Abstract
Increasing evidence suggests the toxicity of intracellular amyloid β-protein (Aβ) to neurons, as well as the involvement of oxidative stress in Alzheimer disease (AD). Here we show that normobaric hyperoxia (exposure of cells to 40% oxygen for five days), and consequent activation of macroautophagy and accumulation of Aβ within lysosomes, induced apoptosis in differentiated SH-SY5Y neuroblastoma cells. Cells under hyperoxia showed: (1) increased numbers of autophagic vacuoles that contained amyloid precursor protein (APP) as well as Aβ monomers and oligomers, (2) increased reactive oxygen species production, and (3) enhanced apoptosis. Oxidant-induced apoptosis positively correlated with cellular Aβ production, being the highest in cells that were stably transfected with APP Swedish KM670/671NL double mutation. Inhibition of γ-secretase, prior and/or in parallel to hyperoxia, suggested that the increase of lysosomal Aβ resulted mainly from its autophagic uptake, but also from APP processing within autophagic vacuoles. The oxidative stress-mediated effects were prevented by macroautophagy inhibition using 3-methyladenine or ATG5 downregulation. Our results suggest that upregulation of macroautophagy and resulting lysosomal Aβ accumulation are essential for oxidant-induced apoptosis in cultured neuroblastoma cells and provide additional support for the interactive role of oxidative stress and the lysosomal system in AD-related neurodegeneration.
Keywords: Alzheimer disease, amyloid β-protein, amyloid precursor protein, apoptosis, autophagy, lysosomes, oxidative stress
Introduction
Alzheimer disease (AD), the most common age-related neurodegenerative disorder, is characterized by extracellular senile plaques, intracellular neurofibrillary tangles and progressive neurodegeneration.1 Senile plaques are mainly composed of amyloid β-protein (Aβ), a 39–43 amino acid peptide formed due to sequential cleavage of the amyloid precursor protein (APP) by β- and γ-secretases.2 Aβ1–40 and Aβ1–42 are the two most common forms of the peptide, of which the latter is more prone to aggregate, more resistant to degradation and more toxic as compared with Aβ1–40.3 Aβ monomers can bind to each other forming oligomers and eventually fibrils.4 Soluble Aβ oligomers are more toxic than monomeric or fibrillar Aβ, being able to induce cognitive deficits and synaptic loss, and thus contribute to the development of AD.5,6
Extracellular Aβ plaques are believed to originate from intracellular Aβ, the level of which may be associated with cognitive deficits that precede the development of plaques.6,7 Intraneuronal Aβ accumulation at early stages of AD occurs before senile plaque formation and can promote neuronal death.8 There is accumulating evidence that the autophagic-lysosomal system, the principal self-clearance machinery,9-11 plays an important role in this process. Three types of autophagy are described in mammalian cells: macroautophagy, microautophagy and chaperone-mediated autophagy. In macroautophagy, portions of the cytoplasm that can include various macromolecules and also organelles, such as mitochondria, are sequestered within nonacidic double membrane vacuoles called autophagosomes. The latter then fuse with lysosomes—acidic vacuoles containing a variety of hydrolytic enzymes—forming autolysosomes (also called secondary lysosomes) where the sequestered material is degraded. Lysosomes that have not yet received material for degradation are sometimes called primary lysosomes. Autophagosomes and autolysosomes are together called autophagic vacuoles. In microautophagy, macromolecules and probably small organelles enter lysosomes through invagination of the membrane, while in chaperone-mediated autophagy specific proteins are delivered to lysosomes by molecular chaperones, such as Hsp73 (reviewed in refs. 12–14).
The involvement of autophagic-lysosomal system in AD follows from several observations. First, neurons from AD patients contain increased numbers of autophagosomes and lysosomes15 and show increased expression of lysosomal hydrolases,16 indicating activation of the autophagic-lysosomal system in this disorder. Second, Aβ generation has been detected within autophagic vacuoles following activation of macroautophagy.17 Third, Aβ shows partial accumulation within neuronal lysosomes in transgenic mice expressing both human mutant APP and mutant presenilin-1.18 Fourth, exogenous Aβ1–42 is internalized by cultured cells and accumulates within lysosomes, causing lysosomal membrane permeabilization and ensuing apoptotic cell death,19,20 in accordance with the previously demonstrated involvement of lysosomes in apoptosis.21,22
Less than 5% of AD cases are familial early onset AD (FAD) associated with mutations that alter APP processing resulting in enhanced Aβ generation and/or aggregation. The majority of all AD cases belong to late-onset, sporadic AD (SAD).23 Oxidative stress is associated with both normal aging and AD.24,25 It is reported that oxidative damage is one of the earliest changes in AD and plays an important role in the development of the disease.26 Furthermore, Aβ has been shown to exert neurotoxicity by increasing neuronal sensitivity to oxidative stress.27-29 In AD, levels of oxidative stress and protein oxidation increase predominantly in cognition-associated Aβ-rich regions, such as the cortex and hippocampus.30 Evidence indicates that a long, gradual accumulation of oxidative damage precedes the appearance of clinical and pathological AD symptoms, including Aβ deposition, neurofibrillary tangle formation, metabolic dysfunction, and cognitive decline.31 Consistent with the role of oxidative stress in AD pathogenesis, some studies report beneficial effects of antioxidant intake on the risk for AD.32
The relationship between oxidative stress and the autophagic-lysosomal system in AD is not well understood. We have previously shown that mild chronic oxidative stress (normobaric hyperoxia) results in increased numbers of autophagic vacuoles and intralysosomal accumulation of Aβ in retinoic acid (RA) differentiated neuroblastoma cells.33 Furthermore, using human embryonal kidney (HEK) cells, we demonstrated that increased cellular Aβ production is associated with enhanced oxidant-induced intralysosomal Aβ accumulation, causing apoptotic cell death through lysosomal destabilization.34 In this study, we investigated the effects of normobaric hyperoxia and APP overexpression on lysosomal Aβ accumulation and cell viability, using RA-differentiated SH-SY5Y neuroblastoma cells. We show that SH-SY5Y cells overexpressing APP are characterized by both enhanced oxidative stress and enhanced macroautophagy, resulting in increased intralysosomal accumulation of monomers and oligomers of Aβ and consequent apoptosis. Moreover, specific inhibition of the lysosomal degradation and γ-secretase suggest that intralysosomal accumulation of Aβ resulted to a large extent from its macroautophagic uptake, although APP processing within autophagic vacuoles can occur as well.17 The deleterious effects of normobaric hyperoxia were more pronounced in cells overexpressing the Swedish FAD mutation (APPswe) compared with those with wild-type APP (APPwt), and were prevented by the inhibition of macroautophagy using 3-methyladenine (3MA) or siRNA against the autophagy-related protein ATG5. These data provide further support for the interactive roles of oxidative stress and autophagy in AD.
Results
Intracellular APP and the lysosomal system in SH-SY5Y neuroblastoma cells stably transfected with APPwt and APPswe
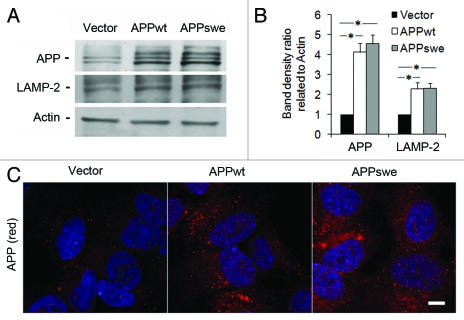
Western blotting of lysates from SH-SY5Y cells transfected with APPwt or APPswe revealed that, under normal conditions, both APPwt and APPswe produced approximately four times more APP than cells transfected with an empty vector (p < 0.05, Fig. 1A and B). This was confirmed by immunofluorescence studies (Fig. 1C).
Figure 1.
