Abstract
Intracellular accumulation of altered proteins, including p62 and ubiquitinated proteins, is the basis of most neurodegenerative disorders. The relationship among the accumulation of altered proteins, autophagy, and spinal cord dysfunction by cervical spondylotic myelopathy has not been clarified. We examined the expression of p62 and autophagy markers in the chronically compressed spinal cord of tiptoe-walking Yoshimura mice. In addition, we examined the expression and roles of p62 and autophagy in hypoxic neuronal cells. Western blot analysis showed the accumulation of p62, ubiquitinated proteins, and microtubule-associated protein 1 light chain 3 (LC3), an autophagic marker, in the compressed spinal cord. Immunohistochemical examinations showed that p62 accumulated in neurons, axons, astrocytes, and oligodendrocytes. Electron microscopy showed the expression of autophagy markers, including autolysosomes and autophagic vesicles, in the compressed spinal cord. These findings suggest the presence of p62 and autophagy in the degenerated compressed spinal cord. Hypoxic stress increased the expression of p62, ubiquitinated proteins, and LC3-II in neuronal cells. In addition, LC3 turnover assay and GFP-LC3 cleavage assay showed that hypoxic stress increased autophagy flux in neuronal cells. These findings suggest that hypoxic stress induces accumulation of p62 and autophagy in neuronal cells. The forced expression of p62 decreased the number of neuronal cells under hypoxic stress. These findings suggest that p62 accumulation under hypoxic stress promotes neuronal cell death. Treatment with 3-methyladenine, an autophagy inhibitor decreased the number of neuronal cells, whereas lithium chloride, an autophagy inducer increased the number of cells under hypoxic stress. These findings suggest that autophagy promotes neuronal cell survival under hypoxic stress. Our findings suggest that pharmacological inducers of autophagy may be useful for treating cervical spondylotic myelopathy patients.
Keywords: p62, autophagy, cervical spondylotic myelopathy, tiptoe-walking Yoshimura (twy) mice, ubiquitinated proteins
Introduction
Cervical spondylotic myelopathy is the most common cause of spinal cord dysfunction by neurodegeneration in people older than 55. The pathology of cervical myelopathy of the spinal cord consists of irreversible neurodegenerative changes, including neuronal loss, axonal degeneration and myelin destruction.1 Although the underlying pathocellular processes of cervical myelopathy remain uncertain, ischemia of the cord, resulting from mechanical compression, affects the clinical manifestations of myelopathy.2-4
The intracellular accumulation of altered proteins is the basis of most neurodegenerative disorders. Altered proteins are usually organized in the form of toxic multimeric complexes that eventually promote neuronal death. Several reports have described p62, which is also named sequestosome 1 (SQSTM1), as a common component of protein aggregates, which are found in protein aggregation diseases, including Lewy bodies in Parkinson disease, neurofibrillary tangles in Alzheimer disease, and huntingtin aggregates.5-7 p62 is a multifunctional protein that interacts with a central component of the autophagy machinery, autophagic marker microtubule-associated protein 1 light chain 3 (LC3), and transports altered proteins to degradation by autophagy. Autophagy has a major housekeeping function, renewal of cellular structures and removal of altered proteins and damaged organelles.8,9 The expression and role of p62 and autophagy in cervical spondylotic myelopathy has not been clarified. In addition, the role of p62 and autophagy in hypoxic neuronal cells has not been examined well. In this report, we examined the expression of p62 and autophagy markers in the chronically compressed spinal cord using tiptoe-walking Yoshimura (twy) mice, which are an animal model of cervical spondylotic myelopathy.10 In addition, we examined the expression and roles of p62 and autophagy in hypoxic neuronal cells.
Results
Accumulation of p62, ubiquitinated proteins, and LC3 in compressed spinal cord
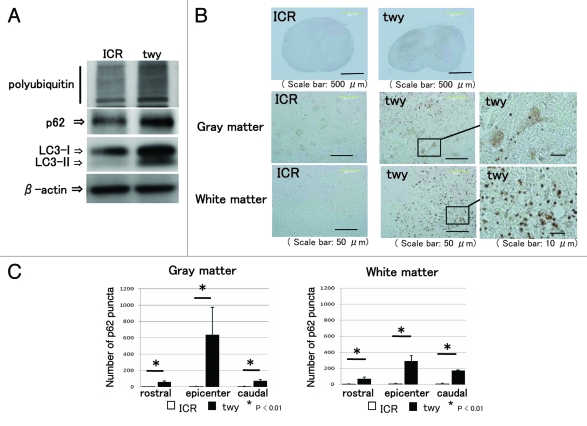
We examined the expression of p62, ubiquitinated proteins, and LC3 in the compressed spinal cord. Western blot analysis indicated that the expression levels of ubiquitinated proteins and p62 were upregulated in the compressed spinal cord of twy mice compared with the spinal cord of ICR mice (Fig. 1A). p62 binds to ubiquitinated proteins and LC3, and p62 and the ubiquitinated proteins are subsequently degraded by autophagy.11,12 Western blot analysis showed that the expression of LC3-I and LC3-II was increased in the compressed spinal cord of twy mice (Fig. 1A). These findings suggest that the accumulation of p62, ubiquitinated proteins, and LC3 in the compressed spinal cord.
Figure 1.
