Abstract
In this paper we explore the effect public policy on the extent to which genes may influence smoking desistance. Using a sample of adult twins (nmz=363, ndz=233) from a large population registry, we estimate Cox proportional hazards models that describe similarity in the timing of smoking desistance among adult twin pairs and we show that identical twin pairs are significantly more likely to quit smoking within a similar time frame compared to fraternal twin pairs. Importantly, we then show that genetic factors for smoking desistance increase in importance following restrictive legislation on smoking behaviors that occurred in the early and mid 1970s. These findings support the social push perspective and make important contributions to the social demography and genetic epidemiology of smoking as well as to the gene-environment interaction literatures.
INTRODUCTION
Given that smoking remains the single most important cause of premature mortality – the source of 443,000 deaths per year during 2000 to 2004 in the United States (Adhikari et al. 2008) – it has enormous importance for understanding the levels and patterns of population mortality (Rogers et al. 2005). Smoking contributes to mortality differences across cohorts (Preston and Wang 2006), nations (Pampel 2002), and socioeconomic groups (Jha et al. 2006). Such influences will not disappear soon. The decline in regular smoking incidence caused large changes in the relative size of the smoking population, but the rate of decline has slowed more recently (Mendez and Warner 2004). Indeed, the United States appears to be only halfway to the goal of eliminating this public health hazard (Fiore and Baker 2009). Along with raising public health concerns, the frustratingly slow progress presents a puzzle about population health and longevity: Despite widespread acceptance of the harm of smoking, high cigarette prices, social disapproval, restrictions on places to light up, and strong desires among users to quit, nearly one fifth of the American adult population still smokes (Thorne et al. 2008).
The demographic attention to the composition of populations at risk may provide some insight into these trends, particularly into the difficulties of getting smokers to quit. Although entry into smoking draws from the general population, exit from smoking draws from a continuously changing population of regular smokers. With declining incidence of smoking, those least committed or likely to become addicted to the habit may now either never start or soon quit, while those most attracted or easily addicted more often take up the habit and have more trouble quitting. As a result, those most reticent to exit from smoking status, whose symptoms of nicotine withdrawal are felt most acutely, may come to make up an increasingly larger portion of the smoking population.
Said differently, the composition of the smoking population with regard to genetic propensities to smoke or become addicted may, in particular, have changed. In general, environmental and genetic factors equally contribute to an individual’s risk of becoming a regular smoker; heritability and environmental influence for regular smoking typically range between .4 and .6 (Carmelli et al. 1992; Hall, Madden, and Lynskey 2002; Li et al. 2003; Sullivan and Kendler 1999) and numerous candidate genes have been identified (for a review see Munafo et al. 2004). However, genetic influences on smoking vary considerably across different social environments (Boardman 2009; Boardman et al. 2008; Timberlake et al. 2006). The environmental moderation of genetic factors is anticipated by the gene-by-environment interaction (GxE) paradigm (Eaves, Silberg and Erkanli 2003; Shanahan and Hofer 2005), and there is relatively consistent evidence that environments may control or enable genetic tendencies to consume cigarettes. This is particularly true when the “environment” is characterized in broad historical periods that have varied social norms, public policies, and institutional constraints and that influence the degree to which genes differentiate between smokers and non-smokers (e.g., Boardman, Blalock, and Pampel 2010; Kendler, Thornton, and Pederson 2000).
Recent decades clearly reflect changes in norms and constraints on smoking that relate closely to policy (Pampel 2005). Cigarette consumption increased more than fivefold from 1920 to 1960, leveled off during the mid 1960s, and then consistently declined following the mid 1970s. At the peak around 1966, roughly one-half of men and one-third of women in the United States smoked regularly (Forey, Hamling, and Lee 2009). The timing of this peak roughly corresponded with first report by the Surgeon General regarding the dangers of smoking in 1964 which ultimately led to the Federal Cigarette Labeling and Advertising Act of 1965. This required that all cigarette packages contain a printed copy of the statement: “Caution: Cigarette Smoking May Be Hazardous to Your Health.” Although the scientific community had declared that smoking was unequivocally hazardous to one’s health, it was not until the mid 1970s until formal restrictions were placed on smoking behaviors. In 1973, Arizona became the first state to pass a comprehensive law that limited smoking in public places. More restrictive sets of laws followed, including the 1975 Minnesota Clean Indoor Air Act, which required restaurants to have nonsmoking sections. Twelve years later Aspen, Colorado, became the first city to formally ban all cigarette smoking in restaurants. The push for bans in all restaurants was bolstered by the nineteenth Surgeon General’s Report (USDHHS 1986), which argued that the “simple separation of smokers and nonsmokers within the same airspace may reduce but cannot eliminate nonsmoker exposure to environmental tobacco smoke.” By the end of 2007, the number of states requiring restaurants to be smoke-free increased to 21 and the number of states without smoking restrictions for restaurants decreased from 19 to 9 (Tynan, Babb, and MacNeil 2008). The implementation of anti-smoking policies coincided with steady movement toward the acceptance of the harm to health of smoking and rejection of the habit by opinion leaders and large parts of the population.
As others have shown (Boardman et al. 2010), this historical context provides a critical backdrop for understanding the genetics of smoking and the often-overlooked gene-environment interaction. As both prevalence and incidence have declined in recent years, smokers who continue in the face of external pressures, social sanctions, and anti-tobacco policies have become a more select group. The change thus raises two questions: Has the impact of genetic characteristics on the likelihood of being in the smoking population across the changed over time? And if so, have changes in the smoking environment reduced or heightened genetic influences on this behavior? Answering these questions can shed light on the nature of gene-environment interactions.
The current study extends this period-moderation perspective by examining smoking desistance as the phenotype. Researchers report heritability estimates of successful quitting to be in the range of .3 to .5 (Xian et al. 2003), but no existing work has examined period effects on the genetics of smoking desistance. Genes implicated in smoking onset are quite different from those associated with smoking desistance (Broms et al. 2006), and we suspect the period effects on the genetics of smoking desistance may differ as well.
