Abstract
Glucagon-like peptide-1 (GLP-1) acts at the G protein-coupled receptor, GLP-1R, to stimulate secretion of insulin and to inhibit secretion of glucagon and gastric acid. Involvement in mucosal secretory physiology has received negligible attention. We aimed to study involvement of GLP-1 in mucosal chloride secretion in the small intestine. Ussing chamber methods, in concert with transmural electrical field stimulation (EFS), were used to study actions on neurogenic chloride secretion. ELISA was used to study GLP-1R effects on neural release of acetylcholine (ACh). Intramural localization of GLP-1R was assessed with immunohistochemistry. Application of GLP-1 to serosal or mucosal sides of flat-sheet preparations in Ussing chambers did not change baseline short-circuit current (Isc), which served as a marker for chloride secretion. Transmural EFS evoked neurally mediated biphasic increases in Isc that had an initial spike-like rising phase followed by a sustained plateau-like phase. Blockade of the EFS-evoked responses by tetrodotoxin indicated that the responses were neurally mediated. Application of GLP-1 reduced the EFS-evoked biphasic responses in a concentration-dependent manner. The GLP-1 receptor antagonist exendin-(9–39) suppressed this action of GLP-1. The GLP-1 inhibitory action on EFS-evoked responses persisted in the presence of nicotinic or vasoactive intestinal peptide receptor antagonists but not in the presence of a muscarinic receptor antagonist. GLP-1 significantly reduced EFS-evoked ACh release. In the submucosal plexus, GLP-1R immunoreactivity (IR) was expressed by choline acetyltransferase-IR neurons, neuropeptide Y-IR neurons, somatostatin-IR neurons, and vasoactive intestinal peptide-IR neurons. Our results suggest that GLP-1R is expressed in guinea pig submucosal neurons and that its activation leads to a decrease in neurally evoked chloride secretion by suppressing release of ACh at neuroepithelial junctions in the enteric neural networks that control secretomotor functions.
Keywords: gastrointestinal hormones, neurogenic chloride secretion, enteric nervous system
glucagon-like peptide-1 (GLP-1) is a gut-derived hormone released from l-type enteroendocrine cells after a meal (28). It attracts interest because of its incretin effect, which results in enhanced insulin secretion even before blood sugar levels become elevated (4, 26). This suggests that it might be an efficacious therapeutic agent in the treatment of diabetes mellitus (4, 28, 47). Moreover, GLP-1 has significant gastrointestinal regulatory functions (26). It suppresses motility in general (1, 32, 39, 44, 48) and retards gastric emptying (3, 32, 48) by action in the antral pump (43) and gastric reservoir (2, 16). GLP-1 inhibits gastric and pancreatic secretory functions in humans (50) and inhibits gastric acid secretion evoked by either pentagastrin or sham feeding (41, 45, 51). GLP-1 effectively inhibits vagally evoked acid secretion but is ineffective for hormonal stimulation (45, 51). Inhibition of acid secretion by GLP-1 is lost after vagotomy, which would be consistent with suppression acetylcholine (ACh) release from vagal efferent innervation of gastric parietal glands.
GLP-1 acts at a specific transmembrane G protein receptor, the GLP-1 receptor (GLP-1R). Messenger RNA for GLP-1R is expressed in nodose ganglia and in the gastrointestinal tract (8, 9, 27, 43). It is expressed by nitrergic neurons in the myenteric plexus of mouse intestine (1). Nevertheless, detailed evaluation of GLP-1R expression in the enteric nervous system (ENS) is lacking. We aimed to identify the classes of neurons in the submucosal plexus of guinea pig small intestine that express GLP-1R.
Interest in the biological actions of GLP-1 has focused on pancreatic endocrine and gastric secretion with little attention given to a possible role for GLP-1 in neurogenic control of the intestinal secretory glands. Our second aim was to study actions of GLP-1 on neurally evoked mucosal secretion of NaCl and H2O in guinea pig small intestine.
Neurogenic control of movement of NaCl, H2O and HCO3− into the intestinal lumen is basic for maintenance of physiological osmolarity and pH and for understanding mechanisms involved in diarrhea and constipation. The ENS, in concert with the sympathetic division of the autonomic nervous system, is responsible for neurogenic moment-to-moment control of H2O and electrolyte transport across the mucosal epithelium (15, 52, 54). Secretomotor neurons in the submucosal plexus, which release ACh and vasoactive intestinal polypeptide (VIP) at the neuroepithelial junctions, are the final motor pathways from the ENS for control of secretion (14, 33, 42).
We investigated a putative role for GLP-1 in neurogenic secretion. GLP-1 action on neurally evoked chloride secretion, immunohistochemical localization of the GLP-1R in the submucosal plexus, and its influence on stimulus-evoked release of ACh from secretomotor neurons were studied.
MATERIALS AND METHODS
Methods used in the present study are unchanged from those we reported earlier for a closely similar study of GLP-2 (5). Consequently, the presentation of methods summarizes and quotes from the earlier article (5).
Tissue preparation.
Male albino Hartley guinea pigs (300–400 g) were killed instantly by stunning, followed by exsanguination from the cervical vessels according to procedures reviewed and approved by the Ohio State University Laboratory Animal Care and Use Committee and United State Department of Agriculture inspectors. Ileal segments were removed and maintained in ice-cold Krebs solution containing (in mM) 120.9 NaCl, 5.9 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 14.4 NaHCO3, 2.5 CaCl2, and 11.5 glucose.
