Abstract
Background
Exenatide twice daily is a first-in-class glucagon-like peptide receptor agonist approved for the treatment of type 2 diabetes. The objective of this analysis was to evaluate the safety profile of exenatide twice daily and to compare its profile with that of a pooled comparator (placebo and insulin) in patients with type 2 diabetes.
Methods
Data from 19 completed, randomized, controlled clinical trials of exenatide twice daily (5 μg and 10 μg) were pooled and analyzed; the pooled data included 5594 intent-to-treat patients who were followed for 12–52 weeks. Incidence rates, exposure-adjusted incidence rates, and 95% confidence intervals around risk differences between groups were calculated.
Results
Baseline demographics and exposure time were comparable between groups (exenatide, N = 3261; pooled comparator, N = 2333; mean exposure time 166–171 days). Transient, mild- to-moderate nausea was the most frequent adverse event with exenatide (36.9% versus 8.3% in the pooled comparator). The incidence of hypoglycemia (minor or major) with concomitant sulfonylurea (exenatide 26.5%, pooled comparator 20.7%) was higher than that without sulfonylurea (exenatide 3.1%, pooled comparator 2.7%) in all groups. Serious adverse events, discontinuations due to serious adverse events, and deaths were reported with similar frequency in the exenatide and pooled comparator groups. Composite exposure-adjusted incidence rates were not statistically different between groups for pancreatitis, renal impairment, or major adverse cardiac events; there was a difference in incidence rates for benign thyroid neoplasm (0.3% versus 0%).
Conclusion
Overall, this analysis, representing over 1500 patient-years of exposure, demonstrated that exenatide twice daily was safe and generally well tolerated in patients with type 2 diabetes. The incidence of most adverse events, including serious adverse events, was similar in both exenatide-treated and comparator-treated patients. The most distinct differences between groups were in gastrointestinal-related adverse events, which is consistent with other therapies within the glucagon-like peptide class.
Keywords: exenatide, safety, adverse events, risk difference
Background
The glucagon-like peptide (GLP)-1 receptor agonist, exenatide, administered twice daily was approved by the US Food and Drug Administration in 2005 for the treatment of type 2 diabetes. In blinded, placebo-controlled and open-label, comparator controlled, Phase III clinical trials, exenatide twice daily significantly reduced glycosylated hemoglobin (HbA1c, mean −1.0%) in patients with type 2 diabetes who were unable to achieve adequate control with maximally effective doses of metformin, sulfonylurea, and/or thiazolidinedione.1 In addition to its glycemic effects, exenatide twice daily was associated with weight loss (−1 kg to −4 kg) and improvements in several cardiometabolic risk factors over 12 to 52 weeks in these trials.
As part of ongoing monitoring of the safety of exenatide, the goal of the current study was to provide an integrated analysis of the safety data from controlled clinical trials of exenatide twice daily, including a comparison of the exenatide-treated population with a population of pooled comparators who were treated with placebo or insulin. The data for this retrospective analysis were pooled from all completed, randomized, controlled Phase III clinical trials conducted by the manufacturer (Amylin Pharmaceuticals, Inc. with Eli Lilly and Company), which included 19 trials of 5594 patients with type 2 diabetes treated for 12 to 52 weeks. Primary and secondary efficacy outcomes have been previously reported for these studies.2–20
Methods
Study participants
Patients from 19 completed, randomized, controlled clinical trials, including 13 double-blind superiority trials comparing exenatide with placebo and six open-label noninferiority studies comparing exenatide with insulin were included in this analysis (Figure 1). For each participating study site, a common clinical protocol was approved by an institutional review board in accordance with the principles described in the Declaration of Helsinki, including all amendments through the South African revision of 1996.21 Patients provided written informed consent before participation.
Figure 1.
Selection of trials for pooled analysis. Of the 61 clinical trials with available data, 19 placebo-controlled or comparator-controlled studies met the criteria for inclusion in the pooled analysis.
Abbreviations: BID, twice daily; GAA-I, α-glucosidase inhibitor; INS, insulin; ITT, intent-to-treat; Met, metformin; SFU, sulfonylurea; TZD, thiazolidinedione; w/wout, with/without.
Patients were between the ages of 18 and 75 years and had a baseline HbA1c of ≤11.0%, a body mass index of 25–45 kg/m2, and a history of stable body weight (≤10% variability) for at least 3 months. Patients were excluded from these studies if they had used weight loss drugs, had evidence of a significant medical condition, or had used specific anti-glycemic agents, corticosteroids, investigational drugs, drugs known to affect gastrointestinal motility, or transplantation drugs within the 3 months prior to study screening.
Patients with type 2 diabetes were treated continuously for 12–52 weeks with exenatide twice daily (5 μg or 10 μg) alone or in combination with metformin, sulfonylurea, and/or thiazolidinedione. In patients who received 10 μg exenatide twice daily, treatment included a 4-week lead-in period with 5 μg exenatide twice daily, followed by a dose increase to 10 μg twice daily for the duration of the trial. Patients in the comparator group received insulin (biphasic insulin aspart or insulin glargine; open-label trials) or placebo (with or without metformin, sulfonylurea, and/or thiazolidinedione background therapy; blinded trials).
Statistical analysis
Baseline demographics were summarized for the intent-to-treat population in each treatment group. The intent-to-treat population included all randomized subjects who received at least one dose of study medication. Adverse events were reported by Preferred Term (using the Medical Dictionary for Regulatory Activities (MedDRA v 13.0) and organized by organ system class. Adverse events were also analyzed by serious adverse events, frequently occurring adverse events, study drug-related adverse events, and adverse events of interest (eg, gastrointestinal-related adverse events, hypoglycemia, thyroid neoplasm, pancreatitis, and cardiovascular adverse events). Mild-to-moderate nausea was defined as an event of nausea with an intensity of mild or moderate as determined by the investigator.
Hypoglycemic episodes were classified as minor if the event was associated with symptoms consistent with hypoglycemia and a blood glucose concentration of <54 mg/dL (3.0 mmol/L); events were considered major if the event resulted in loss of consciousness, seizure, coma, or other change in mental status consistent with neuroglycopenia (as judged by the investigator or physician) in which symptoms resolved after administration of intramuscular glucagon or intravenous glucose, or the event required third-party assistance because of severe impairment in consciousness or behavior and was accompanied by a blood glucose concentration of <54 mg/dL (3.0 mmol/L).
For the analysis of major adverse cardiac events (MACE), 3945 patients from eight randomized placebo-controlled and four active comparator-controlled trials were included.2–12,15,22 A customized MACE definition included terms reflective of cardiovascular mortality, stroke, myocardial infarction, acute coronary syndrome, and revascularization procedures. The risk ratio (RR) for the exposure-adjusted incidence rate and its 95% confidence interval (CI) were calculated for MACE. It should be noted that patients were excluded from study participation if uncontrolled hypertension or a significant history of cardiac disease was present at the time of screening, or if cardiac disease was present within 1 year of screening.
