Abstract
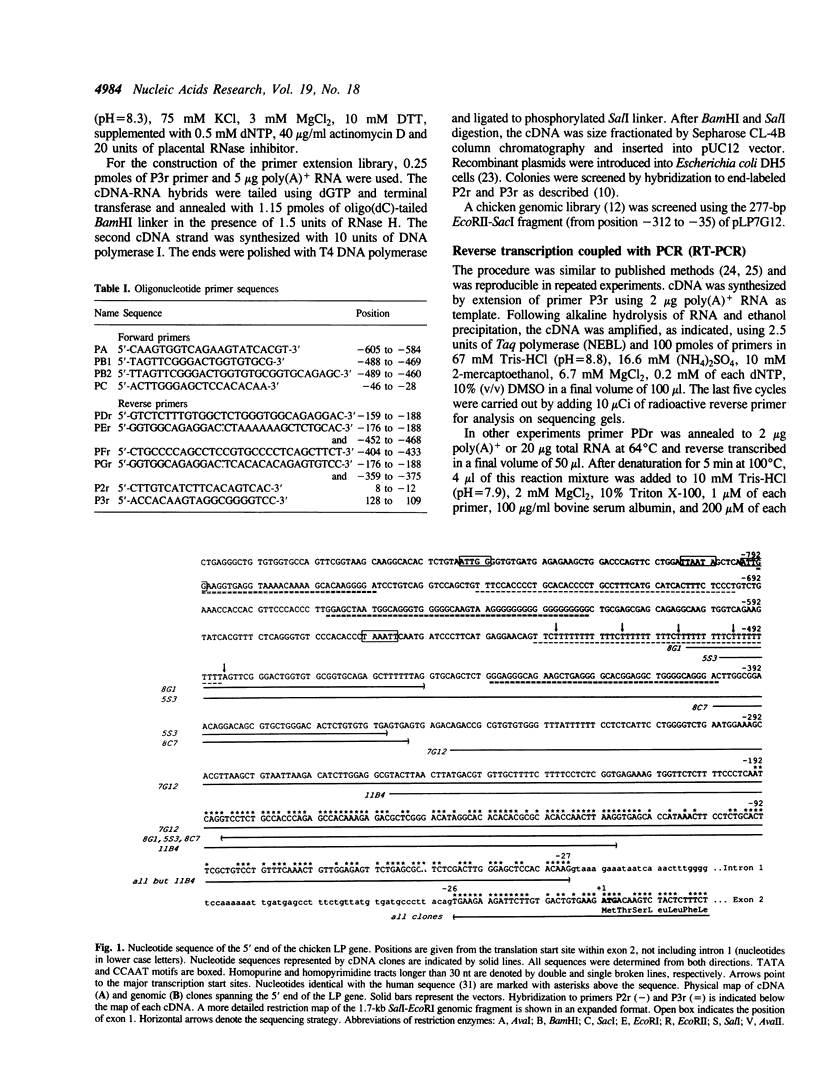
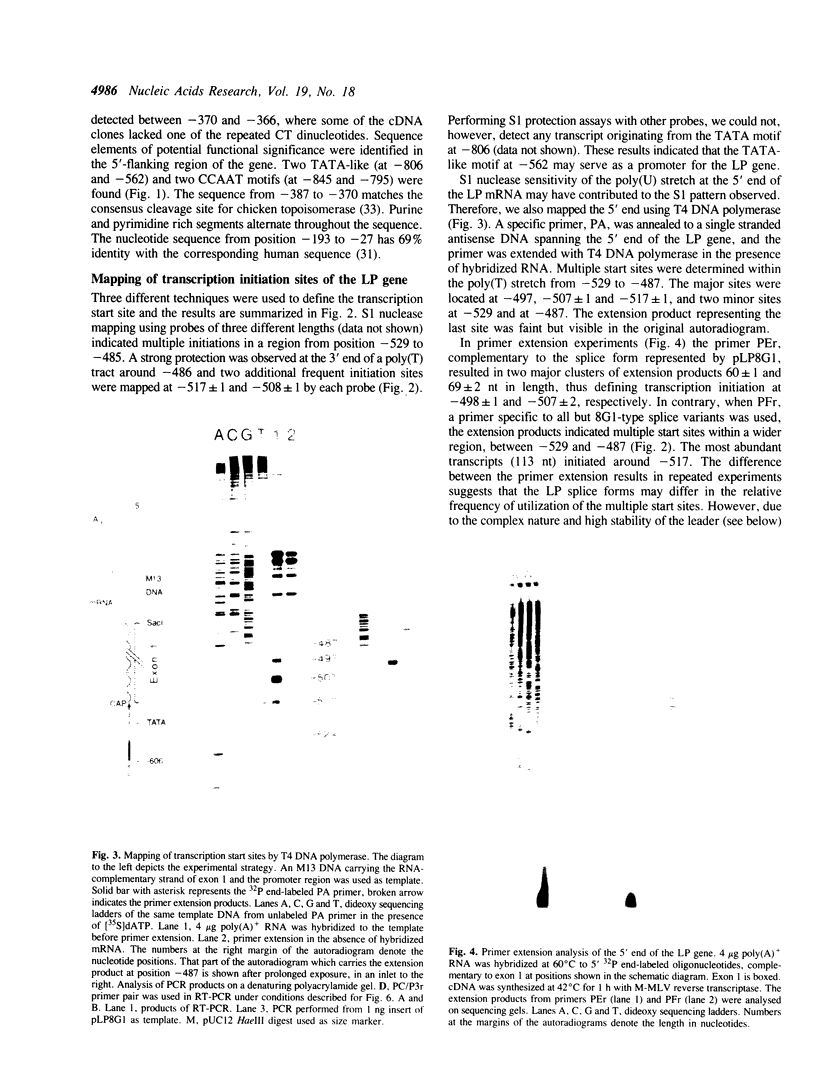
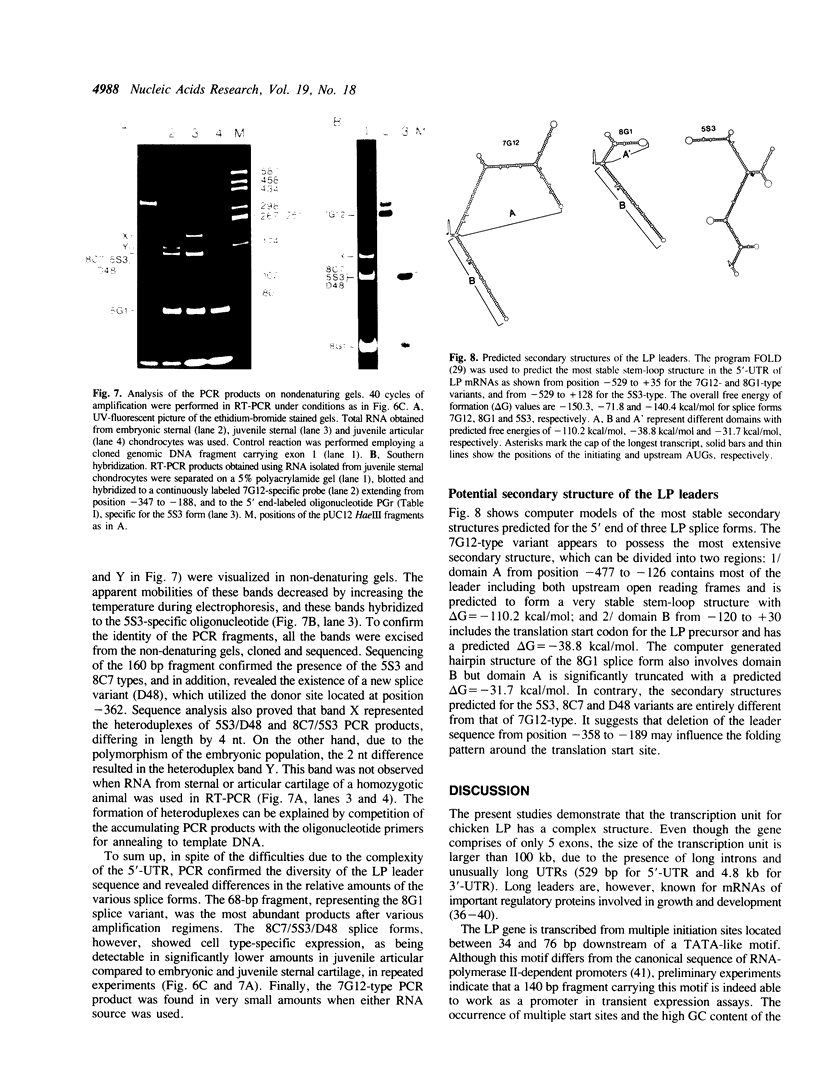
We report here the isolation of the 5' end and the promoter region of the gene for chicken cartilage link protein, and demonstrate extensive heterogeneity of the leader sequence arising from differential utilization of multiple splice sites within the 5'-most exon. The 500-base pairs (bp) exon 1 consists of solely untranslated sequence and is followed by an intron greater than 33 kilobase pairs (kb). Together, the five exons predict a gene size longer than 100 kb. Multiple transcription initiation sites were mapped 34, 46, 56, 66 and 76 bp downstream of a TATA-like motif. Sequence analysis revealed that in addition to the non-spliced variant, multiple mRNA species were generated by alternative splicing resulting in the exclusion of 92, 166, 170, 174 and 263 nucleotides (nt), respectively, from exon 1. Polymerase chain reaction confirmed the existence of various splice forms, and showed cell type- and developmental stage-specific expression for one group of them. Secondary structure predictions indicated that the leaders of the splice forms could form stable hairpin structures with different free energies of formation (up to delta G = -110 kcal/mol), suggesting translational control. The splice variant detected in the largest amount had the least stable predicted hairpin (delta G = -31.7 kcal/mol).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andino R., Rieckhof G. E., Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990 Oct 19;63(2):369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Argraves W. S., Deák F., Sparks K. J., Kiss I., Goetinck P. F. Structural features of cartilage matrix protein deduced from cDNA. Proc Natl Acad Sci U S A. 1987 Jan;84(2):464–468. doi: 10.1073/pnas.84.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. R., Caterson B. The isolation and characterization of the link proteins from proteoglycan aggregates of bovine nasal cartilage. J Biol Chem. 1979 Apr 10;254(7):2387–2393. [PubMed] [Google Scholar]
- Bucher P., Trifonov E. N. Compilation and analysis of eukaryotic POL II promoter sequences. Nucleic Acids Res. 1986 Dec 22;14(24):10009–10026. doi: 10.1093/nar/14.24.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- De Luca S., Heinegård D., Hascall V. C., Kimura J. H., Caplan A. I. Chemical and physical changes in proteoglycans during development of chick limb bud chondrocytes grown in vitro. J Biol Chem. 1977 Oct 10;252(19):6600–6608. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F., Kiss I., Sparks K. J., Argraves W. S., Hampikian G., Goetinck P. F. Complete amino acid sequence of chicken cartilage link protein deduced from cDNA clones. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3766–3770. doi: 10.1073/pnas.83.11.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhia J., Hardingham T. E. The primary structure of human cartilage link protein. Nucleic Acids Res. 1990 Mar 11;18(5):1292–1292. doi: 10.1093/nar/18.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fife R. S., Caterson B., Myers S. L. Identification of link proteins in canine synovial cell cultures and canine articular cartilage. J Cell Biol. 1985 Apr;100(4):1050–1055. doi: 10.1083/jcb.100.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell S., Baker J., Caterson B., Heinegård D., Rodén L. Link protein and a hyaluronic acid-binding region as components of aorta proteoglycan. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1823–1831. doi: 10.1016/s0006-291x(80)80111-2. [DOI] [PubMed] [Google Scholar]
- Goetinck P. F., Stirpe N. S., Tsonis P. A., Carlone D. The tandemly repeated sequences of cartilage link protein contain the sites for interaction with hyaluronic acid. J Cell Biol. 1987 Nov;105(5):2403–2408. doi: 10.1083/jcb.105.5.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grens A., Scheffler I. E. The 5'- and 3'-untranslated regions of ornithine decarboxylase mRNA affect the translational efficiency. J Biol Chem. 1990 Jul 15;265(20):11810–11816. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Hu M. C., Davidson N. Mapping transcription start points on cloned genomic DNA with T4 DNA polymerase: a precise and convenient technique. Gene. 1986;42(1):21–29. doi: 10.1016/0378-1119(86)90146-0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Glick A., Sporn M. B., Roberts A. B. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989 Jan 5;264(1):402–408. [PubMed] [Google Scholar]
- Kiss I., Deák F., Holloway R. G., Jr, Delius H., Mebust K. A., Frimberger E., Argraves W. S., Tsonis P. A., Winterbottom N., Goetinck P. F. Structure of the gene for cartilage matrix protein, a modular protein of the extracellular matrix. Exon/intron organization, unusual splice sites, and relation to alpha chains of beta 2 integrins, von Willebrand factor, complement factors B and C2, and epidermal growth factor. J Biol Chem. 1989 May 15;264(14):8126–8134. [PubMed] [Google Scholar]
- Kiss I., Deák F., Mestrić S., Delius H., Soos J., Dékány K., Argraves W. S., Sparks K. J., Goetinck P. F. Structure of the chicken link protein gene: exons correlate with the protein domains. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6399–6403. doi: 10.1073/pnas.84.18.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K., Sullivan M., Yamada Y. Structure of the promoter of the rat type II procollagen gene. J Biol Chem. 1985 Apr 10;260(7):4441–4447. [PubMed] [Google Scholar]