Gene-Environment Interplay and Smoking
Two competing gene-environment interaction models speak to changes in the smoking environment and the genetics of smoking. The social control model hypothesizes that normative and institutional controls restrict individual behavior, and as a result, two individuals within highly controlled environments may behave similarly despite genetic differences. In this case, phenotypic similarity is simply a function of the social controls linked to laws and legal enforcement, moral codes, religious control, highly organized educational settings, or broad forms of stratification that limit particular individuals’ mobility. For example, Timberlake et al. (2006) show that religious participation reduces the additive genetic influences on smoking onset among adolescents; their estimated 60% heritability nearly drops to zero for those who report that religion is “more important than anything else.” Economic incentives are equally powerful; Boardman (2009) shows that states with higher excise taxes per pack of cigarettes have the lowest observed heritability of regular smoking. Again, the taxes serve as an instrument of social control which ultimately dampens genetic influences.
This hypothesis predicts that as anti-smoking pressures intensify and smoking becomes less common, genetic influences on smoking weaken. Early warnings and publicity in the 1960s about the harm of smoking led those with weaker genetic propensities to avoid starting to smoke or to quit soon, leaving those with stronger genetic propensities to begin and continue smoking. This initial change in the population of smokers created strong genetic influence on quitting. In more recent decades, however, the anti-smoking environment and associated disincentives have become strong enough to affect those with genetic propensities. Reduced entrance into smoking and higher quitting among hardcore smokers tends to reduce the predominance of genetically prone smokers in the smoking population and moderate genetic influences.
Alternatively, the social push model (Raine 2002) hypothesizes that public policies can actually highlight genetic influences by minimizing “noise” that has the potential to overwhelm and hide genetic expression. According to this model, genetic associations are most clearly observable in benign environments lacking social factors that encourage genetically influenced addictive behaviors. When social noise is minimized by policies or other anti-smoking influences, it allows for “biology to shine through” (Raine 2002:14). Conversely, when social factors “push” certain behaviors, biological factors become harder to identify. Therefore, as social forces emerge that discourage smoking or remove the positive reinforcement of smoking, it reduces social influences or non-genetic noise and increases the salience of genetic influences.
According to the social push model, the implementation of increasingly restrictive anti-tobacco policies and the acceptance of anti-smoking norms should do more to concentrate smoking among those with genetic vulnerabilities to smoking. Rather than most benefitting those with physiological and genetic traits that increase nicotine addiction (as posited by the social control hypothesis), recent changes may help other types of smokers the most. As a result, those with genetic propensities toward addiction, those who are most easily induced to start smoking and have the most difficulty in quitting, tend to become concentrated in the smoking population. This changing composition of the population at risk increases the salience of genetic influences.
Historical Context as “Environment”
The gene-environment perspective has been extended to include macro events measured by historical periods as structuring the genetics of smoking behaviors. Kendler et al. (2000) demonstrate that the heritability for tobacco use changes predictably over time. While heritability estimates for men were relatively consistent across the three cohorts born between 1910-1924, 1925-1939, and 1940-1958 (h2~.60), none of the variance in tobacco use was due to genetic factors in the first cohort of women, but by the third cohort, there were no gender differences in the heritability estimates. They argue that women’s smoking behaviors were highly controlled during the first cohort, but absent these controls, genetic tendencies to use tobacco emerged.
Boardman et al. (2010) estimate additive genetic influences on having been a regular smoker at one time among U.S. adults born between 1920 and 1970 and show that the genetic influences on regular smoking reached a minimum (h2 ~ .05) at the same time as the peak of smoking in the United States (roughly 1964). However, genetic influences on regular smoking increased sharply until the mid 1970s and then declined again to levels reported by other studies. Boardman et al. (2010) argue that the first change (after the minimum in 1964) was due to the first Surgeon General’s Report regarding the dangers of smoking. That is, a non-causal social push mechanism was in place in which genetic resilience facilitated the relatively easy avoidance of smoking by some, but genetic vulnerability made it more difficult for certain people to avoid smoking and genetic factors thus came to more heavily influence regular smoking. This changed in the mid 1970s when the first legislation came on board to limit the places where people could smoke and to increase taxes on cigarettes. These real controls, they argue, had a causal influence on the genetics of smoking at this time.
In this study, our goal is to extend the notion that historical trends may influence genetic influence by examining a more specific smoking phenotype, smoking desistance. Previous studies focused on having ever been a regular smoker among all adults (Boardman et al. 2010; Kendler et al. 2000), while this study focuses on quitting among adults who became regular smokers. If the composition of smokers changes over time, the influence of genetic factors on quitting among the differentially selected group may well change and do so in ways that moderate genetic influences (according to the social control hypothesis) or that strengthen genetic influences (according to the social push hypothesis).
No study has yet to explore macro-level historical changes on the genetic determination of desistance or the transition from regular smoker to non-smoker. We argue that smoking desistance denotes a stronger test of the social control vs. the social push model when the environment is characterized as a time period. If the social and institutional controls related to smoking desistance increased significantly starting in 1973, then the social control model would anticipate that the genetic influences on smoking desistance would decrease after this point. However, the social push model suggests that these forces would have a greater influence on the less genetically vulnerable, leaving the composition of smokers to be more influenced by genetic factors. Hence, following 1973, the genetic influences on smoking desistance should increase. The goal of this paper is to evaluate the relevance of these competing perspectives as related to this important behavior during this critical point in time.
METHODS
Data
Over 6,000 twin pairs were ascertained from a large population-based birth registry for the Commonwealth of Virginia, supplemented by twins recruited through a national mailer sent to the American Association of Retired Persons. Sample ascertainment and structure are described in detail by Truett et al. (1994) and Eaves et al. (1999). A large questionnaire on “Health and Life Styles” was first administered to the population in the late 1980s, which contained a broad set of items on health and clinical traits including the onset of regular smoking and the timing of smoking desistance. We only use twin pairs in which both members of the pair reported to have smoked daily at some point in their lives. Further, because of our emphasis on the 1960s and 1970s, we reduce our sample to twin pairs in which the first to quit did so between the years of 1960 and 1980. Meeting these criteria, our reduced sample consists of 363 MZ pairs and 233 same-sex DZ pairs from the larger study.
