Abstract
Calorie restriction (CR) is a component of most weight loss interventions and a potential strategy to slow aging. Accurate determination of energy intake and %CR is critical when interpreting the results of CR interventions; this is most accurately achieved using the doubly labeled water method to quantify total energy expenditure (TEE). However, the costs and analytical requirements of this method preclude its repeated use in many clinical trials. Our aims were to determine 1) the optimal TEE assessment time points for quantifying average energy intake and %CR during long-term CR interventions and 2) the optimal approach for quantifying short-term changes in body energy stores to determine energy intake and %CR during 2-wk DLW periods. Adults randomized to a CR intervention in the multicenter CALERIE study underwent measurements of TEE by doubly labeled water and body composition at baseline and months 1, 3, and 6. Average %CR achieved during the intervention was 24.9 ± 8.7%, which was computed using an approach that included four TEE assessment time points (i.e., TEEbaseline, months 1, 3, and 6) plus the 6-mo change in body composition. Approaches that included fewer TEE assessments yielded %CR values of 23.4 ± 9.0 (TEEbaseline, months 3 and 6), 25.0 ± 8.7 (TEEbaseline, months 1 and 6), and 20.9 ± 7.1% (TEEbaseline, month 6); the latter approach differed significantly from approach 1 (P < 0.001). TEE declined 9.6 ± 9.9% within 2–4 wk of CR beginning and then stabilized. Regression of daily home weights provided the most reliable estimate of short-term change in energy stores. In summary, optimal quantification of energy intake and %CR during weight loss necessitates a TEE measurement within the first month of CR to capture the rapid reduction in TEE.
Keywords: total energy expenditure, doubly labeled water, body energy stores
caloric restriction (CR), a reduction in energy intake (EI) from habitual levels, is a key intervention in many clinical weight loss trials and, more recently, as a potential means to slow the aging process (8, 20). Determining a dose-response relationship between CR and physiological changes requires accurate and precise assessment of EI at baseline (BL) and throughout the intervention. Furthermore, because physiological changes may differ depending on whether weight loss is achieved by CR alone, exercise alone, or CR plus exercise, an accurate assessment of EI is important to help distinguish effects that are attributable to CR from those attributable to exercise.
Many of the current options for estimating EI and %CR in clinical trials have limited accuracy. Self-reported EI is recognized to be inaccurate, with a bias toward underreporting, particularly among obese individuals (10, 27). Weight change is also an imperfect quantitative indicator of EI and %CR, particularly with interventions that combine CR and exercise (23).
Average daily EI can be closely approximated from total daily energy expenditure (TEE) when body weight is stable (11, 27). TEE can be assessed using the doubly labeled water (DLW) method (21), which is the most accurate method and the gold standard for quantifying TEE and EI in free-living individuals (9). When body weight is changing, EI is not equal to TEE but can be approximated using the energy balance equation: EI = TEE + change in body energy stores (23). Although estimation of TEE by DLW during weight loss is accurate and precise (3), measurement of changes in energy stores is less accurate because the magnitude of changes in fat mass (FM) and fat-free mass (FFM) is small relative to the measurement error during 2-wk DLW periods (3). DLW periods longer than two isotope tracer half-lives (∼2 wk in adults) are not recommended because isotope enrichments become too low to allow accurate quantification of isotope elimination rates. Because DLW is generally used only before and at the end of an intervention, assumptions must be made about TEE during intervals between TEE assessments. Furthermore, the costs and analytical requirements of the DLW method preclude its repeated use in many clinical trials. Therefore, our primary aim was to determine which TEE assessment time points are optimal for estimating energy intake and %CR during long-term CR interventions. A secondary aim was to determine the optimal approach for quantifying short-term changes in body energy stores during 2-wk DLW periods.
METHODS
Participants.
Participants included healthy, weight-stable, nonobese volunteers enrolled in phase 1 of the multicenter clinical trial “CALERIE” (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy). The interventions were conducted from 2002 to 2005 at three clinical sites: Pennington Biomedical Research Center (Baton Rouge, LA), Tufts University (Boston, MA), and Washington University School of Medicine (St. Louis, MO). Participant characteristics, inclusion and exclusion criteria, and the CR interventions are described in detail elsewhere (1, 7, 17). Briefly, eligible subjects with body mass index between 23.5 and 29.99 kg/m2 were randomized to one of several CR regimens, an exercise regimen, a combination of CR and exercise, or to a control group for a 6- or 12-mo period. To allow comparison across study sites, the current analysis includes only subjects enrolled in CR interventions without an exercise component (n = 54), and the duration is limited to the first 6 mo of the intervention. Of these 54 participants, 40 met secondary criteria for inclusion in the current analysis based on complete data for TEE, body composition, and body weight at BL and months 1, 3, and 6. Each study was approved by the site's Institutional Review Board. All participants gave written informed consent before enrollment.
CR interventions.
CR prescriptions at the three sites ranged from 16 to 30% (i.e., a 16–30% reduction in EI relative to BL EI). The mean prescribed %CR during the 6-mo CR interventions was 27.9 ± 5.3%. BL EI was assumed to equal BL TEE determined by the doubly labeled water (DLW) method during two consecutive 2-wk periods. Participants received intensive behavioral support and counseling to facilitate adherence to the CR interventions and were advised not to change their daily physical activity.