Levels of APP and LAMP-2 in RA-differentiated SH-SY5Y neuroblastoma cells stably transfected with vector, APPwt or APPswe. (A) Representative western blots for APP (using the 6E10 antibody), LAMP-2 and actin (loading control). (B) Quantification of APP and LAMP-2 band optical densities normalized against actin levels. The intracellular content of both APP and LAMP-2 was significantly increased in APPwt and APPswe cells as compared with vector controls (*p < 0.05; n = 3). (C) Confocal microscopy images of SH-SY5Y cells immunostained for APP (red fluorescence). Nuclei were stained by DAPI (blue fluorescence). APP immunoreactivity was apparently higher in APPwt and APPswe cells as compared with vector controls (n = 3). Bar, 20 μm.
Levels of LAMP-2 were approximately 2-fold increased in APPwt and APPswe cells compared with vector-transfected cells, (p < 0.05, Fig. 1A and B), suggesting an upregulation of the lysosomal system in APP-overexpressing cells.
Increased production of APP and Aβ under hyperoxia
When SH-SY5Y cells were exposed to hyperoxia (40% oxygen) for 5 d, the levels of intracellular APP increased as compared with those observed under normoxic (8% oxygen) conditions. This was the case for all cell lines studied, with significantly higher levels seen in APPwt and APPswe cells, p < 0.05. (Fig. 2A and B). As an indication of non-amyloidogenic APP cleavage,35 the ratio of αAPP fragments in conditioned media against full intracellular APP (holoAPP) was calculated. After cultivation of cells in either normoxia or hyperoxia for 5 d, the media was collected, and both secreted αAPP and holoAPP were detected by western blotting using 6E10 antibodies. Under normoxia, the ratio αAPP/holoAPP was considerably higher in APP-overexpressing cells than in vector-transfected cells. For APPwt cells, αAPP levels and αAPP /holoAPP ratio were twice as high as for APPswe cells (Fig. 2C and D). Following exposure to hyperoxia, αAPP levels and the αAPP/ holoAPP ratio decreased significantly in all cell types (p < 0.05, Fig. 2C and D), suggesting that oxidative stress decreased α-secretase activity.
Figure 2.
Increased amounts of intracellular APP and Aβ, and decreased amounts of secreted αAPP in RA- differentiated SH-SY5Y cells exposed to hyperoxia or normoxia (40% O2 vs. 8% O2) for 5 d. (A) Representative western blots for holoAPP (probes with 6E10 antibodies) and actin (loading control). (B) Quantification of APP bands normalized against actin levels. The content of APP significantly increased in APPwt and APPswe cells after exposure to hyperoxia (*p < 0.05; n = 4). (C) Representative western blot and quantification of αAPP (probed with 6E10 antibodies) in conditioned media from cell cultures (*p < 0.05; n = 4). (D) The ratio of secreted αAPP levels to holoAPP significantly decreased in APPwt and APPswe cells (*p < 0.05; n = 4), suggesting a decreased α-secretase activity after exposure to hyperoxia. (E and F) Confocal microscopy images of APPswe cells exposed to normoxia or hyperoxia and immunostained for (E) Aβ1–42 (red fluorescence) or (F) Aβ oligomers (using A11 antibody, red fluorescence). Nuclei were stained by DAPI (blue fluorescence). Note the increased immunoreactivity in hyperoxia-exposed cells for both Aβ1–42 and Aβ oligomers (n = 3). Bars, 30 μm.
Immunofluorescence microscopy using antibodies against Aβ1–42 or A11 (anti-Aβ oligomers) showed increased immunoreactivity in all cell lines exposed to hyperoxia for 5 d. These increases were particularly notable in APPswe cells (Fig. 2E and F).
APP overexpressing cells show increased reactive oxygen species production and decreased viability
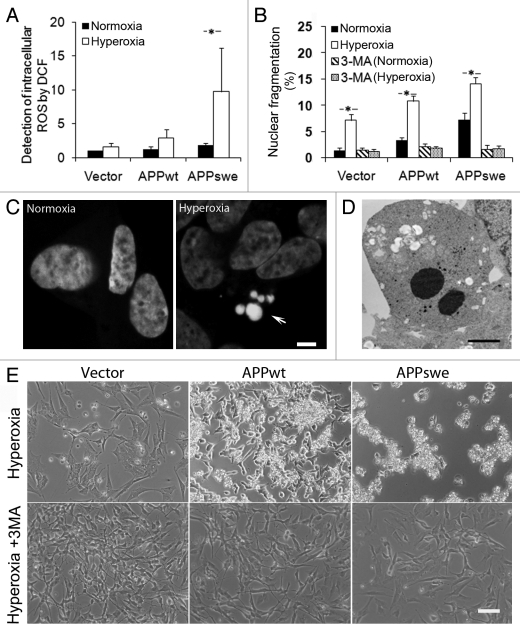
We next studied the sensitivity to hyperoxia of APPwt and APPswe cells, by analyzing intracellular ROS production and apoptosis induction. Intracellular ROS levels (as assessed by DCF oxidation) significantly increased under hyperoxia in all cell lines studied, with the highest in APPswe cells and the lowest in vector controls (Fig. 3A). Interestingly, the addition of the autophagic inhibitor 3MA to the culture medium decreased ROS generation by approximately 50% in all cell lines exposed to hyperoxia (p < 0.05, data not shown).
Figure 3.
Inhibition of macroautophagy prevented apoptosis induced by APP overexpression and hyperoxia. RA-differentiated SH-SY5Y cells transfected with vector, APPwt and APPswe were investigated under hyperoxia and normoxia (40% O2 vs. 8% O2) for 5 d, with or without the autophagic sequestration inhibitor 3MA (1 mM). (A) Intracellular amounts of ROS were measured by DCF oxidation using flow cytometry. ROS levels increased after exposure to hyperoxia, most significantly in APPswe cells. (*p < 0.05; n = 4). (B) Percentage of cells with condensed or fragmented nuclei (assessed by DAPI staining) increased in all three cell lines after hyperoxia exposure for 5 d (*p < 0.05; n = 3), and were the highest in APPswe cells (Vector < APPwt < APPswe). This effect of hyperoxia was prevented by 3MA. (C) Images of DAPI-stained nuclei show normal and fragmented (apoptotic) nuclei in APPswe cells cultured in normoxia and hyperoxia, respectively. Arrow indicates nuclear fragmentation. Bar, 5 μm. (D) Transmission electron microscopy of APPswe cells shows nuclear fragmentation after exposure to hyperoxia. Bar, 2 μm. (E) Phase contrast images of vector-transfected, APPwt and APPswe cells cultured in hyperoxia, with or without 3MA for 7 d. Hyperoxia-exposed cultures show increased numbers of dead cells. This effect was inhibited by 3MA in all cell lines (compare with B). Experiments were performed 3 times. Bar, 100 μm.
For the assessment of apoptosis, nuclear morphology was analyzed by fluorescence microscopy of cells stained with 4′, 6-diamidino-2-phenylindole (DAPI), and by electron microscopy (Fig. 3B–E). Even under normoxia, APPswe and APPwt cells showed a higher proportion of condensed or fragmented nuclei when compared with vector-transfected cells (Fig. 3B). The percentage of apoptotic cells dramatically increased in all cell lines after exposure to hyperoxia, with the highest in APPswe cells and the lowest in vector controls (p < 0.05, Fig. 3B). Inhibition of autophagy by 3MA decreased apoptosis to approximately the same level in all groups (p < 0.05, Fig. 3B and E), suggesting an essential role of autophagy in the hyperoxia-induced apoptosis seen in APP-overexpressing cells.
Hyperoxia enhanced macroautophagy in APP overexpressing cells
The autophagic-lysosomal system was studied by western blotting and immunocytochemistry using antibodies to the lysosomal membrane marker LAMP-2, the autophagic marker LC3 (I and II), as well as total and phosphorylated p70S6K. Even under normoxic conditions, cells overexpressing APP were characterized by increased levels of LAMP-2 and LC3-II when compared with vector controls (Fig. 4A–D). Under hyperoxia, both LAMP-2 and LC3-II increased dramatically in all cell lines, reaching the highest levels in APPswe cells (Fig. 4A–D). In agreement with the western blotting data, immunofluorescence microscopy showed an increased number and size of LAMP-2 and LC3-positive vacuoles, respectively, in all cell lines exposed to hyperoxia, but especially in APPswe cells (Fig. S1). Moreover, the ratio of phosphorylated p70S6K (P-p70S6K) to total p70S6K decreased in all cells lines after exposure to hyperoxia, and was the lowest in APPswe cells (Fig. 4E and F). These findings suggest that both APP overexpression and hyperoxia enhanced macroautophagy, resulting in the upregulation of the lysosomal system.