Measures
Study participants were asked about their lifetime smoking habits. For those who responded that they smoked at least 1-5 cigarettes a day for some period of time, researchers asked “at what age did you start smoking?” They then asked “do you smoke now” and “if you have stopped smoking how old were you when you stopped?” These measures were used to calculate a pairwise measure indicating the length of time (in years) for a twin to quit smoking after his or her sibling had quit. Evidence for genetic influences on the cessation of smoking would be found by a reduced time to desistance among MZ twins compared to DZ twins.
Several important control variables are also included in the multivariate analyses. First, the pair based regression models assume genetic influences are present when the coefficient for identical compared to fraternal twins is positive and significant. However, identical twins are also more likely to share similar social environments compared to dizygotic twins which may have important implications for identifying genetic influences on substance use (Kendler and Gardner 1998). Accordingly, we include a measure that summarizes the typical frequency of visits between twins. This score ranges from 1 (daily visits) to 7 (never see one another). We use the average of the responses of the twins to this measure as a crude control for co-socialization as adults. As shown in Table 1, identical twins report more frequent visits (mean = 4.1) compared to fraternal twins (mean = 4.4; p< .008). Second, the regression models adjust for average education of the pair and a measure describing the difference in education between the twins (absolute value). That is, educational influences on smoking are very strong (Pampel 2005) and educational concordance among twins will cause twins to resemble one another’s smoking habits. This association may contain some genetic components but the social influences of this process make it difficult to properly identify genetic influences independent of these confounding environmental processes. As shown in Table 1, there are no differences in average education by zygosity; however, there are large differences in educational discordance by zygosity. Fraternal twins differed, on average, by 1.4 years but identical twins only differed by 0.8 years (p< .0001). We also control for the average educational level of the pair. Third, smoking intensity is highly heritable and the cessation of smoking is different for light, moderate, and heavy smokers. Further, smoking intensity is itself a highly heritable phenotype (Boardman 2009). Although these measures do not differ by zygosity, to adjust for these influences, we control for average number of cigarettes smoked by the pair and the difference (absolute value) in the number of cigarettes smoked by the pair.
Table 1.
Descriptive Statistics of Variables Used in the Analysis
| Variable | Full Sample | Identical twins | Fraternal Twins | Pr.< |
|---|---|---|---|---|
| Sex [Female = 1] | 0.535 | 0.534 | 0.536 | 0.961 |
| Duration of 2nd to quit | 10.451 (6.716) | 10.088 (6.792) | 11.017 (6.572) | 0.099 |
| Year of birth | 1931.737 (14.413) | 1931.039 (14.437) | 1932.824 (14.338) | 0.140 |
| Difference in age of initiation | 3.331 (4.392) | 3.242 (4.438) | 3.468 (4.325) | 0.541 |
| Age started (2nd to quit) | 18.639 (4.879) | 18.634 (4.853) | 18.648 (4.929) | 0.972 |
| Age when the 1st of the pair quit | 38.953 (13.508) | 39.543 (13.571) | 38.034 (13.385) | 0.184 |
| Pair smoking amount (average) | 14.779 (8.875) | 15.028 (8.994) | 14.391 (8.691) | 0.393 |
| Difference in smoking amount | 8.077 (8.713) | 7.879 (9.042) | 8.386 (8.183) | 0.488 |
| Average years of education | 13.419 (2.002) | 13.471 (2.062) | 13.339 (1.908) | 0.433 |
| Difference in education | 1.013 (1.403) | 0.771 (1.199) | 1.391 (1.605) | 0.000 |
| Frequency of visiting among pair | 4.251 (1.402) | 4.128 (1.443) | 4.442 (1.315) | 0.008 |
| Sample size | 596 | 363 | 233 |
Note: See Truett et al. (1994) and Eaves et al. (1999) for additional information about this study. Standard deviations for continuous variables are reported in parentheses. Significance test for differences between identical and fraternal twins are indicated in final column.
Source: Data on twin pairs come from a large population-based birth registry for the Commonwealth of Virginia and the American Association of Retired Persons. This study only uses data in which both members of the twin pair report regular smoking at some point during the study.
Statistical Analysis
The results presented in Tables 1 and 2 are illustrative because they demonstrate a greater concordance among MZ compared to DZ desistance. However, this comparison is problematic because the measurement of desistance includes both observed values of duration in which the co-twin stops and duration in which the desistance is unobserved and is thus right censored. This means that the measure of “quit within two years of one another” (Table 2) includes those who quit within three years of one another with those in which the co-twin has still not quit. The Cox proportional hazards model (Cox 1972) is a flexible and semi-parametric regression model that models the duration until quitting for the 2nd twin while simultaneously dealing with the censoring problem.
| (1) |
| (2) |
Table 2.
Pairwise Concordance in the Timing of Smoking Desistance by Zygosity
| Proportion of co-twins who quit prior to censoring | Mean years difference in quitting including censored durations | N | |
|---|---|---|---|
| Identical twins (MZ) | .647 | 10.08 (6.79) | 363 |
| Fraternal twins (DZ) | .554 | 11.02 (6.57) | 233 |
| pr.(MZ=DZ) | <.022 | <.049 |
Note: See Truett et al. (1994) and Eaves et al. (1999) for additional information about this study. Standards deviations are reported in parentheses.
Source: Data on twin pairs come from a large population-based birth registry for the Commonwealth of Virginia and the American Association of Retired Persons. This study only uses data in which both members of the twin pair report regular smoking at some point during the study.
The most basic model is presented in equation 1 which specifies the hazard for the ith pair as a function of duration. This model does not specify the form of the baseline hazard and but allows the covariates (x1, x2, xk, etc.) to influence the hazard linearly. As such, the linear prediction for the ith pair is given in equation 2. The ratio of the linear prediction for observation a and observation b is known as the hazard ratio (e.g, eηa / eηb) which is why this model is generally referred to has a proportional hazards model. We use the package coxph (Therneau and Lumley 2009) in R 2.90 to estimate all proportional hazards models.
As described above, we expect greater concordance in smoking cessation among MZ compared to DZ twins, thus we expect a positive and significant slope for the dummy variable coded MZ=1 and DZ=0. Importantly, we also expect that the magnitude of this coefficient will change in the later half of our study window (e.g., 1970-1980), with the magnitude decreasing according to the social control hypothesis and increasing according to the social push hypothesis. We therefore estimate three Cox proportional hazards models: a) full sample (n = 596); b) those twins who first quit between 1960 and 1970 (n = 281); and c) those twins who first quit between 1971 and 1980 (n = 315). These estimates are presented in Table 3.