Energy expenditure.
Total daily energy expenditure (TEE) was measured by the DLW method (24) during two consecutive 2-wk periods at BL and during 2-wk periods preceding the month 1, month 3, and month 6 time points (i.e., M1, M3, and M6) of the CR intervention, as shown in Fig. 1. Two baseline urine samples were collected at the clinical sites before an oral dose of doubly labeled water was administered (0.20 g of H218O, 0.12 g of 2H2O/kg total body water). Subjects voided 1.5 and 3 h after dosing; postdose urine samples were collected at 4.5 h, 6 h, day 7 (2 samples), and day 14 (2 samples). Abundances of 2H and 18O in urine samples and in dilutions of the isotope doses were measured in duplicate, using isotope ratio mass spectrometry at the Pennington Biomedical Research Center. Isotope elimination rates (kh and ko) were calculated using linear regression, and CO2 production was calculated using the equations of Schoeller (24), with the modifications of Racette et al. (16).
Fig. 1.
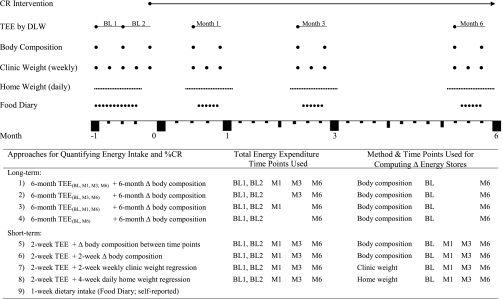
Study timeline and description of 9 approaches for computing average 6-mo energy intake and %calorie restriction (CR). TEE, total daily energy expenditure; DLW, doubly labeled water; BL, baseline; M1, month 1; M3, month 3; M6, month 6.
Because of the proximity of the BL1, BL2, and M1 TEE measurements, isotopic enrichment remained elevated at the beginning of the BL2 and M1 TEE periods. Therefore, the predose urine samples from BL1 were analyzed with the BL2 and M1 sample sets to determine isotopic enrichment above baseline for the calculation of isotope elimination rates. Likewise, the predose BL1 urine samples were used to determine the time zero enrichment for the dilution space calculations, but the values were adjusted for the actual predose enrichments measured at BL2 and M1. TEE was computed using respiratory quotient values of 0.858 at baseline, 0.840 at M1, 0.851 at M3, and 0.860 at M6. These group respiratory quotient values were derived from each subject's reported diet composition at each time point and measured changes in body composition between time points.
Resting metabolic rate was measured by indirect calorimety in the morning after an overnight fast and an inpatient stay in the metabolic research unit at each study site at baseline, M3, and M6; a subsample also was assessed at M1.
Body composition.
FM and FFM were measured at the beginning and end of each 2-wk TEE measurement period by either dual-energy X-ray absorptiometry, as described previously (17, 18), or air displacement plethysmography (BOD POD; Life Measurement, Concord, CA). The BOD POD measurements were made in duplicate using methods described previously (14).
Body weight.
All body weight measurements were made in the morning in the fasted state after voiding. Two methods were used: clinic weights and home weights. Clinic weights were measured each week by research personnel at the study clinics using calibrated scales that were accurate to ±50 g. Home weights were measured and recorded by participants in their homes, using calibrated scales provided by the study. Home weights were obtained daily for ≤4 wk, beginning 1 wk before and ending 1 wk after each 2-wk TEE measurement period.
Self-reported food intake.
Participants received detailed instructions on how to measure and record all foods and beverages in daily food diaries. Research dietitians reviewed these diaries with participants at the end of each recording period and analyzed them for nutrient content using either Nutrition Data System for Research (NDS-R; software versions 4.05, 4.06, and 5.0; Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) or Moore's Extended Nutrient Database (MENu; Pennington Biomedical Research Center, Baton Rouge, LA). EI was calculated from two consecutive 6-day food records at baseline and 6-day food records at months 1, 3, and 6 of the CR intervention, corresponding to the TEE measurement periods. Only participants with food records for ≥6 days at baseline and ≥5 days at each subsequent time point were included in this analysis.
Quantifying change in body energy stores.
The average daily change in energy stores (ΔES) was computed from either body composition or body weight using six different approaches. Long-term ΔES was computed in two ways: 1) change in body composition from baseline to month 6 divided by the exact number of days between measurements and 2) change in clinic weight from baseline to month 6 divided by the exact number of days between measurements. Short-term ΔES was computed in four ways: 1) change in body composition between TEE time points (i.e., BL to M1, M1 to M3, M3 to M6) divided by the number of days between time points, 2) change in body composition during each 2-wk TEE assessment period divided by 14 days, 3) regression of weekly clinic weights during each 2-wk TEE assessment period, and 4) regression of daily home weights for 4 wk surrounding each 2-wk TEE assessment. Changes in body composition were converted to ΔES using 9.3 and 1.1 kcal/g as the energy coefficients of FM and FFM, respectively: ΔES (kcal/day) = ΔFM (g/day) × 9.3 kcal/g + ΔFFM (g/day) × 1.1 kcal/g. Changes in body weight (g/day) were converted to ΔES (kcal/day) using the energy coefficient 7.4 kcal/g: ΔES (kcal/day) = Δweight (g/day) × 7.4 kcal/g.
