Abstract
This study aimed to apply novel high-resolution manometry with eight-sector radial pressure resolution (3D-HRM technology) to resolve the deglutitive pressure morphology at the esophagogastric junction (EGJ) before, during, and after bolus transit. A hybrid HRM assembly, including a 9-cm-long 3D-HRM array, was used to record EGJ pressure morphology in 15 normal subjects. Concurrent videofluoroscopy was used to relate bolus movement to pressure morphology and EGJ anatomy, aided by an endoclip marking the squamocolumnar junction (SCJ). The contractile deceleration point (CDP) marked the time at which luminal clearance slowed to 1.1 cm/s and the location (4 cm proximal to the elevated SCJ) at which peristalsis terminated. The phrenic ampulla spanned from the CDP to the SCJ. The subsequent radial and axial collapse of the ampulla coincided with the reconstitution of the effaced and elongated lower esophageal sphincter (LES). Following ampullary emptying, the stretched LES (maximum length 4.0 cm) progressively collapsed to its baseline length of 1.9 cm (P < 0.001). The phrenic ampulla is a transient structure comprised of the stretched, effaced, and axially displaced LES that serves as a “yield zone” to facilitate bolus transfer to the stomach. During ampullary emptying, the LES circular muscle contracts, and longitudinal muscle shortens while that of the adjacent esophagus reelongates. The likely LES elongation with the formation of the ampulla and shortening to its native length after ampullary emptying suggest that reduction in the resting tone of the longitudinal muscle within the LES segment is a previously unrecognized component of LES relaxation.
Keywords: esophageal manometry, peristalsis, esophageal bolus transit, lower esophageal sphincter
formation of the phrenic ampulla initiates the final phase of bolus transit through the esophagus. The globular-shaped ampulla forms at the caudal end of the esophagus in conjunction with transient esophageal shortening resultant from deglutitive contraction of the longitudinal muscle (4, 7, 19). The bolus is then emptied from the ampulla into the subdiaphragmatic stomach. Ampullary emptying is mechanistically distinct from peristalsis in that luminal clearance is slower and the bolus flow across the hiatus is driven by a sustained hydrostatic pressure rather than a propagated (peristaltic) contraction (13). Videofluoroscopy synchronized with manometry has been invaluable in analyzing the mechanics of ampullary emptying. Correlation between fluoroscopy and manometry is facilitated by placement of mucosal endoclips to mark landmarks such as the squamocolumnar junction (SCJ). The formation of the ampulla can be visualized videofluoroscopically and related to the intraluminal pressures in the tubular esophagus, the ampulla, and at the esophagogastric junction (EGJ). Although this picture may seem complete, the genesis of the ampulla and its anatomic correlate in the resting esophagus are incompletely understood.
Initially described by Clouse and Staiano (5), high-resolution manometry (HRM) and esophageal pressure topography (EPT) have greatly aided the mechanistic analysis of peristalsis. Their seminal work revealed a segmental architecture to peristalsis composed of three contractile segments (S1, S2, and S3) (5). More recent work, also relying on this methodology, focused on bolus transit across the EGJ and revealed a novel physiological landmark above the EGJ, the contractile deceleration point (CDP) (16). The CDP appeared relevant to ampullary emptying in that it was both a spatial and temporal landmark of the termination of peristalsis. With respect to the segmental architecture of peristalsis, the CDP is localized within segment 3 (6, 16), and temporally coincides with the formation of the ampulla. However, the spatial relevance of CDP is unclear.
As often occurs with advances in technology, questions are answered only to unearth new ones. Such is the case with HRM, especially when applied to the EGJ. Although HRM largely solved artifact problems in the quantification of EGJ relaxation related to axial sphincter movement, radial pressure asymmetry in the region of the EGJ arose as another methodological confounder. This especially pertains to the extrinsic compression of the esophagus imparted by the crural diaphragm (CD). Three-dimensional (3D)-HRM, a technology that provides enhanced axial and radial pressure resolution, was developed specifically to address this issue (12). Hence, the aim of this study was to apply novel 3D-HRM technology to resolve the deglutitive pressure morphology at the EGJ in relation to anatomical landmarks. High-fidelity recordings of the EGJ with enhanced spatial and radial resolution may potentially clarify the functional makeup of the phrenic ampulla before, during, and after emptying.
MATERIALS AND METHODS
Subjects.