Renal impairment included all preferred terms defined as renal failure- or renal impairment-related, pancreatitis included both acute and chronic pancreatitis, and thyroid neoplasm included benign neoplasm of thyroid gland and malignant thyroid neoplasm. Additionally, hypersensitivity reactions were assessed relative to positive or negative antibody titers to exenatide in a separate analysis of 2225 patients treated with exenatide twice daily from 12 controlled trials.23
Incidence rates, exposure-adjusted incidence rate per 100 patient-years (PY), risk differences (RD) in incidence rate or exposure-adjusted incidence rate between groups (exenatide incidence rate/exposure-adjusted incidence rate – pooled comparator incidence rate/exposure-adjusted incidence rate), and 95% CI around the differences were calculated for adverse events.
Results
Patient characteristics and exposure
In this pooled dataset of patients with type 2 diabetes, 5594 patients were followed for 12–52 weeks, including 3261 patients receiving exenatide, and 1325 and 1008 patients receiving placebo and insulin comparators, respectively. Figure 1 lists the demographic and baseline characteristics for each treatment group. Demographics were generally similar between the exenatide and pooled comparator groups and there were no notable differences in baseline HbA1c, body mass index, or duration of diabetes. However, in the placebo-controlled group, there was a relatively high proportion (26%) of Asian patients because the placebo-controlled trials included three studies of Asian populations.10,11,17 Additionally, the mean exposures to study drug were also similar between cohorts, ie, 166 days in the exenatide twice-daily group and 171 days in the pooled comparator group (Figure 2).
Figure 2.
Mean treatment exposure. Cumulative duration of exposure (proportion of patients within each duration shown) and mean treatment exposure for the exenatide (166 days) and pooled comparator (171 days) groups.
Abbreviation: BID, twice daily.
Treatment-emergent adverse events and discontinuations
Treatment-emergent adverse events were defined as any untoward medical event that either occurred or worsened at any time after the first administration of study medication, through study termination or early termination. Although 81% of exenatide-treated patients and 69% of placebo/comparator-treated patients experienced an adverse event, few patients discontinued due to adverse events (8% in the exenatide group and 2% in the pooled comparator group). Study drug-related adverse events occurred in 48% of patients treated with exenatide compared with 16% of patients in the pooled comparator group (Table 1). Gastrointestinal-related adverse events (51% versus 21%; RD 30; 95% CI: 27.8–32.6) were the most frequently reported treatment-emergent adverse events associated with exenatide treatment (Tables 2 and 3). Of the other frequent adverse events, only dizziness (5% versus 3%; RD 2; 95% CI: 0.9–3.0) occurred significantly more frequently in the exenatide group than in the pooled comparator group.
Table 1.
Summary of treatment-emergent adverse events and discontinuations
| Patients with treatment-emergent AEs | Exenatide BID (N = 3261) | Pooled comparator (N = 2333) | Risk difference |
|---|---|---|---|
|
|
|||
| n (%) | n (%) | (95% CI)b | |
| With one or more AEs | 2653 (81.4) | 1613 (69.1) | 12.3 (9.9, 14.5) |
| With study drug-related AEsa | 1569 (48.1) | 372 (15.9) | 32.2 (29.9, 34.4) |
| With GI-related AEs | 1677 (51.4) | 495 (21.2) | 30.2 (27.8, 32.6) |
| With serious AEs | 119 (3.6) | 90 (3.9) | −0.3 (−1.2, 0.8) |
| With serious drug-related AEsa | 14 (0.4) | 5 (0.2) | 0.2 (−0.1, 0.5) |
| Deaths | 2 (<0.1) | 3 (<0.1) | 0 (−0.2, 0.1) |
| Discontinued due to AEs | 255 (7.8) | 43 (1.8) | 6.0 (4.9, 7.0) |
| Discontinued due to drug-related AEsa | 207 (6.3) | 18 (0.8) | 5.5 (4.7, 6.5) |
| Discontinued due to serious AEs | 25 (0.8) | 17 (0.7) | 0.1 (−0.4, 0.5) |
| Discontinued due to serious drug-related AEsa | 5 (0.2) | 2 (0.1) | 0.1 (−0.1, 0.2) |
| Discontinued due to GI-related AEs | 173 (5.3) | 7 (0.3) | 5.0 (4.2, 5.8) |
Notes:
Determined by the investigator to be possibly, probably, or definitely drug-related;
RD = Exenatide IR (%) minus pooled comparator IR (%).
Abbreviations: AEs, adverse events; BID, twice daily; CI, confidence interval; GI, gastrointestinal.
Table 2.
Summary of treatment-emergent adverse events by system organ class
| System organ classa | Exenatide BID (N = 3261) n (%) |
Pooled comparator (N = 2333) n (%) |
Risk difference (95% CI)b |
|---|---|---|---|
| Blood and lymphatic system disorders | 34 (1.0) | 17 (0.7) | 0.3 (−0.2, 0.8) |
| Cardiac disorders | 68 (2.1) | 53 (2.3) | −0.2 (−1.0, 0.6) |
| Congenital, familial, and genetic disorders | 2 (<0.1) | 1 (0.0) | <0.1 (−0.1, 0.1) |
| Ear and labyrinth disorders | 49 (1.5) | 52 (2.2) | −0.7 (−1.5, 0.0) |
| Endocrine disorders | 10 (0.3) | 2 (<0.1) | 0.2 (−0.0, 0.4) |
| Eye disorders | 90 (2.8) | 65 (2.8) | 0 (−0.9, 0.8) |
| Gastrointestinal disorders | 1677 (51.4) | 495 (21.2) | 30.2 (27.8, 32.6) |
| General disorders and administration site conditions | 586 (18.0) | 283 (12.1) | 5.9 (4.0, 7.7) |
| Hepatobiliary disorders | 24 (0.7) | 15 (0.6) | 0.1 (−0.3, 0.5) |
| Immune system disorders | 50 (1.5) | 29 (1.2) | 0.3 (−0.3, 0.9) |
| Infections and infestations | 971 (29.8) | 731 (31.3) | −1.5 (−4.0, 0.9) |
| Injury, poisoning, and procedural complications | 234 (7.2) | 153 (6.6) | 0.6 (−0.7, 2.0) |
| Investigations | 231 (7.1) | 94 (4.0) | 3.1 (1.9, 4.2) |
| Metabolism and nutrition disorders | 1128 (34.6) | 719 (30.8) | 3.8 (1.3, 6.3) |
| Musculoskeletal and connective tissue disorders | 471 (14.4) | 356 (15.3) | −0.9 (−2.7, 1.1) |
| Neoplasms, benign, malignant, and unspecified | 36 (1.1) | 18 (0.8) | 0.3 (−0.2, 0.8) |
| Nervous system disorders | 602 (18.5) | 332 (14.2) | 4.3 (2.3, 6.2) |
| Pregnancy, puerperium, and perinatal conditions | 1 (0.0) | 0 (0.0) | 0 (−0.0, 0.1) |
| Psychiatric disorders | 153 (4.7) | 77 (3.3) | 1.4 (0.4, 2.4) |
| Renal and urinary disorders | 69 (2.1) | 53 (2.3) | −0.2 (−0.9, 0.6) |
| Reproductive system and breast disorders | 64 (2.0) | 34 (1.5) | 0.5 (−0.2, 1.2) |
| Respiratory, thoracic, and mediastinal disorders | 313 (9.6) | 220 (9.4) | 0.2 (−1.4, 1.7) |
| Skin and subcutaneous tissue disorders | 260 (8.0) | 163 (7.0) | 1.0 (−0.4,2.4) |
| Social circumstances | 5 (0.2) | 1 (0.0) | 0.2 (−0.0, 0.3) |
| Surgical and medical procedures | 86 (2.6) | 47 (2.0) | 0.6 (−0.2, 1.4) |
| Vascular disorders | 107 (3.3) | 78 (3.3) | 0 (−1.0, 0.9) |
Notes:
Events listed by system organ class are indicated by number (n) of patients who experienced an adverse event;
RD = Exenatide IR (%) minus pooled comparator IR (%).