Quantifying EI.
Nine approaches were evaluated for quantifying EI and %CR, as outlined in Fig. 1. Approaches 1–8 utilized TEE and changes in body energy stores, whereas approach 9 was based on self-reported dietary intake using food diaries. Four long-term approaches for determining average 6-mo EI and %CR were based on average 6-mo TEE plus 6-mo change in body composition (using the long-term ΔES approach as described above). Average 6-mo TEE was calculated as a time-weighted average that included up to four TEE assessment time points (i.e., baseline, months 1, 3, and 6) and used the precise number of days between time points for each participant.
Four short-term approaches for determining average 6-mo EI and %CR were based on EI values at baseline, month 1, month 3, and month 6 computed from TEE assessments at those time points combined with short-term changes in energy stores (using short-term ΔES approaches c, d, e, or f). Average 6-mo EI was calculated as a time-weighted average that included all four EI values (i.e., baseline, month 1, month 3, and month 6) and used the precise number of days between time points for each participant.
For the eight TEE-based approaches, baseline EI was computed as the average TEE from two consecutive baseline TEE assessment periods; EI was assumed to equal TEE because subjects were weight stable at baseline.
Quantifying %CR.
Mean 6-mo %CR was calculated as the percentage decrease in EI relative to baseline EI for each of the nine EI quantification approaches according to the following equation: %CR(mean) = [1 − EI(mean)/EI(BL)] × 100.
Statistical analysis.
Results are reported as means ± SD unless otherwise noted. Confidence intervals are presented for %CR during the 6-mo intervention. Repeated-measures ANOVA using SAS Proc Mixed was used to test for equality of means of EI and %CR across the different approaches. A heterogeneous compound symmetry covariance structure was assumed to account for unequal variances for the different approaches. We tested overall differences for all approaches vs. the reference as well as pairwise comparisons for each approach with the reference. This analysis is considered exploratory, so no adjustments were made for multiple comparisons. Agreement between individual %CR values obtained by quantification approach 1 and approaches 2, 3, and 5 was assessed graphically by the method of Bland and Altman. All analyses were performed with SAS software version 9.2 (SAS Institute, Cary, NC).
RESULTS
As shown in Table 1, 40 participants with complete data during 6 mo of CR were included in the analyses. The age range was 24–59 yr; all but three participants were classified as overweight. Average body weight values at baseline, month 1, month 3, and month 6 were 79.3 ± 10.0, 77.2 ± 9.7, 73.6 ± 9.1, and 70.7 ± 8.8 kg, respectively. Average 6-mo weight change was −8.6 ± 3.7 kg (range: −3.3 to −19.9 kg). Fat mass comprised 61.3 ± 43.8% of the lost weight at month 1, 82.6 ± 23.3% at month 3, and 85.5 ± 17.3% at month 6. Figure 2 illustrates the relative changes in body weight, body composition, TEE, and resting metabolic rate observed during the 6-mo CR interventions. As shown, TEE declined 9.6 ± 9.9% (P < 0.0001) within 1 mo of CR beginning and then stabilized. TEE values at baseline, month 1, month 3, and month 6 averaged 2,761 ± 479, 2,482 ± 416, 2,471 ± 454, and 2,447 ± 410 kcal/day, respectively. Changes in resting metabolism accounted for 25.8% of the decrease in TEE at month 1, 23.0% at month 3, and 43.0% at month 6.
Table 1.
Participant characteristics at baseline
| All (n = 40) | Females (n = 30) | Males (n = 10) | |
|---|---|---|---|
| Age, yr | 38.8 ± 8.5 | 38.6 ± 8.1 | 39.6 ± 10.3 |
| Weight, kg | 79.3 ± 10.0 | 75.6 ± 7.5 | 90.5 ± 8.1 |
| Body mass index, kg/m2 | 27.6 ± 1.6 | 27.6 ± 1.7 | 27.6 ± 1.6 |
| %Fat mass | 35.0 ± 7.7 | 38.4 ± 5.3 | 24.8 ± 3.8 |
| TEE, kcal/day | 2,761 ± 479 | 2,559 ± 295 | 3,369 ± 410 |
| Resting metabolic rate, kcal/day | 1,550 ± 251 | 1,464 ± 190 | 1,811 ± 232 |
| Physical activity level* | 1.79 ± 0.19 | 1.76 ± 0.18 | 1.87 ± 0.20 |
Values are means ± SD.
TEE, total daily energy expenditure.
Physical activity level is calculated as TEE/resting metabolic rate.
Fig. 2.
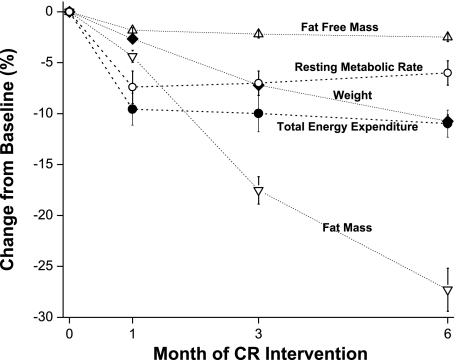
Changes in body weight, body composition, TEE, and resting metabolic rate after 1, 3, and 6 mo of CR.