Accumulation of p62, ubiquitinated protein, and LC3 in compressed spinal cord. (A) The level of protein expression in spinal cords obtained from tiptoe-walking Yoshimura (twy) mice and control mice was examined by western blot analysis. Western blot analysis showed the increased expression of p62, ubiquitinated protein, and LC3 in twy mice. (B) Immunohistochemical examination was performed using cervical spinal cord prepared from ICR mice and twy mice. Immunohistochemical examinations revealed that p62 immunoreactivity was observed as a punctate structure in the gray matter and white matter of the spinal cord of twy mice. (C) The number of p62 puncta was significantly increased in the 2-mm rostral site, the epicenter site, and the 2-mm caudal site of the gray matter and white matter in spinal cords of twy mice. These experiments were performed in triplicate with similar results (*p < 0.01; error bars represent SD).
Upregulation of p62 in degenerated areas of spinal cord
Nissl staining showed that the number of neurons was significantly decreased in the gray matter of the epicenter site and the 2-mm caudal site in the spinal cords of twy mice (Fig. S1A and C). In addition, Luxol fast blue (LFB) staining showed that axons were demyelinated and degenerated in the white matter of the 2-mm rostral site, the epicenter site, and the 2-mm caudal site in the spinal cords of twy mice (Fig. S1B and D). Furthermore, immunohistochemical examination using the hypoxic marker hypoxia-inducible factor-1 α (Hif1a) showed that Hif1a was increased in the nucleus of twy mice spinal cords (Fig. S2A and B). These findings suggest that compressed spinal cords of twy mice are demyelinated, degenerated, and hypoxic. In order to examine the expression of p62 in the compressed spinal cords, we performed immunohistochemistry. Immunohistochemical examination revealed that p62 immunoreactivity was exhibited as a puncta structure in the gray matter and white matter of the spinal cord of twy mice (Fig. 1B). To confirm the immunoreactivity of p62, we performed immunohistochemical analysis by using a different antibody that recognizes another epitope of p62. p62 immunoreactivity for these two antibodies was the same (Fig. S2B). The number of p62 puncta was significantly increased in the gray and white matter of the 2-mm rostral site, the epicenter site, and the 2-mm caudal site of the spinal cords of twy mice (Fig. 1C).
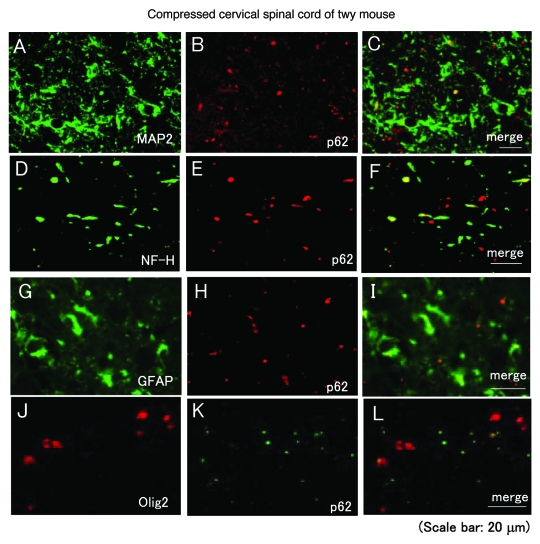
p62 is expressed in neurons, axons, astrocytes, and oligodendrocytes
In order to determine the cells containing p62, we performed an immunohistochemical examination using cell markers. An immunohistochemical examination showed that p62 was detected in MAP2-positive neurons (Fig. 2A–C), NF-H positive axons (Fig. 2D–F), GFAP-positive astrocytes (Fig. 2G–I), and olig2-positive oligodendrocytes (Fig. 2J–L).
Figure 2.
p62 is expressed in neurons, axons, astrocytes, and oligodendrocytes. Immunohistochemical examinations were performed using compressed spinal cords prepared from twy mice. Immunohistochemical examinations showed that p62 was detected in MAP2-positive neurons (A–C) (MAP2: green, p62: red), NF-H positive axons (D–F) (NF-H: green, p62: red), GFAP-positive astrocytes (D–F) (GFAP: green, p62: red), and olig2-positive oligodendrocytes (G–I) (olig2: red, p62: green). These experiments were performed in triplicate with similar results.
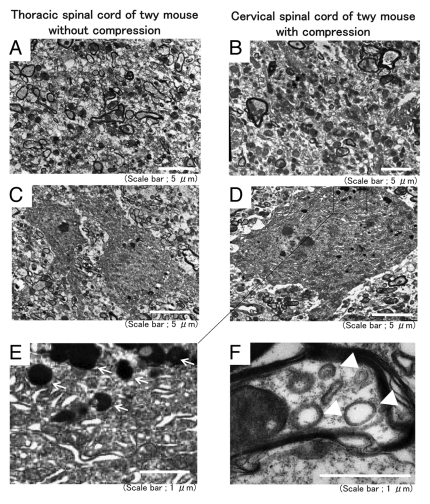
Compressed spinal cord exhibits autolysosomes and autophagosomes
Since the visualization of autophagic vesicles by electron microscopy is still considered the gold standard for demonstrating autophagy, we performed electron microscopic examinations.13 An electron microscopic examination of compressed spinal cord showed that the number of axons was decreased in the compressed cervical spinal cord compared with the thoracic spinal cord in the twy mouse (Fig. 3A and B). Neurons exhibited autolysosomes, which are a feature of autophagy, in the compressed spinal cord (Fig. 3D and E) but normal thoracic spinal cords (Fig. 3C).14 The compressed spinal cord exhibited the double-membrane structures in axons that are characteristic of autophagosomes (Fig. 3F). These findings suggest the presence of autophagy in the degenerated compressed spinal cord.
