Abstract
Pure nucleotide precursor pools are a prerequisite for high-fidelity DNA replication and the suppression of mutagenesis and carcinogenesis. ITPases are nucleoside triphosphate pyrophosphatases that clean the precursor pools of the non-canonical triphosphates of inosine and xanthine. The precise role of the human ITPase, encoded by the ITPA gene, is not clearly defined. ITPA is clinically important because a widespread polymorphism, 94C>A, leads to null ITPase activity in erythrocytes and is associated with an adverse reaction to thiopurine drugs. We studied the cellular function of ITPA in HeLa cells using the purine analog 6-N hydroxylaminopurine (HAP), whose triphosphate is also a substrate for ITPA. In this study, we demonstrate that ITPA knockdown sensitizes HeLa cells to HAP-induced DNA breaks and apoptosis. The HAP-induced DNA damage and cytotoxicity observed in ITPA knockdown cells are rescued by an overexpression of the yeast ITPase encoded by the HAM1 gene. We further show that ITPA knockdown results in elevated mutagenesis in response to HAP treatment. Our studies reveal the significance of ITPA in preventing base analog-induced apoptosis, DNA damage and mutagenesis in human cells. This implies that individuals with defective ITPase are predisposed to genome damage by impurities in nucleotide pools, which is drastically augmented by therapy with purine analogs. They are also at an elevated risk for degenerative diseases and cancer.
Introduction
The human genome is constantly attacked by exogenous or endogenous DNA damaging agents. An accumulation of DNA damage increases genome instability and mutagenesis, which predisposes cells to neoplasia, as well as degenerative diseases [1], [2]. A prominent cause of endogenous DNA damage decreasing the fidelity of DNA replication is contamination of the nucleotide precursor pool with non-canonical nucleotides [3], [4]. These contaminants of the precursor pool include deoxy- and ribonucleoside triphosphates of inosine (ITP/dITP), xanthine (XTP/dXTP), 8-oxo-guanine (8-O-GTP/8-O-dGTP) and others, generated either as byproducts of cellular metabolism or by deamination or oxidation of bases in natural nucleotides. Non-canonical nucleotides contain analogs of the normal nitrogen bases (base analogs), which gives some of them the unique property of ambiguous base pairing during replication [5], [6], [7]. Incorporated base analogs in DNA are repaired by the cellular repair systems, which can result in the accumulation of DNA breaks [8], [9]. If base analogs in DNA escape the repair systems, their capacity for ambiguous base pairing will lead to the accumulation of mutations in the subsequent replication rounds [10], [11]. Taking into consideration the harmful effects of base analog incorporation, it is not surprising that cells have developed elaborate enzymatic systems that protect from base analog-induced DNA damage [12], [13]. These systems function at two levels. The first level involves the interception of non-canonical nucleotides in the precursor pool and their cleavage into di- or monophosphates. The second level involves detection of improper bases after incorporation and their direct removal from DNA. The former is achieved by a class of enzymes called nucleoside triphosphatases (NTPases) [3]. One such NTPase is evolutionary conserved Inosine Triphosphate Pyrophosphatase (ITPA) [14].
ITPA is a human ITPase, whose function is to cleave inosine triphosphate (ITP) and xanthine triphosphate (XTP) as well as their deoxyribose forms into monophospates. This prevents the incorporation of the nucleotide inosine (dITP), which contains the base analog hypoxanthine, and dXTP into DNA [15]. ITPA is expressed in many human tissues [15], [16]. The importance of ITPases is underscored by severe genome instability phenotypes caused by deletion of the ITPA homologs in bacteria, yeast and mice. A mutant of the bacterial ITPase, rdgB, is synthetically lethal in combination with defects in recombination [8], [9], [17]. The deletion of the budding yeast ITPase, HAM1, results in a drastic elevation of mutagenesis induced by the model purine base analog hydroxylaminopurine (HAP) [18]. The most severe phenotype for ITPase deletion is observed in mice. The majority of the progeny with ITPase knockout (genotype Itpa−/−) are inviable [19]. The mice that survive suffer from growth retardation and die before weaning from cardiac failure. Fibroblasts obtained from the ITPase knockout mice accumulated DNA single-strand breaks and chromosomal abnormalities [20]. Therefore, in bacteria, yeast and mice, the ITPase function plays an important role in maintaining genomic integrity.
The precise cellular function of the human ITPase, ITPA, is not clearly defined. There is a polymorphism in the ITPA gene in the human population. Several alleles cause atypical ITPase activity [21], [22], [23]. Clinically, the most relevant polymorphism is the ITPAc.94C>A missense mutation that results in a substitution of proline with threonine at position 32 (P32T). The allelic frequency of this mutation ranges from 5 to19%, with the highest frequency found in the Asian population [22]. Homozygotes for the P32T mutation have no ITPase activity in erythrocytes, whereas heterozygotes have approximately one-fourth ITPase activity. Although ITPA deficiency in humans appears to be benign, the administration of thiopurine therapy leads to adverse drug reactions in these individuals [21], [24], [25].
To analyze the role of ITPA in human cells, we forced nucleotide pool contamination by 6-hydroxyadenine (hydroxylamonipurine, abbreviated HAP). The dHAPTP is as good a substrate for ITPA as ITP or XTP [26]. HAP is a potent mutagen that can mispair with C or T and induce GC to AT and AT to GC transitions [27], [28]. Unlike most mutagens, HAP in non-recombinogenic in yeast and its mutagenic action is independent of translesion synthesis DNA polymerase, Pol ζ [29], [30]. Nevertheless, it is clastogenic in mammalian cells [31]. Most likely, HAP is activated to deoxynucleoside triphosphate by a combination of salvage and de novo purine biosynthesis pathways [26], but definite genetic identification of the responsible enzymes has only been obtained for the first step, conversion of the base to HAPMP by phosphoribosyltransferases in bacteria and yeast (Stepchenkova and Schaaper, personal communication and [32]). The dHAPTP is readily incorporated into DNA by the replicative DNA polymerases of bacteria and eukaryotes [27], [33]and is repaired in bacteria by the same systems as dITP and dXTP [9], thereby enabling us to extrapolate the results obtained with HAP to natural base analogs. Thus, the use of HAP provides us with a good tool to investigate the protective effects of ITPA against nucleotide pool contamination.