Fifteen normal subjects (9 females and 6 males, 21–52 yr old) recruited from a pool of asymptomatic volunteers were studied. None of the subjects had a history of prior gastrointestinal surgery, significant medical disease, or using medications for upper gastrointestinal symptoms (antacids, H2-receptor antagonists, proton pump inhibitors, prokinetics, or anticholinergics). All subjects gave written informed consent. The study protocol was approved by the Northwestern University Institutional Review Board.
3D-HRM.
Manometry studies were done using a novel solid-state assembly (ManoScan 3D; Given Imaging, Los Angeles, CA) (12). This hybrid assembly (4.2 mm outer diameter) incorporated an array of 12 3D high-resolution sensors between 28 proximal and 4 distal circumferential sensors (Fig. 1). The novelty of the 3D array was of increased axial pressure resolution and completely preserved radial pressure resolution. Both the 3D array and the conventional segments utilized individual sensing elements that were 2.5 mm in length. In the 3D array, the sensors were spaced 7.5 mm apart. In the conventional HRM segments, each sensor was 10 mm apart on center. With respect to radial pressure sensitivity, each axial level within the 3D array consisted of eight sensors dispersed 45° apart circumferentially with each functioning as an independent pressure sensor. On the other hand, each sensor within the conventional HRM segments averaged the pressure signals from 12 circumferential sensors into a single recorded pressure. Consequently, a 9-cm segment of conventional HRM yielded 9 averaged pressure readings compared with 96 independent pressure readings in the 9-cm 3D-HRM segment. Each sensing element, whether in the conventional or 3D array, could record pressure transients in excess of 6,000 mmHg/s with an accuracy of ± 1 mmHg after thermal compensation. The data acquisition frequency was 35 Hz. All pressure measurements were referenced to atmospheric pressure.
Fig. 1.
The three-dimensional high-resolution manometry (3D-HRM) segment of the manometric assembly. The 3D-HRM stiff segment consisted of 12 sensing loci, housed as 6 pairs and spanning 9 cm. However, regardless of whether within the same housing or in the adjacent housing, the sensors were 7.5 mm apart. Each axial 3D-HRM locus contained 8 independent pressure sensors radially dispersed 45° apart. The assembly also incorporated circumferentially sensitive sensors above and below the 3D-HRM segment. Each circumferentially sensitive high-resolution sensor averaged the signal of 12 radially dispersed sensing elements with the circumferential sensors being spaced 10 mm apart. The entire manometry assembly measures a total of 128 independent pressure signals.
Manometric data were displayed as pressure topography plots using either the proprietary ManoView software (Given Imaging) or MATLAB (The MathWorks, Natick, MA). MATLAB was used to depict the HRM and 3D-HRM EPTs because of its greater flexibility in illustrating EGJ pressure morphology.
Study protocol.
Studies were done after at least a 6-h fast. After a brief interview and examination, subjects were asked to complete dysphagia and reflux questionnaires. Dysphagia was assessed using the Hospital Odynophagia Dysphagia Questionnaire (HODQ, maximal score: 50; 95th percentile cutoff in controls: 2). Reflux symptoms were measured using the Reflux Disease Questionnaire (RDQ, maximal score for heartburn or regurgitation: 20; normative cutoff: <8). Subjects then underwent esophagogastroduodenoscopy (EGD) in the left lateral decubitus position to evaluate for esophagitis or hiatal hernia. Moderate sedation with 5–10 mg midazolam and 75–200 μg fentanyl was administered before and during the procedure as necessary. A diagnostic gastroscope was used (Olympus GIF type H180J; Olympus, Tokyo, Japan), and an endoclip was placed at the SCJ (Resolution Clip; Boston Scientific, Natick, MA).
Following recovery from sedation (at least 2 h after the EGD), patients underwent transnasal placement of the manometry assembly. The pressure transducers were calibrated before intubation at 0 and 300 mmHg using externally applied pressure. The assembly was positioned to record from the hypopharynx to the stomach with the segment of 3D array straddling the EGJ. The assembly was fixed in place by taping it to the nose. The study protocol included recordings of three 5-ml barium swallows with the barium injected with a syringe into the mouth and swallowed as one bolus. The interval between swallows varied from 20 to 40 s. During the recording, the subjects were supine on a fluoroscopy table and shielded with a lead apron and a lead collar. Real-time fluoroscopic images were recorded through a video module (ManoScanV; Given Imaging) on the computer and synchronized with concurrent 3D-HRM recordings.
Data analysis.