Figure 4.
Enhancement of macroautophagy by overexpression of APP and hyperoxia. Vector-transfected, APPwt and APPswe SH-SY5Y cells were exposed to hyperoxia or normoxia (40% O2 vs. 8% O2) for 5 d. (A) Western blotting of LAMP-2 and actin (loading control). (B) Densitometry of LAMP-2 (normalized to actin levels) indicates significant upregulation of the lysosomal system in all cell lines exposed to hyperoxia, particularly in APPwt and APPswe cells (*p < 0.05; n = 3). (C) Western blotting of LC3 and actin (loading control). (D) Densitometry of LC3-II (vs. actin) showed significant activation of macroautophagy by hyperoxia in all cell lines, particularly in APPwt and APPswe cells (*p < 0.05; n = 5). (E) Western blotting of total and phosphorylated p70S6K (pThr389). Actin was used as loading control. (F) P-p70S6K to total p70S6K densitometry ratio was decreased in APPwt and APPswe cells in both normoxia and hyperoxia (*p < 0.05; n = 4).
We next investigated the possibility that the increase of LC3-II seen in APP-overexpressing and hyperoxia-exposed cells was associated with decreased autophagy flux due to impaired lysosomal degradation. Cells were exposed to the lysosomal protease inhibitors E64d and Pepstatin A, both inhibiting LC3-II degradation.14,36 This resulted in an even higher increase of LC3-II, suggesting a true upregulation of macroautophagic activity (Fig. 5A–D).
Figure 5.
Autophagy flux measurements suggest enhancement of macroautophagy. (A) Western blotting of LC3 in cells cultured with or without lysosomal enzyme inhibitors E64d (10 μM) and Pepstatin A (Pep.A; 10 μg/ml) in normoxic conditions. (B) Increased LC3-II levels (normalized to actin) after lysosomal enzyme inhibition (*p < 0.05; n = 4). (C) Western blotting of LC3 in cells cultured with or without lysosomal enzyme inhibitors E64d (10 μM) and Pepstatin A (10 μg/ml) in hyperoxic conditions [same groups as in (A)]. (D) Increased LC3-II levels (normalized to actin) after lysosomal enzyme inhibition (*p < 0.05; n = 4). The elevated levels of LC3-II after lysosomal enzyme inhibition indicate an increased autophagic flux.
Furthermore, activation of macroautophagy by APP overexpression and hyperoxia was demonstrated by electron microscopy (Fig. 6). In both empty vector and APPswe transfected cells an increase in the number and size of autophagic vacuoles after exposure to hyperoxia was detected (Fig. 6A–D). This was reversed by the treatment of cells with 3MA (Fig. 6E and F). Various forms of autophagic vacuoles induced by hyperoxia in SH-SY5Y cells are shown in Figure S2. Similar types of vacuoles (reflecting different stages of autophagic degradation) have also been described in AD neurons with an upregulated lysosomal system.37
Figure 6.
Electron micrograghs of autophagic vacuoles in RA-differentiated SH-SY5Y cells. Vector-transfected (A, C and E) and APPswe (B, D and F) cells were cultured in normoxia (A and B), hyperoxia (C and D), or hyperoxia combined with 3MA exposure (E and F), respectively. Exposure to hyperoxia dramatically increased the number and size of autophagic vacuoles in all cell lines, especially in APPswe cells. This effect of hyperoxia was prevented by 3MA (1 mM). AV, autophagic vacuoles; Mt, mitochondria; N, nucleus. Bar, 1 μm. n = 3.
Enhancement of intralysosomal APP accumulation by hyperoxia
To study the relationship between APP and the autophagic-lysosomal system, cells were double immunostained for APP (using a N-terminal antibody, LN27) and LC3 or cathepsin D, respectively. Colocalization of APP with LC3 or cathepsin D-positive vacuoles was significantly higher in APP-overexpressing cells compared with vector controls, with the highest levels seen in APPswe cells. Hyperoxia greatly increased the number of APP-positive autophagic vacuoles in all cell lines studied (Fig. 7). Exposure of cells to 3MA (either in normoxia or hyperoxia) dramatically reduced the number of APP-positive autophagic vacuoles (Fig. 7C), suggesting that APP accumulates intralysosomally through macroautophagy.
Figure 7.
APP accumulation in autophagic-lysosomal compartment was enhanced by hyperoxia and prevented by 3MA (1 mM). (A) Confocal microscopy images of APPswe cells exposed to hyperoxia and double immunostained for APP (red fluorescence) and LC3 (green fluorescence). Nuclei were stained by DAPI (blue fluorescence). Arrows indicate colocalization of APP and autophagic vacuoles. Bar, 50 μm. (B) Confocal microscopy images of APPswe cells exposed to hyperoxia and double immunostained for APP (red fluorescence) and for cathepsin-D (green fluorescence). Nuclei were stained by DAPI (blue fluorescence). Arrows show colocalization of APP and lysosomes. Bar, 30 μm. (C) Quantification of cells with APP containing autophagic vacuoles in SH-SY5Y neuroblastoma cultures exposed to normoxia or hyperoxia, with or without 3MA. The percentage of cells with APP-containing autophagic vacuoles was higher in APP-overexpressing cells than in vector controls (Vector < APPwt < APPswe, p < 0.05; n = 4). Hyperoxia significantly increased the percentage of cells with APP containing autophagic vacuoles in all three cell lines, an effect that was prevented by 3MA treatment, (*p < 0.05; n = 4). (D) Quantification of cells with intralysosomal accumulation of APP. Cells overexpressing APP showed increased numbers of APP-positive lysosomes (Vector < APPwt < APPswe, *p < 0.05; n = 4) under normoxia or hyperoxia.
Intralysosomal Aβ accumulation due to APP overexpression and hyperoxia
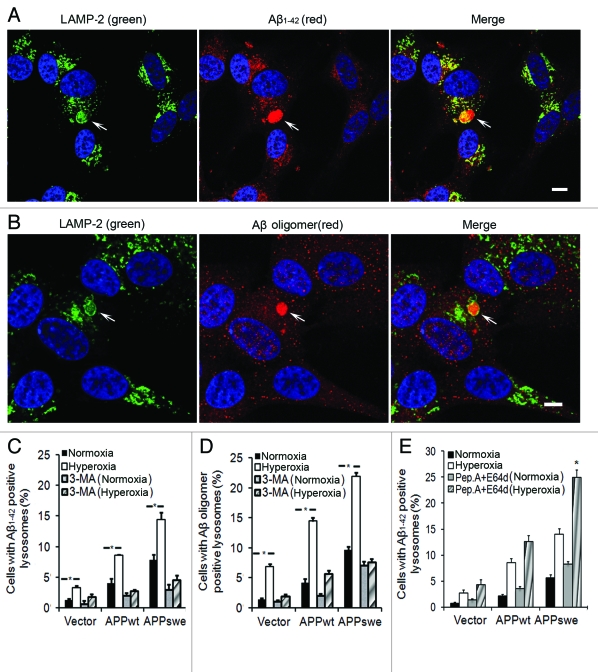
Using double immunofluorescence and confocal microscopy, we next studied whether Aβ1–42 or Aβ oligomers are localized to lysosomes (LAMP-2 positive compartments). Figure 8A and B show examples of the micrographs obtained for Aβ1–42 or Aβ oligomers, respectively, in APPswe cells. As seen in Figure 8C, under normal conditions, the number of cells with Aβ-positive lysosomes was significantly higher in APPwt and especially in APPswe cells. Exposure to hyperoxia increased the content of Aβ-positive lysosomes proportionally in all cell lines (Fig. 8C). This increase was associated with enlarged size of Aβ-containing lysosomes, in which Aβ granules were surrounded by ring-like profiles of LAMP-2 (Fig. 8A). Exposure of cells to 3MA, in either normoxia or hyperoxia, dramatically reduced the number of Aβ1–42-positive lysosomes in all cell lines (Fig. 8C). When cells were double immunostained for Aβ1–40 and LAMP-2, similar results were obtained (data not shown).