A comparison of six approaches for computing changes in body energy stores is shown in Table 2. Average ΔES based on a 6-mo change in body composition (approach a) was −422 ± 180 kcal/day. As shown in Fig. 3 for individual subjects, there was a strong relationship between ΔES computed from the 6-mo change in body composition and ΔES computed from the 6-mo change in body weight (r2 = 0.877, intercept = 30.0, slope = 0.85). Of the short-term methods employed, regression of daily home weights over 4 wk (approach f, −424 ± 181 kcal/day) yielded a ΔES estimate that agreed very well with approach a. In contrast, 2-wk changes in body composition (approach d) and a 2-wk clinic weight regression (approach e) produced the most variable ΔES results that differed significantly (P < 0.01) from approach a.
Table 2.
Daily changes in body energy stores during CR interventions computed using 6 different approaches
| Daily Change in Body Energy Stores, kcal/day |
||||
|---|---|---|---|---|
| Approaches for Computing Change in Body Energy Stores | M1 | M3 | M6 | BL to M6 |
| Long term | ||||
| 6-Mo Δbody composition (approach a) | −422 ± 180 | |||
| 6-Mo Δweight (approach b) | −388 ± 163 | |||
| Short term | ||||
| ΔBody composition between time points (approach c) | −621 ± 548 | −594 ± 241 | −281 ± 173 | −422 ± 180 |
| 2-Wk Δbody composition (approach d) | −891 ± 470 | −669 ± 432 | −268 ± 613 | −615 ± 307‡ |
| 2-Wk weekly clinic weight regression (approach e)* | −659 ± 409 | −563 ± 464 | −304 ± 402 | −515 ± 243‡ |
| 4-Wk daily home weight regression (approach f)† | −657 ± 331 | −403 ± 233 | −232 ± 335 | −424 ± 181 |
Values are means ± SD; n = 40 participants for approaches a, b, c, e, and f and n = 38 for approach d. See methods for calculations.
CR, calorie restriction; M1, month 1; M3, month 3; M6, month 6; BL, baseline.
Mean no. of body weight values included was 3.3 ± 0.5.
Mean no. of body weight values included was 18.7 ± 2.9.
P < 0.01 for comparison with approach a.
Fig. 3.
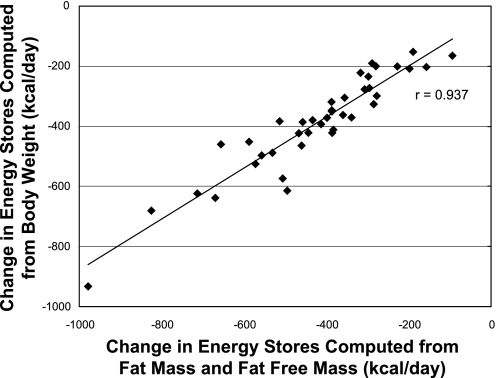
Association between daily change in body energy stores computed from body weight and daily change in body energy stores computed from fat mass and fat-free mass. Body weight and body composition were measured at baseline and after 6 mo of CR. ♦Body weight.
EI and %CR results computed according to the nine quantification approaches are shown in Table 3. Approach 1 serves as the reference approach and includes all four TEE assessment time points and a 6-mo change in body composition; average 6-mo EI and %CR were computed to be 2,063 ± 373 kcal/day and 24.9 ± 8.7%, respectively. To explore the consequences of reducing the number of TEE measurements, we computed EI and %CR after excluding the month 1 and/or month 3 TEE values. Exclusion of the month 3 TEE time point (approach 3) did not compromise the results, yielding 6-mo EI and %CR values that agreed most closely with approach 1. In contrast, exclusion of both interim TEE measurements produced unacceptably high EI and, therefore, low %CR values relative to approach 1. Bland-Altman plots depicting the difference in individual %CR values between approach 1 and approaches 2, 3, and 4 are shown in Fig. 4.
Table 3.