Abbreviations: BID, twice daily; CI, confidence interval.
Table 3.
Frequent (≥5%) treatment-emergent adverse events
| Preferred terma | Exenatide BID (N = 3261) n (%) |
Pooled comparator (N = 2333) n (%) |
Risk difference (95% CI) |
|---|---|---|---|
| Nausea | 1202 (36.9) | 193 (8.3) | 28.6 (26.6, 30.6) |
| Vomiting | 442 (13.6) | 67 (2.9) | 10.7 (9.3, 12.0) |
| Diarrhea | 346 (10.6) | 127 (5.4) | 5.2 (3.8, 6.6) |
| Dizziness | 173 (5.3) | 78 (3.3) | 2.0 (0.9, 3.0) |
| Headache | 275 (8.4) | 173 (7.4) | 1.0 (−0.4, 2.4) |
| Nasopharyngitis | 288 (8.8) | 219 (9.4) | −0.6 (−2.1, 1.0) |
| Upper respiratory tract infection | 193 (5.9) | 137 (5.9) | 0 (−1.2, 1.3) |
Notes: Events listed by preferred term (MedDRA v 13.0 terms) are indicated by number (n) of patients who experienced an adverse event.
Abbreviations: BID, twice daily; CI, confidence interval.
Gastrointestinal-related adverse events were the most common reason for study discontinuation: 5.3% of patients in the exenatide group and 0.3% of patients in the pooled comparator group withdrew due to gastrointestinal-related adverse events (Table 1).
Serious adverse events
There was no difference in the rate of serious adverse events in either group (4% for both exenatide and pooled comparator groups). Study drug-related serious adverse events occurred at a frequency of 0.4% in the exenatide group and 0.2% in the insulin group (detailed listing in Table 4). Study drug-related serious adverse events leading to discontinuation occurred in five (0.2%) exenatide-treated patients and two (0.1%) patients treated with insulin or placebo (Table 1). There were two (<0.1%) deaths in the exenatide group and three (<0.1%) deaths in the pooled comparator group (Table 1); however, the adverse events that led to patient deaths (ie, atrial fibrillation and myocardial infarction in exenatide-treated patients, stroke in an insulin-treated patient, and myocardial infarction in two placebotreated patients) were not considered by the investigator to be related to study drug.
Table 4.
Study drug-relateda serious adverse events by preferred term
| Preferred termb | Study | Intensity | Outcome | Discontinued study due to this adverse event |
|---|---|---|---|---|
| Exenatide BID | ||||
| Hypertension | Heine et al5 | Moderate | Recovered | No |
| Vomiting | Heine et al5 | Severe | Recovered | No |
| Injection-site cellulitis | Nauck et al9 | Severe | Unknown | Yes |
| Nausea | Nauck et al9 | Severe | Unknown | Yes |
| Anorexia | Nauck et al9 | Severe | Unknown | No |
| Weight loss | Nauck et al9 | Severe | Unknown | No |
| Hyperglycemia | Davis et al8 | Severe | Recovered | Yes |
| Allergic alveolitis | Zinman et al6 | Severe | Recovered | No |
| Gastroesophageal reflux disease | DeFronzo et al14 | Severe | Not resolved | No |
| Gastroenteritis | DeFronzo et al14 | Severe | Recovered | No |
| Hemorrhoidal hemorrhage | DeFronzo et al14 | Severe | Recovered | No |
| Allergic dermatitis | Gallwitz et al19 | Severe | Not resolved | Yes |
| Accidental overdose | Buse et al20,66 | Mild | Recovered | No |
| Gastritis | DeFronzo et al3 | Severe | Recovered | Yes |
| Presyncope | Kendall et al4 | Severe | Recovered | No |
| Acute pancreatitis | Kadowaki et al17 | Moderate | Recovered | Yes |
| Hypoglycemia | Gao et al10 | Severe | Recovered | No |
| Acute myocardial infarction | Davies et al13 | Severe | Recovered | No |
| Supraventricular tachycardia | Davies et al13 | Severe | Recovered | No |
| Insulin glargine | ||||
| Ureteric calculus | Heine et al5 | Severe | Recovered | No |
| Angiodema | Heine et al5 | Severe | Recovered | No |
Notes:
Determined by the investigator to be possibly, probably, or definitely related to study drug;
events listed by preferred term (MedDRA v 13.0 terms) are the individual events observed.
Abbreviation: BID, twice daily.
Adverse events of interest
Because of specific safety concerns highlighted by post-marketing safety surveillance and case reports, several adverse events of interest were explored.
Gastrointestinal-related adverse events
Gastrointestinal-related adverse events were reported for 51% of patients treated with exenatide twice daily and in 21% of patients treated with a comparator (RD 30; 95% CI: 27.8–32.6) and were the most common adverse events by organ system class (Table 2). Of the gastrointestinal-related adverse events, nausea (37% versus 8%; RD 29; 95% CI: 26.6–30.6), vomiting (14% versus 3%; RD 11; 95% CI: 9.3–12.0), and diarrhea (11% versus 5%; RD 5; 95% CI: 3.8–6.6) occurred at a significantly greater frequency in the exenatide group than in the pooled comparator group (Table 3). The exposure-adjusted incidence rate of gastrointestinal-related adverse events was 195 per 100 PY in the exenatide group and 53 per 100 PY in the pooled comparator group (RD 142; 95% CI: 131.4–152.3). One serious adverse event each of nausea and vomiting were determined to be related to the study drug by the investigator at the time of the event (Table 4). Gastrointestinal-related adverse events were also among the most common adverse events associated with study withdrawal in the exenatide group (Table 1).