Table 3.
Zygosity Differences in the Duration to Smoking Desistance Following Co-Twin Desistance by Period
| All years | 1960-1964 | 1965-1969 | 1970-1974 | 1975-1980 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ba | Pr(>|z|)b | b | Pr(>|z|) | b | Pr(>|z|) | b | Pr(>|z|) | b | Pr(>|z|) | |
| Year of birth | 0.021 | 0.047 | -0.198 | 0.016 | 0.002 | 0.981 | 0.047 | 0.547 | -0.035 | 0.585 |
| Gender [Male = 0] | ||||||||||
| Female | -0.105 | 0.343 | -0.494 | 0.052 | 0.128 | 0.569 | -0.231 | 0.304 | -0.030 | 0.894 |
| Zygosity [Fraternal twins = 0] | ||||||||||
| Identical twins | 0.174 | 0.057 | 0.070 | 0.389 | 0.253 | 0.145 | -0.108 | 0.323 | 0.507 | 0.014 |
| Pair controls | ||||||||||
| Difference in age of initiation | -0.023 | 0.105 | -0.041 | 0.126 | -0.009 | 0.763 | -0.004 | 0.902 | -0.075 | 0.054 |
| Age started (2nd to quit) | -0.001 | 0.967 | 0.033 | 0.192 | -0.046 | 0.088 | 0.004 | 0.886 | 0.039 | 0.206 |
| Age when the 1st of the pair quit | 0.028 | 0.010 | -0.193 | 0.020 | 0.016 | 0.853 | 0.048 | 0.546 | -0.029 | 0.664 |
| Average years of education | -0.075 | 0.073 | -0.172 | 0.054 | -0.081 | 0.316 | -0.056 | 0.580 | -0.001 | 0.990 |
| Difference in education | -0.014 | 0.029 | -0.015 | 0.328 | 0.011 | 0.365 | -0.048 | 0.000 | -0.006 | 0.694 |
| Pair smoking amount (average) | -0.004 | 0.489 | -0.009 | 0.520 | -0.012 | 0.399 | 0.006 | 0.664 | -0.008 | 0.517 |
| Difference in smoking amount | 0.045 | 0.109 | 0.034 | 0.631 | 0.036 | 0.503 | 0.009 | 0.887 | 0.100 | 0.099 |
| Frequency of visiting among pair | -0.047 | 0.232 | -0.042 | 0.667 | -0.055 | 0.513 | 0.002 | 0.979 | -0.035 | 0.668 |
| Likelihood ratioc | 31.780 | 0.001 | 17.38 | .097 | 11.810 | 0.378 | 15.000 | 0.182 | 17.800 | 0.086 |
| Pseudo R- Square | 0.052 | 0.139 | 0.084 | 0.092 | 0.090 | |||||
| Number of observations | 596 | 116 | 135 | 156 | 189 | |||||
Note: Data on twin pairs come from a large population-based birth registry for the Commonwealth of Virginia and the American Association of Retired Persons (see Truett et al. (1994) and Eaves et al. (1999) for additional information about this study). This study only uses data in which both members of the twin pair report regular smoking at some point during the study.
Cell entries are Cox proportional hazards estimates which describe similarity in the timing of smoking desistance and the corresponding p-values.
The p-values for zygosity are one-tailed tests of significance.
All likelihood ratio tests have 11 degrees of freedom.
We then estimate similar models for each year of our study between 1960 and 1980. Because of small sample sizes for year of first cessation in our study, we calculate time-specific estimates for a moving sample with a window of five years. For example, a sample for 1970 includes pairs in which the first to quit occurred between 1968 and 1972. These estimates are presented in Table 4. Finally, it is important to note that smoking intensity (number of cigarettes per day among smokers) was at it’s highest for birth cohorts in the 1910s-1920s. The regular smokers in our study may be a select group because we may only have information on the lightest smokers among the regular smokers; the heaviest smokers did not make it in to the survey because of early mortality. If there was an issue of selective attrition due to smoking intensity then we should see lower levels of smoking intensity in our sample compared to national averages; those that smoked the most may have never made it in to our study. To investigate this concern, we compared the reported smoking intensity from our sample to a comparable cohort of smokers 32 years before the 1987 survey. Haenszel et al. (1956; as reported in Forey et al. (2009)) report an average of 18 cigarettes per day for 25-35 year old males in 1955. Mortality due to smoking is an incredibly rare event among adults who are this young. These males were born between 1920 and 1930. We then calculated the smoking intensity for this same birth cohort (those that were between the ages of 57 and 67 in 1987) from the Virginia 30k respondents in our study. We estimated an average of 18.3 cigarettes per day per smoker. We found similar results (comparable to the national levels) for women suggesting that there is little to any effect of selective attrition by smoking intensity due to mortality among the respondents of this survey. Based on these results, we are confident that our sample of smokers is representative of US smokers at this time.
Table 4.
Yearly Cox PH Model Estimates for the Duration of Smoking Discordance
| Year | bmza | Pr(|b|=0) | r2 | n |
|---|---|---|---|---|
| 1960 | 0.125 | 0.641 | 0.224 | 100 |
| 1961 | 0.288 | 0.252 | 0.242 | 102 |
| 1962 | 0.070 | 0.772 | 0.139 | 116 |
| 1963 | -0.068 | 0.768 | 0.098 | 119 |
| 1964 | 0.180 | 0.478 | 0.109 | 127 |
| 1965 | 0.165 | 0.486 | 0.112 | 125 |
| 1966 | 0.085 | 0.706 | 0.074 | 140 |
| 1967 | 0.253 | 0.269 | 0.084 | 135 |
| 1968 | 0.168 | 0.471 | 0.079 | 139 |
| 1969 | 0.009 | 0.968 | 0.069 | 140 |
| 1970 | 0.073 | 0.754 | 0.099 | 144 |
| 1971 | 0.066 | 0.790 | 0.093 | 143 |
| 1972 | -0.108 | 0.656 | 0.092 | 156 |
| 1973 | 0.011 | 0.962 | 0.162 | 165 |
| 1974 | -0.172 | 0.453 | 0.126 | 169 |
| 1975 | -0.013 | 0.954 | 0.105 | 165 |
| 1976 | 0.335 | 0.155 | 0.083 | 162 |
| 1977 | 0.499 | 0.044 | 0.094 | 154 |
| 1978 | 0.453 | 0.093 | 0.085 | 150 |
| 1979 | 1.008 | 0.001 | 0.131 | 154 |
| 1980 | 0.829 | 0.007 | 0.120 | 163 |
Note: See Truett et al. (1994) and Eaves et al. (1999) for additional information about this study.