Energy intake and %CR during 6-mo CR interventions quantified using 9 different approaches
| Energy Intake, kcal/day |
%CR |
||||||
|---|---|---|---|---|---|---|---|
| Approaches for Quantifying Energy Intake and %CR | BL‡ | M1 | M3 | M6 | Average | Average | 95% CI |
| Long-term approaches | |||||||
| 6-Mo TEE(BL, M1, M3, M6) + 6-mo Δbody composition (approach 1)† | 2,761 ± 479 | 2,063 ± 373 | 24.9 ± 8.7 | 22.2, 27.7 | |||
| 6-Mo TEE(BL, M3, M6) + 6-month Δbody composition (approach 2) | 2,761 ± 479 | 2,108 ± 394 | 23.4 ± 9.0 | ||||
| 6-Mo TEE(BL, M1, M6) + 6-mo Δbody composition (approach 3) | 2,761 ± 479 | 2,062 ± 383 | 25.0 ± 8.7 | 22.2, 27.8 | |||
| 6-Mo TEE(BL, M6) + 6-mo Δbody composition (approach4) | 2,761 ± 479 | 2,182 ± 408§ | 20.9 ± 7.1§ | 18.6, 23.2 | |||
| Short-term approaches | |||||||
| 2-Wk TEE + Δbody composition between time points (approach 5) | 2,761 ± 479 | 1,861 ± 643 | 1,877 ± 446 | 2,166 ± 444 | 2,042 ± 374 | 25.7 ± 9.2 | 22.7, 28.6 |
| 2-Wk TEE + 2-Wk Δbody composition (approach 6) | 2,761 ± 479 | 1,611 ± 558 | 1,794 ± 440 | 2,178 ± 773 | 1,862 ± 393§ | 32.8 ± 11.7§ | 28.8, 36.7 |
| 2-Wk TEE + 2-Wk weekly clinic weight regression (approach 7) | 2,761 ± 479 | 1,823 ± 517 | 1,909 ± 631 | 2,142 ± 620 | 1,955 ± 426§ | 29.0 ± 11.4§ | 25.3, 32.6 |
| 2-Wk TEE + 4-Wk daily home weight regression (approach 8) | 2,761 ± 479 | 1,825 ± 458 | 2,068 ± 463 | 2,215 ± 602 | 2,045 ± 395 | 25.8 ± 8.5 | 23.1, 28.5 |
| 1-Wk dietary intake (food diary, self-reported; approach 9) | 2,325 ± 570 | 1,900 ± 392 | 2,021 ± 371 | 1,988 ± 396 | 1,981 ± 352§ | 11.5 ± 19.9§ | 4.4 ± 18.6 |
Values are means ± SD; n= 40 participants for approaches 1–5 and 7, n = 36 for approach 6, and n = 33 for approach 9. See methods for description.
CI, confidence interval.
TEE by doubly labeled water.
BL energy intake was assumed to equal baseline TEE for approaches 1–8; prescribed %CR averaged 27.9 ± 5.3 during the CR interventions.
P < 0.01 for comparison with approach 1.
Fig. 4.
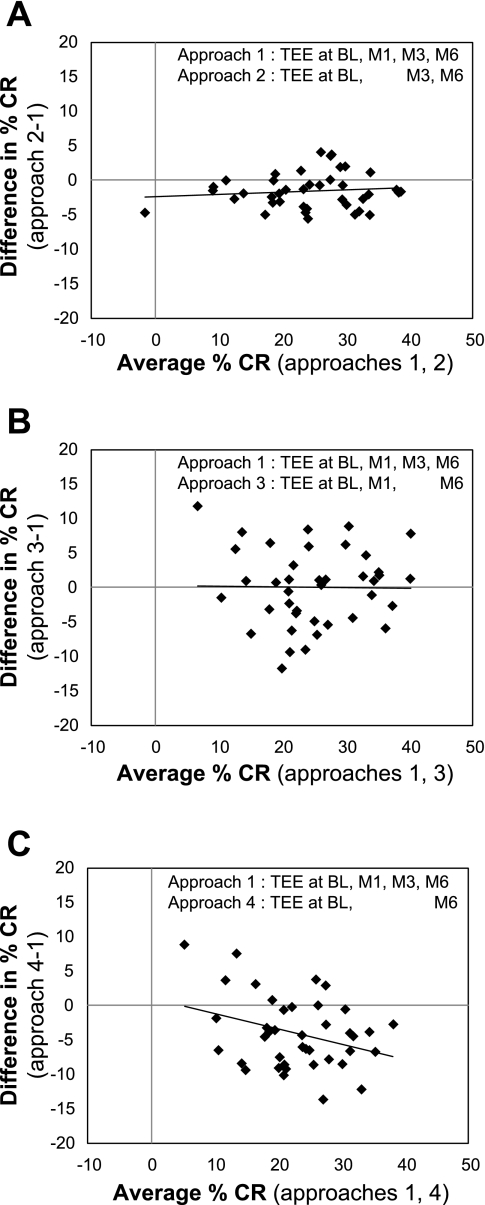
Bland-Altman comparisons of %CR computed using the reference approach that incorporated 4 TEE assessment time points with alternative approaches based on 2 or 3 TEE time points. TEE was assessed using the DLW method. %CR was computed using a time-weighted average TEE plus 6-mo change in body composition. Time points included BL, M1, M3, and M6.
Two additional long-term approaches for computing EI and %CR were explored (results not included in Table 3). Use of 6-mo change in weight instead of body composition in approach 1 did not significantly affect EI or %CR (2,097 ± 362 kcal/day and 23.7 ± 8.3% CR, respectively). In contrast, using only baseline TEE plus a 6-mo change in body composition yielded unacceptably high EI (2,339 ± 452 kcal/day) and, therefore, low %CR values (15.4 ± 5.8%) relative to approach 1 because metabolic adaptation was not accounted for.