Figure 8.
Intralysosomal accumulation of Aβ was enhanced by hyperoxia (5-d exposure) and prevented by 3MA (1 mM). (A) Confocal microscopy images of APPswe cells double immunostained for Aβ1–42 (red fluorescence) and LAMP-2 (green fluorescence). Nuclei were stained by DAPI (blue fluorescence). Arrows indicate the colocalization of Aβ1–42 and lysosomes. Bar, 50 μm. (B) Confocal microscopy images of APPswe cells double immunostained for Aβ oligomers (A11 antibody, red fluorescence) and LAMP-2 (green fluorescence). Nuclei were stained by DAPI (blue fluorescence). Arrows show the colocalization of Aβ oligomers and lysosomes. Bar, 20 μm. (C) Quantification of cells containing intralysosomal Aβ1–42 in cultures exposed to normoxia or hyperoxia, with or without 3MA. The percentage of cells with Aβ-containing lysosomes was higher in APP-overexpressing cells than in vector controls (Vector < APPwt < APPswe, p < 0.05). Hyperoxia significantly increases the percentage of cells with Aβ-positive lysosomes in all three cell lines and this increase was prevented by 3MA. (*p < 0.05; n = 4). (D) Quantification of cells with intralysosomal accumulation of Aβ oligomers. Cells overexpressing APP showed increased numbers of Aβ-positive lysosomes (Vector < APPwt < APPswe). Hyperoxia significantly increased the percentage of cells with lysosomes containing Aβ oligomers in all three cell lines and this increase was prevented by 3MA. (*p < 0.05; n = 4). (E) Quantification of cells containing intralysosomal Aβ1–42 in cultures exposed to normoxia or hyperoxia, with or without lysosomal inhibitors (E64d and Pepstatin A) (*p < 0.05; n = 3).
Analogous studies were performed using specific antibodies for Aβ oligomers (A11). As seen in Figure 8B and D, the results were comparable to those obtained with Aβ1–42 and Aβ1–40 antibodies. These data demonstrate that both APP overexpression and hyperoxia induced intralysosomal accumulation of Aβ (both monomeric and oligomeric) through the macroautophagic pathway.
Suppression of lysosomal proteases by E64d and pepstatin A resulted in further increase of intralysosomal Aβ1–42 (Fig. 8E). This suggests that intralysosomal Aβ accumulation resulting from APP overexpression + hyperoxia is likely associated with enhanced autophagic uptake of APP and/or Aβ, rather than with the inhibition of lysosomal degradation.
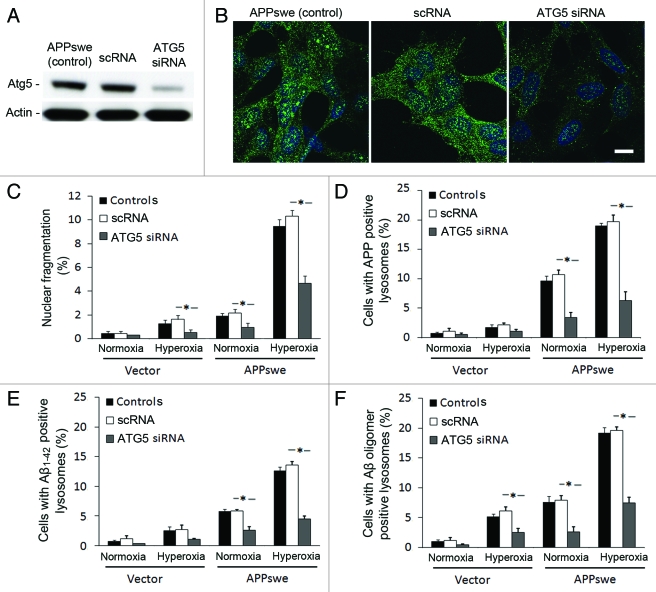
To further support this hypothesis, we exposed APPswe cells to siRNA against ATG5, a protein specifically required for macroautophagy.38 As seen in Figure 9A, ATG5 was downregulated to 75% by siRNA. After exposure to hyperoxia, LC3 immunostaining showed a granular pattern in controls (regular APPswe), as well as in negative scramble siRNA controls (scRNA), while in ATG5 siRNA cells, the staining pattern was diffuse with reduced brightness (Fig. 9B). Moreover, downregulation of ATG5 resulted in similar degree of (1) cell death protection (Fig. 9C); (2) decreased number of APP containing lysosomes (Fig. 9D); and (3) decreased number of Aβ (Aβ1–42 and Aβ oligomers) containing lysosomes (Fig. 9E and F). These results were similar to those observed in cells with 3MA-suppressed macroautophagy (Figs. 3, 7 and 8) and altogether strengthen the role of macroautophagy in Aβ toxicity.
Figure 9.
Downregulation of ATG5 by siRNA protected cells from apoptosis and decreased the intralysosomal accumulation of APP and Aβ. (A) Western blot of ATG5 in APPswe cells (control), cells with scramble RNA transfection (scRNA) or ATG5 siRNA transfection. (B) Single immunostaining of LC3 (green fluorescence) in APPswe cells (control), cells with scRNA, and with ATG5 siRNA after 3 d exposure to hyperoxia. Both control and scRNA cells showed brighter and more granular staining of LC3 compared with ATG5 siRNA cells. Bar, 40 μm. (n = 3). Nuclei were stained by DAPI (blue fluorescence). (C–F) Vector and APPswe cells (controls), cells with scRNA, or ATG5 siRNA were exposed to normoxia and hyperoxia for 3 d. The number of cells with: nuclear fragmentation (C) and with APP (D), Aβ1–42 (E), or Aβ oligomer (F) -positive lysosomes were counted. ATG5 siRNA protected cells from death (C), and decreased lysosomal accumulation of APP (D), Aβ1–42 (E), and Aβ oligomers (F) (*p < 0.05; n = 3).
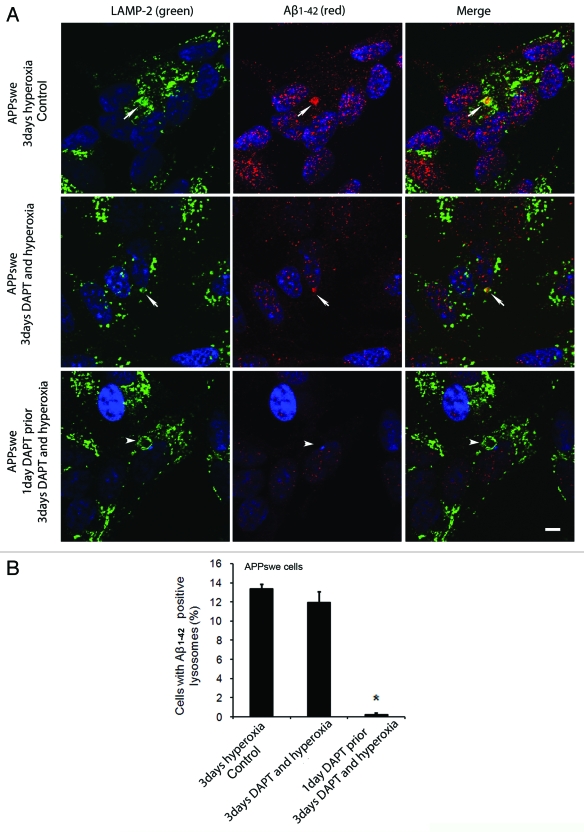
To study the possible involvement of intralysosomal APP processing in Aβ accumulation within the lysosomal compartment, the γ- secretase inhibitor DAPT (500 nM) was administered before or simultaneously with autophagy induction by hyperoxia. Figures 10 and 11 show the results obtained in APPswe cells for Aβ1–42 and Aβ oligomers, respectively. When cells were exposed to DAPT and hyperoxia for 3 d, only a minor decrease in the number of cells with Aβ- (both monomers and oligomers) containing lysosomes was noted, as compared with cells exposed to hyperoxia alone (Figs. 10 and 11). However, when such treatment (DAPT + hyperoxia) was preceded by a 24 h administration of DAPT under normoxic conditions (i.e., before induction of autophagy), a dramatic decrease of Aβ-containing lysosomes was observed (Figs. 10 and 11). Similar results were found in vector-transfected and APPwt cells (data not shown). These results suggest that under hyperoxia a substantial part of the Aβ probably accumulates intralysosomally through autophagic uptake. However, since we also noted an overall decrease of Aβ levels (both cytosolic and lysosomal) after simultaneous inductions of autophagy and γ- secretase inhibition, the co-existence of intralysosomal APP processing is also plausible.
