Abstract
Enterochromaffin (EC) cells of the diffuse neuroendocrine cell system secrete serotonin (5-HT) with activation of gut motility, secretion, and pain. These cells express adenosine (ADORA) receptors and are considered to function as mechanosensors. Physiological pathways mediating mechanosensitivity and adenosine responsiveness remain to be fully elucidated, as do their roles in inflammatory bowel disease (IBD) and neoplasia. Pure (98–99%) FACS-sorted normal and IBD human EC cells and neoplastic EC cells (KRJ-I) were studied. IBD-EC cells and KRJ-I overexpressed ADORA2B. NECA, a general ADORA receptor agonist, stimulated, whereas the A2B receptor antagonist MRS1754 inhibited, 5-HT release (EC50 = 1.8 × 10−6 M; IC50 = 3.7 × 10−8 M), which was associated with corresponding alterations in intracellular cAMP levels and pCREB (Ser133). Mechanical stimulation using a rhythmic flex model induced transcription and activation of Tph1 (tryptophan hydroxylase) and VMAT1 (vesicular monoamine transporter 1) and the release of 5-HT, which could be inhibited by MRS1754 and amplified by NECA. Secretion was also inhibited by H-89 (PKA inhibitor) while Tph1 and VMAT1 transcription was regulated by PKA/MAPK and PI3K-mediated signaling. Normal and IBD-EC cells also responded to NECA and mechanical stimulation with PKA activation, cAMP production, and 5-HT release, effects reversible by MRS1754. EC cells express stimulatory ADORA2B, and rhythmic stretch induces A2B activation, PKA/MAPK/IP3-dependent transcription, and PKA-dependent secretion of 5-HT synthesis and secretion. Receptor expression is amplified in IBD and neoplasia, and 5-HT release is increased. Determination of factors that regulate EC cell function are necessary for understanding its role as a mechanosensory cell and to facilitate the development of agents that can selectively target cell function in EC cell-associated disease.
Keywords: adenosine, Crohn's disease, enterochromaffin cell, serotonin
the enterochromaffin (EC) cell is the most numerous neuroendocrine cell in the epithelia lining the lumen of the gastrointestinal (GI) tract. These cells play a key role in the regulation of gut secretion, motility, pain, and nausea through paracrine regulation of neighboring mucosal cells and activation of intrinsic primary afferent neurons (IPANs) of the enteric nervous system (ENS) and the central nervous system (CNS) via vagal afferents (18). The monoamine neurotransmitter serotonin [5-hydroxytryptamine (5-HT)] has proven central in EC cell regulatory function, and these cells synthesize, store, and release the vast majority (95%) of the body's store of this amine (18). EC cells function as “taste buds of the gut” and represent sensory transducers responding to mechanical events, luminal acidification, or nutrients such as glucose and short-chain fatty acids, bile salt, tastants, and olfactants (6, 15, 22–24, 31, 42, 47). In addition, EC cell secretion can also be activated by more diffuse inputs including neural, bacterial, and immunological inputs (8, 19). Specifically, inflammation and the development of inflammatory bowel diseases (IBD) is associated with altered EC cell 5-HT release (19, 28).
The intestinal epithelium undergoes repetitive stretching and contraction during peristalsis and is also subjected to strain during villus shortening that accompanies mucosal repair (3). Since the EC cell contains sensory elements that are activated by stretch or mechanical forces, these effects should mediate a biological response (4). Deformation of the intestinal mucosa in vivo reflects a complex pattern of ring and sleeve contractions occurring at different frequencies and rhythms. Ring contractions have been observed to occur at an average frequency ranging from 7 contractions/min in the ileum to 12 contractions/min in the duodenum (39). Half a century ago, Bulbring in 1959 reported that pressure applied to the mucosal epithelium induces 5-HT release (6). More recently, this observation has been confirmed by demonstration that EC cells respond with 5-HT release upon mechanical stimulation (41) and that local reflex contractions of the gut circular muscle are strongly correlated with 5-HT release (4). This effect was caused primarily by the contraction of the smooth muscle and subsequent deformation of the mucosa, supporting the notion that the EC cell is a site of convergence for mechanical forces (e.g., deformation, shear, tension) that contribute to the release of 5-HT during motor reflexes (4). However, it is unclear what mechanisms regulate this biological response and whether this is increased in EC cell-associated diseases, e.g., Crohn's disease, colitis, or carcinoids.
Adenosine triphosphate (ATP) and its metabolic products, including adenosine diphosphate (ADP) and adenosine, are released in response to mechanical forces and play a prominent role in the mechanosensory signaling of intestinal cells (34). There exists four classes of purinoreceptors: P1, which includes the high-affinity adenosine receptors (ADORA) A1 and A2A and the low-affinity receptors A2B and A3; P2, which includes P2X/ligand-gated ion channels; P3; and P4 (34). Endogenous adenosine can activate inhibitory A3/A1 receptor-Gαq/PLC/IP3-Ca2+ and excitatory A2A/A2B receptor-AC/cAMP signaling pathways in pancreatic BON cells to regulate amine release (34). Adenosine itself is upregulated in inflammatory conditions (35), and ADORA receptors seem to be differently regulated in IBD mucosa (1, 35, 43). Targeting ADORAs is associated with modulating epithelial or immune function (14, 35). Little, however, is known about the effects of strain and adenosine on transcription and secretory pathways in normal and inflammation-targeted EC cells as well as in transformed EC cells (carcinoids).