Short-term EI quantification approaches that utilized changes in body composition between TEE time points (approach 5) or 4-wk daily home weight regression (approach 8) agreed well with approach 1 (Table 3). In contrast, approaches that relied on 2-wk changes in body composition (approach 6) or 2-wk clinic weight regression (approach 7) to compute average 6-mo EI and %CR did not agree with the reference approach.
DISCUSSION
Using data compiled from a multicenter study of CR (CALERIE), we tested various approaches for determining energy intake and %CR during a 6-mo period of CR. The results of these analyses have important clinical applicability for weight loss studies and other trials in which energy intake is restricted relative to the weight maintenance energy requirement, such as CALERIE. Three important observations of the current study are that 1) optimal quantification of average energy intake and %CR during a weight loss intervention necessitates a TEE measurement within the first 2–4 wk to capture the rapid reduction in daily energy expenditure that occurs early during CR, 2) the optimal approach for determining free-living energy intake and %CR at a single point in time during active weight loss includes a TEE measurement combined with regression of daily weights for 4 wk surrounding the TEE assessment period, and 3) body weight is a valid substitute for body composition when determining long-term changes in body energy stores during weight loss.
Although it has been known for many years that metabolic rate and 24-h energy expenditure decline quickly upon initiation of an energy-restricted diet, previous studies (2, 22) utilized room calorimeters, more restrictive diets (i.e., ≥50% CR), and relatively small sample sizes. The current study is the first to demonstrate that TEE in free-living individuals decreases significantly within the first 2–4 wk of beginning a moderate (i.e., 25%) CR diet. Similar to the observations of de Boer et al. (2), in which 24-h energy expenditure decreased 8.9% by days 6 and 7 of a 58% CR diet, we observed a 9.6% decrease in TEE by weeks 2–4 of a 25% CR diet. However, whereas 24-h energy expenditure continued to decline and reached 85% of the baseline value at week 6 in the study of de Boer et al. (2), TEE in the current study remained at 90% of baseline TEE at months 3 and 6, which was likely due to the more mild level of CR.
The reference approach for computing average 6-mo EI and %CR in the current study included measurement of TEE at four time points (baseline and months 1, 3, and 6) and changes in body composition over the 6-mo period of CR. We compared this approach with several alternative approaches that included fewer TEE assessments or different approaches for computing changes in body energy stores. Because TEE declined rapidly upon initiation of CR, inclusion of a TEE measurement during month 1 provided the best estimate of average energy intake and %CR during the 6-mo CR intervention. As expected, exclusion of both month 1 and month 3 TEE results yielded discrepant estimates of EI and %CR compared with the reference approach. In the absence of interim TEE measurements, average EI and %CR can be estimated by weighting the baseline and final TEE values to account for the rapid decrease in TEE that occurs with CR. In the current study, weighting the baseline and final TEE values as and of the average month 6 TEE, respectively, produced EI and %CR values that were similar to the reference approach. However, this assumption would not be valid in cases where TEE either declines continuously during the CR intervention or increases due to weight regain or engagement in higher levels of physical activity. Furthermore, because individuals differ in the degree to which TEE declines in response to CR (12, 29), as well as in the time course of response, measuring TEE at an interim time point enables the most valid individual estimate of EI.
Estimates of long-term changes in energy stores based on body composition and body weight yielded group EI and %CR estimates that were similar and highly correlated within individuals. This suggests that the easier and less costly weight-based approach can substitute adequately for body composition measurements in some circumstances. However, for certain populations or with interventions that combine CR and exercise, the actual ratio of changes in fat mass to changes in fat-free mass may cause the true energy coefficient of weight change to differ from 7.4 kcal/g. Furthermore, the relative contributions of fat mass and fat-free mass to weight change are influenced by sex, degree of adiposity, and type of exercise intervention. In addition, during periods of weight regain (a common phenomenon following weight loss), the ratio of changes in fat mass to fat-free mass may differ from that during weight loss.
We also explored the influence of using serial short-term measurements of body weight and body composition to quantify changes in energy stores. The advantage of this approach is that it enables one to compute EI at a single time point during a weight loss intervention; the disadvantages include the potentially large errors in estimating daily change in body energy stores and the assumption that changes in energy stores during the 2-wk TEE assessment period accurately reflect changes that occur between TEE assessments. As expected, regression of daily body weights proved to be a better indicator of short-term changes in body energy stores than body composition measurements at the beginning and end of each 2-wk TEE period due to the small magnitude of changes in FM and FFM relative to measurement error. Importantly, the EI quantification approach based on regression of daily home weights yielded EI and %CR results that were not statistically different from the reference approach, whereas approaches that computed EI and %CR using 2-wk body composition changes or regression of weekly clinic weights produced results that differed significantly from the reference approach. These results are consistent with the findings of Hall and Chow (6), who demonstrated that daily body weight measurements can be used to estimate an individual's change in energy intake if the assessment period exceeds 28 days. Although their mathematical model provides accurate and precise estimates of changes in EI during weight loss, these authors point out that determining an individual's EI and adherence to a diet necessitates an accurate assessment of baseline EI, which can only be achieved using DLW. In support of this assertion, we found that estimates of baseline EI computed using the Dietary Reference Intakes Estimated Energy Requirement (EER) formulae (9) [as proposed in the model of Hall and Chow (6)] differed substantially from baseline EI determined by DLW, with the degree of error dependent upon the physical activity coefficient used in each EER formula (range: −25.6 to +0.6% error for “low active”, −17.4 to +14.2% error for “active”). Individualizing the physical activity level can minimize this error.