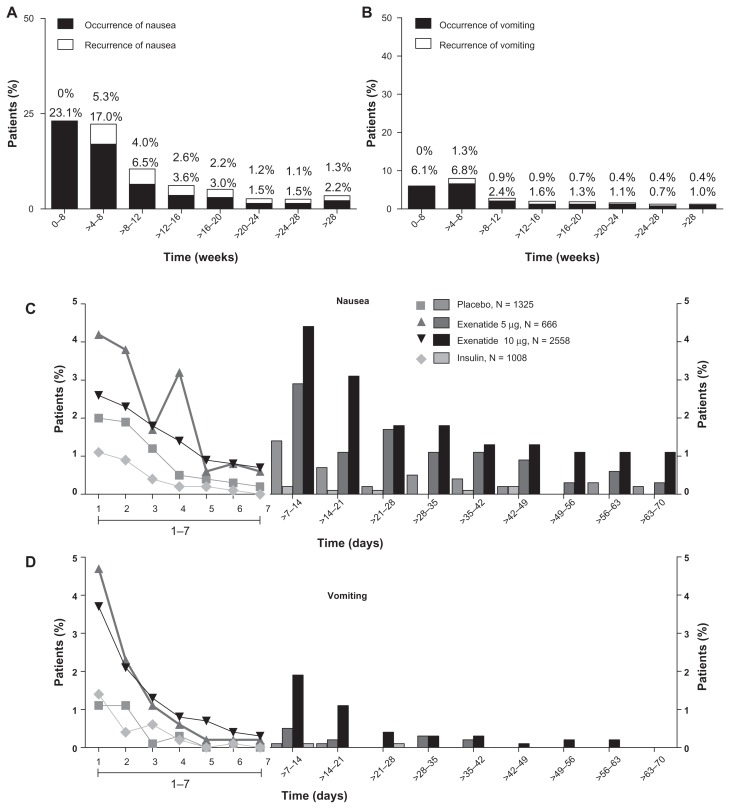
The incidences of nausea and vomiting decreased over time in the exenatide group, regardless of dose. The occurrence of nausea decreased from 23.1% during the interval from week 0 to week 4 to 2.2% after week 28, with a substantial reduction in incidence rate after 8 weeks of treatment (6.5% during the interval from week 8 to week 12, Figure 3A). Vomiting decreased from 6.1% during the interval from week 0 to week 4 to 1.0% after week 28 (Figure 3B). There were few recurrences of nausea and vomiting events. An analysis of the duration of nausea and vomiting demonstrated that the nausea and vomiting events associated with exenatide treatment twice daily were dosedependent and that these events generally resolved in 1–2 days in most patients (Figure 3C and D).
Figure 3.
Incidence, recurrence, and duration of nausea and vomiting over time. Nausea and vomiting with exenatide twice daily 10 μg (4-week lead-in period with 5 μg exenatide twice daily, followed by dose increase to 10 μg twice daily for the duration of the trial; n = 2558), placebo (n = 1325), and insulin (n = 1008). (A) Occurrence and recurrence of nausea over time (grouped into 4-week intervals). (B) Occurrence and recurrence of vomiting over time. Each event is attributed to a defined period according to the event onset date, and recurrence of nausea/vomiting is defined as an event with onset during the defined period and any of the previous periods. Percentages are based on number of subjects who remained in the trial during the defined period. (C) Duration of nausea. (D) Duration of vomiting. The duration of the nausea/vomiting event is calculated as the resolution date (or the last participation date if event is ongoing at the time of study termination) minus the event onset date plus 1.
Hypoglycemia
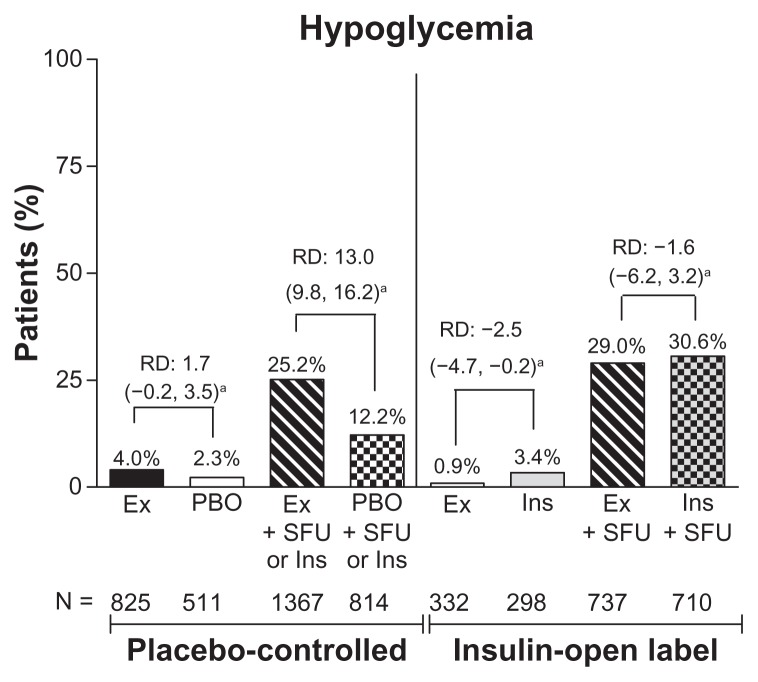
The incidence of hypoglycemia was similar between the groups without sulfonylurea use. In a separate analysis of hypoglycemic events by concomitant sulfonylurea use, the incidence of hypoglycemia was low in patients who did not use a concomitant sulfonylurea (exenatide 3.1%, pooled comparator 2.7% [RD 0.4; 95% CI:−1.1–1.9] vs. with sulfonylurea use, 26.5% and 20.7%, respectively [RD 5.8; 95% CI: 3.0–8.6]). Analysis by placebo-controlled or comparator-controlled groups and concomitant sulfonylurea showed significant differences between the exenatide and placebo subgroups in patients who received a sulfonylurea (25% versus 12%; 95% CI: 9.8–16.2) and between the exenatide and insulin subgroups who did not receive a sulfonylurea (0.9% versus 3.4%; 95% CI: −4.7 to −0.2, Figure 4). Thus, of the patients who experienced hypoglycemia, 99% of patients in the exenatide group and 93% of patients in the pooled comparator group were receiving sulfonylurea or insulin at the time of the hypoglycemia event. Major hypoglycemia was rare in all treatment groups.
Figure 4.
Incidence of hypoglycemia by treatment. Percentage of patients who experienced hypoglycemia (minor or major).
Note: a95% confidence interval for the risk difference (exenatide incidence rate [%] minus pooled comparator incidence rate [%]).
Abbreviations: RD, risk difference; Ex, exenatide; BID, twice daily; Ins, insulin; PBO, placebo; SFU, sulfonylurea. PBO + SFU or Ins refers to placebo with SFU or with background insulin.