EC cells comprise only ∼0.5–1% of the mucosal cells in the small bowel, and a technique to isolate EC cells to high purity has recently been described (29, 38). Additionally, only one well characterized neoplastic human cell line derived from an EC cell exists (38, 45). This cell line, KRJ-I, demonstrates similar signaling pathways, enzyme activity, and secretory products (e.g., 5-HT) as the normal EC cell (30, 38), and this, coupled with similar responsiveness to neural and luminal agents (31, 38), indicates this cell line represents a good model to study EC cell function in vitro.
To test the effects of mechanical forces in vitro, a technique has been developed to model the repetitive deformation of an intestinal epithelial monolayer. Cells are cultured on flexible-bottomed wells and are subjected to rhythmic deformation (10 cycles/min, with an average strain of 10%) that is considered to be within physiological ranges during gut peristalsis (3). Although rotational shaking at >100 cycles/min has been studied (34), the physiological relevance of this model is unclear.
In the present study, we hypothesized that strain (via adenosine) modulates the activation of EC cell ADORA receptors to mediate EC cell 5-HT synthesis, secretion, and transcription, principally through PKA/cAMP and MAPK signaling pathways. We first examined the ADORA receptor profile in normal EC cells, EC cells isolated from Crohn's disease, and the KRJ-I cell line and then delineated the signaling pathways associated with adenosine and mechanical (rhythmic flex) forces focusing on ADORA2B. Thereafter, we examined signaling and secretion in isolated normal and IBD-EC cells and confirmed adenosine/ADORA2B were involved in regulating secretory responses to mechanical stimulation.
MATERIALS AND METHODS
Compounds.
5′-(N-ethylcarboxamido) adenosine (NECA) (Sigma-Aldrich, St. Louis, MO) is a general adenosine receptor agonist, SCH442416 (Tocris Bioscience) is a selective A2A antagonist, and MRS1754 hydrate (Sigma) is a specific A2B receptor antagonist. The PKA inhibitor H-89, the cAMP inhibitor 2′,5′-dideoxyadenosine (2′,5′,-dd-Ado), the MEK1 inhibitor PD98059, and the PI3K inhibitor Wortmannin (all Sigma) were used to study activation of intracellular pathways. Compounds were dissolved in DMSO, and dilutions were made in Ham's F-12 medium (GIBCO).
Tissue samples.
Tissue was collected from 15 patients (eight men, seven women; median age [range] = 51 yr [29–67 yr]). Crohn's tissue (n = 8) was obtained from patients who had undergone hemi- or colectomies for Crohn's ileitis (n = 2) or colitis (n = 6). Only grossly affected tissue was studied. Macroscopically “normal” tissue was obtained from patients undergoing surgery for diverticulitis (n = 3) or hemicolectomies for colon cancer (colon, n = 4). All tissue was collected between 2008 and 2010 at the Yale University Department of Surgery. The institutional review board has designated this as nonhuman subjects research.
Normal and IBD-EC cell isolation.
Crohn's disease (n = 8) and normal colon (n = 7) mucosa were used for the isolation and study of short-term cultured EC cells (28, 31). EC cells were isolated from the colon following mucosal stripping and enzymatic digestion using a combination of Nycodenz gradient fractionation and acridine orange uptake, and cell FACS were sorted as described (38). In previous studies, we obtained preparations of >98% pure EC cells (28, 31, 38). Approximately 1 × 106 cells were obtained per mucosal sample, a quantity sufficient for real-time PCR, short-term culture and mechanical strain and secretion studies (28, 31, 38).
KRJ-I cell culture.
KRJ-I cells were cultured in Ham's F-12 media supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 mg/ml) (Sigma) at 37°c with 5% CO2. Cells were cultured for 24 h before any experiments. Basal 5-HT concentrations in the cell medium of untreated cells were 3–4 ng/ml. In initial experiments, real-time PCR was undertaken to confirm ADORA expression. Cells were then incubated with NECA, SCH442416, or MRS1754 (10−11-10−4 M) for 60 min to determine effects on basal-line (nonmechanically stimulated) 5-HT release and cAMP activation.
Flexercell strain experiments.
Normal and IBD-EC cells or KRI-I cells were plated on collagen-coated, flexible-bottomed wells (Flex I plates; Flexercell International, Hillsborough, NC). Normal and IBD-EC cells were cultured for 8 h before experiments, whereas KRJ-1cells were precultured for 24 h. Cells were subjected to cycles of stretch and relaxation by a computer-driven, vacuum-operated Flexercell strain unit FX-4000 (Flexercell International). Rhythmic deformation was applied at a frequency of 10 cycles/min, with 10% average radial elongation of cells, for 0–6 h as previously described (3). In some experiments, KRJ-1 cells were exposed to rhythmic deformation with or without preincubation with ADORA receptor modulating compounds (NECA, 1 × 10−5 M; SCH442416, 1 × 10−6 M; MRS1754, 1 × 10−7 M; controls, Ham's F12 medium alone).
PKA/cAMP signaling pathway analysis.
Cultured cells were stimulated with NECA (1 × 10−5 M) or mechanically stressed (Flexercell) for 1 h. In some studies, ADORA antagonists were used. PKA activity and cAMP were quantified using SuperArray ELISA kits (SA Biosciences) as previously described (27).
Real-time PCR.
RNA from either nonstressed or mechanically stressed (Flexercell) cells was extracted using TRIZOL (Invitrogen), cleaned (Qiagen, RNeasy kit), and converted to cDNA using the high-capacity cDNA archive kit (Applied Biosystems) (26, 27). Real-time PCR was performed using the ABI 7900 sequence detection system. Real-time PCR analyses were performed in triplicate using assays-on-demand primers as described (29). Amplification of each gene (ADORA1, 2A, 2B, 3, Tph1, VMAT1) was normalized to the three reference genes (ALG9, TFCP2, and ZNF410) using a geNorm protocol as described (33).