Source: Data on twin pairs come from a large population-based birth registry for the Commonwealth of Virginia and the American Association of Retired Persons. This study only uses data in which both members of the twin pair report regular smoking at some point during the study and in which one member of the pair reports quitting.
Cell entries are Cox proportional hazards estimates which describe similarity in the timing of smoking desistance by the year in which the first member of the pair initiated quitting. See text for a full description of the methods used to calculate the parameter estimates.
RESULTS
Table 2 provides tentative evidence for genetic influences on smoking desistence using two related measures by zygosity. In total, nearly 65% of the identical twins reported smoking desistence following the desistence of their twin. This same measure among dizygotic twins was only 55%. This difference is statistically significant (p < .022) and the greater degree of concordance for the desistence phenotype among identical twins provides a baseline indication that genetic factors may be related quitting smoking. The same conclusion is reached when examining zygosity differences in the time it takes for the 2nd of the pair to quit smoking following the desistence of their co-twin. On average, MZ pairs quit within 10 years of one another but this number increases to 11 years among DZ pairs (p < .049). Among the pairs in which both members quit (no censored values) the mean difference in the timing of quitting is 8.5 years for fraternal twins and 7.5 for identical twins (p <.12).
Table 3 presents the results from five Cox proportional hazards models using the full samples and then examining the association by period of desistance. As described above, an attempt to adjust for age and cohort influences is evident in the statistical controls for year of birth, difference in years in which the members of the pair began smoking, the age at which the target person started smoking (which indirectly measures duration of smoking for the 2nd twin prior to the age at which the 1st of the pair quit), and the age at which the first member of the pair quit smoking. We also include controls for education and smoking intensity to rule out other confounding factors. The primary parameter of interest is the coefficient for MZ (b = 0.174, p < .057). This positive coefficient suggests that MZ pairs are a “greater risk” of quitting in a shorter period of time (after their sibling quit) compared to DZ pairs. In other words, MZ pairs exhibit a greater concordance for smoking desistance than do same-sex DZ pairs; we take this excess concordance as tentative evidence for genetic influences on this complex phenotype.
Importantly, this same model is repeated for the following four periods in which the first of the pair quit: 1960-1964; 1965-1969; 1970-1974; 1975-1980. As shown, the value of the coefficient in the first model is an average genetic influence across the four periods but this masks the observation that there is very little evidence for genetic influences in the first three periods but markedly higher influence for the final period (b = 0.507, p <.014). A similar association can be seen by comparing the coefficients for differences in education across the periods. On average, educational discordance was negatively associated with duration of desistence for the second member of the pair (b = -0.014, p< .029). In other words, twin pairs who resemble one another with respect to their education tend to quit smoking within a similar time frame compared to twin pairs who differ from one another in their education. This association is strongest in the third period (1970-1974) but subsides in the final period. In other words, this crude indicator of the social environmental is highest when the genetic factors are the lowest and non-existent when genetic factors are most noticeable. Taken together, these findings provide strong support for the social push hypothesis.
To further explore period influence on the genetics of smoking desistance, we then repeated this model for each year between 1960 and 1980. Using a moving window of five years (see Table 4 and Figure 1), these results further support the social push hypothesis for smoking desistance. As hypothesized, the first upward tick in the genetic influences on smoking desistance occurs following the years 1973-1975, the years in which the first formal restrictions of smoking in public places began to be implemented. As shown, the genetic influences on smoking desistence increase roughly five-fold during the last two years compared to the average influence across the entire study. Although evidence of health risks had already emerged and warnings on cigarette packages had already been required, this was the first time public smoking behaviors were sanctioned and limited. The policy changes thus coincided with increases in the extent to which genetic risk characterizes the population of smokers. The results indicate the mid 1970s as a turning point in the observed genetic influences on smoking desistance.
Figure 1. Yearly Changes in the Genetic Influences on Smoking Desistance.
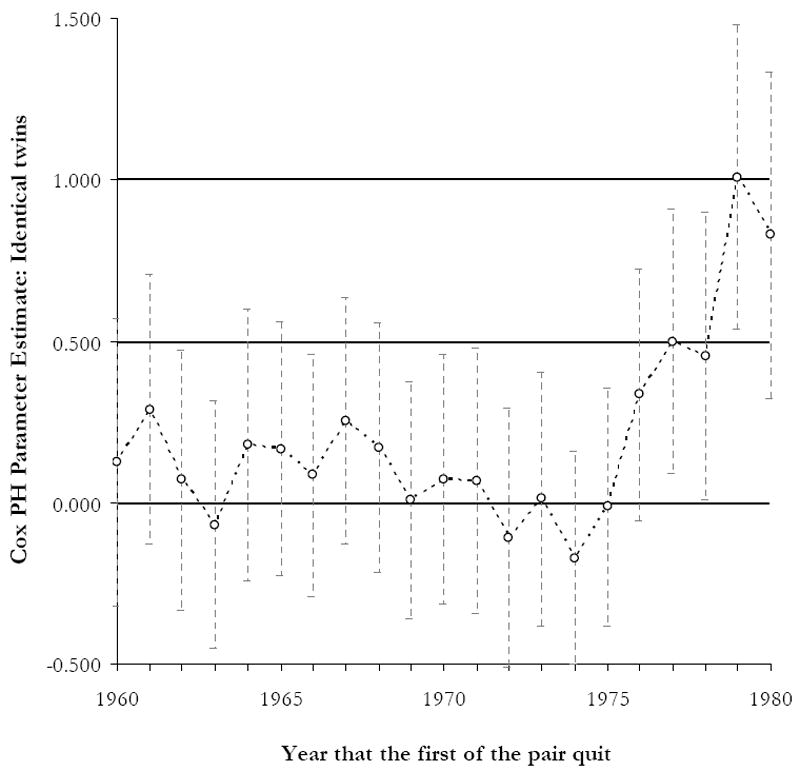
Note: Estimates derived from Table 4. See text for a full description of the methods used to calculate the parameter estimates.