Immunohistochemistry.
Immunohistochemical methods were standard and essentially the same as reported in detail for our earlier study of GLP-2 (5). Table 1 lists sources for primary and secondary antibodies and the optimal dilutions used. Whole mounts of the submucosal plexus were placed in PBS containing 10% normal donkey serum and 0.3% Triton X-100 for 30 min at room temperature to minimize nonspecific binding and increase tissue permeability and were then incubated overnight at 4°C in primary antibodies for GLP-1R or a mixture of primary antibodies for double labeling. Competition with the control peptide (catalog no. LS-P1205; Lifespan Biosciences) established antibody specificity for GLP-1R. The GLP-1R antibody was preabsorbed with the control peptide for 2 h and then used for immunohistochemical localization of the receptor. After being washed with PBS, the tissues were incubated with a single secondary antibody or a mixture of appropriate secondary antibodies conjugated with fluorescein isothiocyanate (FITC) or indocarbocyanin (Cy3) at room temperature for 1 h, followed by washing in PBS and mounting with Vectashield (Vector, Burlingame, CA). Fluorescence labeling was examined with a Nikon Eclipse 90i fluorescence microscope (Nikon Instruments, Melville, NY). Photomicrographs were acquired with a CoolSnap HQ2 monochrome digital camera and MetaMorph software (Molecular Devices, Sunnyvale, CA), stored on disk, and analyzed with MetaMorph. Images were minimally adjusted for brightness and contrast with MetaMorph. Immunoreactivity (IR) for the GLP-1R and enteric neurochemical codes and numbers of double-labeled cells were assessed in randomly chosen ganglia situated throughout the preparations.
Table 1.
Codes and sources of primary and secondary antibodies used in the study
| Antigen | Host | Code | Dilution | Source |
|---|---|---|---|---|
| Anti-Hu | Mouse | A21271 | 1:50 | Molecular Probes |
| GLP-1R | Rabbit | LS-A1205 | 1:100 | Lifespan Biosciences |
| ChAT | Goat | AB144P | 1:100 | Chemicon |
| NPY | Sheep | AB1583 | 1:1,500 | Chemicon |
| Somatostatin | Rat | MAB354 | 1:200 | Chemicon |
| VIP | Sheep | AB1581 | 1:1,000 | Chemicon |
| Rabbit IgG | Donkey Cy3 | 711-165-152 | 1:500 | Jackson |
| Mouse IgG | Donkey FITC | 715-095-150 | 1:100 | Jackson |
| Goat IgG | Donkey FITC | 705-095-003 | 1:100 | Jackson |
| Sheep IgG | Donkey FITC | 713-095-147 | 1:100 | Jackson |
| Rat IgG | Donkey FITC | AP189F | 1:100 | Chemicon |
Anti-Hu, anti-human neuronal protein; GLP-1R, glucagon-like peptide-1 receptor; ChAT, choline acetyltransferase; NPY, neuropeptide Y; VIP, vasoactive inhibitory peptide.
Ussing chamber methods.
The muscularis externa, including the myenteric plexus, was removed by microdissection to obtain flat-sheet preparations for the Ussing chamber studies. The flat-sheet preparations of submucosa/mucosa and submucosal plexus were mounted between halves of Ussing flux chambers, which had a total cross-sectional area of 0.64 cm2. The tissues were bathed on both sides, with 10 ml of oxygenated (95% O2 and 5% CO2) Krebs solution, maintained at 37°C by circulation from a temperature-controlled water bath. Each Ussing chamber had a pair of Ag-AgCl electrodes connected through Krebs-agar bridges to calomel half-cells for the measurement of transmural potential difference (PD). A second pair of electrodes was connected to an automated voltage-clamp amplifier, which was a source of short-circuit current (Isc) to maintain zero potential difference between the PD-sensing bridges. Current strength necessary for changing the transepithelial PD by 2.5 mV was used to monitor tissue conductance as a determinant of tissue viability. Isc (measured in μA/cm2) was monitored by a voltage-clamp apparatus (DVC-1000; World Precision Instruments, Sarasota, FL) and displayed with a chart recorder (Dash IV; Astro-Med, West Warwick, RI). Electrical field stimulation (EFS) applied between pairs of aluminum foil electrodes placed on the submucosal surface of the tissue at the intersection between the two halves of the Ussing chamber was used to fire all submucosal neurons, including secretomotor neurons (13). Grass SD 88 stimulators (Grass Instruments, Quincy, MA) supplied current to the electrodes. The stimulus parameters were stimulus strength, 1–5 mA; frequency, 10 Hz; pulse duration, 0.5 ms; and total stimulation duration, 90 s. Changes in Isc during EFS were quantified as the difference between the peak response and baseline Isc before stimulation. Blockade of the EFS-evoked responses by tetrodotoxin (1 μM) confirmed earlier findings and was evidence that the responses were neurally mediated (13, 22, 30, 31).
Experimental protocol.