Figure 3.
Compressed spinal cord showed autolysosomes and autophagosomes. Electron microscopic examinations were performed using compressed cervical spinal cord and thoracic spinal cord without compression prepared from twy mice. Electron microscopic examinations showed that axons were decreased at compressed sites of the cervical spinal cord (B) compared with the thoracic spinal cord (A) in twy mice. Neurons showed autolysosomes in the compressed spinal cord (D and E) but thoracic spinal cord (C). The compressed spinal cord showed double-membrane structures, which are characteristic of autophagosomes in the axons (F).
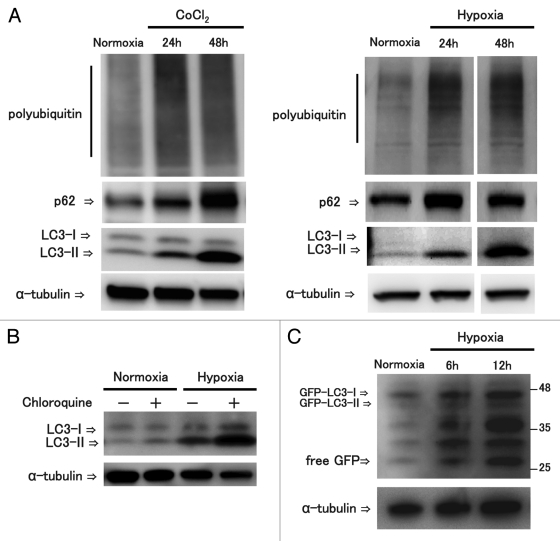
Hypoxic stress increased the expression of p62, ubiquitinated proteins, LC3-II, and autophagy flux in neuronal cells
Because hypoxia caused by ischemia of the cord affects the clinical manifestations of myelopathy resulting from mechanical compression,2-4,15 we examined the expression of p62 and ubiquitinated proteins in neuronal cells under hypoxic stress. We used the CoCl2-chemical hypoxia model and the anaeropack hypoxia model. Both hypoxic stresses increased hypoxic markers, Hif1a and Glut1 (Fig. S3A). Hypoxia models showed that hypoxic stress increased the expression of polyubiquitinated proteins, p62 protein, and LC3-II in neuronal cells (Fig. 4A). In order to assess autophagy flux, we performed LC3 turnover assay by examining the LC3-II expression level in the presence of the lysosomal inhibitor chloroquine. LC3 turnover assay showed that chloroquine treatment increased the amount of LC3-II expression under hypoxic stress (Fig. 4B). To confirm the autophagy flux, we performed western blot analysis of the free green fluorescence protein (GFP) fragments resulting from the degradation of GFP-LC3 within the autolysosome. It has been reported that the response of GFP to lysosomal degradation is more resistant than that of LC3; therefore, free GFP has been suggested to be an indicator of functional autophagy flux.16,17 Western blot analysis showed that hypoxic stress increased the free GFP fragment in neuronal cells (Fig. 4C). Furthermore, histochemical examination showed the number of GFP-LC3 puncta was increased in hypoxic conditions (Fig. S3B). In addition, the number of GFP-LC3 puncta was significantly increased in the presence of chloroquine under normoxic or hypoxic conditions (Fig. S3C). These findings suggest that hypoxic stress increased p62 and autophagy in neuronal cells.
Figure 4.
Hypoxic stress increased the expression of P62, ubiquitinated proteins, LC3-II, and autophagy flux in neuronal cells. For hypoxia examinations, neuronal cells were treated with the CoCl2 induced hypoxic stress or the anaeropack induced hypoxic stress. Western blot analysis showed that hypoxic stress increased the expression of ubiquitinated proteins, p62 and LC3-II in neuronal cells (A). To examine the autophagy flux, LC3 turn over assay was performed. LC3 turnover assay showed that chloroquine, a lysosomal inhibitor, treatment increased the amount of LC3-II under hypoxic stress (B). Following transfection of GFP-LC3, GFP-LC3 cleavage assay was performed. Western blot analysis showed that hypoxic stress increased the free GFP fragment in neuronal cells. These experiments were performed in triplicate with similar results.
Forced expression of p62 decreased neuronal cell proliferation
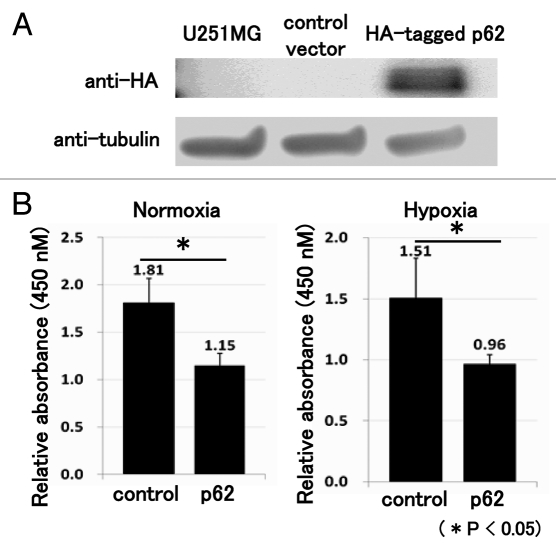
In order to examine the effect of p62 accumulation in neuronal cells, we performed a WST assay following the forced expression of p62. We confirmed the transfection efficiency by the anti-HA antibody (Fig. 5A). The WST assay showed that the forced expression of p62 decreased the number of neuronal cells under normoxia and hypoxic stress (Fig. 5B).