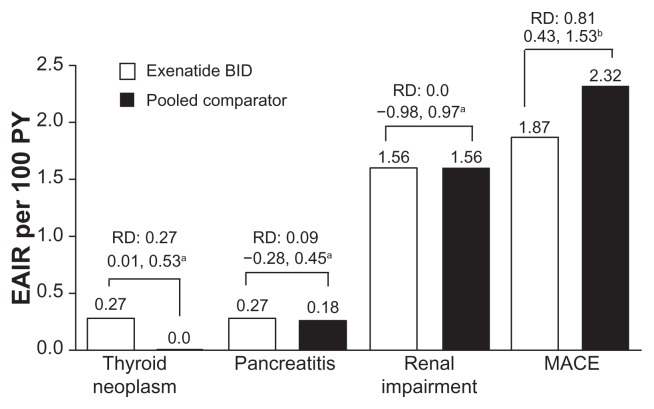
Renal impairment, thyroid neoplasm, and pancreatitis
Renal impairment-related adverse events, including acute renal failure, were infrequently reported (1.6 per 100 PY for both groups) and there was no significant difference between groups (95% CI: −1.0 to 1.0, Figure 5). Thyroid neoplasms, including benign and malignant, were rare. There was a difference in the exposure-adjusted incidence rate of thyroid neoplasms between groups, ie, 0.3 per 100 PY (incidence rate < 0.1%) in the exenatide group and 0 per 100 PY in the pooled comparator group (RD 0.3; 95% CI: 0.01–0.53, Figure 5). None of the neoplasms were malignant. Acute pancreatitis was rare in the patients examined in this analysis; neither the incidence rate (0.1% in both the exenatide and pooled comparator groups (95% CI: −0.2 to 0.1) nor the exposure-adjusted incidence rate of pancreatitis (exenatide, 0.27 per 100 PY versus pooled comparator, 0.18; RD 0.1; 95% CI: −0.28 to 0.45) was significantly different between the groups (Figure 5).
Figure 5.
Adverse events of interest. Exposure-adjusted incidence rate and risk difference of thyroid neoplasm, pancreatitis, and renal impairment (exenatide twice daily n = 3261, pooled comparator n = 2333) and major adverse cardiac events analysis (n = 2316, n = 1629, respectively). Thyroid neoplasm includes benign neoplasm of thyroid gland and malignant thyroid neoplasm. Pancreatitis includes acute pancreatitis and chronic pancreatitis. Renal impairment includes renal failure. Major adverse cardiac events include stroke, myocardial infarction, cardiac mortality, acute coronary syndrome, and revascularization procedures.
Notes: a95% confidence interval for the risk difference (exenatide incidence rate [%] minus pooled comparator incidence rate [%]); bmajor adverse cardiac events analysis: risk ratio and 95% confidence interval for the risk ratio.
Abbreviations: EAIR, exposure-adjusted incidence rate; BID, twice daily, MACE, major adverse cardiac events; PY, patient years.
Cardiovascular-related adverse events
MACE occurred infrequently, with an exposure-adjusted incidence rate of 1.9 per 100 PY with exenatide treatment and 2.3 per 100 PY with comparators (RR 0.81; 95% CI: 0.43–1.53, Figure 5).22 Three cardiovascular events (hypertension, myocardial infarction, and subventricular tachycardia) in two patients were designated study drug-related serious adverse events by the investigator in the exenatide group.5,13
Antibody formation and immune-related and injection site-related events
A separate analysis was performed to characterize antibody formation with exenatide and the association between antibody formation and injection-site-related adverse events.23 Antibody titers were characterized from 12 controlled studies of patients who received exenatide twice daily for up to one year (exenatide, n = 2225; pooled comparator [placebo, insulin, or thiazolidinedione], n = 1591). A patient was defined as having treatment-emergent anti-exenatide antibodies if antibodies were present after the first exenatide injection following absence of antibodies or missing antibody measurement at baseline, or if the titer increased by at least three dilutions from a detectable baseline measurement. The incidence of positive antibody titer was 35% (31% low titer [≤125] and 5% higher titer [≥625]) at endpoint (12–52 weeks). Antibody titers peaked early (between weeks 6 and 16) and declined over time (25% of patients had positive titers for antibodies at 52 weeks and 17% had positive titers at 3 years). Overall, there was no difference in the incidence of all potentially immune-related adverse events between treatment groups (7.9% in the exenatide group and 7.4% in the pooled comparator group). A subset of patients had a slightly greater incidence of general disorders and administration site conditions associated with a positive antibody titer (2.8%) than with a negative titer (0.7%).
Analysis of the incidence rates of injection-site-related adverse events in the full analysis (19 studies) indicated that these reactions were rare in both groups (data not shown); injection site erythema (0.6% versus 0%; RD 0.5; 95% CI: 0.3–0.8), injection-site pruritus (0.8% versus 0.1%; RD 0.7; 95% CI: 0.4–1.0), injection-site urticaria (0.1% versus 0%; RD 0.1; 95% CI: 0.0–0.2), injection-site rash (0.4% versus 0%; RD 0.4; 95% CI: 0.2–0.6), and injection-site reaction (0.3% versus 0%; RD 0.3; 95% CI: 0.1–0.5) were greater in the exenatide group compared with the pooled comparator group. The incidence of all other injection-site reactions was similar between groups.
Discussion
In this analysis of 19 randomized, placebo-controlled and active comparator-controlled clinical trials, no specific safety signals of concern were identified with the use of exenatide twice daily. Overall, exenatide twice daily was safe and generally well tolerated. The most common treatment-emergent adverse events associated with exenatide treatment were gastrointestinal, and were mild and transient. The rate of serious adverse events and discontinuations due to serious adverse events were low overall and occurred at a similar rate in both the exenatide and pooled comparator groups. The results of long-term extension studies of exenatide twice daily (up to 3 years of treatment)24–29 and postmarketing surveillance (over 1.6 million PY of exposure since launch) support these findings.
The present analysis included only randomized, controlled clinical trials of exenatide twice daily versus placebo or insulin, but several uncontrolled, open-label extension studies of exenatide twice daily have reported similar safety outcomes.24–29 In these open-label extension studies, gastrointestinal-related adverse events, particularly nausea and vomiting, were frequently reported and were the most common adverse events leading to study discontinuation. Further, these studies showed a low rate of serious adverse events and infrequent occurrence of hypoglycemia in the absence of concomitant sulfonylurea use. Of these trials, detailed, comparable safety data were reported for the 3-year endpoint of a large, open-label extension trial of 527 intent-to-treat patients treated with 10 μg exenatide twice daily.26 In this trial, 11% of patients discontinued the study due to adverse events; in the present pooled analysis, 8% of exenatide patients discontinued due to adverse events. Nausea was reported by 59% of patients in the extension trial compared with 36.9% of patients in the current analysis, and most discontinuations due to adverse events were attributed to nausea in both the extension trial and our analysis. Hypoglycemia was reported by 40% and 30% of patients in the extension trial and current analysis, respectively (regardless of concomitant sulfonylurea use), but most cases were mild-to-moderate in severity. These findings were also reported in a 2009 systematic review by Norris et al,1 which also assessed safety results from eight of the studies included in the current analysis.