The preparations equilibrated for 30 min at 37°C before application of EFS. Stimulation was then begun at 5-min intervals and continued until reproducible responses were recorded. Stimulation was continued to obtain control data and was then repeated in the presence of progressively increasing concentrations of GLP-1 (0.1 nM–1 μM). Incubation time for each concentration was 5 min. Pharmacological analysis consisted of application of GLP-1 in the presence of one of four different receptor antagonists: 1) the GLP-1 receptor antagonist exendin-(9–39) (10 nM); 2) the muscarinic receptor antagonist scopolamine (1 μM); 3) the nicotinic receptor antagonist hexamethonium (100 μM), or 4) a selective VIP type I antagonist, VPAC (1 μM). Each agent was placed into the chamber 30 min before addition of GLP-1.
ACh release.
Methods for measurement of EFS-evoked release of ACh in Ussing chambers were similar to those of Yau et al. (55) and Javed and Cooke (33), with the exception that these workers measured release of [3H]ACh, whereas we determined ACh concentrations using ELISA. Flat-sheet preparations were placed in Ussing chambers with each half-chamber filled with agar to reduce the volume to 300 μl. Electrode and fluid ports were blocked with agar. The preparations were incubated with GLP-1 or GLP-1 followed by exendin-(9–39) applied in combination for 30 min. Transmural EFS was applied in each protocol. Contents of the chambers were collected immediately at the end of stimulation and stored at −20°C for subsequent analysis.
ACh concentration in each sample was determined by fluorescence immunoassay (Amplex red acetylcholine/acetylcholinesterase assay kit, catalog no. A12217; Invitrogen, Carlsbad, CA) and a fluorescence microplate reader (SpectraMax M2; Molecular Devices). Fluorescence was measured with excitation at 560 ± 10 nm and detection at 590 ± 10 nm. ACh release was expressed as micrograms per square centimeter of tissue.
Chemicals.
The following reagents were used. Carbachol, scopolamine, and hexamethonium were purchased from Sigma (St Louis, MO). GLP-1 and exendin-(9–39) were obtained from Tocris (Ellisville, MO). The VPAC1 receptor antagonist [Ac-His1, d-Phe2, Lys15, Arg16, Leu27]-VIP(3–7)-GRF(8–27) was obtained from Phoenix Pharmaceuticals (Burlingame, CA).
Data analysis and statistics.
Preparations from the same animal were studied at the same time in four Ussing chamber setups. Data are means ± SE; n values refer to the number of animals. Statistical significance was determined with paired Student's t-test between control and experimental populations or ANOVA followed by Bonferroni post hoc test, when appropriate. Differences were considered significant at P < 0.05. Labeled neurons in 30 submucosal ganglia were counted in the immunohistochemical studies. Total number of neurons, labeled with a specific neuronal marker, and the percent overlap of those markers with GLP-1R were determined.
RESULTS
Baseline Isc was 150 ± 1.9 μA/cm2 (n = 18), and the corresponding conductance was 37.2 ± 1.2 mS/cm2. Application of GLP-1 (0.1 nM–1 μM) to the serosal side of the preparations evoked no change in the baseline Isc (137.2 ± 2.5 μA/cm2, n = 9, P > 0.05) and did not alter the total tissue conductance (34.7 ± 2.2 mS/cm2, n = 9, P > 0.05).
Exposure to the muscarinic receptor agonist carbachol (10 μM) evoked a maximal increase in Isc of 52.2 ± 5.0 μA/cm2 (n = 3) within 3 min. Responses to carbachol were unaffected by application of 10 nM GLP-1 (50.9 ± 6.9, n = 3, P > 0.05).
Transmural EFS.
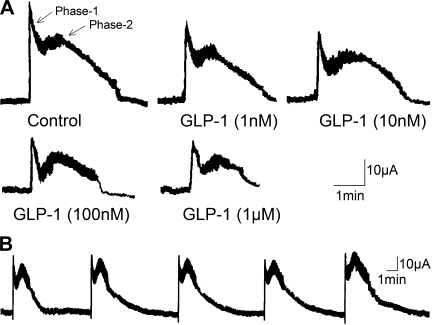
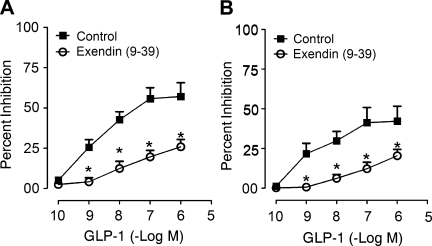
Transmural EFS evoked biphasic increases in Isc, which consisted of a spike-like rapid rise (phase 1) followed by a second, sustained phase 2 plateau-like response (Fig. 1A). No significant “rundown” of the stimulus-evoked responses appeared during 30 min following equilibration of the responses (Fig. 1B). GLP-1 (0.1 nM–1 μM), placed in the serosal side of the chamber, evoked concentration-dependent reductions in both phase 1 and phase 2 of the EFS-evoked responses (Figs. 1 and 2). This action of GLP-1 was suppressed following placement of the GLP-1R antagonist exendin-(9–39) (10 nM) in the chambers (Fig. 2).
Fig. 1.
Biphasic responses to electrical field stimulation are suppressed by glucagon-like peptide-1 (GLP-1) in a concentration-dependent manner. A: concentration-dependent attenuation of stimulus-evoked biphasic responses. B: consecutive responses evoked over 30 min served as controls. Stimulus parameters: 1–5 mA, 10 Hz, pulse width 0.5 ms, duration 90 s.