5-HT ELISA assays.
5-HT release was measured using a commercially available 5-HT ELISA assay (BA 10–0900; Rocky Mountain Diagnostics, Colorado Springs, CO). The range for this assay is 15–2,500 pg/ml and has previously been used successfully by our group to measure 5-HT release in EC cell culture (29, 38).
Protein extraction and Western blot analysis.
Whole cell lysates were prepared using ice-cold lysis buffer [×10 RIPA lysis buffer (Millipore) complete protease inhibitor (Roche), phosphatase inhibitor set 1 and 2 (Calbiochem), 100 mM PMSF (Roche), 200 mM Na3VO4 (Acros Organics), 12.5 g/ml SDS (American Bioanalytical)], and the protein amount of the supernatant was quantified using the BCA protein assay kit (Thermo Fisher Scientific). After denaturation (SDS sample buffer), total protein lysates (20 μg) were separated on an SDS-PAGE gel (10%), transferred to PVDF membranes (Bio-Rad, Hercules, CA; pore size 0.45 mm) and incubated with primary antibodies (Cell Signaling Technology, Danvers, MA) overnight at 4°C. A horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology) followed by immunodetection using the Supersignal West Pico Luminol/Enhancer solution (Thermo Scientific) was undertaken (32). In these studies, pCREB(Ser133) (9191; Cell Signaling), TPH1 (110-57629; BD Biosciences), pTPH-1 (30574; Abcam), and VMAT1 (58170; Abcam) were measured. The optical density of the appropriately sized bands was quantified using ImageJ software (NIH), and protein expression was normalized to β-actin (Sigma-Aldrich).
Statistics.
Results (n = 4–6/experiment) were expressed as means ± SE. All statistical analyses were performed using Prism 4 (GraphPad Software, San Diego, CA). When indicated, dose-response curves were calculated, and the EC50 or IC50 was determined. Results were compared between control and stimulated cells using the Mann-Whitney test. Differences between mRNA transcript and protein levels were analyzed using the two-way nonparametric test (Kruskal-Wallis). A P value of <0.05 was considered significant.
RESULTS
ADORA profile.
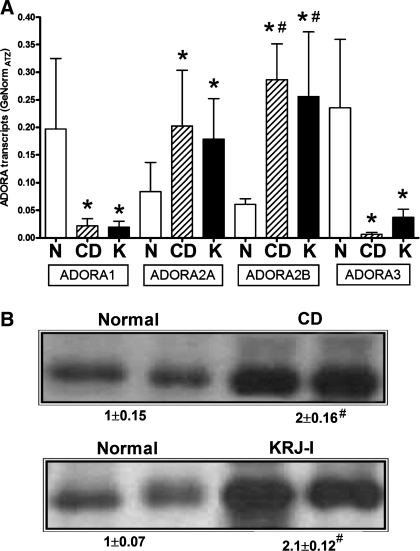
Real-time PCR identified that the normal EC cell expressed transcripts for both the inhibitory ADORAs (ADORA1/3) as well as the excitatory ADORA2A/2B (Fig. 1A). IBD-EC cells as well as KRJ-I cells also expressed this receptor profile; however, expression of the 2A and 2B receptors was significantly elevated (P < 0.05) in these samples compared with ADORA1/3, suggesting a selective overexpression of stimulatory receptors in these conditions. ADORA2B transcripts were also identified to be significantly elevated in IBD-EC and KRJ-I cells compared with normal EC cells. Analysis of protein expression confirmed overexpression (approximately twofold) of ADORA2B in IBD-EC cells and KRJ-I (Fig. 1B). ADORA2A was not overexpressed at a protein level (data not shown). These results suggest EC cells may respond to adenosine-mediated ADORA activation, and the response may be amplified in disease conditions.
Fig. 1.
Transcripts (A) and protein expression (B) for adenosine receptors (ADORA) in EC cells from different diseases. Normal EC cells (N) were characterized by similar expression of all four ADORA subtypes. EC cells from Crohn's Disease (CD) and KRJ-I cells (K) also expressed all four subtypes, but expression of both the stimulatory type 2 receptors was elevated compared with the inhibitory ADORA1/3. Western blot confirmation of ADORA2B expression in IBD-EC cells and KRJ-I cells demonstrated greater than twofold elevation compared with normal EC cells. Values are means ± SE; n = 4. *Significant difference vs. ADORA 1/3 (P < 0.05). #Significant difference vs. normal (P < 0.05).
Effect of adenosine and ADORA2B antagonists on EC cell cAMP signaling and 5-HT secretion under nonstrained conditions.
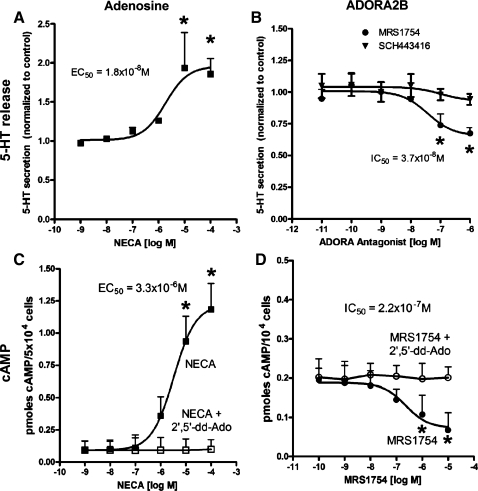
A concentration-dependent stimulatory effect of NECA (EC50 = 1.8 × 10−6 M) was noted for 5-HT secretion in nonstrained EC cells (Fig. 2A). In contrast, MRS1754 inhibited basal 5-HT secretion in a concentration-dependent manner (IC50 = 3.7 × 10−8 M) (Fig. 2C), comparable to results previously obtained in HEK-293 cells stably transfected with plasmids encoding the human A2B receptor (21). No effects were noted when SCH442416 was used. The secretory effects were associated with concentration-dependent alterations in cAMP (NECA: EC50 = 3.3 × 10−6 M; MRS: IC50 = 2.2 × 10−7 M) (Fig. 2, B and D), suggesting that 5-HT secretion is principally mediated via ADORA2B activation and cAMP signaling in this cell line.