Figure 5.
The forced expression of p62 decreased the number of neuronal cells. Following transfection of HA-tagged p62 expression vector, western blot analysis revealed that cells transfected with the HA-tagged p62 expression vector reacted positively with the anti-HA antibody (A). Three days after transfection, a WST assay was performed. The WST assay showed that forced expression of p62 decreased the number of neuronal cells under normoxia and hypoxic stress (B). These experiments were performed in triplicate with similar results (*p < 0.05; error bars represent SD).
Autophagy increased the number of neuronal cells under hypoxic stress
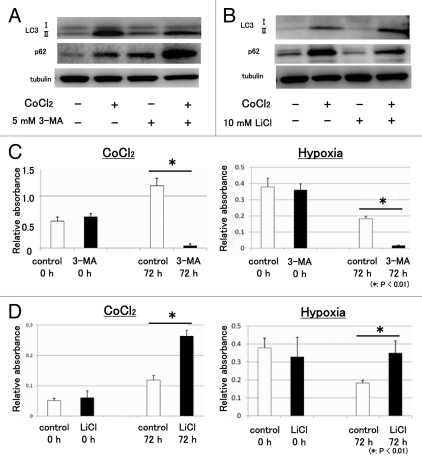
Autophagy contributes to the degradation of damaged proteins such as damaged mitochondria, midbody rings, peroxisomes, microbes, and p62.18-22 In order to examine the function of autophagy in neuronal cells under hypoxic stress, we used 3-MA, an autophagy inhibitor, and LiCl, an autophagy inducer.23 Western blot analysis showed that 3-MA decreased the expression of LC3-II under hypoxic stress. In addition, western blot analysis showed that 3-MA increased the expression of p62 under normoxia and hypoxic stress (Fig. 6A). In contrast, LiCl increased the expression of LC3-II under hypoxic stress. In addition, LiCl decreased the expression of p62 under normoxia and hypoxic stress (Fig. 6B). These findings suggest that autophagy degraded p62 under hypoxic stress. The WST assay showed that 3-MA treatment decreased the number of neuronal cells under hypoxic stress (Fig. 6C). In contrast, LiCl treatment increased the number of neuronal cells under hypoxic stress (Fig. 6D). These findings suggest that autophagy promoted neuronal cell survival under hypoxic stress.
Figure 6.
Autophagy increased the number of neuronal cells under hypoxic stress. In order to examine the function of autophagy in neuronal cells under hypoxic stress, we used 3-methyladenine (3-MA), an autophagy inhibitor, and lithium chloride (LiCl), an autophagy activator. (A) Western blot analysis showed that 3-MA (5 mM) decreased the expression of LC3-II under hypoxic stress. 3-MA increased the expression of p62 under normoxia and hypoxic stress. (B) LiCl (10 mM) increased the expression of LC3-II under hypoxic stress. LiCl decreased the expression of p62 under normoxia and hypoxic stress. (C) The WST assay showed that 3-MA treatment decreased the number of neuronal cells under hypoxic stress induced by CoCl2 and hypoxia. (D) LiCl treatment increased the number of neuronal cells under hypoxic stress. These experiments were performed in triplicate with similar results (*p < 0.01; error bars represent SD).
Discussion
Compression on the spinal cord microvasculature causes hypoxia and infarction of the gray matter and demyelination in the posterior and lateral columns.24 The precise molecular mechanisms for the progressive loss of neurons, oligodendrocytes, and demyelination in the spinal cord in cervical spondylotic myelopathy remains uncertain, although there is speculation that hypoxia caused by ischemia, excitotoxicity, and oxidative stress could result in the induction of apoptosis in cervical spondylotic myelopathy.25 The pathology of patients with cervical spondylotic myelopathy has been reported to include Wallerian degeneration of the posterior columns cephalad to the site of compression and of the corticospinal tracts caudal to the site of compression.15,26,27 We showed that the expression of p62 puncta was increased with neuronal loss in the gray matter of the caudal and epicenter sites. In white matter, the p62 puncta were observed to spread to the epicenter site and to the caudal site of the compressed site, along with demyelination and degeneration. These findings suggest that the distribution of p62 was consistent with the pathologic neurodegenerative changes of cervical spondylotic myelopathy. p62 has been reported to be a common component of protein aggregation in neurodegenerative diseases and to cause neuronal loss and axon degeneration in the brain.6,28,29 These findings suggest that the accumulation of p62 in chronically compressed spinal cord is related to the destructive pathologic changes. In addition, p62 is a stress response protein that is upregulated by exposure to sodium arsenite, cadmium, ionophores, proteasome inhibitors, and the overexpression of abnormal protein aggregation.7,28,30-32 Consistent with these findings, we showed that hypoxic stress upregulated p62 in neuronal cells. In addition, the forced expression of p62 decreased the number of neuronal cells. These findings suggest that the upregulation of p62 contributed to neuronal loss in the compressed spinal cord.
p62 associates with polyubiquitinated proteins and aggregates through its ubiquitin-binding domain.11,33,34 We showed that, in addition to p62, ubiquitinated proteins were upregulated in compressed spinal cord. In addition, we showed that ubiquitinated proteins were upregulated in neuronal cells under hypoxic stress. Ubiquitinated protein aggregates are observed in almost every neurodegenerative disease, which is suggestive of possible dysfunction in the ubiquitin proteasome system. The significance of the accumulation of ubiquitinated protein aggregates in the central nervous system of those affected with different neurodegenerative diseases is unclear.35 Further examinations of the function of ubiquitinated proteins in compressed spinal cord are needed.