Fig. 2.
The GLP-1 receptor antagonist exendin-(9–39), at 10 nM, inhibited the action of GLP-1 to suppress both phase 1 and phase 2 of electrical field stimulation of chloride secretion. A: action of exendin-(9–39) on stimulus-evoked phase 1. B: action of exendin-(9–39) on stimulus-evoked phase 2. Data are means ± SE for preparations from 5 animals. *P < 0.05 for exendin-(9–39) relative to GLP-1 alone.
Exposure to 1 μM scopolamine, a muscarinic receptor antagonist, alone abolished phase 1 and significantly reduced phase 2 of the EFS-evoked responses. In the presence of scopolamine, GLP-1 (0.1 nM–1 μM) failed to modify further the EFS-evoked secretory responses (Fig. 3A).
Fig. 3.
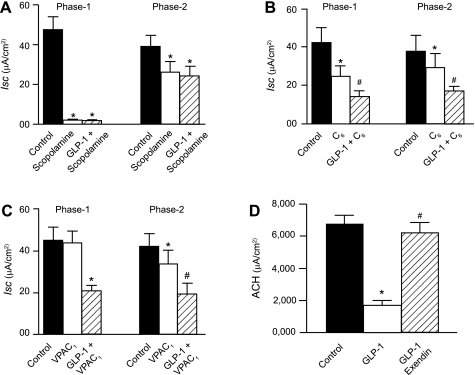
Pharmacological analysis of GLP-1 action on phases 1 and 2 of short-circuit current (Isc) responses to electrical field stimulation and evoked release of acetylcholine (ACh). A: the muscarinic receptor antagonist scopolamine suppressed phase 1 to a greater extent than phase 2. GLP-1 suppressed phase 1 to a greater extent than phase 2. No suppression of phase 2 by GLP-1 occurred in the presence of scopolamine. B: the nicotinic receptor antagonist hexamethonium (C6) suppressed both phase 1 and phase 2. Application of GLP-1 in the presence of C6 decreased further the amplitude of phase 1 and phase 2 responses. C: the vasoactive intestinal peptide (VIP) receptor antagonist VPAC1 suppressed phase 2 but not phase 1 responses. Suppression by GLP-1 was unaffected by the presence of VPAC1. D: the GLP-1 receptor antagonist exendin-(9–39) suppressed inhibition of stimulus-evoked ACh by GLP-1. Data are means ± SE for preparations from 6 animals. Concentrations: GLP-1, 10 nM; scopolamine, 1 μM; C6, 100 μM; VPAC1, 1 μM; exendin-(9–39), 10 nM. *P < 0.05 for GLP-1 relative to control. #P < 0.05 for GLP-1 relative to C6 or VPAC1. #P < 0.05 for GLP-1 relative to exendin (9–39).
Application of the nicotinic receptor antagonist hexamethonium (100 μM) in the bathing medium on the serosal side of the preparation reduced both phase 1 and phase 2 of the EFS-evoked responses. In the presence of hexamethonium, GLP-1, in a concentration range from 0.1 nM to 1 μM, continued to inhibit the first and second phases of the EFS-evoked secretory responses (Fig. 3B).
The presence of the VIP receptor antagonist VPAC1 (1 μM) in the bathing medium on the serosal side of the preparation did not modify phase 1 but reduced significantly phase 2 of the EFS-evoked responses. In the presence of VPAC1, GLP-1 (0.1 nM–1 μM) continued to suppress both the first and second phases of the secretory responses (Fig. 3C). Suppression of phase 2 of EFS-evoked Isc by VIP receptor antagonists is accepted as evidence that phase 2 is mediated in part by release of VIP from secretomotor neurons at intestinal neuroepithelial junctions (14, 42). We confirmed that incubation with VPAC1, before placement of VIP in the Ussing chambers, suppressed VIP-evoked increases in Isc (data not shown).
ACh release.
Because functional data suggest that GLP-1 acts to suppress release of ACh from secretomotor neurons at their junctions with the epithelium, we measured the amount of ACh released from the submucosal/mucosal preparations evoked by EFS in the absence or presence of GLP-1 to test the hypothesis further. GLP-1 (10 nM) significantly reduced the amount of EFS-evoked ACh release (Fig. 3D). Pretreatment with 10 nM exendin-(9–39) reversed the action of GLP-1 to suppress stimulus-evoked release of ACh (Fig. 3D).
Immunofluorescence.
IR for the GLP-1R in the submucosal plexus was localized to the cytoplasm of the neurons (Fig. 4). GLP-1R-IR was colocalized with IR for the protein, Hu, in over 50% of the neurons (Fig. 4, A1–A3). The nuclear protein Hu is part of a family of conserved RNA-binding proteins that includes HuC, HuD, and HuR (49). It is a useful immunohistochemical tool because it labels all ENS neurons (36). No IR fluorescence was seen when the GLP-1R antibody was preadsorbed with its control peptide (not shown).
Fig. 4.