Fig. 2.
Effect of adenosine receptor modulating compounds on 5-HT release and cAMP production in nonstrained KRJ-I cells. The general ADORA receptor agonist NECA stimulated 5-HT secretion with a maximal 1.9-fold increase at 10−5 M (A), and dose-dependently increased intracellular cAMP (approximately sixfold, 10−5 M), which was reversed by preincubation with 2′,5′-dideoxyadenosine (2′,5′-dd-Ado 10−5 M; C). The A2B receptor antagonist MRS1754, in contrast, inhibited 5-HT secretion with a maximal 33% decrease at 1 × 10−6 M (B) and inhibited cAMP production (maximal effect at 10−6 M) (D). The A2A antagonist, SCH442416, had no effect. Values are means ± SE; n = 4. *Significant difference vs. control (P < 0.05).
Flexercel strain (mechanically stressed) model and ADORA2B.
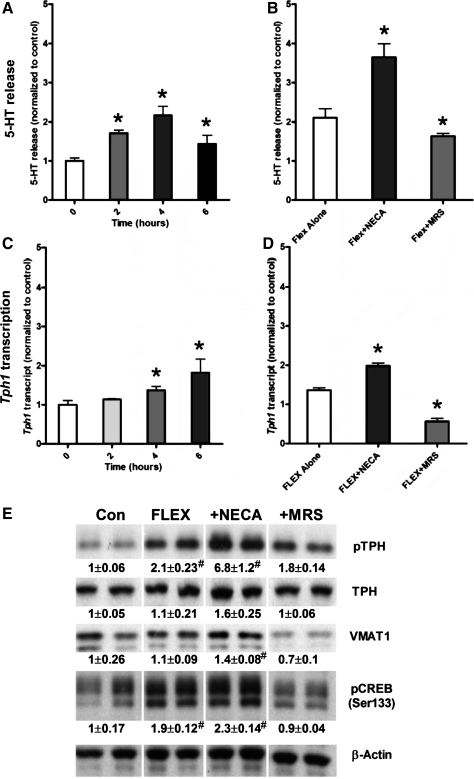
Short-term cultured KRJ-I cells were subjected to rhythmic deformation at 10 cycles/min with an average strain of 10%, and secretion of 5-HT, as well as transcription of the rate-limiting step in synthesis of this amine-tryptophan hydroxylase 1 (Tph1), were measured after 2, 4, and 6 h. An increase in 5-HT (>1.5-fold vs. controls; P < 0.05) was observed after 2 h of mechanical stress (Fig. 3A); the greatest increase was observed after 4 h of strain, and amine release corresponded to increased transcription of Tph1 (Fig. 3C). In parallel studies, cells were pre-incubated with either NECA (10−5 M) or MRS1754 (10−7 M) for 15 min and then flexed for 4 h. This time point was sampled for both secretion and transcription because peak secretion of secretory products as well as elevated transcription was observed after 4 h of flex. NECA stimulated (P < 0.05) secretion of 5-HT compared with release from flex-strained control cells, whereas MRS1754 inhibited (P < 0.05) the release of these products (Fig. 3B). Both MRS1754 and NECA had analogous effects on the transcription of Tph1 (Fig. 3D). We also examined activation of TPH1 and VMAT1 by Western blot. TPH1 is activated following phosphorylation by MAPK (31, 38), whereas VMAT1 regulates 5-HT uptake into secretory vesicles (13), both critical features of 5-HT synthesis and secretion. One-hour flex was associated with increased pTPH1, approximately twofold compared with nonstressed cells (Fig. 3E). Addition of NECA significantly increased TPH1 phosphorylation (approximately sevenfold), whereas MRS1754 reduced levels similar to control (1.8-fold). Examination of the ratio of pTHP1:TPH1 identified this also to be increased in the model, identifying enzyme activation as a function of flex. VMAT1 was altered by a combination of flex and NECA (1.4-fold), and increased levels were reduced by MRS1754 to values less than control (0.7-fold). Examination of CREB activation, a transcription factor that regulates Tph1 expression (16, 48) and a known target of PKA/cAMP signaling (11, 20) (Fig. 3E) was then undertaken to evaluate whether this was one of the factors involved in mechanical stress-mediated signal transduction. Like TPH1 activation, 1-h flex was associated with increased pCREB approximately twofold compared with nonstressed cells. Addition of NECA did not significantly increase CREB phosphorylation, but MRS1754 reduced levels to control (0.9-fold). These results indicate that the flex-induced transcriptional alterations and 5-HT synthesis/secretion occurred via PKA/pCREB and ADORA2B-dependent mechanisms.
Fig. 3.
Effect of Flexercell strain on 5-HT secretion, Tph1 transcription, TPH1 activation, and VMAT1 and CREB (Ser133) phosphorylation. Rhythmic flex affected both the secretion of 5-HT (maximum 2.2-fold at 4 h) and transcription of Tph1 (maximum 1.8-fold at 6 h) in a time-dependent manner (A and C). In cells preincubated with NECA (10−5 M) for 15 min before stretch for 4 h, 5-HT secretion and Tph1 transcription were amplified, whereas 15 min preincubation with MRS1754 (10−7 M) inhibited flex-induced of 5-HT secretion and Tph1 transcription (B and D). Flex stimulated pTPH1 (approximately twofold) and pCREB (approximately twofold), effects amplified by NECA and inhibited by MRS1754. VMAT1 was increased by a combination of flex stimulation and NECA (1.6-fold), which was completely reversed by MRS1754 (E). All data are normalized to nonflexed controls. Values are means ± SE; n = 4–6. *Significant difference vs. normal subjects (#P < 0.05).