Figure 10 (See opposite page).
Effects of γ-secretase inhibition by DAPT on intralysosomal accumulation of Aβ1–42. (A) double immunostaining of LAMP-2 (green) and Aβ1–42 (red) in APPswe cells after 3 d exposure to hyperoxia only (controls), 3 d exposure to hyperoxia and DAPT (500 nM) or 1 d DAPT pretreatment followed by 3 d exposure to hyperoxia and DAPT . Arrows show colocalization of Aβ1–42 with lysosomes while arrowheads indicate enlarged lysosomes without any Aβ1–42 accumulation. After DAPT treatment, the general amount of Aβ1–42 (cytosolic and lysosomal) and the size of Aβ1–42 containing lysosomes were decreased compared with controls. Bar, 30 μm. n = 3. Nuclei were stained by DAPI (blue fluorescence). (B). The percentage of cells with large Aβ1–42 positive lysosomes significantly decreased after 1 d of DAPT pretreatment followed by 3 d exposure to hyperoxia and DAPT, in comparison to controls (*p < 0.05; n = 3).
Figure 11.
Effects of γ-secretase inhibition by DAPT on intralysosomal accumulation of Aβ oligomers. (A) Double immunostaining of LAMP-2 (green) and Aβ oligomers (red) in APPswe cells after 3 d exposure to hyperoxia (controls), 3 d exposure to hyperoxia and DAPT (500 nM) or 1 d DAPT pretreatment followed by 3 d exposure to hyperoxia and DAPT. Arrows show colocalization of Aβ oligomers with lysosomes while arrowheads indicate enlarged lysosomes without accumulation of Aβ oligomers. After DAPT treatment, the general amount of Aβ oligomers (cytosolic and intralysosomal) and the size of Aβ oligomer containing lysosomes were decreased compared with controls. Bar, 30 μm. n = 3. Nuclei were stained by DAPI (blue fluorescence). (B). The percentage of cells with large lysosomes containing Aβ oligomers significantly decreased after 1 d DAPT pretreatment followed by 3 d exposure to hyperoxia and DAPT, in comparison to controls (*p < 0.05; n = 3).
Aβ aggregation is pH-dependent, and oligomerization seems to be more rapid at a low pH.39 To investigate if Aβ oligomerization takes place inside the lysosomes, or if Aβ oligomers are delivered through autophagy, we increased lysosomal pH with NH4Cl and exposed cells to hyperoxia for 3 d. No significant changes in the number of cells with Aβ oligomer-containing lysosomes were found after NH4Cl treatment, although a slight decrease (approximately 10%) was noted. These results suggest that, under hyperoxia, the majority of intralysosomal Aβ oligomers were taken up through autophagic pathway.
Finally, a possible involvement of endocytosis in the intralysosomal Aβ accumulation was assessed by double immunostaining for Aβ1–42 and Rab5. Although hyperoxia was associated with increased endocytosis, as suggested by increased number and size of Rab5-positive granules, suggesting membrane distribution of Rab5 (Fig. 12A), no apparent colocalization of Rab5 and Aβ (Aβ1–42 and Aβ oligomers) was noted (Fig. 12B and C).
Figure 12.
Hyperoxia enhanced endocytosis without inducing Aβ localization to endosomes. (A) Single immunostaining of Rab5 (green fluorescence) in vector, APPwt and APPswe cells under normoxia or hyperoxia. Under normoxia, Rab5 showed a preferentially diffuse (cytosolic) distribution pattern, while after exposure to hyperoxia it becomes more granular (membrane bound), suggesting an upregulation of early endosomes after oxidative stress. Bar, 60 μm. n = 3. Nuclei were stained by DAPI (blue fluorescence). (B and C) Double immunostaining showed no colocalization between Aβ1–42 (B, red fluorescence) or Aβ oligomers (C, red fluorescence) and Rab5 (B and C, green fluorescence) in APPswe cells after exposure to hyperoxia (arrow and magnified image), suggesting that most of intralysosomal Aβ was not delivered by endocytosis. Bar, 40 μm. n = 3. Nuclei were stained by DAPI (blue fluorescence).
Discussion
According to the free radical theory of aging, normal aerobic metabolism results in unavoidable ROS-induced molecular damage leading to aging and age-associated disease, including AD.24,40 In our study, RA-differentiated SH-SY5Y human neuroblastoma cells that overexpress APP (APPwt or APPswe) were exposed to chronic mild oxidative stress by normobaric hyperoxia (40% ambient oxygen). These cells produced increased quantities of APP and Aβ and showed decreased viability under oxidative stress conditions, as compared with vector control cells. Both APPwt and APPswe cells, especially the latter, also showed enhanced intracellular ROS production and increased macroautophagy. These effects were particularly pronounced under hyperoxia, but some minor oxidative damage was also observed under normal oxygen conditions. The enhanced sensitivity of cells to oxidant-induced apoptosis was paralleled by increased intralysosomal accumulation of Aβ, both monomeric and in form of oligomers. Moreover, inhibition of macroautophagy by either 3MA treatment or downregulation of ATG5 prevented ROS production, intralysosomal accumulation of Aβ and cell death. These results together suggest the important role of macroautophagy in Aβ toxicity.
Our results raised a question as to what extent the intralysosomal Aβ accumulation was a result of increased autophagic uptake of Aβ or enhanced intralysosomal processing of APP. Indeed, increased macroautophagy resulted in the intralysosomal accumulation of APP, some of which is probably processed to Aβ as previously described.17 We showed that inhibition of γ-secretase during autophagic induction by hyperoxia caused only a minor decrease in the number of lysosomes containing Aβ, either monomers or oligomers. In contrast, inhibition of γ-secretase prior to autophagy induction resulted in a substantial decrease of Aβ in the lysosomes, suggesting that, in our paradigm, a large part of intralysosomal Aβ was delivered through macroautophagy. However, since simultaneous inductions of autophagy and γ-secretase inhibition also caused some decrease of Aβ levels in the lysosomes and cytosol, intralysosomal production of Aβ from APP (previously demonstrated by Yu et al.17) probably occurs as well. Such a possibility is supported by our finding of enhanced intralysosomal APP accumulation due to hyperoxia.
The lack of colocalization of Aβ and Rab5 suggests that the intralysosomal Aβ did not originate from previously secreted Aβ that could enter cells via endocytosis. Nevertheless, Aβ has been found in many intracellular sites, such as mitochondria, lysosomes, endosomes, cytosol, ER and Golgi complexes.6 Any of these intracellular sites could be the source for macroautophagic delivery of Aβ to lysosomes seen in our system.