DISCUSSION
It is increasingly obvious that a complete understanding of complex behaviors such as smoking and smoking desistance requires detailed information about both biological and social factors. Previous research has shown that regular smoking is highly heritable, but recent work has demonstrated that heritability estimates are contingent upon the social and institutional characteristics of the environment (Boardman et al. 2008; Boardman 2009; Timberlake et al. 2006). The analysis offers new insight into the nature of these contingencies for smoking desistance. We do not find evidence for the social control model for smoking desistance. That is, environmental changes from the 1960s to the 1970s did not reduce the heritability of smoking desistance. We did, however, find evidence supporting the social push model in which increasing social trends changed the composition of smokers such that those who remained in the smoking population were distinguished by increased genetic vulnerability relative to those in previous years.
We add to the existing gene-environment interplay literature in two ways. First, building on previous work (Boardman et al. 2010; Kendler et al. 2000), we demonstrate that historical time periods can be characterized as distinct social environments that moderate genetic influences on behavior. This point is made by Rutter (2006:60), who argues that “there is not, and cannot be, any absolute value for the strength of genetic influences on a trait” and “heritability figures are necessarily specific to populations and to time periods.” Despite the support for this perspective among leading genetic epidemiologists, little work has been done to specify the mechanisms responsible for periodic highs and lows in social versus genetic causes for health behaviors in the population. In this paper, temporal changes in the genetic epidemiology of smoking coincide with changes in smoking norms, the costs of smoking, and legal limits placed upon smokers. This historical context thus provides a useful background to evaluate existing gene-environment interaction theory.
Importantly, the findings on changes in heritability across historical periods suggest that current efforts to characterize genetic influences on behaviors like smoking should be careful to consider that samples are drawn from populations at a particular moment in a smoking trend. That is, suppose a sample of regular smokers was taken in 2010 and then followed to monitor their smoking duration, quit attempts, and successful desistance. If researchers used family-based association methods across the human genome to predict successful quitting, it is possible that very few or even a different set of SNPs or DNA sequences would be identified than if this same study had been done in 1960. Not only does it appear that the heritability for smoking desistance was smaller in the 1960s compared to the 1970s, compromising the likelihood of identifying causal loci, but also that the composition of smokers and the SNPs linked to smoking desistance in the 1960s may be quite different from those in later decades.
Second, we extend this literature by considering smoking desistance as the primary smoking phenotype. Although the heritability of successful quitting is shown to be roughly .40 (Xian et al. 2003), there remains very little behavioral genetic research on smoking desistance and almost no work has examined gene-environment interactions with respect to the likelihood that a current smoker will quit. We argue that this desistance phenotype is more closely linked to physiological processes associated with nicotine addiction and that zygosity differences in the concordance of quitting, as opposed to concordance in smoking onset, sheds new light on this complex issue. This is important because entry into smoking draws from the general population but exit from smoking draws from the regular smoking population. Smokers make up a continuously changing population; thus the most reticent to exit from smoking status may also be those for whom symptoms of nicotine withdrawal are felt most acutely.
Important implications for case-control studies of the genetics of smokers versus nonsmokers follow from changes in the relative sizes of genetically resilient and vulnerable smokers. Over time, cases may be an increasingly select group of people compared to controls who are drawn from a much larger pool of nonsmokers. In other words, even under the most strict association models, identifying causal genetic factors may be somewhat problematic for behaviors like smoking with clear social trends. In much the same way, changes in the genetic vulnerability of smokers may mean that conclusions drawn from early randomized trials of effective cessation interventions may no longer apply to the current population of smokers.
This perspective helps to account for the differences observed in the Boardman et al. (2010) study in which the authors show that the heritability of the onset of regular smoking decreases in the mid 1970s. Not only are onset and cessation different phenotypes, but the social mechanisms for entry and exit are very different. Similarly, genes implicated in smoking onset are quite different from those implicated in smoking desistance (Broms et al. 2006). As such, it is not surprising that the period effects on the genetics of smoking desistance are different as well. It is well known that genes linked to novelty seeking and risk taking are associated with smoking onset, whereas smoking desistance and successful quitting are strongly associated with loci that regulate nicotine metabolism (Erhniger et al. 2007; Lerman et al. 1999).
It follows that social policies designed to address the social and genetic epidemiology of smoking need to be clear about the etiology of these different smoking phenotypes. That is, effective smoking policy needs to consider the current location within a particular trend when developing effective policies. Social policy affects the incidence, but it also differentially affects the trade off between social and genetic causes of the behavioral phenotype; the causes of entry, exit, and continuation into the smoking population are likely to be very different over time so the policies should adjust accordingly. For example, restrictions on smoking in public places, anti-tobacco ads, and increased costs of purchasing cigarettes helped many smokers stop. However, as genetic factors become increasingly important, these policies may decline in effectiveness. They may be effective in prodding social smokers with genetic resilience to quit but do less to help genetically vulnerable smokers quit. Thus, Franks et al. (2007) find that rising cigarette prices have done less in the last decade than in earlier decades to reduce smoking among the most vulnerable socioeconomic groups. Many therefore call for policies designed to assist people in quitting that are targeted at the physiological and social-psychological mechanisms of addiction among disadvantaged groups (Sorenson et al. 2004).