p62 associates with LC3-II through its LC3-interacting region.34 Recently, it has been reported that the suppression of neuronal autophagy leads to the accumulation of p62 and damaged mitochondria, neurodegenerative changes, progressive axon degeneration, and neuronal cell death.12,34,36-41 We showed that LC3-II an autophagy marker is upregulated in compressed spinal cord and neuronal cells under hypoxic stress. In addition, electron microscopic examinations showed the existence of autolysosomes and autophagosomes in compressed spinal cord. Furthermore, autophagy flux is upregulated by hypoxic stress in neuronal cells. These findings suggest that autophagy is upregulated in the compressed spinal cord. Autophagy is activated in neurons exposed to hypoxic or excitotoxic stimuli as well as after closed head injury or cerebral ischemia.42-46 It is still controversial, however, whether enhanced autophagy in neurodegeneration represents a mechanism of cell death or a rescue mechanism, since there is evidence that autophagy can function both as a survival and as a death mechanism.47,48 It has been reported that the upregulation of autophagic protein degradation protects against neurological dysfunction in neurodegenerative disease models, including polyglutamine disease and Parkinson disease.49,50 Furthermore, protective roles of autophagy have been reported in traumatic brain injury.46,51,52 Conversely, autophagy has been shown to promote cell death in neuronal injury.53,54 In order to address this question, we treated neuronal cells with 3-MA, an autophagy inhibitor, or LiCl, an autophagy inducer. Although it should be considered that the pharmacological approach that we used to modulate autophagy may generate changes in other cell signaling, our findings suggest that autophagy participated in neuronal cell protection.
We performed real-time PCR to examine whether hypoxic stress increase p62 transcription. Real-time PCR showed that the expression of p62 and LC3 mRNA was not increased under hypoxic stress (data not shown). It has been reported that p62 and LC3 are constitutively transcribed in sufficient amounts, and their post-transcriptional modifications and associations with other autophagy machinery, rather than transcription, appears to be critical for protein accumulation.13,55 Further examinations should be performed to clarify the molecular mechanisms of hypoxia-associated accumulations of autophagy-associated proteins.
In conclusion, we showed the accumulation of p62 and LC3-II in the chronically compressed spinal cord. The forced expression of p62 and the inhibition of autophagy decreased the number of neuronal cells. Induction of autophagy participates in neuronal cell protection. These findings suggest that the pharmacological induction of autophagy may be a useful approach for treating cervical spondylotic myelopathy patient.
Materials and Methods
Mouse model of chronic spinal cord compression
The twy mouse displays spontaneous calcified deposits posterolaterally at the C1-C2 vertebral level. The deposits compress the cervical cord progressively with age, resulting in profound motor paresis 16–24 weeks after birth. This experiment was conducted on ten 24-week-old male twy mice (Central Institute for Experimental Animals), which display neurological dysfunction, and ten 24-week-old imprinting control region (ICR) mice, which served as controls.
Tissue preparation
For immunohistochemical analysis, the mice (n = 5 per group) were perfused with normal saline and fixed with 4% paraformaldehyde (Wako, 162-16065) under intraperitoneal anesthesia. The spinal cords were embedded in paraffin and 4-µm transverse sections were prepared. We divided the cervical spinal cord into three segments: the rostral site, which is the segment between C1 and C2 that is 2 mm rostral to the compressive lesion; the epicenter site, the most compressed segment, which is between C2 and C3; and the caudal site, the segment between C3 and C4 that is 2 mm caudal to the compressive lesion. For western blot analysis, the 10-mm cervical spinal cords of twy and ICR mice (n = 3 per group) were dissected and homogenized in T-PER (Pierce Protein Research Products, 78510). All experimental procedures were performed in compliance with the Guidelines for the Care and Use of Animals described in the American Journal of Physiology and with the Guidelines established by the Institute of Laboratory Animal Sciences, Faculty of Medicine, Kagoshima University. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize possible alternatives to in vivo techniques.
Histological analysis
Sections prepared from twy and ICR mice (n = 5 per group) were stained with Luxol fast blue (LFB; Muto Pure Chemicals Co., 080618) and areas of myelination in the white matter of the spinal cord were examined. Nissl staining by 10% cresyl violet (Muto Pure Chemicals Co., 080818) was also performed in order to evaluate the number of neural cells in the gray matter. An image analysis system (Mitani Corporation, Lumina Vision) was used to count the number of Nissl-positive cells and the percentage of LFB-positive areas.
Antibodies
The following primary antibodies were used: rabbit anti- p62 (Wako, 018-22141), rabbit anti-p62 (Sigma Aldrich, P0067), mouse anti-LC3 (Cosmo Bio Co, CTB-LC3-1-50), mouse anti-ubiquitinated protein (Millipore, 04-263), mouse anti-microtubule associated protein-2 (MAP2; Chemicon, MAB3418), mouse anti-phosphorylated high molecular weight neurofilament protein (NF-H; Abcam, 7795-100), mouse anti-glial fibrillary acidic protein (GFAP; Dako, M0761), goat anti-olig2 (Santa Cruz Biotechnology, 19969) rabbit anti-Hif1a (Abnova, PAB12138), rabbit anti-Glut1 (Bioworld Technology, BS1149), rabbit anti-GFP (Abcam, ab290), rabbit β-actin (Cell Signaling, 4967), and mouse anti-tubulin (Santa Cruz Biotechnology, 23948). The following secondary antibodies were used: Alexa Fluor 568 goat anti-rabbit IgG (Molecular Probes, 514959), Alexa Fluor 488 donkey anti-mouse IgG (Molecular Probes, A21202), Alexa Fluor 546 rabbit anti-goat IgG (Molecular Probes, A21085), Fluorescein-5-isothiocyanate (FITC) goat anti-rabbit IgG (MP Biomedicals, 55646), HRP conjugated anti-rabbit IgG (Cell Signaling, 7074S), and HRP conjugated anti-mouse IgG (Cell Signaling, 7076).