In agreement with previous studies that documented low accuracy of self-reported EI (13, 15), the food diary data in the current study revealed underreporting of EI compared with TEE-determined EI at baseline. Self-reported EI remained lower than the reference approach when averaged across the 6-mo CR intervention, although the degree of underestimation was relatively small due to the extensive training and practice in monitoring food intake during the CR intervention. As expected, %CR based on food diary data was highly variable and did not agree very well with the reference approach. It is noteworthy that objective measurements using DLW and body composition changes indicate that, on average, adherence to the CR intervention was excellent, with 25.5% CR achieved compared with 27.9% prescribed.
Del Corral et al. (4) presented an alternative method of assessing dietary adherence in weight loss regimens that is also based on measurements of TEE and changes in body energy stores. In their model, adherence is computed as the ratio of actual daily energy loss to expected energy loss; actual daily energy loss is calculated from fat mass and fat-free mass by the same approach as in our study, and expected daily energy loss is calculated by subtracting prescribed EI from average TEE, with average TEE based on pre- and postintervention measurements: % adherence = 100 × [TEE(average of BL, final) − EIactual]/[TEE(average of BL, final) − EIprescribed].
In contrast, in the current study we computed %CR as the reduction in EI relative to baseline EI, and adherence can be expressed as the ratio of actual %CR to prescribed %CR: %adherence = 100 × [(1 − EIactual/EIBL ) × 100/(%CRprescribed)].
Because TEE declines quickly upon initiation of CR (2, 22) due to weight loss and metabolic adaptation (19), %CR and %adherence calculated using our approaches will generally be higher than those based on the method of Del Corral et al. (4) Inclusion of exercise during weight loss may attenuate the decline in TEE, thereby yielding more similar adherence results between the two methods.
Recently, comprehensive physiological models have been developed to predict changes in body weight and body composition in response to CR. These models have also been used to estimate the changes in EI that account for an observed degree and time course of weight loss for individual subjects and for groups (5, 25, 26). These approaches have great potential value for gauging individual adherence during a CR intervention, where the complexities of EI estimates from TEE and changes in body composition in real time are limited. Furthermore, the individual estimates of EI in these models require validation in larger numbers of subjects. Therefore, quantification of EI from assessments of TEE and changes in body energy stores is the preferred approach for estimating %CR across a variety of populations. TEE-based energy intake data from a wider range of CR and weight-loss trials could be used to refine and extend physiological models to improve their ability to estimate EI from weight changes.
A limitation of the current study is the absence of a comparative measurement of EI that is 100% accurate and precise. Although the DLW method is the gold standard for quantifying TEE and energy intake in free-living individuals (9), the relatively small analytical and physiological errors result in a combined error of ∼5% (28), which may be magnified when EI is computed during periods of weight loss or weight regain. The duplicate baseline DLW periods and the stringent procedures for isotope dosing, sample acquisition, isotope analyses, and body weight measurement in the current study were designed to minimize errors and optimize the accuracy and precision of this method.
In summary, numerous models may be used to quantify EI and %CR during CR interventions. We presented eight approaches that incorporate TEE measured by DLW and changes in body energy stores computed six different ways. The optimal approach for quantifying average EI and %CR during a 6-mo CR intervention relies on TEE measurements at baseline and during the first few weeks of CR to capture the rapid reduction in daily energy expenditure; long-term changes in body energy stores can be computed from body composition or body weight. The most reliable approach for determining free-living energy intake (and %CR) at a single time point during active weight loss relies on a TEE measurement combined with an estimate of daily change in body energy stores computed from either regression of daily weights over a 4-wk period or a change in body composition during the preceding months. These long- and short-term approaches can be used to determine adherence to weight loss interventions and to enhance the interpretation of results when actual %CR differs from the level prescribed.
GRANTS
This research was supported by National Institute on Aging Cooperative Agreements U01-AG-020487, U01-AG-020478, U01-AG-020480, and U01-AG-022132 and National Institutes of Health Grants MO1-RR-00036, P60-DK-020579-30, and P30-DK-056341. L. M. Redman was supported by a Neil Hamilton-Fairley Training Fellowship awarded by the National Health and Medical Research Council of Australia (ID 349553). This trial was registered at clinicaltrials.gov as NCT00099138, NCT00099151, and NCT00099099.
DISCLOSURES
None of the authors has a personal or financial conflict of interest.