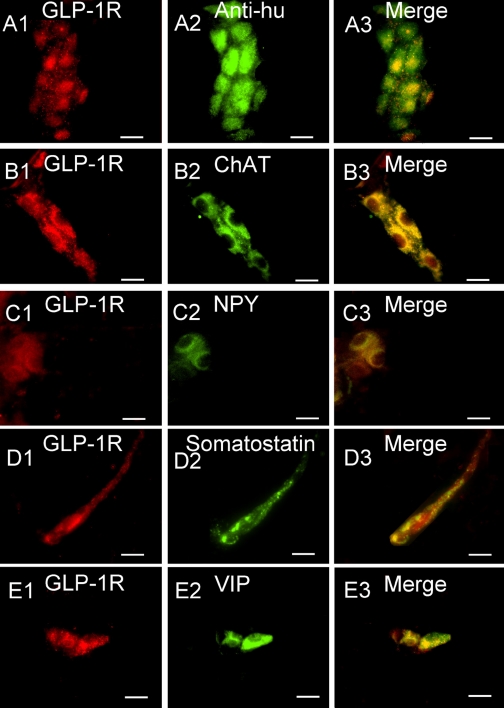
Expression of immunoreactivity (IR) for the GLP-1 receptor (GLP-1R) in whole mounts of guinea pig small intestinal submucosal plexus. A1–A3: coexpression of GLP-1R-IR with anti-Hu-IR, which marks all enteric neurons, reveals expression of GLP-1R-IR restricted to neuronal cell bodies. B1–B3: coexpression of GLP-1R-IR with choline acetyltransferase (ChAT)-IR, which is a neurochemical marker for cholinergic secretomotor neurons. C1–C3: coexpression of GLP-1R-IR with neuropeptide Y (NPY)-IR, which is a neurochemical marker for secretomotor/nonvasodilator motor neurons when coexpressed with ChAT-IR. D1–D3: coexpression of GLP-1R-IR with somatostatin-IR, which is a neurochemical marker for interneurons that provide inhibitory input to secretomotor neurons. E1–E3: coexpression of GLP-1R-IR with VIP-IR, which is a neurochemical marker for noncholinergic secretomotor/vasodilator motor neurons. Scale bars, 20 μm.
Application of double-labeling immunofluorescence methods showed that GLP-1R colocalized with 66.91 ± 4.3% (n = 4) of neurons that expressed choline acetyltransferase-IR (ChAT-IR) (Fig. 4, B1–B3) and with 60.60 ± 6.2% (n = 3) of neuropeptide Y-IR (NPY-IR) neurons (Fig. 4, C1–C3). GLP-1R-IR was coexpressed with somatostatin-IR in 54.8 ± 6.6% (n = 4) of the neurons (Fig. 4, D1–D3). GLP-1R-IR colocalized VIP-IR in 42.0 ± 7.8% (n = 3) of the neurons (Fig. 4, E1–E3).
DISCUSSION
GLP-1 influences gastric, insulin, and glucagon secretion (29, 40, 41, 45, 50, 51). Our results suggest, for the first time, that GLP-1 might also be involved in intrinsic neuroendocrine signaling that regulates mucosal secretion of electrolytes, H2O, and mucus and, therefore, luminal liquidity, pH, and protection in the small intestine.
Transmucosal EFS.
Finding of no effect of GLP-1 on baseline Isc and conductance indicates a lack of direct action on epithelial ion transport, per se. Instead, GLP-1 suppression of EFS-evoked Isc suggests inhibition of neurogenic chloride secretion. This action appeared to be receptor mediated because it was concentration dependent and suppressed by exendin-(9–39), which is a potent GLP-I receptor antagonist and a valuable tool for investigating the actions of GLP-I (21). Blockade of GLP-1R by exendin-(9–39) changed neither baseline Isc nor EFS-evoked responses, which suggests that GLP-1 has no direct action on enterocytes or paracellular conduction pathways. Moreover, it suggests absence of any spontaneous release of GLP-1 from intramural sources in the preparations in vitro.
The carbachol-evoked responses reflect direct stimulation of muscarinic receptors on enterocytes, because the responses are not suppressed by neural blockade with tetrodotoxin and are blocked by the muscarinic antagonist scopolamine (see Fig. 3A) (12, 33). Lack of effect of GLP-1 on carbachol-evoked stimulation of Isc suggests that GLP-1 inhibitory action on EFS-evoked Isc took place at submucosal secretomotor neurons and/or at other ENS neurons that provided excitatory synaptic input to the secretomotor neurons.
The EFS evoked biphasic increases in Isc in the current study were typical of earlier reports (13, 20). They mimicked observations in a variety of other species, including mice (11), rabbits (30), and humans (31). The biphasic responses in guinea pig flat-sheet preparations reflect release of multiple neurotransmitters from secretomotor neurons and release of transmitters from interneurons that supply excitatory synaptic input to the secretomotor neurons. ACh and VIP are the main neurotransmitters released by the secretomotor neurons. The action of ACh is at epithelial muscarinic M3 receptors and signal transduction involves elevation of cytosolic Ca2+ in the enterocytes and activation of protein kinase C (12, 17, 18, 38). Signal transduction for VIP differs from ACh. It involves stimulation of adenylyl cyclase, elevation of cAMP, and stimulation of PKA in the enterocytes (19, 46). Phase 1 of the EFS-evoked response in the guinea pig is mediated mainly, if not solely, by the release of ACh. Phase 2 has a peptidergic component that includes VIP as a major contributor (14, 15).