Taken together, the results of this study in conjunction with other existing studies (Boardman et al. 2010; Kendler et al. 2000) are sufficient to demonstrate the value of extending the gene-environment interaction approach to broad historical periods characterized by different incentives and constraints (both institutional and normative) on smoking behaviors among adults. There are, however, several limitations of our study that should be considered when interpreting the results. First, smoking cessation is a complex phenotype. We use a simple binary variable indicating whether or not a respondent quit smoking and the year in which they did so. As others have shown (Hatziandreu et al. 1990), the transition to quitting smoking involves many attempts of different durations and very different patterns of long term success. However, a standard measure of successful smoking cessation is going at least 6 months without a cigarette (Lancaseter et al. 2006). Thus, while the path to successful cessation may be quite different and may represent a distinct phenotype, our age-based measure of quitting denotes a valid measure of smoking termination and it is unlikely that the respondents designating an age of quitting in our study will re-initiate smoking. To date, very little research has characterized the genetic etiology of smoking desistence and those that do emphasize a simple binary characterization of successful quitting (Xian et al. 2003). Future research would benefit from a more nuanced measure of quitting that involves the complex patterns of attempts and durations of quit attempts prior to successful termination. These different trajectories are likely to be highly heritable and their moderation by social factors would give further information about the important role of the social environment in this complex series of social behaviors. Second, our study is limited to smokers in the 1960s and 1970s. The increasing level of taxes, further reductions on social spaces in which smoking is allowed, and strong social norms against private and public smoking denote a fundamentally different social context in which smoking takes place. Future research should consider continuing this line of inquiry into the current decade and expanding the geographic scope to different regions of the country and to different countries. The later point is particularly relevant to policy makers because of the different scope and different timing of policy interventions across countries.
CONCLUSION
Demographers and epidemiologists have demonstrated that smoking prevalence affects overall population health and mortality as well as variations in health and mortality across cohorts, genders, nations, and socioeconomic groups. Despite the well-publicized and widely accepted evidence of the harm of smoking, the importance of its influence has persisted. Indeed, changes in the composition of the population at risk for quitting suggest reasons for slowing progress against tobacco. While starting to smoke draws from the more general population of nonsmokers, stopping draws from a continuously narrowing population of smokers. Based on the assumption that policies and related environmental changes affect the composition of smokers, which in turn affects the determinants of cessation, this study examined changes in genetic influences on quitting.
Our perspective on changing composition fits with arguments about gene-environment interaction. Clinical and psychological scholarship has progressed over the years to the point where it is now well understood that genetic diversity is one of the components that contributes to differences in the risk for substance use disorders, including smoking onset, amount of smoking, and smoking desistance (Li et al. 2003). But genetic factors are clearly not the entire story. The social and the physical environment, the interaction between parent and child, peers, role models, and the macro environment, such as public policy, are clearly and demonstratively important in human variability. While useful in general to understanding initiation of smoking, age of onset, addiction, reward and penalty, this integrative perspective has special utility for understanding cessation. Environmentally induced changes in the composition of smokers should strongly shape the influence of genetic factors on quitting.
In the analyses presented here, we have demonstrated contingencies in the genetic contributions to smoking desistance by showing that the macro environment, measured by historical periods, changes those most at risk for continued tobacco smoking. Consistent with social push arguments, genetic influence prove stronger in the 1970s than the 1960s. Because this approach treats the biometric analyses of smoking desistance as an interaction between the person and the macro environment as well as individual environment, it provides information on how to identify the influence of genes in determining variation in smoking desistance.
Footnotes
This paper is part of a larger study funded by the National Institute of Child Health and Human Development (K01 HD 50336). Research funds were also provided by the NIH/NICHD funded CU Population Center (R21 HD 051146-01). The authors would like to thank the anonymous reviewers and Jane Menken their insightful comments on earlier drafts of this paper.
Contributor Information
Jason D. Boardman, Department of Sociology and Institute of Behavioral Science, University of Colorado
Casey L. Blalock, Department of Sociology and Institute of Behavioral Science, University of Colorado
Fred C. Pampel, Department of Sociology and Institute of Behavioral Science, University of Colorado
Peter K. Hatemi, Department of Political Science, University of Iowa
Andrew C. Heath, Midwest Alcoholism Research Center, Washington University, St. Louis
Lindon J. Eaves, Virginia Institute for Psychiatric and Behavioral Genetics, Medical College of Virginia, Richmond
References
- Adhikari B, Kahende J, Malarcher A, Pechacek T, Tong V. Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses – United States, 2000-2004. Morbidity & Mortality Weekly Report. 2008;57:1226–28. [PubMed] [Google Scholar]
- Boardman JD, Blalock CL, Pampel FC. Trends in the genetic influences on smoking. Journal of Health & Social Behavior. 2010;51(1):108–123. doi: 10.1177/0022146509361195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD. State-Level Moderation of Genetic Tendencies to Smoke. American Journal of Public Health. 2009;99:480–86. doi: 10.2105/AJPH.2008.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Saint Onge JM, Haberstick BC, Timberlake DS, Hewitt JK. Do Schools Moderate the Genetic Determinants of Smoking? Behavior Genetics. 2008;38:234–46. doi: 10.1007/s10519-008-9197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Silventoinen K, Madden PAF, Heath AC, Kaprio J. Genetic Architecture of Smoking Behavior: A Study of Finnish Adult Twins. Twin Research and Human Genetics. 2006;9:64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic Influence on Smoking: A Study of Male Twins. New England Journal of Medicine. 1992;327:829–33. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression Models and Life Tables (with Discussion) Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- Eaves L, Silberg J, Erkanli A. Resolving Multiple Epigenetic Pathways to Adolescent Depression. Journal of Child Psychology and Psychiatry. 2003;44:1006–14. doi: 10.1111/1469-7610.00185. [DOI] [PubMed] [Google Scholar]