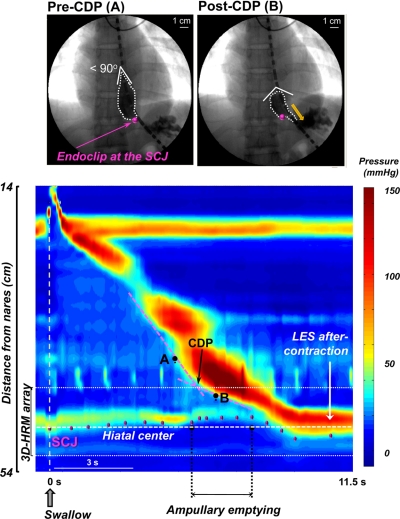
Esophageal emptying was analyzed using the videofluoroscopic recordings with particular focus on the events of ampullary emptying as exemplified by the fluoroscopic still images in Fig. 2. A glaring distinction in the fluoroscopy images obtained pre- and post-CDP is in the morphology of the bolus tail, widening from an acute to an obtuse angle. Ampullary formation was arbitrarily established as the time when the angle of the inverted “V” of the bolus clearing wave (formed by the point of luminal closure and the walls encapsulating the barium bolus 1 cm distal) achieved 90° (13). The axial location of the SCJ was ascertained by following the relative positions of the endoclip and 3D-HRM housing, both clearly visible on fluoroscopic images. Fluoroscopic magnification was corrected by using the known size of the manometric assembly housing.
Fig. 2.
The transition from peristaltic to ampullary emptying seen in selected videofluoroscopic images (top) and in esophageal pressure topography (EPT) with key landmarks identified (bottom). During peristalsis, the inverted “V” of the clearing wave was <90°. This progressively widened as the bolus assumed a globular shape with development of the phrenic ampulla. The EPT is illustrated in the conventional high-resolution manometry (HRM) mode with the segment of the 3D-HRM array indicated. The onset of ampullary emptying coincided closely with the contractile deceleration point (CDP), localized by the intersection of the pink tangents skirting the contractile complex. Videofluoroscopic tracking of the endoclip relative to the manometric housing allowed localization of the squamocolumnar junction (SCJ) on the EPT plots (magenta dots). The hiatal center (white dashed line) was also localized fluoroscopically and correlated with EPT by relation to the manometric housing. LES, lower esophageal sphincter.
Manometric recordings provided EPT landmarks beginning with upper esophageal sphincter relaxation at the start of the swallow and ending with return to baseline at the level of the EGJ (Fig. 2). The CDP was identified as the location at which two tangential lines skirting the 30-mmHg isobaric contour intersect, with the first tangent being distal to the transition zone and the second tangent originating at the EGJ where the contractile front terminated (16). Spatial measurements were summarized relative to the position of the hiatal center, defined by the CD contraction immediately before the start of each swallow sequence.
EGJ pressure morphology was assessed during the period of bolus clearance using the circumferentially averaged and nadir radial pressures detected by the eight radially dispersed sensors to contrast the conventional HRM approach against the 3D-HRM approach. The latter approach was intended to pinpoint the radian of lowest EGJ pressure that creates a channel for bolus flow otherwise masked by circumferential averaging (12). Deglutitive EGJ relaxation and the associated EGJ pressure gradient were assessed both ways at axial locations centered at the hiatus and at 7.5-mm increments above and below. Pressure changes during bolus clearance were assessed at ampullary formation and completion of bolus clearance, as well as at 25, 50, and 75% of emptying time to control for the differences in the duration of the clearance process among subjects.
The pressure morphology of the high-pressure zone at the lower esophageal sphincter (LES) was assessed at the completion of ampullary emptying, during LES aftercontraction, and at baseline using nadir radial pressures at the SCJ, 7.5, 15, 22.5, and 30 mm above the SCJ and gastric pressure 30 mm below the SCJ. The length of the high-pressure zone was determined by the distance between the SCJ and the temporally corresponding location of intraesophageal or intragastric pressure in any circumferential sector. Hence, the proximal and distal limits of the LES were defined as the locations of circumferential pressure increase relative to the adjacent esophagus and stomach.
Statistical analysis.
Data were summarized as median (5th-95th percentile), unless stated otherwise, and compared using nonparametric statistical tests. A P < 0.05 was considered significant.
RESULTS
Subject demographics.
On endoscopic assessment, Los Angeles grade A esophagitis was seen in one subject with the remainder being normal. No hiatal hernia was evident in any subject. Of the 15 subjects, 14 had a normal HODQ score (≤2) and one had a borderline abnormal score of nine. All 15 subjects had normal RDQ scores of two or less.