Western blot analysis
Western blot analysis was performed as previously reported.56 Approximately 20 μg of protein were loaded onto 4–12% NuPAGE precast gels (Invitrogen Corporation, NP0322) and transferred to nitrocellulose membranes. The membranes were blocked in Tris-buffered saline/Tween 20 (TBST) buffer [0.01 M TRIS-HCl (Nacalai Tesque, 35406-91), pH 7.5, 0.15 M NaCl (Nacalai Tesque, 313-20), and 0.05% Tween 20 (Sigma Aldrich, 129K0041)] containing 3% milk for 1 h at room temperature. The membrane was incubated with the primary antibody overnight at 4°C, followed by secondary horseradish peroxidase-labeled antibody. Detection was performed using the ECL detection system (GE Healthcare, 396509). Protein bands were visualized with ImageQuant (Fujifilm LAS, 4000 mini).
Immunohistochemistry
Immunohistochemistry was performed as previously reported.57 Sections were treated with methanol (Wako, 137-01823) containing 3% hydrogen peroxide (Wako, 081-04215) for 10 min. The sections were then washed in phosphate-buffered saline (PBS) containing 0.1% Triton X-100 (Wako, 581-81705) for 5 min, blocked with 10% milk for 1 h at room temperature, and incubated with the primary antibody overnight at 4°C. The sections were incubated with peroxidase-labeled polymer-HRP conjugated to anti-rabbit immunoglobulin (Dako, K4003) for 1 h at room temperature and then visualized with 0.02% diaminobenzidine (Thermo Scientific, 1856090). As a negative control, the same procedures were performed without the primary antibody. The number of p62 puncta was counted in the anterior, lateral, and posterior funiculus as well as anterior and posterior horns at rostral, epicenter, and caudal sites, respectively. The images were taken at 20× magnification using a microscope (AX80TR; Olympus, Tokyo Japan). The images were taken with an image analysis system Lumina Vision connected to a microscope (Olympus Corporation, AX80TR). For fluorescence staining, the cells were counterstained with DAPI (Wako, 340-07971). Immunohistochemistry with each second antibody alone without the primary antibody was performed as a control. The sections were examined with a confocal fluorescence microscope (Carl Zeiss, LSM 700).
Electron microscopy
For electron microscopy, twy mice were anesthetized and subjected to intracardiac perfusion with physiological saline. The compressed cervical cord and uncompressed thoracic cord were removed and immersed in 3% glutaraldehyde (Katayama Chemical, A1972) in 0.1 M phosphate buffer (pH 7.4) overnight. The samples were then postfixed with 1% OsO4 (Wako, CDK7227) in 0.1 M phosphate buffer (pH 7.4), rinsed in 10% saccharose (Wako, CEQ013) three times (10 min each), and stained en bloc in 3% aqueous uranyl acetated (Kanto Chemical Co., K21278873) for 1 h at room temperature. Samples were then dehydrated in an ascending series of ethanol concentrations, replaced by propulene oxide (Wako, KWH4855), and embedded in epoxy resin (Nisshin EM Co. Ltd., 3601). Ultrathin sections were stained with uranyl acetate and Reynold’s lead citrate,58 and observed using an electron microscope (Hitachi H-7100, Japan).
Cell culture
The immortalized mouse neural precursor cell line, MEB5, and the U251MG human glioma cell line were provided by the Health Science Research Resource Bank (HSRRB). MEB5 cells were grown in high glucose Dulbecco’s modified Eagle’s medium (DMEM; Sigma Aldrich, D6429) supplemented with 5 ug/mL insulin (Sigma Aldrich, I1882), 10 ng/mL EGF (Pepro Tech Inc., 315-09), 50 ug/mL transferrin (Gibco, 11107), 10 ng/mL biotin (Sigma Aldrich, B4639), and 30 nM Na-selenite (Sigma Aldrich, S5261). U251MG cells were grown in DMEM (Sigma Aldrich, D6046) supplemented with 10% fetal cow serum (Equitech Bio, SFBM30-2239). In most experiments, cells were cultured under normoxic conditions (5% CO2 / 95% air). For hypoxia examinations we used two methods. We used CoCl2 (Sigma Aldrich, c8661), a transition metal that mimics hypoxia. For hypoxia-mimicking experiments, Cells were cultured at 37°C in fresh culture medium in the presence of CoCl2 (250 μM). In addition, we used AnaeroPack System (Mitsubishi Gas Chemical Company, A-07). The AnaeroPack absorbs O2 in the cell culture jar and generates conditions consisting of less than 0.1% O2 concentration. Cells were cultured in fresh culture medium in an AnaeroPack System at 37°C. For autophagy inhibition, cells were cultured in fresh culture medium in the presence of 3-MA (Sigma Aldrich, M9281). For autophagy induction, cells were cultured in fresh culture medium in the presence of LiCl (Nacalai Tesque, 20645-92).