Mild-to-moderate nausea was the most frequently occurring treatment-emergent adverse event in this analysis; there was only one case of severe nausea. The GLP-1 receptor agonists are generally associated with increased gastrointestinal-related adverse events, and it is known that gastrointestinal symptoms are generally more frequent in patients with diabetes than in those without diabetes.30–33 The sensation of nausea with exenatide use is thought to be due to slowed gastric emptying, appetite suppression, or stimulation of neural GLP-1 receptors. However, with continued exenatide use, the frequency and severity of nausea and vomiting typically decreases over time, and dose titration moderates some of these events.1 As in the present analysis, studies of long-term use of exenatide twice daily have shown that nausea generally subsides with continued treatment.24–26,34 Studies of the GLP-1 receptor agonist, liraglutide, have produced similar results with regard to the prevalence of gastrointestinal-related adverse events and the transient occurrence of nausea.32,33 The ability of antiemetics to mitigate nausea associated with antidiabetic agents is unknown given the paucity of data available on this subject in the literature. However, a recent analysis indicated that predosing with an oral antiemetic before a single 10 μg exenatide dose is associated with significant reductions in treatment-emergent nausea and vomiting in premedicated patients (17% and 7%, respectively) compared with nonpremedicated patients (62% and 38%, respectively).35
Although the goal of achieving nearly normal HbA1c levels decreases many of the risks of hyperglycemia-related complications in diabetes, achieving this goal may be hindered by the occurrence of hypoglycemia.36,37 The incidence of hypoglycemia was low, overall, and the rate of these events was similar between exenatide-treated patients and placebo-treated patients. Most events of hypoglycemia occurred with concomitant sulfonylurea use. In the absence of concomitant sulfonylurea use, exenatide-treated patients experienced rates of hypoglycemia similar to those in patients treated with placebo, and lower rates of hypoglycemia than patients treated with insulin. Major hypoglycemia was rare in all treatment groups.
In addition to an increase in pancreatitis in the general population in recent years, which may be due to the greater rates of obesity, hyperlipidemia, and obstruction of the common bile duct by gall stones,38–43 concerns about pancreatitis with antihyperglycemic agents have been emphasized by postmarketing safety surveillance and case reports.44–48 In 2007 and 2008, the US Food and Drug Administration issued a safety alert and update based on postmarketing surveillance that reported a total of 36 cases of acute pancreatitis in patients treated with exenatide.44 However, a definitive diagnosis of pancreatitis was not available in all of these cases, because many of the patients in the reports did not have a computed tomography scan (or other imaging) to confirm the diagnosis; further, most of these patients had at least one other risk factor for pancreatitis. In contrast, large postmarketing studies found no evidence to support an association between exenatide and increased risk of pancreatitis. 49–52 One study of a large cohort of patients (exenatide n = 27,996 and an approximately equal number of matched comparators) from a health insurance claims database found no association between exenatide and pancreatitis; the incidence of acute pancreatitis (0.13%) was comparable for initiators of exenatide relative to the comparator group (0.12%; RR 1.0; 95% CI: 0.6–1.7).49 Additionally, results from an analysis of a national integrated claims database showed that patients with type 2 diabetes had a two-fold higher risk of pancreatitis and that there was no definite association between exenatide use and increased incidence of pancreatitis.50 Other antidiabetic therapies have also been associated with the development of pancreatitis, but such occurrences have been rare.51–53
Obesity and dyslipidemia have been associated with an increased risk of developing pancreatitis and are risk factors for further morbidity and mortality in patients who have pancreatitis.39,41,42 Moreover, patients with type 2 diabetes appear to be at increased risk for pancreatitis, as shown by two large studies of health care databases.54,55 The first, a study of a large US health insurance claims database demonstrated that patients with type 2 diabetes were at 2.8-fold greater risk (95% CI: 2.61–3.06) of pancreatitis compared with a nondiabetic cohort.54 In the second study of a European health database, the risk of developing acute pancreatitis was elevated in patients with type 2 diabetes, with an adjusted hazard ratio of 1.5 (95% CI: 1.31–1.70).55
Events of exenatide-associated renal impairment or renal failure are reported infrequently in published studies.56,57 In the current analysis, the exposure-adjusted incidence rate of renal impairment was 1.6 per 100 PY in both the exenatide and pooled comparator groups. These data are consistent with the results of an analysis of six clinical trials58 that found no association between exenatide twice daily or placebo in renal adverse events and similar changes in kidney function between patients in both groups. However, because exenatide is primarily cleared via renal mechanisms,59 a labeled warning remains stating that exenatide twice daily should not be used in patients with severe renal impairment (creatinine clearance < 30 mL/minute) or end-stage renal disease, should be used with caution in patients with renal transplantation, and caution should be exercised when initiating or escalating doses of exenatide in patients with moderate renal impairment (creatinine clearance 30 to 50 mL/minute). Additionally, because exenatide may induce nausea and vomiting with transient hypovolemia, treatment may worsen renal function.
Thyroid cancers have become a topic of interest with the GLP-1 receptor agonists, because sustained GLP-1 agonism has been associated with an increased incidence of C-cell adenomas and carcinomas in rodents,60 although the clinical relevance of the animal data is unknown. In particular, there appears to be a species-specific difference in thyroid C-cell response to GLP-1 receptor agonists that causes C-cell secretion of calcitonin and hyperplasia in rodents.60 In the present analysis, all occurrences of thyroid neoplasm were benign and very rare; the exposure-adjusted incidence rate of any thyroid neoplasm was 0.3 per 100 PY with exenatide compared with no occurrences of thyroid neoplasm with placebo/insulin (RD 0.3; 95% CI: 0.01–0.53). Thyroid neoplasm has also rarely been reported with liraglutide, and studies monitoring calcitonin have indicated similar levels with liraglutide and comparators.61–63
Overall, major adverse cardiovascular events were uncommon in this analysis. Recently, an association has been suggested between use of GLP-1 receptor agonists and improvement in cardiovascular risk factors. In an analysis of cardiovascular-related events from a large insurance database, exenatide treatment twice daily was associated with a lower 10-year risk of cardiovascular-related adverse events and hospitalizations than were other antidiabetic treatments.64 However, data from controlled, adequately powered clinical trials with prospectively blinded adjudication of cardiovascular events are needed to evaluate whether exenatide has cardioprotective effects; one such trial is underway (EXSCEL trial, ClinicalTrials.gov Identifier NCT01144338).
During treatment with GLP-1 receptor agonists, antibodies to treatment may develop in some patients, as has been observed with other peptide therapeutics.61,65 Antibody formation to therapeutic peptides is common, even when the peptide is identical to the endogenous human form. Analysis of antibodies showed that 37% of exenatide-treated patients developed antibodies to exenatide after 30 weeks of treatment; this rate fell to 17% after 3 years.23 In studies of liraglutide, antibodies developed in approximately 8% of patients who received liraglutide treatment for up to 26 weeks.66 Analysis of cross-reactivity in a subset of antibody-positive patients showed that treatment-emergent antibodies to exenatide did not cross-react with human GLP-1 or glucagon. In the LEAD-6 trial of liraglutide, 4.4% (five of 113) of antibody-positive samples cross reacted with GLP-1. However, because no baseline data were provided for the LEAD-6 patients, it is unknown whether the cross-reactivity was pre-existing or treatment-emergent.67
It should also be noted that previous studies have indicated that a low antibody titer is not predictive of safety or efficacy issues.23 However, results of the subanalysis presented in this manuscript indicate that there was no difference between groups in potentially immune-related adverse events overall, and only a slight increase in the occurrence of some injection-site-related adverse events in patients with a positive antibody titer to exenatide.