Characteristics of distal esophageal emptying.
Figure 2 illustrates a typical swallow shown in a conventional HRM EPT format with representative concurrent videofluoroscopic images. The fluoroscopic images highlight the change in the shape of the bolus before and after the CDP. The location of the SCJ, derived from fluoroscopic images, is superimposed on the EPT showing that the SCJ demarcated the distal LES margin and moved in the orad direction away from the hiatal center during bolus clearance. The orad excursion was most pronounced during ampullary emptying.
The onset of bolus transit across the EGJ varied among subjects spanning the period from 1 s before to 1.5 s after formation of the phrenic ampulla. The CDP occurred 0.6 (−0.3 to +1.6) s after the onset of trans-EGJ flow, overlapping this event in time. Before the CDP, peristalsis propagated at a rate of 4.5 (3.1–7.0) cm/s. After the CDP, luminal clearance progressed at a rate of 1.1 (0.4–1.9) cm/s. Table 1 tracks the movement of the SCJ during ampullary emptying showing that the orad SCJ excursion reached 1.5 cm above the hiatal center at the onset of ampullary emptying and at the time of the CDP. Spatially, the CDP was located 4.0 (1.8–4.9) cm above the SCJ. As ampullary emptying progressed (∼25% into emptying time), the SCJ reached its maximal orad excursion of 1.6 cm (Table 1) with negligible descent during the remainder of the emptying period. Once the ampullary emptying was completed, the SCJ descended and effectively marked the distal margin of the LES during the aftercontraction and baseline periods.
Table 1.
Squamocolumnar junction excursions during esophageal emptying
| Time Landmark | Orad Distance From the Hiatal Center, cm |
|---|---|
| Swallow start | 0 (−1.4 to 0.7) |
| Bolus entry into the distal esophagus | 0.6 (−1.4 to 1.0) |
| CDP | 1.5 (−0.4 to 2.0) |
| Onset ampullary emptying | 1.5 (−0.5 to 2.1) |
| 25% Emptying time | 1.6 (0–2.3) |
| 50% Emptying time | 1.4 (0–2.2) |
| 75% Emptying time | 1.4 (−0.3 to 1.9) |
| Complete ampullary emptying | 0.9 (−0.5 to 1.3) |
| Postemptying | −0.1 (−1.9 to 1.0) |
Values are given as median (5th–95th percentile).
CDP, contractile deceleration point.
3D pressure morphology of the post-CDP contraction.
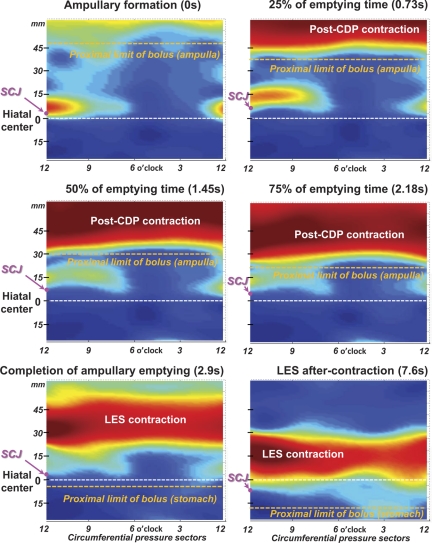
Figure 3 illustrates typical pressure topography from along the 9-cm 3D-HRM array obtained at landmark times before, during, and after ampullary emptying. As in Fig. 2, the position of the SCJ obtained from the synchronized fluoroscopy demarcates the distal margin of the LES. Note that the ampulla forms in the 4-cm segment of esophagus proximal to the SCJ, which is situated just proximal to the hiatus throughout the period of emptying. As bolus flow into the stomach proceeds, the ampulla progressively shrinks in both radial and axial dimensions. Coincident with the collapse and disappearance of the ampulla, a high-pressure zone was reconstituted, albeit in an elevated and elongated state, spanning almost 5 cm in length at the completion of bolus transfer (Fig. 3, bottom left). This contracting segment surely encompassed the LES and likely some adjacent esophagus functioning as a physiological sphincter. Subsequently, during the ensuing 4.7 s, the SCJ gradually descends and the LES regains its resting configuration, eventually spanning a length of ∼2.5 cm. Summary data on the process of sphincter shortening for the entire set of subjects in the postemptying period are given in Fig. 4. Accordingly, the high-pressure zone length measure (Fig. 4) shortened from a median of 4.0 (1.8–5.6) cm at the completion of bolus clearance to an aftercontraction length of 2.5 (1.3–4.3) cm and baseline length of 1.9 (0.6–3.7) cm (Fig. 4C).