AUTHOR CONTRIBUTIONS
S.B. Racette, S.K.D., E.C.H., S.B. Roberts, E.R., and J.P.D. did the conception and design of the research; S.B. Racette, S.K.D., J.P.D., and L.R. performed the experiments; S.B. Racette, M.B., J.P.D., and L.R. analyzed the data; S.B. Racette, S.K.D., M.B., E.C.H., S.B. Roberts, E.R., C.P., J.P.D., W.E.K., J.R., and L.R. interpreted the results of the experiments; S.B. Racette prepared the figures; S.B. Racette, S.K.D., E.C.H., and L.R. drafted the manuscript; S.B. Racette, S.K.D., M.B., E.C.H., S.B. Roberts, E.R., C.P., J.P.D., W.E.K., J.R., and L.R. edited and revised the manuscript; all authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge other CALERIE investigators and team members who contributed to these studies: Pennington Biomedical Research Center: Andrew Deutsch, Paula J. Geiselman, Frank Greenway, Leonie Heilbronn, Enette Larson-Meyer, Michael Lefevre, Marlene Most-Windhauser, and Steven Smith; Tufts University: Cheryl H. Gilhooly, Paul J. Fuss, and Gerard E. Dallal; Washington University School of Medicine: John O. Holloszy, Hassan Arif, Ali Ehsani, Luigi Fontana, Samuel Klein, Christopher Koenig, Kathleen A. Obert, Kenneth B. Schechtman, Morgan Schram, Karen Steger-May, Richard I. Stein, Dennis T. Villareal, Laura Weber, and Edward P. Weiss; Duke Clinical Research Institute: Connie Bales. We would also like to acknowledge helpful comments on DLW methods from William Wong, Baylor College of Medicine.
REFERENCES
- 1. Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 85: 1023–1030, 2007 [DOI] [PubMed] [Google Scholar]
- 2. de Boer JO, van Es AJ, Roovers LC, van Raaij JM, Hautvast JG. Adaptation of energy metabolism of overweight women to low-energy intake, studied with whole-body calorimeters. Am J Clin Nutr 44: 585–595, 1986 [DOI] [PubMed] [Google Scholar]
- 3. de Jonge L, DeLany JP, Nguyen T, Howard J, Hadley EC, Redman LM, Ravussin E. Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. Am J Clin Nutr 85: 73–79, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Corral P, Chandler-Laney PC, Casazza K, Gower BA, Hunter GR. Effect of dietary adherence with or without exercise on weight loss: a mechanistic approach to a global problem. J Clin Endocrinol Metab 94: 1602–1607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab 298: E449–E466, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall KD, Chow CC. Estimating changes in free-living energy intake and its confidence interval. Am J Clin Nutr 94: 66–74, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295: 1539–1548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol 42: 709–712, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine, Food, and Nutrition Board Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies, 2005 [Google Scholar]
- 10. Johnson RK, Soultanakis RP, Matthews DE. Literacy and body fatness are associated with underreporting of energy intake in US low-income women using the multiple-pass 24-hour recall: a doubly labeled water study. J Am Diet Assoc 98: 1136–1140, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Larsson CL, Westerterp KR, Johansson GK. Validity of reported energy expenditure and energy and protein intakes in Swedish adolescent vegans and omnivores. Am J Clin Nutr 75: 268–274, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Martin LJ, Su W, Jones PJ, Lockwood GA, Tritchler DL, Boyd NF. Comparison of energy intakes determined by food records and doubly labeled water in women participating in a dietary-intervention trial. Am J Clin Nutr 63: 483–490, 1996 [DOI] [PubMed] [Google Scholar]
- 14. McCrory MA, Gomez TD, Bernauer EM, Molé PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc 27: 1686–1691, 1995 [PubMed] [Google Scholar]
- 15. Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, Beresford SA, Caan B, Thomson C, Satterfield S, Kuller L, Heiss G, Smit E, Sarto G, Ockene J, Stefanick ML, Assaf A, Runswick S, Prentice RL. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am J Epidemiol 167: 1247–1259, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol Endocrinol Metab 267: E585–E590, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 61: 943–950, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab 92: 865–872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E; Pennington CALERIE Team Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 4: e4377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal 14: 275–287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberts SB. Use of the doubly labeled water method for measurement of energy expenditure, total body water, water intake, and metabolizable energy intake in humans and small animals. Can J Physiol Pharmacol 67: 1190–1198, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Rumpler WV, Seale JL, Miles CW, Bodwell CE. Energy-intake restriction and diet-composition effects on energy expenditure in men. Am J Clin Nutr 53: 430–436, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Schoeller DA. The energy balance equation: looking back and looking forward are two very different views. Nutr Rev 67: 249–254, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr 118: 1278–1289, 1988 [DOI] [PubMed] [Google Scholar]
- 25. Thomas DM, Martin CK, Heymsfield S, Redman LM, Schoeller DA, Levine JA. A simple model predicting individual weight change in humans. J Biol Dyn 5, 1–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr 92: 1326–1331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab 281: E891–E899, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Trabulsi J, Troiano RP, Subar AF, Sharbaugh C, Kipnis V, Schatzkin A, Schoeller DA. Precision of the doubly labeled water method in a large-scale application: evaluation of a streamlined-dosing protocol in the Observing Protein and Energy Nutrition (OPEN) study. Eur J Clin Nutr 57: 1370–1377, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Velthuis-te Wierik EJ, Westerterp KR, van den Berg H. Impact of a moderately energy-restricted diet on energy metabolism and body composition in non-obese men. Int J Obes Relat Metab Disord 19: 318–324, 1995 [PubMed] [Google Scholar]