Our protocol was to evoke the biphasic increases in Isc, add GLP-1 in the serosal compartment of the chamber, and reapply EFS. Results obtained with this protocol support a hypothesis that phase 1 of the EFS-evoked responses is mediated primarily by secretomotor release of ACh and that phase 2 includes a component of VIP release in parallel with ACh. Our data suggest that GLP-1 acts to suppress the cholinergic component in both phases 1 and 2 with marginal, if any, effect on VIP release. Consistent with this was the finding that GLP-1 did not further suppress phase 2 after blockade of the cholinergic component by scopolamine. Moreover, when the VIPergic component of phase 2 was suppressed by the selective VIP antagonist VPAC1, exposure to GLP-1 still reduced the phase 2 response. When considered in view of the results showing no effect of GLP-1 on Isc after neural blockade by tetrodotoxin, these results suggest that the action of GLP-1, to suppress EFS-evoked secretion, reflects inhibition of presynaptic/prejunctional release of ACh by ENS neurons.
EFS, when applied to mucosal preparations in Ussing chambers, fires most all of the cell bodies of all classes of ENS neurons in the preparations and therefore their axonal output either to synapses on cell bodies of neighboring neurons or at neuroeffector junctions (10). Blockade by hexamethonium (i.e., C6) of nicotinic synaptic connections among neighboring neurons in the ENS microcircuitry partially suppressed both phases 1 and 2 of the EFS-evoked responses (see Fig. 3). The responses, remaining after nicotinic blockade, reflected direct stimulation of secretomotor neurons and release of ACh at the neuroepithelial junctions. Suppression of the residual responses by GLP-1 suggests inhibition of ACh release at the neuroepithelial junctions. Together, these results support the overall conclusion that GLP-1 acts to inhibit ACh release at synaptic connections between ENS neurons and at the junctions of secretomotor neurons with the epithelium.
Immunofluorescence.
Our immunofluorescence results for localization of GLP-1R in the submucosal plexus are consistent with a conclusion that GLP-1R suppresses neurogenic secretion by inhibiting neuronal ACh release. Neuronal cell bodies, which expressed ChAT-IR, NPY-IR, somatostatin-IR, or VIP-IR, all expressed GLP-1R-IR. ChAT-IR and NPY-IR, in the same neuron, is a neurochemical code for the class of secretomotor neurons that do not send collaterals to innervate periglandular arterioles (25, 54). ChAT-IR neurons that contain calretinin are cholinergic secretomotor/vasodilator neurons (7, 25). VIP-IR is a marker for noncholinergic secretomotor/vasodilator neurons that receive sympathetic noradrenergic inhibitory input. Somatostatin-IR identifies interneurons in the ENS that supply inhibitory synaptic input to secretomotor neurons (23, 37). Expression of GLP-1R by cholinergic secretomotor/nonvasodilator neurons and cholinergic secretomotor/vasodilator neurons can account for suppression of the cholinergic component of EFS-evoked phases 1 and 2 of the secretory responses when GLP-1 was present in the tissue chamber.
Expression of GLP-1R by noncholinergic secretomotor/vasodilator neurons is inconsistent with our finding that GLP-1 had little or no effect on the VIPergic component of the stimulus-evoked phase 2 response. The discrepancy might be explained by the existence of VIPergic secretomotor neurons known to evoke mucosal HCO3− secretion (34). Expression of GLP-1R by the neurons that contained VIP might be a reflection of differences in parallel neural pathways, one for Cl− secretion and the other for HCO3−.
ACh release.
Cholinergic neurons make up about 50% of the submucosal neurons in the guinea pig small intestine (6, 24). Finding that GLP-1R was expressed by cholinergic secretomotor neurons suggests a neuronal action for GLP-1. This and finding that suppression of EFS-evoked biphasic increases in Isc by GLP-1 or a muscarinic receptor antagonist lead us to suggest that the action of GLP-1 on neurogenic Cl− secretion is suppression of neuronal release of ACh. Finding that GLP-1 suppressed stimulus-evoked ACh release and suppression of this action by a selective GLP-1R antagonist supports the suggestion.
Suppression of EFS-evoked ACh release could result from an inhibitory action of GLP-1 on the excitability of cholinergic secretomotor neurons, or it could result from binding at inhibitory presynaptic/prejunctional GLP-1Rs at axonal ACh release sites. Intracellular electrophysiological recording of GLP-1 action on electrical and synaptic behavior in submucosal neurons will be necessary to clarify the specific mechanism by which GLP-1 suppresses ACh release in the ENS.
Physiological significance of the action of GLP-1 in the small intestine is suggested to be a mechanism involved in minute-to-minute adjustment of the output of cholinergic secretomotor/vasodilator neurons. By adjusting secretomotor/vasodilator output, the negative feedback control networks in the ENS can regulate the osmolarity of the intraluminal milieu and thereby contribute to maintenance of an optimal environment for pancreatic and brush border digestive enzymes to work as digestion proceeds.
Conclusion.