It has been suggested that the acidic lysosomal environment can promote formation of Aβ oligomers and protofibrils39 and that intralysosomally accumulated Aβ damages lysosomal membranes, resulting in the leak of acid hydrolases into the cytosol and apoptotic death as described in our previous study.34 In support of these results, we showed that the prevention of intralysosomal Aβ (Aβ1–42 and oligomeric Aβ) accumulation by macroautophagy inhibition rescued the cells. Surprisingly, the increase of lysosomal pH using NH4Cl resulted only in a slight decrease in intralysosomal oligomeric Aβ. It is possible that NH4Cl treatment did not affect Aβ oligomerization outside the lysosomal compartment, and these oligomers would have been delivered to the lysosomes through autophagy. Also, it has been demonstrated that membranes of different cellular compartments, such as the lysosomal membrane, can initiate Aβ aggregation regardless of pH.41 Thus, when Aβ gradually accumulates intralysosomally, its concentrations can finally reach levels resulting in aggregation.42
Ling et al.43 demonstrated that the lysosomal system in Aβ expressing Drosophila flies lose degradative capacity with aging and that autophagy induction in the aged flies decreased their survival. In the human brain, there is a massive accumulation of undegraded material within autophagic vacuoles in dystrophic neurons, indicating an impaired lysosomal function.17,44 Thus, it is possible that the increased intralysosomal Aβ seen in hyperoxia could be the consequence of deficient lysosomal degradation. To explore this possibility, we inhibited lysosomal proteases with E64d and pepstatin A, which resulted in enhanced accumulation of intralysosomal Aβ. Since E64d and pepstatin A are also β- and γ-secretase inhibitors, respectively,45,46 we found an overall decrease in cytosolic Aβ (data not shown). These results are in agreement with the conception that at least some lysosomal degradative capacity was still present under hyperoxia.
Why do APP-overexpressing cells show elevated ROS production and increased macroautophagy? The most plausible explanation is that Aβ acts as a pro-oxidant.27,28,47 Because APPwt and APPswe cells produce more Aβ than vector controls, they would also generate more ROS under the same environmental conditions. Oxidative stress, in turn, can increase APP and Aβ formation, as shown in this report and our earlier observation,34 giving rise to an amplifying oxidative stress - Aβ loop. As a consequence, the APPwt and APPswe cells would also show higher autophagic activity, which upregulates in an attempt to repair ROS-induced molecular damage. Hence, APP-overexpressing cells experience triple pathogenic effects of increased Aβ production, increased oxidative stress, and increased macroautophagy—all contributing to intralysosomal Aβ accumulation and consequent apoptotic cell death.
An inhibitor of autophagic sequestration, 3MA, decreased the content of APP- and Aβ-containing lysosomes in APPwt and APPswe cells approximately to the level seen in vector control cells, indicating that APP and Aβ (including toxic Aβ oligomers) accumulate within the lysosomal compartment through macroautophagy. Also, 3MA decreased intracellular levels of ROS in all three cell lines exposed to hyperoxia, probably suggesting the involvement of substances delivered to lysosomes through autophagy, for example, transition metals in ROS generation. Moreover, 3MA dramatically increased the survival of hyperoxia-exposed cells, making the percentage of apoptotic cells practically the same in all three cell lines studied. Macroautophagy inhibition by knocking down ATG5 confirmed these results in APPswe cells. Thus, in our model of investigation, prevention of intralysosomal Aβ accumulation by inhibition of macroautophagy was beneficial against oxidant-induced damage to APP-overexpressing neuroblastoma cells. Other studies demonstrated an autophagy-dependent reduction in Aβ load,42,48 and the importance of autophagic degradation of Aβ for cell survival.42,49-52 These observations do not contradict with our results, showing activation of macroautophagy in response to oxidative damage to cellular components. In our model, autophagic induction results in intralysosomal Aβ accumulation due to the fact that Aβ uptake by sequestration and/or its formation from APP within autophagic vacuoles exceed lysosomal degradative capacity. One possible reason for lysosomal degradative insufficiency is oxidative modification of material inside lysosomes, making it indigestible.53
Normally, macroautophagy sequesters damaged organelles and large protein aggregates into autolysosome where cargos are efficiently degraded, and autophagy vesicles are rarely observed in healthy neurons.9,54 In the early stage of AD, moderately active autophagic-lysosomal system is needed for increased organelle and protein turnover in damaged and regenerating neurons, promoting their survival.55 However, in advanced AD, oxidative stress induces overactive autophagy, and age-dependent lysosomal degradative failure can cause Aβ overload of lysosomes, resulting in apoptosis. Since the autophagic-lysosomal system plays different roles in healthy and diseased neurons,10 possible therapies based on autophagy modulation require careful targeting of specific steps involved in the pathway to achieve efficient digestion.37 The macroautophagy inducer rapamycin has been reported to reduce Aβ and tau pathology and improve learning and memory in animal model of AD, when lysosomes maintained good digestive function.51 However, when lysosomal digestive ability is suppressed, stimulation of autophagy may accelerate the course of the disease, while its inhibition may prevent the intralysosomal accumulation of Aβ and other toxic materials, promoting cell survival.
In summary, our results indicate that macroautophagy-generated intralysosomal increase of Aβ is essential for oxidant-induced apoptosis, providing aditional support for the interactive role of oxidative stress and the lysosomal system in AD-related neurodegeneration.
Materials and Methods
Cells and culture conditions
Human SH-SY5Y neuroblastoma cells were obtained from the American Type Culture Collection (ATCC, CRL-2266™) and stably transfected with an empty pcDNA 3.1 vector containing a cytomegalovirus promoter, or wild-type APP695 (APPwt), or APP Swedish KM670/671NL double mutation (APPswe) using Lipofectamine TM2000 according to the manufacturer’s (Invitrogen, 11668027) instructions. Overexpression of APP was confirmed by western blotting and fluorescence microscopy (Fig. 1).
Transfected cells were cultured in Minimum Essential Medium with Glutamax (Invitrogen, 32561-037) containing 10% fetal bovine serum and 200 μl/ml geneticin (for selection of transfected cells, Invitrogen, 10131035) in the atmosphere of 8% O2, 87% N2 and 5% CO2 at 37°C, (normal conditions) in 75 cm2 plastic culture flasks (Corning, 430641). For differentiation, neuroblastoma cells were exposed to 10 µM all-trans RA (Sigma, R-2625) for 14 d. The medium was changed every second d.
Differentiated cells were plated in plastic Petri dishes at a density of 104 cells per cm2. After 24 h, the culture medium was changed to the serum-free OptiMEM1 (Invitrogen, 51985-026) supplemented with 10 µM RA and 200 μg/ml geneticin. The cells were divided into two groups: (1) cells cultured under normoxia, i.e., 8% O2, 87% N2 and 5% CO2; (2) cells cultured under hyperoxia, i.e., 40% O2, 55% N2 and 5% CO2 (chronic oxidative stress).
In some experiments, for the increase of lysosomal pH, cultures were treated with 10 mM ammonium chloride (NH4Cl, Sigma, 443093) for 3 d.
Inhibition of macroautophagy
3-methyladenine (3MA; Sigma, P0899) and ATG5 siRNA (Qiagen, 1027416) were used to inhibit autophagic sequestration. For 3MA treatment (1 mM, 5 d), differentiated cells were plated on coverslips in plastic Petri dishes at a density of 104 cells per cm2. After 24 h, the culture medium was changed to the serum-free OptiMEM1 supplemented with 10 µM RA and 200 μg/ml geneticin and 1 mM 3MA. After 5 d, cells and culture medium were used for analysis.
For macroautophagy inhibition by RNA interference, siRNA against ATG5 (Quiagen, 1027416) or negative scramble siRNA controls, scRNA (Qiagen, 1022076) were used. Cells were seeded on coverslips in 12-well plates in serum medium and transfected with 25 nM ATG5 siRNA for 48 h by using HiPerFect Transfection Reagent (Qiagen, 301704) following the manusfacturer’s protocol. Before hyperoxia exposure, the medium was changed to serum free OptiMEM1 containing 25 nM ATG5 siRNA. The efficacy of RNA interference was assessed by western blot analysis of Atg5 and LC3.