WST assay
We performed a WST assay to measure the metabolic activity of viable cells. Cells were incubated with a substrate for WST-1 (Roche Applied Science, 1644807) for 4 h, washed with PBS, and lysed in order to release formazan from the cells. The cells were then analyzed in a Safire microplate reader (Bio-Rad Laboratories, Model 680).
Vector transfection
The p62 expression vector was kindly provided by Dr. Sudo T.59 Transfection of the expression vector was performed as recommended in the supplier’s protocol using FuGENE 6 as previously reported.60 (Roche Applied Science, 1815091)
LC3 turnover assay
MEB5 cells were incubated in normoxic or hypoxic conditions for 48 h in the presence and absence of 10 μM chloroquine (lysosomal inhibitor, Sigma Aldrich, C6628-25) followed by western blot analysis using LC3 antibodies.
GFP-LC3 cleavage assay
U251MG cells were transfected with GFP-LC3 (Invitrogen, P36235) for 48 h. Cells were incubated under normoxia or hypoxic conditions for 6 h and 12 h. Then, cells were collected and western blot analysis was performed using GFP antibody.
Quantification of GFP-LC3 puncta
The number of GFP-LC3 puncta was quantified in the presence and absence of chloroquine (10 μM), which inhibits lysosomal degradation. U251MG cells were transfected with GFP-LC3 (Invitrogen, P36235) for 48 h and subjected to the indicated normoxia or hypoxia conditions for 12 h. Cells were fixed with 4% paraformaldehyde (Nacalai Tesque, 26123-55) for 30 min. The images of single GFP-LC3-expressing cells were taken at 40× magnification using a microscope (AX80TR; Olympus, Tokyo Japan). The number of GFP-LC3 puncta in a single cell was counted with an image analysis system Lumina Vision system. At least 50 cells were counted for three different trials.
Statistical analyses
The data was examined for statistical differences with an unpaired Student’s t-test. Statistical analyses were performed using Excel (Microsoft Corporation) with an add-in software package (OMS Publishing, Inc., Statcel2). Values in the figures are given as mean [standard deviation (SD)]. For all data, p values < 0.05 were considered significant.
Supplementary Material
Supplementary PDF file supplied by authors.
Acknowledgments
We are grateful to Hui Gao for excellent technical assistance. We wish to thank the Joint-research laboratory of Kagoshima University Graduate School of Medical and Dental Sciences. This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) (C) 19591725, (C) 20591786, (C) 21591919, (C) 21591920, and (C) 22591663.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/17892
References
- 1.Kameyama T, Hashizume Y, Ando T, Takahashi A, Yanagi T, Mizuno J. Spinal cord morphology and pathology in ossification of the posterior longitudinal ligament. Brain. 1995;118:263–78. doi: 10.1093/brain/118.1.263. [DOI] [PubMed] [Google Scholar]
- 2.Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy. Effects of ischemia and compression of the canine cervical spinal cord. J Neurosurg. 1975;43:9–17. doi: 10.3171/jns.1975.43.1.0009. [DOI] [PubMed] [Google Scholar]
- 3.Hukuda S, Wilson CB. Experimental cervical myelopathy: effects of compression and ischemia on the canine cervical cord. J Neurosurg. 1972;37:631–52. doi: 10.3171/jns.1972.37.6.0631. [DOI] [PubMed] [Google Scholar]
- 4.Rao RD, Currier BL, Albert TJ, Bono CM, Marawar SV, Poelstra KA, et al. Degenerative cervical spondylosis: clinical syndromes, pathogenesis, and management. J Bone Joint Surg Am. 2007;89:1360–78. doi: 10.2106/00004623-200706000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Kuusisto E, Salminen A, Alafuzoff I. Early accumulation of p62 in neurofibrillary tangles in Alzheimer's disease: possible role in tangle formation. Neuropathol Appl Neurobiol. 2002;28:228–37. doi: 10.1046/j.1365-2990.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- 6.Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, et al. p62 Is a common component of cytoplasmic inclusions in protein aggregation diseases. Am J Pathol. 2002;160:255–63. doi: 10.1016/S0002-9440(10)64369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaoka U, Kim K, Jana NR, Doi H, Maruyama M, Mitsui K, et al. Increased expression of p62 in expanded polyglutamine-expressing cells and its association with polyglutamine inclusions. J Neurochem. 2004;91:57–68. doi: 10.1111/j.1471-4159.2004.02692.x. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–41. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- 9.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–52. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosoda Y, Yoshimura Y, Higaki S. A new breed of mouse showing multiple osteochondral lesions–twy mouse. Ryumachi. 1981;21(Suppl):157–64. [PubMed] [Google Scholar]
- 11.Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol. 2004;24:8055–68. doi: 10.1128/MCB.24.18.8055-8068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–14. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, et al. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–68. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine. 1998;23:2730–7. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosokawa N, Hara Y, Mizushima N. Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett. 2006;580:2623–9. doi: 10.1016/j.febslet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 19.Pohl C, Jentsch S. Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol. 2009;11:65–70. doi: 10.1038/ncb1813. [DOI] [PubMed] [Google Scholar]
- 20.Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci USA. 2008;105:20567–74. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M, et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol. 2009;11:1233–40. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584:1374–8. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–11. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu YL, Leong JC, Fang D, Woo E, Huang CY, Lau HK. Cervical myelopathy due to ossification of the posterior longitudinal ligament. A clinical, radiological and evoked potentials study in six Chinese patients. Brain. 1988;111:769–83. doi: 10.1093/brain/111.4.769. [DOI] [PubMed] [Google Scholar]