Cholinergic neurons, including secretomotor neurons, in the submucosal plexus of guinea pig small intestine express receptors for GLP-1. Stimulation of the GLP-1 receptors suppresses mucosal Cl− secretion evoked by electrical stimulation of neurons in the submucosal plexus. Suppression of mucosal Cl− secretion results from GLP-1 action to attenuate release of ACh from secretomotor neurons.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK 37238 and R01 DK 57075 (to J. D. Wood) and a travel grant (SIF 2010) from the Italian Physiology Society (to S. Baldassano).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.B., F.M., and J.D.W. conception and design of research; S.B., G.-D.W., and J.D.W. performed experiments; S.B., G.-D.W., and F.M. analyzed data; S.B., G.-D.W., F.M., and J.D.W. interpreted results of experiments; S.B., G.-D.W., and J.D.W. prepared figures; S.B. and J.D.W. drafted manuscript; S.B., G.-D.W., F.M., and J.D.W. edited and revised manuscript; S.B., G.-D.W., F.M., and J.D.W. approved final version of manuscript.
REFERENCES
- 1. Amato A, Cinci L, Rotondo A, Serio R, Faussone-Pellegrini MS, Vannucchi MG, Mule F. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil 22: 664–e203, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Andrews CN, Bharucha AE, Camilleri M, Low PA, Seide BM, Burton DD, Nickander KK, Baxter KL, Zinsmeister AR. Effects of glucagon-like peptide-1 and sympathetic stimulation on gastric accommodation in humans. Neurogastroenterol Motil 19: 716–723, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anvari M, Paterson CA, Daniel EE, McDonald TJ. Effects of GLP-1 on gastric emptying, antropyloric motility, and transpyloric flow in response to a nonnutrient liquid. Dig Dis Sci 43: 1133–1140, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Baldassano S, Liu S, Qu MH, Mule F, Wood JD. Glucagon-like peptide-2 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro. Am J Physiol Gastrointest Liver Physiol 297: G800–G805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bornstein JC, Furness JB. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst 25: 1–13, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 262: 58–70, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137: 2968–2678, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134: 2156–2164, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Carey HV, Cooke HJ, Zafirova M. Mucosal responses evoked by stimulation of ganglion cell somas in the submucosal plexus of the guinea-pig ileum. J Physiol 364: 69–79, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carey HV, Cooke HJ. Influence of enteric nerves on jejunal function of the piebald-lethal mouse (Abstract). Gastroenterology 86: A1040, 1984 [Google Scholar]
- 12. Cooke HJ. Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 246: G263–G267, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Cooke HJ, Shonnard K, Wood JD. Effects of neuronal stimulation on mucosal transport in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 245: G290–G296, 1983 [DOI] [PubMed] [Google Scholar]
- 14. Cooke HJ, Zafirova M, Carey HV, Walsh JH, Grider J. Vasoactive intestinal polypeptide actions on the guinea pig intestinal mucosa during neural stimulation. Gastroenterology 92: 361–370, 1987 [DOI] [PubMed] [Google Scholar]
- 15. Cooke HJ. Neural and humoral regulation of small intestinal electrolyte transport. In: Physiology of the Gastrointestinal Tract. edited by Johnson LR, Christensen J, Jackson MJ, Jacobson ED, Walsh JH. New York: Raven, 1987, p. 1307–1350 [Google Scholar]
- 16. Delgado-Aros S, Kim DY, Burton DD, Thomforde GM, Stephens D, Brinkmann BH, Vella A, Camilleri M. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol 282: G424–G431, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 348–354, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dickinson KE, Frizzell RA, Sekar MC. Activation of T84 cell chloride channels by carbachol involves a phosphoinositide-coupled muscarinic M3 receptor. Eur J Pharmacol 225: 291–298, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Eklund S, Brunsson I, Jodal M, Lundgren O. Changes in cyclic 3′5′-adenosine monophosphate tissue concentration and net fluid transport in the cat's small intestine elicited by cholera toxin, arachidonic acid, vasoactive intestinal polypeptide and 5-hydroxytryptamine. Acta Physiol Scand 129: 115–125, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Fang X, Hu HZ, Gao N, Liu S, Wang GD, Wang XY, Xia Y, Wood JD. Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol 536: 113–122, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Fehmann HC, Jiang J, Schweinfurth J, Wheeler MB, Boyd AE, 3rd, Goke B. Stable expression of the rat GLP-I receptor in CHO cells: activation and binding characteristics utilizing GLP-I(7–36)-amide, oxyntomodulin, exendin-4, and exendin(9–39). Peptides 15: 453–456, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Fei G, Wang YZ, Liu S, Hu HZ, Wang GD, Qu MH, Wang XY, Xia Y, Sun X, Bohn LM, Cooke HJ, Wood JD. Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon. Am J Physiol Gastrointest Liver Physiol 296: G823–G832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foong JP, Parry LJ, Gwynne RM, Bornstein JC. 5-HT1A, SST1, and SST2 receptors mediate inhibitory postsynaptic potentials in the submucous plexus of the guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 298: G384–G394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furness JB, Costa M, Keast JR. Choline acetyltransferase- and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res 237: 329–336, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Furness JB. The Enteric Nervous System. Oxford: Blackwell, 2006 [Google Scholar]