Detection of macroautophagy
Detection of macroautophagy was performed by several methods:14 (1) Western blotting of LC3 (performed on 15% SDS page gels). The density of LC3-II band (16 KDa) indicated autophagosome formation (detail in western blot section below). (2) Immunocytochemistry of LC3 for visualization autophagic vacuoles (details in immunocytochemistry section). (3) The ratio of P-p70S6K to p70S6K, representing induction of autophagy (detail in western blot section below). (4) Autophagy flux was evaluated by western blotting of LC3 after exposure of cells to 10 µM E64d (an inhibitor of cysteine proteases, Sigma, E8640) and 10 µg/ml Pepstatin A (an inhibitor of aspartic peptidases, Sigma, P5318) during normoxia or hyperoxia. (5) Transmission electron microscopy: cells in 100 × 20 mm Petri dishes were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer containing 0.1 M sucrose and 3 mM CaCl2, pH 7.4 at room temperature for 30 min then overnight at 4°C. After fixation, cells were rinsed in 0.1 M phosphate buffer, pH 7.4 and centrifuged. Pellets were then post-fixed in 2% osmium tetroxide in 0.1 M phosphate buffer, pH 7.4 at 4°C for 2 h, dehydrated in ethanol followed by acetone and embeded in LX-112 (Lad). Sections were contrasted with uranyl acetate followed by lead citrate and examined in a Tecnai 12 transmission electron microscope (FEI) at 80 kV. Digital images were taken using a Veleta digital camera (Soft Imaging System, GmbH).
Western blot analysis
Detection of α-secretase processed APP (α-APP) was performed as previously described.56 75 µl medium of each sample was loaded to the gels. Immunoblotting was performed as described elsewhere57 using primary antibodies at 1:1000 dilution: anti-Aβ1–17 (6E10, Signet Laboratories, SIG-39320), anti-lysosomal associated membrane protein-2 (CD107b/LAMP-2, Southern Biotechnology, 9840-01), anti-autophagy related protein LC3B (LC3, Novus Biologicals, NB600-1384B), anti-p70 S6 Kinase (Cell Signaling, 9202S), anti-Phospho-p70 S6 Kinase (Cell signaling, 9206S), or anti-actin (Sigma-Aldrich, A 2668). They were followed by anti-rabbit (Amersham, 384927) or anti-mouse (Amersham, 380199) horseradish peroxidase-linked secondary antibodies at 1:2000 dilution. Immunoreactivity was detected by the ECL detection system (Amersham, RPN2209) and exposure to Hyper film MP (Amersham, 28906837). Some immunoblots were stripped using RestoreTM western blot Stripping buffer (Pierce, 21059) at room temperature for 15 min, and then re-blotted with other antibodies. The films were scanned and the quantification of immunoblots was performed using Image J program (available at http://rsbweb.nih.gov/ij/). The relative amount of protein corresponding to an immunoreactive band was calculated as a product of average optical density of the band by its area and expressed in arbitrary units.
Immunofluorescence microscopy
Cells were prepared for immunocytochemistry as described earlier.33 Formaldehyde-fixed cells were incubated with primary antibodies against Aβ1–42 (1:100 dilution, Chemicon, AB5078P), Aβ1–40 (1:100 dilution, Chemicon, AB5074B), amyloid oligomers (A11, 1:100 dilution, Invitrogen, AHB0052), N-terminus of APP (LN27, 1:100 dilution, Zymed Laboratories, 13-0200), CD107b/LAMP-2 (1:400 dilution, Southern Biotechnology, 9840-01), LC3 (1:100 dilution, Novus Biologicals, NB600-1384B), Rab5 (1:200 dilution, BD Biosciences PharMingen, sc 46692), or cathepsin D (1:100, Upstate Biotechnology, 06-467). Alexa Fluor 488 conjugated goat anti mouse IgG or Alexa Fluor 594 conjugated goat anti rabbit IgG (both diluted 1:400, Molecular Probes, A11029 or A11037, respectively) were used as secondary antibodies. For double immunofluorescence, a combination of two different primary antibodies was followed by a combination of two different secondary antibodies. The specimens were mounted in Vectashield containing DAPI (Vector Laboratories, H-1200) and inspected with a Nikon Eclipse E600 W confocal microscope using a 488 nm argon laser and 543 nm helium-neon laser. Cells with one or more Aβ (including Aβ1–40, Aβ1–42 and Aβ oligomers) or APP positive autophagic vacuoles/lysosomes (usually exceeding 1 µm in diameter) were randomly selected and counted under a Nikon Microphot-SA microscope using both phase contrast and fluorescence illuminations. The percentage of these cells was calculated for each specimen and averaged within each experimental group (n = 3). At least 300 randomly selected cells in each specimen (900 cells in one group) were counted.
Detection of cell death
As previously described,33 formaldehyde-fixed cells were mounted in Vectashield containing DAPI. Cells were randomly selected, and those with condensed and/or fragmented nuclei (considered as apoptotic cells) were counted under a Nikon Microphot-SA microscope using both phase contrast and fluorescence illuminations (330–380/420 nm excitation/barrier filter). The percentage of these cells was calculated for each specimen and averaged within each experimental group (n = 3). At least 300 cells in each specimen (900 cells in one group) were counted.
Inhibition of γ-secretase
LY-374973, N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a γ- secretase inhibitor (Sigma, D5942) was used at a concentration of 500 nM to inhibit Aβ production. Three different conditions were used: (1) cells cultured under hyperoxia for 3 d. (2) Cells exposed to hyperoxia and DAPT for 3 d. (3) Cells pretreated with DAPT for 1 d, and then exposed to hyperoxia and DAPT for 3 d.
Measurement of intracellular reactive oxygen species production
Intracellular reactive oxygen species (ROS) production was detected by carboxy-H2DCFDA (DCF, Invitrogen, C-400) oxidation that was assessed by flow cytometry. Cells cultured in 12-well plates were washed, incubated with 10 µM DCF in serum-free medium at 37°C for 15 min. The fluorescence of 10,000 cells was analyzed in an LSR flow cytometer (Becton-Dickinson) using a 488 nm argon laser and CellQuest software.
Statistical analysis
Values are given as mean ± SD. The results were analyzed for statistical significance using the Mann-Whitney U test for two-group comparisons and Kruskal-Wallis test for multigroup comparisons. P values ≤ 0.05 were considered significant.
Supplementary Material
Supplementary PDF file supplied by authors.
Acknowledgments
This work was supported by the Gustav V and Queen Victoria Foundation (J.M.), County Council of Östergötland (J.M., L.Z., M.H.), Stiftelsen Olle Engkvist Byggmästare (L.Z., K.K.), Stiftelsen för Gamla Tjänarinnor (L.Z., A.C.-M.), Gun och Bertil Stohnes Stiftelse (L.Z., A.C.-M.), Lions forskningsfond (L.Z.), Svenska Lundbeckstiftelsen (L.Z.), Karolinska Institute Fund for Geriatric Research (A.C.-M.), Alice och Knut Wallenberg Stiftelse (A.C.-M.), Swedish Alzheimer Foundation (K.K., M.H.) and The Swedish Brain Power (A.C.-M.). The authors thank Lisbeth Hjälle and Åsa-Lena Dackland for excellent technical and flow cytometry assistance, respectively, and Kjell Hultenby’s group for transmission electron microscopy.