- Kozak M. A profusion of controls. J Cell Biol. 1988 Jul;107(1):1–7. doi: 10.1083/jcb.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Glédic S., Périn J. P., Bonnet F., Jollès P. Identity of the protein cores of the two link proteins from bovine nasal cartilage proteoglycan complex. Localization of their sugar moieties. J Biol Chem. 1983 Dec 25;258(24):14759–14761. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson K. E., Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9774–9777. doi: 10.1073/pnas.86.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. C., Park K., Lopez-Casillas F., Kim K. H. Structural features of the acetyl-CoA carboxylase gene: mechanisms for the generation of mRNAs with 5' end heterogeneity. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4042–4046. doi: 10.1073/pnas.86.11.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzella J. M., Blackshear P. J. Regulation of rat ornithine decarboxylase mRNA translation by its 5'-untranslated region. J Biol Chem. 1990 Jul 15;265(20):11817–11822. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Yamasaki T., Noguchi T., Tanaka T., Kono N., Tarui S. Evidence for alternative RNA splicing and possible alternative promoters in the human muscle phosphofructokinase gene at the 5' untranslated region. Biochem Biophys Res Commun. 1990 Jan 30;166(2):637–641. doi: 10.1016/0006-291x(90)90856-i. [DOI] [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. The primary structure of link protein from rat chondrosarcoma proteoglycan aggregate. J Biol Chem. 1986 Mar 15;261(8):3519–3535. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Brown M., Dziewiatkowski D. D. The link protein in proteoglycan aggregates from the Swarm rat chondrosarcoma. J Biol Chem. 1977 Sep 25;252(18):6470–6477. [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Peterson G. L., Smith D. H., Ashkenazi A., Ramachandran J., Schimerlik M. I., Capon D. J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987 May 1;236(4801):600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Cöster L., Hassell J. R. Mammalian eyes and associated tissues contain molecules that are immunologically related to cartilage proteoglycan and link protein. J Cell Biol. 1982 Jun;93(3):910–920. doi: 10.1083/jcb.93.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst F., Rosenberg M. P., Iyer A., Kaul K., Vande Woude G. F. c-mos proto-oncogene RNA transcripts in mouse tissues: structural features, developmental regulation, and localization in specific cell types. Mol Cell Biol. 1987 May;7(5):1629–1637. doi: 10.1128/mcb.7.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Thurieau C., Jollès P. Link protein interactions with hyaluronate and proteoglycans. Characterization of two distinct domains in bovine cartilage link proteins. J Biol Chem. 1987 Sep 25;262(27):13269–13272. [PubMed] [Google Scholar]