Fig. 3.
Instantaneous 3D-HRM pressure morphology at the hiatus during a typical period of ampullary emptying. Each panel corresponds to the specific landmark time indicated, and the 3D-HRM plots preserve the 8-sector 360° pressure topography. The radially dispersed pressure sectors are related to a clock face viewed from below with 12 o'clock corresponding to the lesser curvature of the stomach and 6 o'clock corresponding to the greater curvature. Note the locus of nadir radial pressure centered at about 4 o'clock persisting until completion of the ampullary emptying. This nadir radial pressure, obscured by circumferential averaging in Fig. 2, is the flow permissive path for ampullary emptying. The relative locations of the SCJ (magenta dot) are indicated on the y-axis relative to the hiatus (white dashed line at position 0). The proximal limit of the bolus (orange dashed line), derived from the corresponding fluoroscopic images, is marked at the different stages of ampullary emptying. Note the progressive narrowing of the post-CDP and LES contraction that occurs as the ampulla empties and the LES regains its resting configuration.
Fig. 4.
Reestablishment of the LES high-pressure zone after ampullary emptying. A: the pressure topography along with the fluoroscopic image at the completion of ampullary emptying. Measurements were taken at the completion of ampullary emptying, midway to return to baseline during LES aftercontraction, and with return to baseline. Measurements were made from the 3D array (B) as a distance between the SCJ and the distal limit of intraesophageal pressure. Length values are shown as medians and interquartile ranges (C). *P < 0.01 and **P < 0.001 vs. LES high-pressure zone length at completion of ampullary emptying.
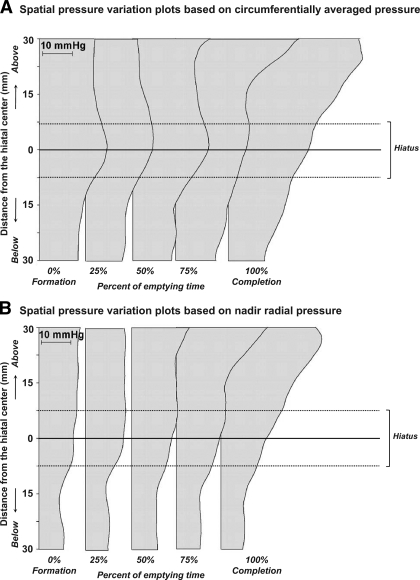
During ampullary emptying, there was marked radial asymmetry of the pressures recorded distal to the post-CDP contraction in Fig. 3 such that there was a pressure maximum at 12 o'clock (facing the lesser gastric curvature) and a distinct sector of nadir radial pressure in the 3 to 6 o'clock direction (6 o'clock denoting the sector of peak CD pressure). This sector of nadir pressure is nearly isobaric with the stomach, making it conducive to bolus flow from the ampulla into the stomach. Figure 5 is a graphical representation of summary data from all subjects on the pressure gradient across the hiatus illustrated as a series of spatial pressure variation plots. Note that the plots in Fig. 5B, based on nadir radial pressures, show an uninterrupted positive EGJ pressure gradient favorable to bolus flow, consistent with the demonstration of flow fluoroscopically. On the other hand, when the spatial pressure variation plots were constructed using circumferentially averaged pressure (Fig. 5A), the median pressures at the hiatus were 4–5 mmHg greater than the pressure 15 mm above the hiatus, a condition that, if true, would prevent flow. Transhiatal pressure gradient data generated by the nadir radial pressure approach vs. the circumferentially averaged method are also summarized in Table 2, again showing the artifactual barrier to flow that appears with use of circumferential pressure averaging. Overall, the nadir radial pressures at the hiatus (Table 2) were lower than those based on circumferential averaging (P < 0.01), and the EGJ pressure gradients were slightly, but not significantly, greater. However, more notably, the median nadir pressures at the hiatus were within 1 mmHg (range 0–0.6 mmHg) of those 15 mm above the hiatus compared with being as much as 5 mmHg greater (range 4.0–5.3 mmHg) when using circumferential averaging (Table 2).
Fig. 5.