Signaling pathways in the flexercel strain (mechanically stressed) model.
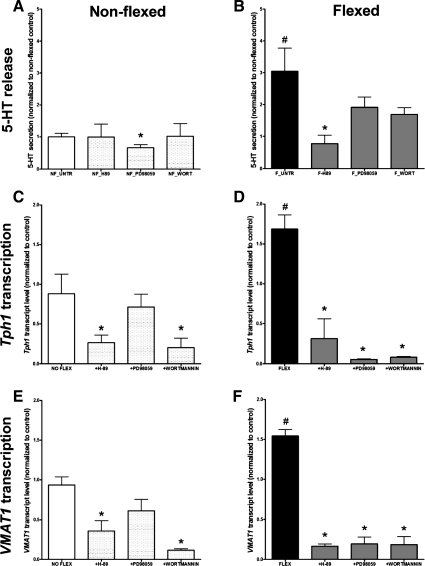
Having determined that ADORA2B receptor activation stimulated 5-HT secretion, Tph1 transcription, and activation of TPH1, VMAT1, and CREB, we next evaluated whether other canonical signaling pathways were involved in these phenomena. The effects of PKA, MEK1, and PI3K inhibition were therefore examined in flexed and nonflexed (control) cells. In nonflexed cells, PD98059 (MEK1 inhibitor) inhibited 5-HT secretion, compared with untreated cells, whereas H-89 and wortmannin inhibited Tph1 and VMAT1 transcription (Fig. 4, A and C), suggesting that MAPK regulates normal secretion and PKA and PKB, Tph1, and VMAT1 transcription. In flexed cells, in contrast, H-89 inhibited 5-HT secretion, whereas all three inhibitors (H-89, PD98059, and wortmannin) significantly decreased both Tph1 and VMAT1 transcription (Fig. 4, C and D). We interpret these results to reflect that rythmic stress activates 5-HT release principally through PKA (and cAMP signaling pathways), whereas Tph1 and VMAT1 transcription is regulated through all three pathways (PKA, PKB, and MAPK). Evidence of pCREB (Figs. 2 and 3) support a role for PKA/cAMP signaling in this process.
Fig. 4.
The effect of signal pathway inhibitors on 5-HT release and Tph1 and VMAT1 transcription in nonflexed and rhythmically flexed KRJ-I cells. In nonflexed cells, 5-HT secretion was regulated by MAPK (A), whereas both Tph1 and VMAT1 transcription were regulated through the PKA and PI3K (PKB) pathways (C). In flexed cells, 5-HT secretion is regulated predominantly through the PKA pathway (B), whereas Tph1 and VMAT1 transcription are regulated through PKA, PI3K, and MAPK signaling (D). Values are means ± SE; n = 4. *Significant difference vs. untreated cells (P < 0.01). #Significant difference vs. nonflexed cells (P < 0.05).
5-HT secretion and ADORA2B signaling in normal and IBD-EC cells.
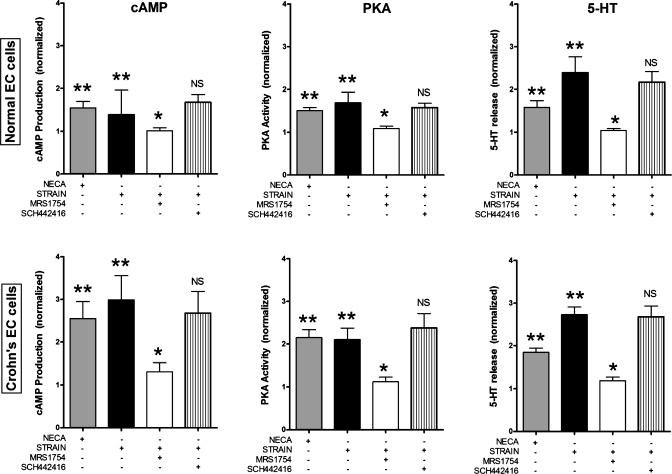
After establishing a flex-based mechanical stress model in KRJ-I and having identified the principal roles for adenosine via ADORA2B in the neoplastic cell, we next examined the effects of adenosine and strain on PKA/cAMP signaling and 5-HT secretion in short-term cultured normal and Crohn's EC cells. Our results (Fig. 5) demonstrate that activation of ADORA2B for 120 min by mechanical strain significantly stimulated both cAMP and PKA levels in Crohn's EC cells and normal EC cells. These effects were specific, could be reversed by MRS1754 but not by SCH442416 (an ADORA2A receptor antagonist), and were elevated in the IBD-EC cell studies. These effects were modeled using NECA (1 × 10−5 M), demonstrating that the strain-mediated effects occurred via an adenosine-mediated response. Alterations in PKA and cAMP were associated with 5-HT release in isolated normal EC cells, 5-HT was secreted 1.5- to 2.3-fold more (P < 0.04) with NECA and flex, respectively, vs. nonstrained cells. This could be reversed by MRS1754 but not by SCH442416, confirming this as a 2B response. Crohn's EC cells responded in an analogous fashion, both to flex and NECA, as well as to the ADORA2 antagonists.
Fig. 5.