- Rao C. D., Pech M., Robbins K. C., Aaronson S. A. The 5' untranslated sequence of the c-sis/platelet-derived growth factor 2 transcript is a potent translational inhibitor. Mol Cell Biol. 1988 Jan;8(1):284–292. doi: 10.1128/mcb.8.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A., Goldstein J. L., Brown M. S. Multiple mRNAs for 3-hydroxy-3-methylglutaryl coenzyme A reductase determined by multiple transcription initiation sites and intron splicing sites in the 5'-untranslated region. J Biol Chem. 1985 Aug 25;260(18):10369–10377. [PubMed] [Google Scholar]
- Rhodes C., Doege K., Sasaki M., Yamada Y. Alternative splicing generates two different mRNA species for rat link protein. J Biol Chem. 1988 May 5;263(13):6063–6067. [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Poole A. R., Mort J. S. The heterogeneity of link proteins isolated from human articular cartilage proteoglycan aggregates. J Biol Chem. 1982 Oct 25;257(20):11908–11914. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzner J. R., Muller M. T. A consensus sequence for cleavage by vertebrate DNA topoisomerase II. Nucleic Acids Res. 1988 Jun 24;16(12):5533–5556. doi: 10.1093/nar/16.12.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe N. S., Dickerson K. T., Goetinck P. F. The chicken embryonic mesonephros synthesizes link protein, an extracellular matrix molecule usually found in cartilage. Dev Biol. 1990 Feb;137(2):419–424. doi: 10.1016/0012-1606(90)90266-l. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Sheehan J. K., Nieduszynski I. A. A study of the interaction between cartilage proteoglycan and link protein. Biochem J. 1987 Dec 15;248(3):943–951. doi: 10.1042/bj2480943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Blackshear P. J. Rat ornithine decarboxylase gene. Nucleotide sequence, potential regulatory elements, and comparison to the mouse gene. J Biol Chem. 1989 May 25;264(15):9016–9021. [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Angel R. M., Papavassiliou A. G., Fernández-Tomás C., Silverstein S. J., Racaniello V. R. Cell proteins bind to multiple sites within the 5' untranslated region of poliovirus RNA. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8299–8303. doi: 10.1073/pnas.86.21.8299. [DOI] [PMC free article] [PubMed] [Google Scholar]