Spatial pressure variation plots of circumferentially averaged (A) and nadir radial pressure (B) at landmark times of ampullary emptying. The median pressure along the hiatal center (solid line at 0 mm) was greater when based on circumferential averaging than nadir radial pressures, creating an artifactual focal high pressure at the hiatal center that appeared to interrupt an otherwise favorable flow-permissive gradient.
Table 2.
Intraluminal pressure gradients across the hiatus during ampullary emptying
| Circumferentially Averaged Pressure at Each Axial Locus, mmHg |
Nadir Radial Pressure at Each Axial Locus, mmHg |
|||||||
|---|---|---|---|---|---|---|---|---|
| Distance from the hiatal center |
EGJ pressure gradient | Distance from the hiatal center |
EGJ pressure gradient | |||||
| Time Landmark | 15 mm Above | 0 ± 7.5 mm | 15 mm Below | 15 mm Above | 0 ± 7.5 mm | 15 mm Below | ||
| Onset ampullary emptying | 19 (13–23) | 26 (14–33) | 14 (10–23) | 3 (−2 to 12) | 14* (8–19) | 14* (7–19) | 7* (5–15) | 5 (1–9) |
| 25% Emptying time | 19 (14–39) | 29 (18–42) | 13 (9–24) | 4 (−2 to 27) | 14* (9–24) | 14* (7–25) | 7* (2–15) | 7 (1–15) |
| 50% Emptying time | 22 (16–30) | 29 (21–39) | 14 (10–24) | 5 (1–16) | 15* (11–24) | 18* (10–23) | 8* (4–16) | 7 (3-14) |
| 75% Emptying time | 25 (17–43) | 27 (19–56) | 16 (9–27) | 7 (0–26) | 17* (10–31) | 18* (8–29) | 8* (4–16) | 9 (2-22) |
Comparison of pressure values obtained with circumferential averaging (top) and based on selecting the nadir radial pressure at each axial locus (bottom). Values are given as median (5th–95th percentile).
EGJ, esophagogastric junction.
P < 0.01 vs. circumferentially averaged pressure.
DISCUSSION
This investigation used concurrent fluoroscopy, an endoclip placed at the SCJ, and a novel 96-element 3D-HRM device to characterize the interaction between pressure and morphology during the final phase of bolus transit through the esophagus. The major findings were that: 1) esophageal contractility dominated by pre-CDP peristalsis terminated ∼4 cm proximal to the SCJ at the CDP, approximating the upper margin of the fluoroscopically defined phrenic ampulla, 2) the ampulla was comprised of the effaced, elevated, and elongated high-pressure zone, 3) emptying of the ampulla occurred by sustained contraction of the circular muscle of the elongated LES evident by the gradual reduction in the dimensions of the bolus compartment, 4) after completion of ampullary emptying, the sphincter reconstituted its resting morphology by continued contraction of its circular muscle in conjunction with longitudinal muscle shortening during SCJ descent, and 5) the measure of nadir radial pressure, rather than circumferentially averaged pressure within the EGJ, most accurately reconciled intraluminal flow permissive pressure gradients with fluoroscopically observed flow across the hiatus during the swallow period.
During pre-CDP peristalsis, there is a well-defined relationship between fluoroscopic imaging and manometric recording wherein site-specific luminal closure corresponds to the upstroke of the pressure wave proceeding distally at an average of 5.1 cm/s (10, 13, 16, 18). Although only the circular muscle contribution to peristalsis is detectable by fluoroscopy or manometry, the coordinated contraction of longitudinal muscle, bunching up the circular muscle layer and thereby increasing its capacity to impart force on the bolus, is demonstrable with studies using either fluoroscopy in conjunction with endoclips or intraluminal ultrasound (1, 11, 13–15, 17). Recent HRM studies done with concurrent fluoroscopy have demonstrated that this mechanism of bolus transport transitions in the distal esophagus at the CDP to a mechanism characterized by formation of the phrenic ampulla with a much slower progression rate. Findings from the current study suggest that the CDP, in fact, approximates the proximal margin of the LES segment.