Effect of adenosine agents and Flexercell strain on normal and IBD-EC cell cAMP and PKA activity. Cyclic AMP production (top) and PKA activity (bottom) in normal EC (left) and isolated Crohn's EC cells (right). Crohn's EC cells had elevated (approximately twofold) responses to NECA or strain compared with normal EC cells. All effects were reversible by the selective ADORA2B antagonist (MRS1754: 1 μM) but not the ADORA2a antagonist (SCH442416: 1 μM). Values are means ± SE; n = 7. *Significant difference vs. strain (P < 0.05). **Significant difference vs. no strain/control (P < 0.05).
DISCUSSION
It has long been suggested that EC cell secretion is activated by mechanical stimulation (6). The lack of pure EC cell preparations and appropriate EC cell lines, however, has hindered specific mechanistic studies of receptor activation, intracellular pathways, transcription, and secretion from these cells. In the present study, using normal human EC cells, cells isolated from Crohn's disease and the KRJ-I cell line, which we have previously demonstrated to represent an appropriate model to study EC cell function (22, 31, 45), we demonstrated that rhythmic Flexercell-mediated deformation resulted in physiological 5-HT synthesis and release via adenosine activation of ADORA2B receptors and PKA/cAMP/pCREB signaling.
Adenosine receptors are expressed in normal (7) and inflamed mucosa (2, 14, 43) and are present in EC cell-derived neuroendocrine tumors (22, 25). We have recently identified that KRJ-I cells express all four ADORAs (by RT-PCR and sequencing); however, the predominant receptors on KRJ-I (identified using selective agonists and antagonists) are stimulatory ADORA2 (22, 25). In the present study, we identified that isolated IBD-EC cells, like normal EC cell and KRJ-I cells, also exhibit transcripts for inhibitory and stimulatory ADORA receptors and confirm that ADORA2B is elevated in expression and is coupled to PKA/cAMP signaling. Functionally, activation of ADORA with NECA resulted in cAMP production (EC50 ∼0.5 × 10−5 M), which could be inhibited by MRS1754 (IC50 ∼1 × 10−7 M). This is similar to an earlier study in a Chinese hamster ovary cell line stably transfected with human adenosine A2B receptor cDNA that exhibited an EC50 value of between 1 and 3.1 μM for the production of cAMP (9). Although it is acknowledged that pharmacologically based observations are limited by the efficacy of the agents studied, our results with agents that have efficacies at pathophysiologically relevant doses (1–10 nM) (44) indicate that stimulatory ADORA2B receptors may play a role in regulating adenosine-mediated signals in EC cells.
In preliminary studies (25), our laboratory examined the utility of rotational strain as a model for mechanical stimulation. Some groups have studied rotational strain and concluded that mechanically induced release, using rotational forces of >250 rpm, of endogenous adenosine mediated 5-HT secretion from BON cells (7, 34). The effect appeared biphasic; low concentrations of adenosine are considered to modulate basal 5-HT release via inhibitory A1/A3 receptors coupled to Gαq/PLC/IP3-Ca2+, whereas stimulation with higher concentrations of adenosine activates 5-HT secretion by binding to A2A/A2B receptor-AC/cAMP-coupled pathways (7, 34). In our hands, a rotational model (using 130 rpm) markedly decreased cell viability, induced a burst of 5-HT release that was not sustained beyond 1 h of rotation, was unaltered by ADORA agonists and antagonists, and most likely was due to a direct leakage through damaged membranes or through lysis of nonviable cells. In contrast, we used a repetitive deformation model (10 cycles/min, with an average strain of 10%) that is considered to be within physiological ranges during gut peristalsis (3). This method has also been used with success to measure 5-HT secretion from pulmonary neuroendocrine cells (PNEC) (30) and the neuroendocrine tumor lung cell line NCI-H727 (40). Using this model, we identified that rhythmic flex strain did not significantly alter LDH release but was associated with time-dependent increases in 5-HT release as well as transcription of Tph1. In general, the peak increase in transcription was observed 1–2 h after peak secretion of the corresponding compound. Flex was associated with CREB phosphorylation, a key transducer of PKA/cAMP signaling and a known regulator of transcription of both Tph1 (16, 48) and VMAT1 (10).
There are a variety of levels of evidence that support a central role for ADORA2B receptor activation in normal and disease-associated EC cell 5-HT synthesis and secretion. The stimulatory effect of the general ADORA agonist, NECA, and inhibitory effect by the specific ADORA2B antagonist MRS1754 on secretion in Crohn's EC cells and KRJ-I indicate that the mechanical activation of 5-HT release is directly mediated by surface-expressed ADORA2B receptors. It should be noted that the inability of MRS1754 to completely inhibit the flex-induced secretion of 5-HT indicates that A2B receptor activation may not be the sole pathway involved. Tph1 and VMAT1 transcription were, however, completely inhibited by MRS1754, suggesting that regulation of the EC cell secretory transcriptome was regulated preferentially through ADORA2B/pCREB signaling. We postulate, given the transcriptome, sequencing, and ADORA agonist/antagonist data (22, 30), that other purinergic regulatory pathways, e.g., via activation of excitatory P2Y1 receptors, of a Gαs-coupled receptor other than ADORA or of a non-G-protein-coupled purinergic receptor (34) may provide additional potential regulatory mechanisms.