- Eaves L, Heath A, Martin N, Maes H, Neale M, Kendler K, Kirk K, Corey L. Comparing the Biological and Cultural Inheritance of Personality and Social Attitudes in the Virginia 30, 000 Study of Twins and Their Relatives. Twin Research. 1999;2:62–80. doi: 10.1375/136905299320565933. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Clegg HV, Collins AC, Corley RP, Crowley T, Hewitt JK, Hopfer CJ, Krauter K, Lessem J, Rhee SH, Schlaepfer I, Smolen A, Stallings MC, Young SE, Zeiger JS. Association of the Neuronal Nicotinic Receptor β2 Subunit Gene (CHRNB2) With Subjective Responses to Alcohol and Nicotine. American Journal of Medical Genetics Part B. 2007;144B:596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Baker TB. Stealing a March in the 21st Century: Accelerating Progress in the 100-Year War against Tobacco Addiction in the United States. American Journal of Public Health. 2009;99:1170–75. doi: 10.2105/AJPH.2008.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forey B, Hamling J, Lee P. International Smoking Statistics. Second Edition. Statistics and Computing Ltd and Nicholas Wald of the Wolfson Institute of Preventative Medicine; London: 2009. [02/15/10]. Available online at: http://www.pnlee.co.uk/ISS.htm. [Google Scholar]
- Franks P, Jerant AF, Leigh JP, Lee D, Chiem A, Lewis I, Lee S. Cigarette Prices, Smoking, and the Poor: Implications of Recent Trends. American Journal of Public Health. 2007;97:1873–77. doi: 10.2105/AJPH.2006.090134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenszel W, Shimkin MB, Miller HP. Tobacco smoking patterns in the United States. United States Public Health Service; Washington DC: 1956. Public Health Monograph No. 45. PHS Publication No. 463. [PubMed] [Google Scholar]
- Hall W, Madden P, Lynskey MT. The Genetics of Tobacco Use: Methods, Findings and Policy Implications. Tobacco Control. 2002;11:119–24. doi: 10.1136/tc.11.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziandreu EJ, Pierce JP, Lefkopoulou M, Fiore MC, Mills SL, Novotny TE, Giovino GA, Davis RM. Quitting Smoking in the United States in 1986. Journal of the National Cancer Institute. 1990;82:1402–6. doi: 10.1093/jnci/82.17.1402. [DOI] [PubMed] [Google Scholar]
- Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ, Lopez AD. Social Inequalities in Male Mortality, and in Male Mortality from Smoking: Indirect Estimation From National Death Rates in England and Wales, Poland, and North America. The Lancet. 2006;368(9533):367–70. doi: 10.1016/S0140-6736(06)68975-7. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO., Jr Twin Studies of Adult Psychiatric and Substance Dependence Disorders: Are They Biased by Differences in the Environmental Experiences of Monozygotic and Dizygotic Twins in Childhood and Adolescence? Psychological Medicine. 1998;28:625–33. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Pedersen NL. Tobacco Consumption in Swedish Twins Reared Apart and Reared Together. Archives of General Psychiatry. 2000;57:886–92. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of Relapse After Quitting Smoking: A Systematic Review of Trials. Archives of Internal Medicine. 2006;166:828–35. doi: 10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B, Boyd NR, Shields PG. Evidence Suggesting the Role of Specific Genetic Factors in Cigarette Smoking. Health Psychology. 1999;18:14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A Meta-Analysis of Estimated Genetic and Environmental Effects on Smoking Behavior in Male and Female Adult Twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Mendez D, Warner KE. Adult Cigarette Smoking Prevalence: Declining as Expected (Not as Desired) American Journal of Public Health. 2004;94:251–52. doi: 10.2105/ajph.94.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Clark TG, Johnstone EC, Murphy MFG, Walton RT. The Genetic Basis for Smoking Behavior: A Systematic Review and Meta-Analysis. Nicotine & Tobacco Research. 2004;6:1–15. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Pampel FC. Cigarette Use and the Narrowing Sex Differential in Mortality. Population and Development Review. 2002;28:77–104. [Google Scholar]
- Pampel FC. Diffusion, Cohort Change, and Social Patterns of Smoking. Social Science Research. 2005;34:117–39. doi: 10.1016/j.ssresearch.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SH, Wang H. Sex Mortality Differences in the United States: The Role of Cohort Smoking Patterns. Demography. 2006;43:631–46. doi: 10.1353/dem.2006.0037. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial Studies of Antisocial and Violent Behavior in Children and Adults: A Review. Journal of Abnormal Child Psychology. 2002;30:311–26. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Rogers RG, Hummer RA, Krueger PM, Pampel FC. Mortality Attributable to Cigarette Smoking in the United States. Population and Development Review. 2005;31:259–92. doi: 10.1111/j.1728-4457.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Genes and Behavior: Nature–Nurture Interplay Explained. London: Blackwell; 2006. [Google Scholar]
- Shanahan MJ, Hofer SM. Social Context in Gene-Environment Interactions: Retrospect and Prospect. Journals of Gerontology: Series B. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Sorensen G, Barbeau E, Hunt MK, Emmons K. Reducing Social Disparities in Tobacco Use: A Social-Contextual Model for Reducing Tobacco Use among Blue-Collar Workers. American Journal of Public Health. 2004;94:230–39. doi: 10.2105/ajph.94.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The Genetic Epidemiology of Smoking. Nicotine & Tobacco Research. 1999;1:S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Therneau T, Lumley T. Survival: Survival Analysis, Including Penalised Likelihood. R Package Version. 2009;2:35–4. [Google Scholar]
- Thorne SL, Malarcher A, Maurice E, Caraballo R. Cigarette Smoking Among Adults – United States, 2007. Morbidity & Mortality Weekly Report. 2008;57:1221–26. [PubMed] [Google Scholar]
- Timberlake DS, Rhee SH, Haberstick BC, Hopfer C, Ehringer M, Lessem JM, Smolen A, Hewitt JK. The Moderating Effects of Religiosity on the Genetic and Environmental Determinants of Smoking Initiation. Nicotine & Tobacco Research. 2006;8:123–33. doi: 10.1080/14622200500432054. [DOI] [PubMed] [Google Scholar]
- Truett KR, Eaves LJ, Walters EE, Heath AC, Hewitt JK, Meyer JM, Silberg J, Neale MC, Martin NG, Kendler KS. A Model System for Analysis of Family Resemblance in Extended Kinships of Twins. Behavior Genetics. 1994;24:35–49. doi: 10.1007/BF01067927. [DOI] [PubMed] [Google Scholar]
- Tynan N, Babb S, MacNeil A. State Smoking Restrictions for Private-Sector Worksites, Restaurants, and Bars – United States, 2004 and 2007. Morbidity & Mortality Weekly Report. 2008;57:549–52. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services [USDHHS] [4/8/08];The Health Consequences of Involuntary Smoking: A Report of the Surgeon General. 1986 Available online at http://profiles.nlm.nih.gov/NN/B/C/P/M/_/nnbcpm.pdf.
- Xian H, Scherrer JF, Madden PAF, Lyons MJ, Tsuang M, True WR, Eisen SA. The Heritability of Failed Smoking Cessation and Nicotine Withdrawal in Twins Who Smoked and Attempted to Quit. Nicotine & Tobacco Research. 2003;5:245–54. [PubMed] [Google Scholar]


