenic VEGFxxx, and the anti-angiogenic VEGFxxxb isoforms. AUG= start site for translation, UTR=untranslated region, pA = poly adenylation site. C. Protein structure of two major isoforms of each family. This C-terminal splicing leads to an alternative last six amino acids (CDKPRR or SLTRKD). The isoforms are termed according to the amino acid number of the resulting protein (xxx). HSPG=heparin sulphate proteoglycan, R1=VEGF receptor 1, R2 =VEGF Receptor 2 (not to scale).
ACKNOWLEDGEMENTS
The authors would like to thank Child Cancer Foundation (CLIC-Sargent), the Richard Bright VEGF Research Trust, British Heart Foundation and Above and Beyond Charities for their support.
Footnotes
CONFLICTS OF INTEREST: Prof S. Harper and Prof D. Bates hold a patent on VEGF165b isoforms as anti-angiogenic agents.
REFERENCES
- 1.Mueller S, Matthay KK. Neuroblastoma: biology and staging. Curr Oncol Rep. 2009;11:431–438. doi: 10.1007/s11912-009-0059-6. [DOI] [PubMed] [Google Scholar]
- 2.Pastorino F, Di PD, Loi M, Becherini P, Caffa I, Zorzoli A, et al. Recent advances in targeted anti-vasculature therapy: the neuroblastoma model. Curr Drug Targets. 2009;10:1021–1027. doi: 10.2174/138945009789577954. [DOI] [PubMed] [Google Scholar]
- 3.Rossler J, Taylor M, Geoerger B, Farace F, Lagodny J, Peschka-Suss R, et al. Angiogenesis as a target in neuroblastoma. Eur J Cancer. 2008;44:1645–1656. doi: 10.1016/j.ejca.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Beierle EA, Strande LF, Chen MK. VEGF upregulates Bcl-2 expression and is associated with decreased apoptosis in neuroblastoma cells. J Pediatr Surg. 2002;37:467–471. doi: 10.1053/jpsu.2002.30868. [DOI] [PubMed] [Google Scholar]
- 5.Beierle EA, Dai W, Langham MR, Jr., Copeland EM, III, Chen MK. VEGF receptors are differentially expressed by neuroblastoma cells in culture. J Pediatr Surg. 2003;38:514–521. doi: 10.1053/jpsu.2003.50091. [DOI] [PubMed] [Google Scholar]
- 6.Beierle EA, Dai W, Langham MR, Jr., Chen MK. Neuroblastoma, apoptosis, and growth factors. Am Surg. 2003;69:28–31. [PubMed] [Google Scholar]
- 7.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–1908. [PubMed] [Google Scholar]
- 8.Fakhari M, Pullirsch D, Paya K, Abraham D, Hofbauer R, Aharinejad S. Upregulation of vascular endothelial growth factor receptors is associated with advanced neuroblastoma. J Pediatr Surg. 2002;37:582–587. doi: 10.1053/jpsu.2002.31614. [DOI] [PubMed] [Google Scholar]
- 9.Fakhari M, Pullirsch D, Abraham D, Paya K, Hofbauer R, Holzfeind P, et al. Selective upregulation of vascular endothelial growth factor receptors neuropilin-1 and -2 in human neuroblastoma. Cancer. 2002;94:258–263. doi: 10.1002/cncr.10177. [DOI] [PubMed] [Google Scholar]
- 10.Komuro H, Kaneko S, Kaneko M, Nakanishi Y. Expression of angiogenic factors and tumor progression in human neuroblastoma. J Cancer Res Clin Oncol. 2001;127:739–743. doi: 10.1007/s004320100293. [DOI] [PubMed] [Google Scholar]
- 11.Meister B, Grunebach F, Bautz F, Brugger W, Fink FM, Kanz L, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors in human neuroblastoma. Eur J Cancer. 1999;35:445–449. doi: 10.1016/s0959-8049(98)00387-6. [DOI] [PubMed] [Google Scholar]
- 12.Rossler J, Breit S, Havers W, Schweigerer L. Vascular endothelial growth factor expression in human neuroblastoma: up-regulation by hypoxia. Int J Cancer. 1999;81:113–117. doi: 10.1002/(sici)1097-0215(19990331)81:1<113::aid-ijc19>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 14.Cebe SS, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Y, Hoareau-Aveilla C, Oltean S, Harper SJ, Bates DO. The anti-angiogenic isoforms of VEGF in health and disease. Biochem Soc Trans. 2009;37:1207–1213. doi: 10.1042/BST0371207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rennel E, Waine E, Guan H, Schuler Y, Leenders W, Woolard J, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, Magnussen A, Schuler Y, Kelly SP, et al. Recombinant human VEGF165b protein is an effective anti-cancer agent in mice. Eur J Cancer. 2008;44:1883–1894. doi: 10.1016/j.ejca.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 20.Walton JD, Kattan DR, Thomas SK, Spengler BA, Guo HF, Biedler JL, et al. Characteristics of stem cells from human neuroblastoma cell lines and in tumors. Neoplasia. 2004;6:838–845. doi: 10.1593/neo.04310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chlenski A, Liu S, Cohn SL. The regulation of angiogenesis in neuroblastoma. Cancer Lett. 2003;197:47–52. doi: 10.1016/s0304-3835(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 23.Bates DO, Harper SJ. Therapeutic potential of inhibitory VEGF splice variants. Future Oncol. 2005;1:467–473. doi: 10.2217/14796694.1.4.467. [DOI] [PubMed] [Google Scholar]
- 24.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, et al. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms - a novel therapeutic strategy for angiogenesis. J Biol Chem. 2009 doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz R, Pena C, Silva J, Lorenzo Y, Garcia V, Garcia JM, et al. p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int J Cancer. 2008;123:1060–1067. doi: 10.1002/ijc.23619. [DOI] [PubMed] [Google Scholar]
- 28.Segerstrom L, Fuchs D, Backman U, Holmquist K, Christofferson R, Azarbayjani F. The anti-VEGF antibody bevacizumab potently reduces the growth rate of high-risk neuroblastoma xenografts. Pediatr Res. 2006;60:576–581. doi: 10.1203/01.pdr.0000242494.94000.52. [DOI] [PubMed] [Google Scholar]
- 29.Sims TL, Williams RF, Ng CY, Rosati SF, Spence Y, Davidoff AM. Bevacizumab suppresses neuroblastoma progression in the setting of minimal disease. Surgery. 2008;144:269–275. doi: 10.1016/j.surg.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maris JM, Courtright J, Houghton PJ, Morton CL, Kolb EA, Lock R, et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51:42–48. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Smith KM, Chong AL, Stempak D, Yeger H, Marrano P, et al. In vivo antitumor and antimetastatic activity of sunitinib in preclinical neuroblastoma mouse model. Neoplasia. 2009;11:426–435. doi: 10.1593/neo.09166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy CS, Karmakar S, Banik NL, Ray SK. Synergistic efficacy of sorafenib and genistein in growth inhibition by down regulating angiogenic and survival factors and increasing apoptosis through upregulation of p53 and p21 in malignant neuroblastoma cells having N-Myc amplification or non-amplification. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9324-7. [DOI] [PubMed] [Google Scholar]
- 33.Maris JM, Courtright J, Houghton PJ, Morton CL, Gorlick R, Kolb EA, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:581–587. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 34.Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]