Abstract
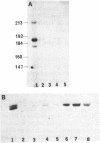
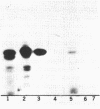
We have used photoaffinity labelling to examine the chloroplast RNA polymerase components which come into contact with nascent transcripts during the in vitro transcription of plastid DNA. The transcripts were synthesized in the presence of a photoactive analogue (4-thio UTP) and alpha-32P-ATP, using enriched pea chloroplast RNA polymerase preparation and a recombinant plasmid containing the plastid 16S rRNA promoter. Brief irradiation of the transcriptional complex crosslinked the photoactive nascent RNA to proximal proteins. Labelling of the transcriptional complex was dependent on 4-thio UTP and template DNA. Two polypeptides of 51 and 54 kDa were consistently crosslinked to the nascent transcripts; about 60% of the total radioactivity of the crosslinked RNA was associated with these polypeptides. In some experiments, two additional polypeptides of 38 and 75 kDa were also found to be associated with about 13% and 17% of the total crosslinked RNA radioactivity, respectively. The UV-crosslinked transcriptional complexes were stable to either DNase or S1 nuclease hydrolysis but partially sensitive to RNase T1. Insensitivity of the complex to hydrolysis with RNase H suggested that the nascent transcripts were not crosslinked to the template. The complexes could also be hydrolysed by proteinase K and thermolysin. No crosslinkage was observed when labelled RNA molecules containing 4-thio UMP residues were added after synthesis to the polymerase preparation. This suggested that the method identified only those polypeptides which came into close contact with the transcript during its synthesis. Antibodies raised against the RNA-protein complex confirmed the presence of the polypeptides in the chloroplast RNA polymerase preparation on Western blots. Preincubation of these antibodies with the chloroplast RNA polymerase inhibited plastid DNA transcription. These data showed that the transcript-binding polypeptides were functional components of the chloroplast transcriptional complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armaleo D., Gross S. R. Purification of the three nuclear RNA polymerases from Neurospora crassa. J Biol Chem. 1985 Dec 25;260(30):16169–16173. [PubMed] [Google Scholar]
- Armaleo D., Gross S. R. Structural studies on Neurospora RNA polymerases and associated proteins. J Biol Chem. 1985 Dec 25;260(30):16174–16180. [PubMed] [Google Scholar]
- Bartholomew B., Dahmus M. E., Meares C. F. RNA contacts subunits IIo and IIc in HeLa RNA polymerase II transcription complexes. J Biol Chem. 1986 Oct 25;261(30):14226–14231. [PubMed] [Google Scholar]
- Bartholomew B., Meares C. F., Dahmus M. E. Photoaffinity labeling of RNA polymerase III transcription complexes by nascent RNA. J Biol Chem. 1990 Mar 5;265(7):3731–3737. [PubMed] [Google Scholar]
- Cadena D. L., Dahmus M. E. Messenger RNA synthesis in mammalian cells is catalyzed by the phosphorylated form of RNA polymerase II. J Biol Chem. 1987 Sep 15;262(26):12468–12474. [PubMed] [Google Scholar]
- Hanna M. M., Meares C. F. Topography of transcription: path of the leading end of nascent RNA through the Escherichia coli transcription complex. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4238–4242. doi: 10.1073/pnas.80.14.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M. M. Photoaffinity cross-linking methods for studying RNA-protein interactions. Methods Enzymol. 1989;180:383–409. doi: 10.1016/0076-6879(89)80113-2. [DOI] [PubMed] [Google Scholar]
- Hu J., Bogorad L. Maize chloroplast RNA polymerase: the 180-, 120-, and 38-kilodalton polypeptides are encoded in chloroplast genes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1531–1535. doi: 10.1073/pnas.87.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleiss M., Ochiai H., Cerutti P. A. Photochemically induced transition of 4-thiouridine to uridine or uridine and cytidine in E. coli transfer ribonucleic acid. Biochem Biophys Res Commun. 1969 Jan 6;34(1):70–76. doi: 10.1016/0006-291x(69)90530-0. [DOI] [PubMed] [Google Scholar]
- Rajasekhar V. K., Sun E., Meeker R., Wu B. W., Tewari K. K. Highly purified pea chloroplast RNA polymerase transcribes both rRNA and mRNA genes. Eur J Biochem. 1991 Jan 1;195(1):215–228. doi: 10.1111/j.1432-1033.1991.tb15697.x. [DOI] [PubMed] [Google Scholar]
- Renart M. F., Sastre L., Díaz V., Sebastián J. Purification and subunit structure of RNA polymerases I and II from Dictyostelium discoideum vegetative cells. Mol Cell Biochem. 1985 Feb;66(1):21–29. doi: 10.1007/BF00231819. [DOI] [PubMed] [Google Scholar]
- Riva M., Schäffner A. R., Sentenac A., Hartmann G. R., Mustaev A. A., Zaychikov E. F., Grachev M. A. Active site labeling of the RNA polymerases A, B, and C from yeast. J Biol Chem. 1987 Oct 25;262(30):14377–14380. [PubMed] [Google Scholar]
- Roberge M., Bradbury E. M. RNA contacts the two large polymerase subunits and a 52-kDa polypeptide in nucleolar RNA polymerase I transcribing complexes. J Biol Chem. 1988 Dec 5;263(34):18553–18557. [PubMed] [Google Scholar]
- Sawadogo M., Sentenac A. RNA polymerase B (II) and general transcription factors. Annu Rev Biochem. 1990;59:711–754. doi: 10.1146/annurev.bi.59.070190.003431. [DOI] [PubMed] [Google Scholar]
- Shuman J. D., Vinson C. R., McKnight S. L. Evidence of changes in protease sensitivity and subunit exchange rate on DNA binding by C/EBP. Science. 1990 Aug 17;249(4970):771–774. doi: 10.1126/science.2202050. [DOI] [PubMed] [Google Scholar]
- Smith H. J., Bogorad L. The polypeptide subunit structure of the DNA-dependent RNA polymerase of Zea mays chloroplasts. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4839–4842. doi: 10.1073/pnas.71.12.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun E., Wu B. W., Tewari K. K. In vitro analysis of the pea chloroplast 16S rRNA gene promoter. Mol Cell Biol. 1989 Dec;9(12):5650–5659. doi: 10.1128/mcb.9.12.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]