Glossary
Abbreviations:
- 3MA
3-methyladenine
- AD
Alzheimer disease
- ATG5
autophagy-related protein 5
- Aβ
amyloid β-protein
- APP
amyloid precursor protein
- NH4Cl
ammonium chloride
- APPwt
wild-type APP695
- APPswe
Swedish KM670/671NL double mutation
- α-APP
α-secretase processed APP
- DAPT
LY-374973, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester
- DCF
carboxy-H2DCFDA
- DAPI
4′ 6-diamidino-2-phenylindole
- E64d
(2S,3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester, EST
- HEK
human embryonic kidney
- LAMP-2
lysosomal associated membrane protein-2
- LC3
microtubule-associated protein 1 light chain 3
- NFT
neurofibrillary tangles
- RA
retinoic acid
- ROS
reactive oxygen species
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/18051
References
- 1.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glabe C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer's disease. J Mol Neurosci. 2001;17:137–45. doi: 10.1385/JMN:17:2:137. [DOI] [PubMed] [Google Scholar]
- 4.Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer's and Parkinson's diseases. Mol Med. 2008;14:451–64. doi: 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 6.LaFerla FM, Green KN, Odo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 7.Koistinaho M, Ort M, Cimadevilla JM, Vondrous R, Cordell B, Koistinaho J, et al. Specific spatial learning deficits become severe with age in beta -amyloid precursor protein transgenic mice that harbor diffuse beta -amyloid deposits but do not form plaques. Proc Natl Acad Sci USA. 2001;98:14675–80. doi: 10.1073/pnas.261562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, et al. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/S0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu Rev Biochem. 2000;69:303–42. doi: 10.1146/annurev.biochem.69.1.303. [DOI] [PubMed] [Google Scholar]
- 10.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–52. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–12. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luzio JP, Poupon V, Lindsay MR, Mullock BM, Piper RC, Pryor PR. Membrane dynamics and the biogenesis of lysosomes. Mol Membr Biol. 2003;20:141–54. doi: 10.1080/0968768031000089546. [DOI] [PubMed] [Google Scholar]
- 14.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu WH, Kumar A, Peterhoff C, Shapiro Kulnane L, Uchiyama Y, Lamb BT, et al. Autophagic vacuoles are enriched in amyloid precursor protein-secretase activities: implications for beta-amyloid peptide over-production and localization in Alzheimer's disease. Int J Biochem Cell Biol. 2004;36:2531–40. doi: 10.1016/j.biocel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Adamec E, Mohan PS, Cataldo AM, Vonsattel JP, Nixon RA. Up-regulation of the lysosomal system in experimental models of neuronal injury: implications for Alzheimer's disease. Neuroscience. 2000;100:663–75. doi: 10.1016/S0306-4522(00)00281-5. [DOI] [PubMed] [Google Scholar]
- 17.Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, et al. Macroautophagy–a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langui D, Girardot N, El Hachimi KH, Allinquant B, Blanchard V, Pradier L, et al. Subcellular topography of neuronal Abeta peptide in APPxPS1 transgenic mice. Am J Pathol. 2004;165:1465–77. doi: 10.1016/S0002-9440(10)63405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ditaranto K, Tekirian TL, Yang AJ. Lysosomal membrane damage in soluble Abeta-mediated cell death in Alzheimer's disease. Neurobiol Dis. 2001;8:19–31. doi: 10.1006/nbdi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 20.Yang AJ, Chandswangbhuvana D, Margol L, Glabe CG. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Abeta1-42 pathogenesis. J Neurosci Res. 1998;52:691–8. doi: 10.1002/(SICI)1097-4547(19980615)52:6<691::AID-JNR8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Terman A, Neuzil J, Kagedal K, Ollinger K, Brunk UT. Decreased apoptotic response of inclusion-cell disease fibroblasts: a consequence of lysosomal enzyme missorting? Exp Cell Res. 2002;274:9–15. doi: 10.1006/excr.2001.5441. [DOI] [PubMed] [Google Scholar]
- 22.Brunk UT, Dalen H, Roberg K, Hellquist HB. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med. 1997;23:616–26. doi: 10.1016/S0891-5849(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 23.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 24.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 25.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–41. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 27.Cedazo-Mínguez A, Huttinger M, Cowburn RF. Beta-VLDL protects against A beta(1-42) and apoE toxicity in human SH-SY5Y neuroblastoma cells. Neuroreport. 2001;12:201–6. doi: 10.1097/00001756-200102120-00006. [DOI] [PubMed] [Google Scholar]
- 28.Akterin S, Cowburn RF, Miranda-Vizuete A, Jimenez A, Bogdanovic N, Winblad B, et al. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 2006;13:1454–65. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–10. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding Q, Dimayuga E, Keller JN. Oxidative damage, protein synthesis, and protein degradation in Alzheimer's disease. Curr Alzheimer Res. 2007;4:73–9. doi: 10.2174/156720507779939788. [DOI] [PubMed] [Google Scholar]
- 31.Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, et al. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–9. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Roberg K, Jerhammar F, Marcusson J, Terman A. Autophagy of amyloid beta-protein in differentiated neuroblastoma cells exposed to oxidative stress. Neurosci Lett. 2006;394:184–9. doi: 10.1016/j.neulet.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Zheng L, Kagedal K, Dehvari N, Benedikz E, Cowburn R, Marcusson J, et al. Oxidative stress induces macroautophagy of amyloid beta-protein and ensuing apoptosis. Free Radic Biol Med. 2009;46:422–9. doi: 10.1016/j.freeradbiomed.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Hooper NM, Turner AJ. The search for alpha-secretase and its potential as a therapeutic approach to Alzheimer s disease. Curr Med Chem. 2002;9:1107–19. doi: 10.2174/0929867023370121. [DOI] [PubMed] [Google Scholar]
- 36.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–5. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 37.Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120:4081–91. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- 38.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 39.Su Y, Chang PT. Acidic pH promotes the formation of toxic fibrils from beta-amyloid peptide. Brain Res. 2001;893:287–91. doi: 10.1016/S0006-8993(00)03322-9. [DOI] [PubMed] [Google Scholar]
- 40.Harman D. Alzheimer's disease pathogenesis: role of aging. Ann N Y Acad Sci. 2006;1067:454–60. doi: 10.1196/annals.1354.065. [DOI] [PubMed] [Google Scholar]
- 41.Waschuk SA, Elton EA, Darabie AA, Fraser PE, McLaurin JA. Cellular membrane composition defines A beta-lipid interactions. J Biol Chem. 2001;276:33561–8. doi: 10.1074/jbc.M103598200. [DOI] [PubMed] [Google Scholar]
- 42.Tian Y, Bustos V, Flajolet M, Greengard P. A small-molecule enhancer of autophagy decreases levels of A{beta} and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 2011;25:1934–42. doi: 10.1096/fj.10-175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling D, Song HJ, Garza D, Neufeld TP, Salvaterra PM. Abeta42-induced neurodegeneration via an age-dependent autophagic-lysosomal injury in Drosophila. PLoS ONE. 2009;4:e4201. doi: 10.1371/journal.pone.0004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 45.Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J Biol Chem. 2008;283:7745–53. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- 46.Tian G, Sobotka-Briner CD, Zysk J, Liu X, Birr C, Sylvester MA, et al. Linear non-competitive inhibition of solubilized human gamma-secretase by pepstatin A methylester, L685458, sulfonamides, and benzodiazepines. J Biol Chem. 2002;277:31499–505. doi: 10.1074/jbc.M112328200. [DOI] [PubMed] [Google Scholar]
- 47.Yatin SM, Varadarajan S, Butterfield DA, Vitamin E. Prevents Alzheimer's Amyloid beta-Peptide (1-42)-Induced Neuronal Protein Oxidation and Reactive Oxygen Species Production. J Alzheimers Dis. 2000;2:123–31. doi: 10.3233/jad-2000-2212. [DOI] [PubMed] [Google Scholar]
- 48.Hung SY, Huang WP, Liou HC, Fu WM. Autophagy protects neuron from Abeta-induced cytotoxicity. Autophagy. 2009;5:502–10. doi: 10.4161/auto.5.4.8096. [DOI] [PubMed] [Google Scholar]
- 49.Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134:258–77. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S, et al. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer's disease. Neuron. 2008;60:247–57. doi: 10.1016/j.neuron.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caccamo A, Majumder S, Richardson A, Strong R, Odo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–20. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–9. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terman A, Gustafsson B, Brunk UT. Autophagy, organelles and ageing. J Pathol. 2007;211:134–43. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- 54.Ling D, Salvaterra PM. A central role for autophagy in Alzheimer-type neurodegeneration. Autophagy. 2009;5:738–40. doi: 10.4161/auto.5.5.8626. [DOI] [PubMed] [Google Scholar]
- 55.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–40. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cedazo-Minguez A, Bonecchi L, Winblad B, Post C, Wong EH, Cowburn RF, et al. Nicergoline stimulates protein kinase C mediated alpha-secretase processing of the amyloid precursor protein in cultured human neuroblastoma SH-SY5Y cells. Neurochem Int. 1999;35:307–15. doi: 10.1016/S0197-0186(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 57.Sandebring A, Dehvari N, Perez-Manso M, Thomas KJ, Karpilovski E, Cookson MR, et al. Parkin deficiency disrupts calcium homeostasis by modulating phospholipase C signalling. FEBS J. 2009;276:5041–52. doi: 10.1111/j.1742-4658.2009.07201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.