Spatially, the CDP was located an average of 4 cm proximal to the SCJ. Temporally, the CDP occurred coincident with formation of the ampulla and defined its proximal margin. Hence, the maximal size of the ampulla, spanning from the CDP to the SCJ, was consistent with previous measurements (13). However, resting LES length, also determined using this 3D-HRM device, averaged only 2.4 cm (12). Consequently, if the ampulla is comprised of an opened sphincter segment, our observations imply that the sphincter is not only effaced radially but also elongated. This may be partially attributable to the recruitment of adjacent esophagus to act as a physiological sphincter and partially to actual elongation. Neuroanatomical observations provide evidence supporting this hypothesis. Nitrinergic inhibitory fibers, absent in the longitudinal muscle of the adjacent esophagus, occur at the level of the LES with density similar to that seen in the circular muscle throughout the distal esophagus and LES (2). This unique inhibitory innervation creates a localized LES “yield zone” wherein the longitudinal muscle of the LES relaxes and elongates during the peristaltic contraction of the more proximal longitudinal muscle. The mechanical effect of the yield zone is to minimize the orad displacement of the SCJ during peristalsis (3). Mathematical modeling suggests that, without the yield zone, the force displacing the cardia in the orad direction would be substantial (20). Assuming that the extent of yield amounts to ∼50% elongation, the size of the stretched and effaced LES closely corresponds to the observed 4-cm length of the ampulla at the time of the CDP. These observations also fit well with the recent demonstration that axial traction on the LES activates neural pathways that elicit LES relaxation, possibly mediated by mechanosensitive enteric neurons in the LES segment (8, 9). Concurrent with this axial traction, the distal esophagus is tethered to the CD by the phrenoesophageal ligament. Further studies into the micromechanics of these structures (and perturbations to them imparted by hiatus hernia) would likely be facilitated with the aid of high-frequency intraluminal ultrasound.
Despite the LES yield zone accommodating some proximal esophageal longitudinal muscle shortening, the SCJ was still elevated 1.5 cm throughout the course of ampullary emptying (Table 1). This suggests that ampullary emptying is associated with tonic contraction of both the longitudinal muscle in the esophageal body and circular muscle of the sphincter, progressively collapsing the bolus compartment in the process (Figs. 2 and 3). Once bolus clearance is completed, the LES reconfigures from its elongated and displaced configuration to its contracted and descended state. Evident in Fig. 4, this is accompanied by narrowing of the LES high-pressure zone on the EPT plot, presumably a result of the longitudinal muscle within the LES segment regaining its resting (contracted) length coincident with reelongation of the adjacent esophagus. At the completion of ampullary emptying, the high-pressure zone extended >3 cm proximal to the SCJ, whereas, with return to baseline 6 s later, its axial length was only ∼1.5 cm. This highlights the disconnect between the resting length of the sphincter and the high-pressure zone generated by the sphincter during emptying. Definitively proving this concept would require tracking an additional endoclip localized at the CDP. We hypothesize that a CDP clip would be localized above the upper LES margin at rest and track closely with the proximal margin of the post-CDP contraction during ampullary emptying.
A unique attribute of the 3D-HRM array utilized in this study was the capability of detecting not only the circumferential average pressure at each axial location but also the eight individual radially dispersed sector pressures. Among the radial sectors, the nadir sector(s) indicate readings obtained facing toward the open lumen or bolus compartment, whereas sectors indicating greater pressures are obtained from points of contact with the luminal wall or areas subject to extrinsic compression (12). Consequently, one would anticipate that nadir radial pressure values would provide a higher-fidelity recording of intraluminal pressure gradients and sphincter relaxation on account of their being less subject to radial pressure asymmetries imparted by extrinsic structures. The realization of this prediction was demonstrated by the comparison between spatial pressure variation plots and relaxation pressures derived from either nadir radial or circumferentially averaged pressures in Fig. 5 and Table 2. The circumferentially averaged data suggest a persistent high pressure at the hiatal center during a period that flow is known to be occurring; this is not seen using the nadir radial pressure data. Hence, the nadir radial pressure values reconcile more accurately flow-permissive gradients and sphincter relaxation measurements recorded manometrically with bolus flow observed fluoroscopically.
In conclusion, the phrenic ampulla is a transient structure that assists bolus clearance into the stomach once the peristaltic contraction reaches at the CDP. Somewhat paradoxically, the ampulla is a manifestation of both proximal longitudinal muscle contraction of the adjacent esophagus and longitudinal muscle elongation within the LES yield zone that occurs in conjunction with the LES circular muscle effacement. Consequently, the phrenic ampulla seen fluoroscopically is most likely comprised of the stretched, effaced, and axially displaced LES. The subsequent post-CDP contractility empties the bolus contained within the ampulla as the circular muscle of the LES contracts. The likely elongation of the LES during formation of the ampulla and narrowing to its native length after ampullary emptying suggest that reduction in the resting tone of the longitudinal muscle within the LES segment is a previously unrecognized component of LES relaxation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-56033 (P. J. Kahrilas) and R01 DK-079902 (J. E. Pandolfino).