- 26. Hellstrom PM. GLP-1: broadening the incretin concept to involve gut motility. Regul Pept 156: 9–12, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Hellstrom PM. GLP-1 playing the role of a gut regulatory compound. Acta Physiol (Oxf) 201: 151–156, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Holst JJ, Orskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 211: 169–174, 1987 [DOI] [PubMed] [Google Scholar]
- 30. Hubel KA. The effects of electrical field stimulation and tetrodotoxin on ion transport by the isolated rabbit ileum. J Clin Invest 62: 1039–1047, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hubel KA, Shirazi S. Human ileal ion transport in vitro: changes with electrical field stimulation and tetrodotoxin. Gastroenterology 83: 63–68, 1982 [PubMed] [Google Scholar]
- 32. Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol Gastrointest Liver Physiol 273: G920–G927, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Javed NH, Cooke HJ. Acetylcholine release from colonic submucous neurons associated with chloride secretion in the guinea pig. Am J Physiol Gastrointest Liver Physiol 262: G131–G136, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Konturek PC, Konturek SJ, Hahn EG. Duodenal alkaline secretion: its mechanisms and role in mucosal protection against gastric acid. Dig Liver Dis 36: 505–512, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-17–36: a physiological incretin in man. Lancet 2: 1300–1304, 1987 [DOI] [PubMed] [Google Scholar]
- 36. Lin Z, Gao N, Hu HZ, Liu S, Gao C, Kim G, Ren J, Xia Y, Peck OC, Wood JD. Immunoreactivity of Hu proteins facilitates identification of myenteric neurones in guinea-pig small intestine. Neurogastroenterol Motil 14: 197–204, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Liu S, Xia Y, Hu H, Ren J, Gao C, Wood JD. Histamine H3 receptor-mediated suppression of inhibitory synaptic transmission in the submucous plexus of guinea-pig small intestine. Eur J Pharmacol 397: 49–54, 2000 [DOI] [PubMed] [Google Scholar]
- 38. McCabe RD, Dharmsathaphorn K. Mechanism of VIP-stimulated chloride secretion by intestinal epithelial cells. Ann NY Acad Sci 527: 326–345, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Miki T, Minami K, Shinozaki H, Matsumura K, Saraya A, Ikeda H, Yamada Y, Holst JJ, Seino S. Distinct effects of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 on insulin secretion and gut motility. Diabetes 54: 1056–1063, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 79: 616–619, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Orskov C, Holst JJ, Nielsen OV. Effect of truncated glucagon-like peptide-1. Endocrinology 123: 2009–2013, 1988 [DOI] [PubMed] [Google Scholar]
- 42. Reddix R, Kuhawara A, Wallace L, Cooke HJ. Vasoactive intestinal polypeptide: a transmitter in submucous neurons mediating secretion in guinea pig distal colon. J Pharmacol Exp Ther 269: 1124–1129, 1994 [PubMed] [Google Scholar]
- 43. Rotondo A, Amato A, Lentini L, Baldassano S, Mule F. Glucagon-like peptide-1 relaxes gastric antrum through nitric oxide in mice. Peptides 32: 60–64, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Schirra J, Wank U, Arnold R, Goke B, Katschinski M. Effects of glucagon-like peptide-1(7–36)amide on motility and sensation of the proximal stomach in humans. Gut 50: 341–348, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schjoldager BT, Mortensen PE, Christiansen J, Orskov C, Holst JJ. GLP-1 (glucagon-like peptide 1) and truncated GLP-1, fragments of human proglucagon, inhibit gastric acid secretion in humans. Dig Dis Sci 34: 703–708, 1989 [DOI] [PubMed] [Google Scholar]
- 46. Schwartz CJ, Kimberg DV, Sheerin HE, Field M, Said SI. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J Clin Invest 54: 536–544, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sinclair EM, Drucker DJ. Proglucagon-derived peptides: mechanisms of action and therapeutic potential. Physiology (Bethesda) 20: 357–365, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellstrom PM. Inhibitory effect of glucagon-like peptide-1 on small bowel motility. Fasting but not fed motility inhibited via nitric oxide independently of insulin and somatostatin. J Clin Invest 102: 764–774, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ule J. Ribonucleoprotein complexes in neurologic diseases. Curr Opin Neurobiol 18: 516–523, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38: 665–673, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Wettergren A, Wojdemann M, Meisner S, Stadil F, Holst JJ. The inhibitory effect of glucagon-like peptide-1 (GLP-1) 7–36 amide on gastric acid secretion in humans depends on an intact vagal innervation. Gut 40: 597–601, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wood JD. Neurotransmission at the interface of sympathetic and enteric divisions of the autonomic nervous system. Chin J Physiol 42: 201–210, 1999 [PubMed] [Google Scholar]
- 53. Wood JD. Enteric Nervous System: The Brain-in-the-Gut. Colloquium Series on Integrated Systems Physiology: From Molecule to Function Princenton, NJ: Morgan & Claypool, 2011 [Google Scholar]
- 54. Xue J, Askwith C, Javed NH, Cooke HJ. Autonomic nervous system and secretion across the intestinal mucosal surface. Auton Neurosci 133: 55–63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yau WM, Dorsett JA, Youther ML. Modulation of submucosal cholinergic neurons by 5-hydroxytryptamine and neuropeptides. Am J Physiol Gastrointest Liver Physiol 259: G1019–G1024, 1990 [DOI] [PubMed] [Google Scholar]