An examination of the signaling pathways linking mechanically mediated ADORA2 activation to cell function identified that only the PKA inhibitor affected 5-HT secretion, whereas PKA, MAPK, and PI3K inhibitors all reduced transcription of Tph1 and VMAT1. In an attempt to limit any off-target mechanistic inhibition, e.g., wortmannin at high concentrations (500 nM) can also inhibit MAPK (12), we used appropriate doses (1 nM) in our experiments to pharmacologically define signaling responses. In the past, we have identified that the cAMP antagonist, 2′5′-dideoxyadenosine, inhibited GPCR-stimulated 5-HT secretion, suggesting that 5-HT secretion is a cAMP/PKA-mediated process (27). We postulate that mechanically stimulated EC cell secretion is principally mediated by cAMP signaling pathways and that this may be amplified by inflammation or disease. In contrast, Tph1 transcript, which exhibits cAMP-responsive elements in its promoter sequence (5), appears also to respond to PKB and MAPK activation. The latter is consistent with the role of the ERK pathway in activating both Tph1 synthesis (27) as well as phosphorylation (and thereby activation) of the enzyme (31, 38). VMAT1 transcription is similarly affected by pathway perturbations, whereas expression of the functional protein itself is decreased by MRS1754. In addition, due to the significant amount of cross talk between the cAMP/cAMP-dependent protein kinase (PKA) and MAPK pathways (17), it is possible that increased adenylyl cyclase activity also stimulates activation of the MAPK pathway through increased phosphorylation of ERK1/2 and thereby increases 5-HT synthesis and secretion, with the caveat that interpretation of pharmacologically based experiments may be complicated by the intensive cross talk and redundancy of signals in any transduction network (46).
In conclusion, rhythmic mechanical strain that mimics normal bowel movements induces EC cell secretion and transcription of EC cell secretory products, responses that are accentuated by inflammation or neoplasia. Under normal conditions, EC cells appear to function as sensors of mechanical stimulation. Stimulation of A2B receptors activates the PKA, PI3K, and MAPK signaling pathways, leading to Tph1 transcription, whereas the PKA/cAMP pathway subsequently regulates 5-HT secretion. These effects are amplified in EC cells isolated from inflamed mucosa. Delineating the signaling pathways by which mechanical forces and adenosine mediate EC cell function may facilitate the understanding of both normal and abnormal EC cell function and thus reveal new therapeutic targets for neuroendocrine cells in intestinal diseases. The increased expression of ADORA2B, in particular, indicates this may be a potential target, particularly as blockade (36) or gene knockout (37) is associated with attenuated colitis in murine models.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-080871 and Kontaktutvalget at St Olavs University Hospital and Faculty of Medicine, NTNU, Trondheim, Norway.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.C., B.S., B.I.G., A.T., and M.K. performed experiments; A.C., B.S., B.I.G., A.G., A.K.S., A.T., I.M.M., and M.K. analyzed data; A.C., B.I.G., A.G., A.K.S., A.T., B.E.S., R.P., I.M.M., and M.K. interpreted results of experiments; A.C., B.S., A.T., and M.K. prepared figures; A.C., B.S., A.G., A.K.S., A.T., B.E.S., R.P., and M.K. drafted the manuscript; A.C., B.S., B.I.G., A.G., A.K.S., A.T., B.E.S., R.P., I.M.M., and M.K. approved the final version of the manuscript; B.S., B.I.G., A.G., A.K.S., B.E.S., R.P., I.M.M., and M.K. conception and design of research; B.I.G., A.G., A.K.S., B.E.S., R.P., and M.K. edited and revised the manuscript.
REFERENCES
- 1. Antonioli L, Fornai M, Colucci R, Awwad O, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Differential recruitment of high affinity A1 and A2A adenosine receptors in the control of colonic neuromuscular function in experimental colitis. Eur J Pharmacol 650: 639–649, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Pharmacological modulation of adenosine system: novel options for treatment of inflammatory bowel diseases. Inflamm Bowel Dis 14: 566–574, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Basson MD, Li GD, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol 168: 476–488, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Bertrand PP. Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol 577: 689–704, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boularand S, Darmon MC, Ravassard P, Mallet J. Characterization of the human tryptophan hydroxylase gene promoter. Transcriptional regulation by cAMP requires a new motif distinct from the cAMP-responsive element. J Biol Chem 270: 3757–3764, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Bulbring E, Lin R. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis: the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol 140: 381, 1958 [PMC free article] [PubMed] [Google Scholar]
- 7. Christofi FL, Kim M, Wunderlich JE, Xue J, Suntres Z, Cardounel A, Javed NH, Yu JG, Grants I, Cooke HJ. Endogenous adenosine differentially modulates 5-hydroxytryptamine release from a human enterochromaffin cell model. Gastroenterology 127: 188–202, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann NY Acad Sci 915: 77–80, 2000 [DOI] [PubMed] [Google Scholar]
- 9. de Zwart M, Link R, von Frijtag Drabbe Kunzel JK, Cristalli G, Jacobson KA, Townsend-Nicholson A, IJzerman AP. A functional screening of adenosine analogues at the adenosine A2B receptor: a search for potent agonists. Nucleosides Nucleotides 17: 969–985, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desnos C, Laran MP, Scherman D. Regulation of the chromaffin granule catecholamine transporter in cultured bovine adrenal medullary cells: stimulus-biosynthesis coupling. J Neurochem 59: 2105–2112, 1992 [DOI] [PubMed] [Google Scholar]