- 25.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- 26.Ogino H, Tada K, Okada K, Yonenobu K, Yamamoto T, Ono K, et al. Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine. 1983;8:1–15. doi: 10.1097/00007632-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Oyanagi K, Takahashi H, Takahashi HE, Ikuta F. Cervical spondylotic myelopathy. Clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine. 1996;21:827–33. doi: 10.1097/00007632-199604010-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kuusisto E, Salminen A, Alafuzoff I. Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport. 2001;12:2085–90. doi: 10.1097/00001756-200107200-00009. [DOI] [PubMed] [Google Scholar]
- 29.Kuusisto E, Kauppinen T, Alafuzoff I. Use of p62/SQSTM1 antibodies for neuropathological diagnosis. Neuropathol Appl Neurobiol. 2008;34:169–80. doi: 10.1111/j.1365-2990.2007.00884.x. [DOI] [PubMed] [Google Scholar]
- 30.Ishii T, Yanagawa T, Yuki K, Kawane T, Yoshida H, Bannai S. Low micromolar levels of hydrogen peroxide and proteasome inhibitors induce the 60-kDa A170 stress protein in murine peritoneal macrophages. Biochem Biophys Res Commun. 1997;232:33–7. doi: 10.1006/bbrc.1997.6221. [DOI] [PubMed] [Google Scholar]
- 31.Nakaso K, Yoshimoto Y, Nakano T, Takeshima T, Fukuhara Y, Yasui K, et al. Transcriptional activation of p62/A170/ZIP during the formation of the aggregates: possible mechanisms and the role in Lewy body formation in Parkinson's disease. Brain Res. 2004;1012:42–51. doi: 10.1016/j.brainres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–91. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donaldson KM, Li W, Ching KA, Batalov S, Tsai CC, Joazeiro CA. Ubiquitin-mediated sequestration of normal cellular proteins into polyglutamine aggregates. Proc Natl Acad Sci USA. 2003;100:8892–7. doi: 10.1073/pnas.1530212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 35.Rogers N, Paine S, Bedford L, Layfield R. Review: the ubiquitin-proteasome system: contributions to cell death or survival in neurodegeneration. Neuropathol Appl Neurobiol. 2010;36:113–24. doi: 10.1111/j.1365-2990.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 36.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–63. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 37.Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang CC, Wang C, Peng X, Gan B, Guan JL. Neural-specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration. J Biol Chem. 2010;285:3499–509. doi: 10.1074/jbc.M109.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr., Iwata J, Kominami E, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci USA. 2007;104:14489–94. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, et al. Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem. 2008;283:22847–57. doi: 10.1074/jbc.M802182200. [DOI] [PubMed] [Google Scholar]
- 41.Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell. 2009;33:505–16. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12:162–76. doi: 10.1038/sj.cdd.4401545. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Han R, Liang ZQ, Wu JC, Zhang XD, Gu ZL, et al. An autophagic mechanism is involved in apoptotic death of rat striatal neurons induced by the non-N-methyl-D-aspartate receptor agonist kainic acid. Autophagy. 2008;4:214–26. doi: 10.4161/auto.5369. [DOI] [PubMed] [Google Scholar]
- 44.Diskin T, Tal-Or P, Erlich S, Mizrachy L, Alexandrovich A, Shohami E, et al. Closed head injury induces upregulation of Beclin 1 at the cortical site of injury. J Neurotrauma. 2005;22:750–62. doi: 10.1089/neu.2005.22.750. [DOI] [PubMed] [Google Scholar]
- 45.Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29:132–41. doi: 10.1016/j.nbd.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–39. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–18. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 48.Erlich S, Shohami E, Pinkas-Kramarski R. Neurodegeneration induces upregulation of Beclin 1. Autophagy. 2006;2:49–51. doi: 10.4161/auto.2156. [DOI] [PubMed] [Google Scholar]
- 49.Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, et al. Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat Chem Biol. 2007;3:331–8. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravikumar B, Rubinsztein DC. Can autophagy protect against neurodegeneration caused by aggregate-prone proteins? Neuroreport. 2004;15:2443–5. doi: 10.1097/00001756-200411150-00001. [DOI] [PubMed] [Google Scholar]
- 51.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–8. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 53.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu CT, Zhu J, Dagda R. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy. 2007;3:663–6. doi: 10.4161/auto.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka M, Setoguchi T, Hirotsu M, Gao H, Sasaki H, Matsunoshita Y, et al. Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br J Cancer. 2009;100:1957–65. doi: 10.1038/sj.bjc.6605060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirotsu M, Setoguchi T, Sasaki H, Matsunoshita Y, Gao H, Nagao H, et al. Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer. 2010;9:5. doi: 10.1186/1476-4598-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–12. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawai K, Saito A, Sudo T, Osada H. Specific regulation of cytokine-dependent p38 MAP kinase activation by p62/SQSTM1. J Biochem. 2008;143:765–72. doi: 10.1093/jb/mvn027. [DOI] [PubMed] [Google Scholar]
- 60.Matsunoshita Y, Ijiri K, Ishidou Y, Nagano S, Yamamoto T, Nagao H, et al. Suppression of Osteosarcoma Cell Invasion by Chemotherapy Is Mediated by Urokinase Plasminogen Activator Activity via Up-Regulation of EGR1. PLoS ONE. 2011;6:e16234. doi: 10.1371/journal.pone.0016234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.