Strengths and limitations
There are several strengths of this analysis, ie, a large number of patients were included in the pooled data set, the trials were randomized and controlled with centralized monitoring and laboratory analyses, and the results were derived from individual patient data. Limitations were that adverse events were not independently adjudicated; the design of this analysis was not adequately powered to detect very rare adverse events (incidence rate < 0.01%), and the duration of the trials may not have been long enough to observe some adverse events, eg, cancers. Additionally, because the pooled comparator included blinded placebo and open-label active comparator (insulin) groups, specific adverse events associated with the active comparator may have been under-represented when combined with placebo-related adverse events, affecting direct comparisons between the exenatide and pooled comparator groups.
Conclusion
This integrated analysis of adverse event data for 5594 patients representing over 1500 PY of exenatide twice daily exposure provided a comprehensive evaluation of the safety and tolerability of exenatide twice daily. The rates of serious adverse events were low overall, and incidences were similar in both the exenatide and pooled comparator groups. Discontinuations due to adverse events occurred more frequently in the exenatide group and were often due to gastrointestinal-related adverse events. The greatest differences in incidences between the exenatide and comparator groups were in gastrointestinal-related adverse events, which were also the most frequently occurring adverse events with exenatide, a commonality among therapies in the GLP-1 receptor agonist class. The occurrence of nausea and vomiting may be perceived as treatment-limiting, but these events were generally mild in intensity, occurred most frequently upon initiation of treatment, and decreased over time. In the absence of concomitant sulfonylurea use, exenatide-treated patients experienced a low incidence of hypoglycemia, with rates of hypoglycemia similar to those in patients treated with placebo and lower than in patients treated with insulin. Exposure-adjusted adverse event rates for pancreatitis, renal-related adverse events, and MACE were low and generally similar between groups; thyroid neoplasm was also rare with exenatide but the incidence was higher than that of the comparator because there were no occurrences of thyroid neoplasm with placebo or insulin. Taken together, the results of this analysis showed that exenatide twice daily was safe and generally well tolerated in patients with type 2 diabetes mellitus.
Acknowledgments
We thank the patients and investigators who participated in the included studies. We also thank Haiying Dong for her contributions to the data analysis, Daniel Braun for his contributions to the individual study designs, and Jeffrey Ferguson, Dawn Nicewarner, and Carmelle Remillard for their contributions to the discussion and editing of the manuscript.
Footnotes
Disclosure
This study was sponsored by Amylin Pharmaceuticals, Inc. and Eli Lilly and Company. LM, CB, KG, and JH are employees and shareholders of Amylin Pharmaceuticals, Inc.
References
- 1.Norris SL, Lee N, Thakurta S, Chan BK. Exenatide efficacy and safety: a systematic review. Diabet Med. 2009;26(9):837–846. doi: 10.1111/j.1464-5491.2009.02790.x. [DOI] [PubMed] [Google Scholar]
- 2.Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 4.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 5.Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559–569. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146(7):477–485. doi: 10.7326/0003-4819-146-7-200704030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29(11):2333–2348. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Davis SN, Johns D, Maggs D, et al. Exploring the substitution of exenatide for insulin in patients with type 2 diabetes treated with insulin in combination with oral antidiabetes agents. Diabetes Care. 2007;30(11):2767–2772. doi: 10.2337/dc06-2532. [DOI] [PubMed] [Google Scholar]
- 9.Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50(2):259–267. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Yoon KH, Chuang L-M, et al. Efficacy and safety of exenatide in patients of Asian descent with type 2 diabetes inadequately controlled with metformin or metformin and a sulphonylurea. Diabetes Res Clin Pract. 2009;83(1):69–76. doi: 10.1016/j.diabres.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki T, Namba M, Yamamura A, et al. Exenatide exhibits dose-dependent effects on glycemic control over 12 weeks in Japanese patients with suboptimally controlled type 2 diabetes. Endocr J. 2009;56(3):415–424. doi: 10.1507/endocrj.k08e-296. [DOI] [PubMed] [Google Scholar]
- 12.Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30(8):1448–1460. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Davies MJ, Donnelly R, Barnett AH, et al. Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the Helping Evaluate Exenatide in patients with diabetes compared with Long-Acting insulin (HEELA) study. Diabetes Obes Metab. 2009;11(12):1153–1162. doi: 10.1111/j.1463-1326.2009.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Triplitt C, Qu Y, et al. Effects of exenatide plus rosiglitazone on β-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin. Diabetes Care. 2010;33(5):951–957. doi: 10.2337/dc09-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill A, Hoogwerf BJ, Burger J, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6. doi: 10.1186/1475-2840-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apovian CM, Bergenstal RM, Cuddihy RM, et al. Effects of exenatide combined with lifestyle modification in patients with type 2 diabetes. Am J Med. 2010;123(5):468, e469–468, e417. doi: 10.1016/j.amjmed.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Kadowaki T, Mitsuyoshi N, Imaoka T, et al. Improved glycemic control and reduced bodyweight with exenatide: a double-blind, randomized, phase 3 study in Japanese patients with suboptimally controlled type 2 diabetes over 24 weeks. J Diabet Investig. 2011;2(3):210–217. doi: 10.1111/j.2040-1124.2010.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liutkus J, Rosas Guzman J, Norwood P, et al. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin. Diabetes Obes Metab. 2010;12(12):1058–1065. doi: 10.1111/j.1463-1326.2010.01251.x. [DOI] [PubMed] [Google Scholar]
- 19.Gallwitz B, Böhmer M, Segiet T, et al. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: a randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care. 2011;34(3):604–606. doi: 10.2337/dc10-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association. World Medical Association Declaration of Helsinki: Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277(11):925–926. [PubMed] [Google Scholar]
- 22.Ratner R, Han J, Nicewarner D, et al. Cardiovascular safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc Diabetol. 2011;10:22. doi: 10.1186/1475-2840-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fineman M, Mace K, Diamant M, et al. Clinical relevance of anti-exenatide antibodies: safety, efficacy, and cross-reactivity with long-term treatment. Diabetes Obes Metab. 2012 doi: 10.1111/j.1463-1326.2012.01561.x. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 24.Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8(4):436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 25.Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther. 2007;29(1):139–153. doi: 10.1016/j.clinthera.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24(1):275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 27.Riddle MC, Henry RR, Poon TH, et al. Exenatide elicits sustained glycaemic control and progressive reduction of body weight in patients with type 2 diabetes inadequately controlled by sulphonylureas with or without metformin. Diabetes Metab Res Rev. 2006;22(6):483–491. doi: 10.1002/dmrr.646. [DOI] [PubMed] [Google Scholar]