DISCLOSURES
No relevant competing financial and other interests exist for Monika A. Kwiatek, Frédéric Nicodème, John E. Pandolfino, or Peter J. Kahrilas.
ACKNOWLEDGMENTS
M. A. Kwiatek contributed to the conception and study design, study supervision, data collection, analysis and interpretation, statistical analysis, manuscript drafting, editing, critical revision, and final approval. F. Nicodème contributed to data analysis and interpretation and manuscript final approval. J. E. Pandolfino contributed to the conception and study design, obtained funding, data interpretation, manuscript editing, critical revision, and final approval. P. J. Kahrilas contributed to the conception and study design, obtained funding, data interpretation, manuscript drafting, editing, critical revision, and final approval.
REFERENCES
- 1. Brasseur JG, Ulerich R, Dai Q, Patel DK, Soliman AM, Miller LS. Pharmacological dissection of the human gastro-oesophageal segment into three sphincteric components. J Physiol 580: 961–975, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christensen J, Fang S, Rick GA. NADPH-diaphorase-positive nerve fibers in smooth muscle layers of opossum esophagus: gradients in density. J Auton Nerv Syst 52: 99–105, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Christensen J, Miftakhov R. Hiatus hernia: a review of evidence for its origin in esophageal longitudinal muscle dysfunction. Am J Med 108, Suppl 4a: 3S–7S, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Clark MD, Rinaldo JA, Jr, Eyler WR. Correlation of manometric and radiologic data from the esophagogastric area. Radiology 94: 261–270, 1970 [DOI] [PubMed] [Google Scholar]
- 5. Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol Gastrointest Liver Physiol 261: G677–G684, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Clouse RE, Staiano A, Bickston SJ, Cohn SM. Characteristics of the propagating pressure wave in the esophagus. Dig Dis Sci 41: 2369–2376, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Dodds WJ, Stewart ET, Hodges D, Zboralske FF. Movement of the feline esophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest 52: 1–13, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dogan I, Bhargava V, Liu J, Mittal RK. Axial stretch: A novel mechanism of the lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 292: G329–G334, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Jiang Y, Bhargava V, Mittal RK. Mechanism of stretch-activated excitatory and inhibitory responses in the lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 297: G397–G405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology 94: 73–80, 1988 [DOI] [PubMed] [Google Scholar]
- 11. Kahrilas PJ, Wu S, Lin S, Pouderoux P. Attenuation of esophageal shortening during peristalsis with hiatus hernia. Gastroenterology 109: 1818–1825, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Kwiatek MA, Pandolfino JE, Kahrilas PJ. 3D-high resolution manometry of the esophagogastric junction. Neurogastroenterol Motil 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin S, Brasseur JG, Pouderoux P, Kahrilas PJ. The phrenic ampulla: distal esophagus or potential hiatal hernia? Am J Physiol Gastrointest Liver Physiol 268: G320–G327, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Mittal RK, Liu J, Puckett JL, Bhalla V, Bhargava V, Tipnis N, Kassab G. Sensory and motor function of the esophagus: lessons from ultrasound imaging. Gastroenterology 128: 487–497, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Nicosia MA, Brasseur JG, Liu JB, Miller LS. Local longitudinal muscle shortening of the human esophagus from high-frequency ultrasonography. Am J Physiol Gastrointest Liver Physiol 281: G1022–G1033, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Pandolfino JE, Leslie E, Luger D, Mitchell B, Kwiatek MA, Kahrilas PJ. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil 22: 395–400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pouderoux P, Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of esophageal shortening during peristalsis. Gastroenterology 112: 1147–1154, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Sugarbaker DJ, Rattan S, Goyal RK. Mechanical and electrical activity of esophageal smooth muscle during peristalsis. Am J Physiol Gastrointest Liver Physiol 246: G145–G150, 1984 [DOI] [PubMed] [Google Scholar]
- 19. Sugarbaker DJ, Rattan S, Goyal RK. Swallowing induces sequential activation of esophageal longitudinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 247: G515–G519, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Yassi R, Cheng LK, Rajagopal V, Nash MP, Windsor JA, Pullan AJ. Modeling of the mechanical function of the human gastroesophageal junction using an anatomically realistic three-dimensional model. J Biomech 42: 1604–1609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]