- 11. DiRocco DP, Scheiner ZS, Sindreu CB, Chan GC, Storm DR. A role for calmodulin-stimulated adenylyl cyclases in cocaine sensitization. J Neurosci 29: 2393–2403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ecay TW, Dickson JL, Conner TD. Wortmannin inhibition of forskolin-stimulated chloride secretion by T84 cells. Biochim Biophys Acta 1467: 54–64, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflügers Arch 447: 636–640, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol 182: 4957–4964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubank S, Harris M, Pappas T, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 284: R1269–R1276, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Garcia-Osta A, Del Rio J, Frechilla D. Increased CRE-binding activity and tryptophan hydroxylase mRNA expression induced by 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) in the rat frontal cortex but not in the hippocampus. Brain Res Mol Brain Res 126: 181–187, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: comradeship and hostility. Cell Signal 20: 1592–1607, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Cote F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137: 1649–1660, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci 16: 3035–3044, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji X, Kim YC, Ahern DG, Linden J, Jacobson KA. [3H]MRS 1754, a selective antagonist radioligand for A(2B) adenosine receptors. Biochem Pharmacol 61: 657–663, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalhan A, Vazquez M, Jasani B, Stott J, Neal J, Gharibi B, Kidd M, Modlin I, Pfragner R, Rees D, Ham J. Adenosine receptor signal pathways in neuroendocrine tumours. In: UK and Ireland Neuroendocrine Tumor Society. London: Bioscientifica, 2008 [Google Scholar]
- 23. Kellum J, Albuerqueque F, Stoner M, Harris R. Stroking human jejunal mucosa induces 5-HT release and Cl− secretion via afferent neurons and 5-HT4 receptors. Am J Physiol Gastrointest Liver Physiol 277: G515–G520, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Kellum J, Jaffe B. Validation and application of a radioimmunoassay for serotonin. Gastroenterolology 70: 516, 1976 [PubMed] [Google Scholar]
- 25. Kidd M, Chin A, Gustafsson B, Siddique ZL, Drozdov I, Sumpio B, Pfragner R, Modlin I. The role of gastrointestinal mechanical forces in the regulation of gut enterochromaffin cell secretion and the implications for irritable bowel disease. Gastroenterology 136: A–4, 2009 [Google Scholar]
- 26. Kidd M, Eick G, Shapiro MD, Camp RL, Mane SM, Modlin IM. Microsatellite instability and gene mutations in transforming growth factor-beta type II receptor are absent in small bowel carcinoid tumors. Cancer 103: 229–236, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kidd M, Eick GN, Modlin IM, Pfragner R, Champaneria MC, Murren J. Further delineation of the continuous human neoplastic enterochromaffin cell line, KRJ-I, and the inhibitory effects of lanreotide and rapamycin. J Mol Endocrinol 38: 181–192, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn's disease. Neurogastroenterol Motil 21: 439–450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kidd M, Modlin I, Eick G, Champaneria M. Isolation, functional characterization, and transcriptome of the Mastomys ileal enterchromaffin cells. Am J Physiol Gastrointest Liver Physiol 291: G778–G791, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kidd M, Modlin I, Gustafsson B, Drozdov I, Hauso O, Pfragner R. Regulation of small intestinal EC cell serotonin release: the role of taste and mechanical force. Gastroenterology 144: A155, 2008 [Google Scholar]
- 31. Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol 295: G260–G272, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Kidd M, Modlin IM, Pfragner R, Eick GN, Champaneria MC, Chan AK, Camp RL, Mane SM. Small bowel carcinoid (enterochromaffin cell) neoplasia exhibits transforming growth factor-beta1-mediated regulatory abnormalities including up-regulation of C-Myc and MTA1. Cancer 109: 2420–2431, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Kidd M, Nadler B, Mane S, Eick G, Malfertheiner M, Champaneria M, Pfragner R, Modlin I. GeneChip, geNorm, and gastrointestinal tumors: novel reference genes for real-time PCR. Physiol Genomics 30: 363–370, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Kim M, Cooke H, Javed N, Carey H, Christofi F, Raybould H. d-Glucose releases 5-hydroxytryptamine from Human BON cells as a model of enterochromaffin cells. Gastroenterology 121: 1400–1406, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci 62: 2647–2657, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kolachala V, Ruble B, Vijay-Kumar M, Wang L, Mwangi S, Figler H, Figler R, Srinivasan S, Gewirtz A, Linden J, Merlin D, Sitaraman S. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol 155: 127–137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology 135: 861–870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Modlin IM, Kidd M, Pfragner R, Eick GN, Champaneria MC. The functional characterization of normal and neoplastic human enterochromaffin cells. J Clin Endocrinol Metab 91: 2340–2348, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Otterson MF, Sarr MG. Normal physiology of small intestinal motility. Surg Clin North Am 73: 1173–1192, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Pan J, Copland I, Post M, Yeger H, Cutz E. Mechanical stretch-induced serotonin release from pulmonary neuroendocrine cells: implications for lung development. Am J Physiol Lung Cell Mol Physiol 290: L185–L193, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Patel BA, Bian X, Quaiserova-Mocko V, Galligan JJ, Swain GM. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst 132: 41–47, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Raybould H, Glatzle J, Robin C, Meyer J, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol 284: G367–G372, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, Huang K, Christofi FL. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis 15: 971–984, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schafer DE, Lust WD, Sircar B, Goldberg ND. Elevated concentration of adenosine 3′:5′-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci USA 67: 851–856, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Siddique ZL, Drozdov I, Floch J, Gustafsson BI, Stunes K, Pfragner R, Kidd M, Modlin IM. KRJ-I and BON cell lines: defining an appropriate enterochromaffin cell neuroendocrine tumor model. Neuroendocrinology 89: 458–470, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Tortora G, Bianco R, Daniele G. Strategies for multiple signalling inhibition. J Chemother 16: 41–43, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Zhu J, Wu X, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol 530: 431–442, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48. Zubenko GS, Jones ML, Estevez AO, Hughes HB, 3rd, Estevez M. Identification of a CREB-dependent serotonergic pathway and neuronal circuit regulating foraging behavior in Caenorhabditis elegans: a useful model for mental disorders and their treatments? Am J Med Genet B Neuropsychiatr Genet 150B: 12–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]