- 28.Nelson P, Poon T, Guan X, et al. The incretin mimetic exenatide as a monotherapy in patients with type 2 diabetes. Diabetes Technol Ther. 2007;9(4):317–326. doi: 10.1089/dia.2006.0024. [DOI] [PubMed] [Google Scholar]
- 29.Poon T, Taylor K, Nielsen L, et al. Effects of exenatide (synthetic exendin-4) on glucose control and safety in patients with type 2 diabetes treated with metformin, a sulfonylurea, or both: an ongoing, open-label phase 3 trial [Abstract] Diabet Med. 2004;21(Suppl 2):13. [Google Scholar]
- 30.Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161(16):1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Park HS, Ko SY, et al. Diabetic factors associated with gastrointestinal symptoms in patients with type 2 diabetes. World J Gastroenterol. 2010;16(14):1782–1787. doi: 10.3748/wjg.v16.i14.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horowitz M, Vilsbøll T, Zdravkovic M, Hammer M, Madsbad S. Patient-reported rating of gastrointestinal adverse effects during treatment of type 2 diabetes with the once-daily human GLP-1 analogue, liraglutide. Diabetes Obes Metab. 2008;10(7):593–596. doi: 10.1111/j.1463-1326.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- 33.Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab. 2009;11(Suppl 3):26–34. doi: 10.1111/j.1463-1326.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 34.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves β-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32(5):762–768. doi: 10.2337/dc08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellero C, Han J, Bhavsar S, et al. Prophylactic use of anti-emetic medications reduced nausea and vomiting associated with exenatide treatment: a retrospective analysis of an open-label, parallel-group, single-dose study in healthy subjects. Diabet Med. 2010;27(10):1168–1173. doi: 10.1111/j.1464-5491.2010.03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCall AL, Cox DJ, Brodows R, et al. Reduced daily risk of glycemic variability: comparison of exenatide with insulin glargine. Diabetes Technol Ther. 2009;11(6):339–344. doi: 10.1089/dia.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright AD, Cull CA, Macleod KM, Holman RR. Hypoglycemia in type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis: UKPDS73. J Diabetes Complications. 2006;20(6):395–401. doi: 10.1016/j.jdiacomp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33(4):323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- 39.Suazo-Baráhona J, Carmona-Sánchez R, Robles-Díaz G, et al. Obesity: a risk factor for severe acute biliary and alcoholic pancreatitis. Am J Gastroenterol. 1998;93(8):1324–1328. doi: 10.1111/j.1572-0241.1998.442_l.x. [DOI] [PubMed] [Google Scholar]
- 40.Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6(4):279–285. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 41.Martínez J, Johnson CD, Sánchez-Payá J, et al. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology. 2006;6(3):206–209. doi: 10.1159/000092104. [DOI] [PubMed] [Google Scholar]
- 42.Torgerson JS, Lindroos AK, Näslund I, Peltonen M. Gallstones, gall-bladder disease, and pancreatitis: cross-sectional and 2-year data from the Swedish Obese Subjects (SOS) and SOS reference studies. Am J Gastroenterol. 2003;98(5):1032–1041. doi: 10.1111/j.1572-0241.2003.07429.x. [DOI] [PubMed] [Google Scholar]
- 43.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371(9607):143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 44.US Food and Drug Administration. Information for Healthcare Professionals: Exenatide (marketed as Byetta), 8/2008 update. [Accessed January 1, 2012]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124713.htm.
- 45.US Food and Drug Administration. Information for Healthcare-Professionals – Acute pancreatitis and sitagliptin (marketed as Januvia and Janumet) [Accessed January 1, 2012]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm183764.htm.
- 46.Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care. 2006;29(2):471. doi: 10.2337/diacare.29.02.06.dc05-2043. [DOI] [PubMed] [Google Scholar]
- 47.Tripathy NR, Basha S, Jain R, Shetty S, Ramachandran A. Exenatide and acute pancreatitis. J Assoc Physicians India. 2008;56:987–988. [PubMed] [Google Scholar]
- 48.Ayoub WA, Kumar AA, Naguib HS, Taylor HC. Exenatide-induced acute pancreatitis. Endocr Pract. 2010;16(1):80–83. doi: 10.4158/EP09104.CRR. [DOI] [PubMed] [Google Scholar]
- 49.Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25(4):1019–1027. doi: 10.1185/03007990902820519. [DOI] [PubMed] [Google Scholar]
- 50.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 2010;33(11):2349–2354. doi: 10.2337/dc10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blomgren KB, Sundström A, Steineck G, Wiholm BE. Obesity and treatment of diabetes with glyburide may both be risk factors for acute pancreatitis. Diabetes Care. 2002;25(2):298–302. doi: 10.2337/diacare.25.2.298. [DOI] [PubMed] [Google Scholar]
- 52.Fimognari FL, Corsonello A, Pastorell R, Antonelli-Incalzi R. Metformin-induced pancreatitis: a possible adverse drug effect during acute renal failure. Diabetes Care. 2006;29(5):1183. doi: 10.2337/diacare.2951183. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Perez A, Schlienger RG, García Rodríguez LA. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care. 2010;33(12):2580–2585. doi: 10.2337/dc10-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32(5):834–838. doi: 10.2337/dc08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girman CJ, Kou TD, Cai B, et al. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab. 2010;12(9):766–771. doi: 10.1111/j.1463-1326.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- 56.Ferrer-Garcia JC, Martinez-Chanza N, Tolosa-Torréns M, Sánchez-Juan C. Exenatide and renal failure. Diabet Med. 2010;27(6):728–729. doi: 10.1111/j.1464-5491.2010.03009.x. [DOI] [PubMed] [Google Scholar]
- 57.Weise WJ, Sivanandy MS, Block CA, Comi RJ. Exenatide-associated ischemic renal failure. Diabetes Care. 2009;32(2):e22–e23. doi: 10.2337/dc08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuttle K, Brown C, Lee D, et al. Effect of exenatide BID on kidney function and adverse events in clinical trials. Diabetes. 2011;60(Suppl 1):A266. [Google Scholar]
- 59.Copley K, McCowen K, Hiles R, et al. Investigation of exenatide elimination and its in vivo and in vitro degradation. Curr Drug Metab. 2006;7(4):367–374. doi: 10.2174/138920006776873490. [DOI] [PubMed] [Google Scholar]
- 60.Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151(4):1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 61.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 62.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 63.Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab. 2011;96(3):853–860. doi: 10.1210/jc.2010-2318. [DOI] [PubMed] [Google Scholar]
- 64.Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34(1):90–95. doi: 10.2337/dc10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buse JB, Garber A, Rosenstock J, et al. Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trials. J Clin Endocrinol Metab. 2011;96(6):1695–1702. doi: 10.1210/jc.2010-2822. [DOI] [PubMed] [Google Scholar]
- 67.Buse J, Montanya E, Sesti G, et al. Frequency and magnitude of antibody formation are lower with liraglutide than exenatide: LEAD-6 results [Abstract] Diabetes Care. 2010;59(Suppl 1):184. [Google Scholar]