In this study, using the cervical carcinoma cell line HeLa and HAP as a model, we demonstrate that ITPA knockdown sensitizes human cells to base analog-induced DNA breakage, mutagenesis and apoptosis. These phenotypes can be rescued by overexpressing the yeast ITPase, HAM1, in the ITPA knockdown cells. Our data suggest that ITPA plays a critical role in protecting human cells against the cytotoxic, genotoxic and mutagenic effects of base analogs. This implies that individuals with defective ITPase are at an elevated risk for degenerative diseases and cancer.
Results
HAP incorporation into DNA of HeLa cells
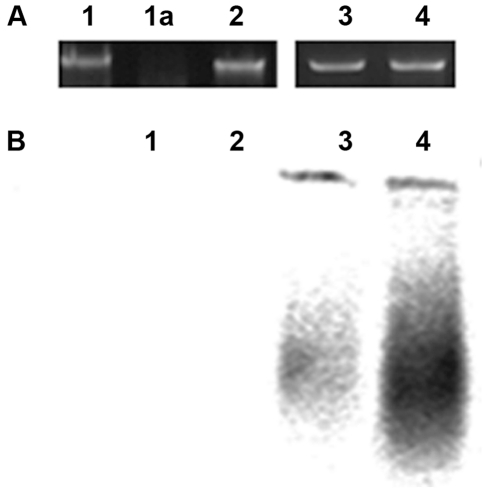
It is known that hypoxanthine bases accumulate at a detectable level in RNA and in DNA in Itpa knockout mice [19], [20]. To find whether HAP is present in DNA of treated HeLa cells, we studied the appearance of endonuclease V-cleavable sites. HAP in DNA is recognized by the product of the bacterial nfi gene, EndoV protein [9]. The enzyme cuts the second bond 3′ to the modified base and leaves free 3′ OH groups [34], [35]. Such DNA will be a substrate for nick translation and therefore, the incorporation of label by E. coli DNA polymerases I would be proportional to the quantity of such nicks [36]. We found that the number of EndoV cleavable sites tremendously increases in DNA isolated from HeLa cells grown in the presence of HAP (Fig. 1). This means that after 24 hours there is a substantial proportion of HAP in DNA, which was not removed by DNA repair in human cells. We previously detected DNA breaks, presumably being intermediate products of repair of HAP, in the Comet assay after the same 24 hours [37]. While sensitive Comet assay detects some breaks, most HAP is still present in DNA at this time. Massive removal of HAP achieved by EndoV in vitro produces a strong signal in nick-translation assay.
Figure 1. HAP treatment leads to the appearance of EndoV sensitive sites in HeLa DNA.
We extracted genomic DNA from HeLa cells grown with or without HAP. Treatment of this DNA with bacterial EndoV creates 3′ nicks, which are substrates for nick-translation (BioProbe® Nick translation kit with bio-16-dUTP (Enzo Life Sciences)) as described in Materials and Methods. A. Agarose gel electrophoresis of nick-translated DNA from HeLa cells. 1- from untreated cells; 1a – from untreated cells digested with DNase; 2 – from cells grown in 2.64 mM HAP; 3- from untreated cells, DNA incubated with Endo V; and 4 - from cells grown in 2.64 mM HAP, DNA incubated with Endo V. B. Detection of newly synthesized biotinylated DNA separated by alkaline agarose electrophoresis. 1- from untreated cells; 2 – from cells grown in 2.64 mM HAP; 3- from untreated cells, DNA incubated with Endo V; and 4 - from cells grown in 2.64 mM HAP, DNA incubated with Endo V.
HAP treatment triggers apoptosis in HeLa cells
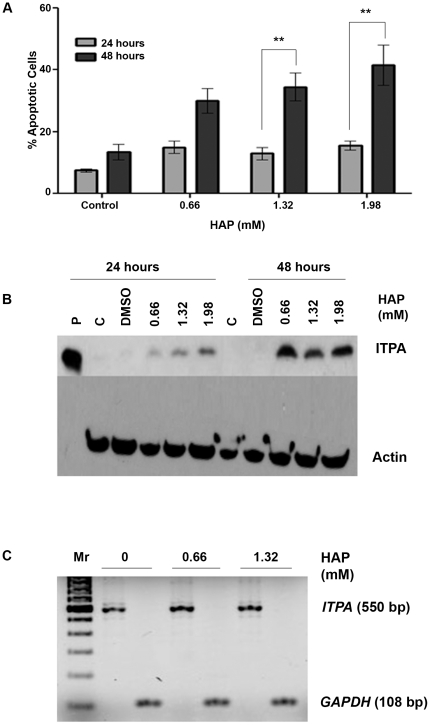
Previously, it has been reported that HAP treatment results in chromosomal fragmentation in human epidermoid cells [38]. This effect appears to be cell line specific, because HAP did not induce a chromosomal catastrophe in HCT116 cells [37]. This suggests that HAP is potentially capable of causing devastating DNA damage in human cells, but this is realized only under certain conditions. We examined whether HAP treatment triggered apoptosis in human cells. To do this, we determined the effects of increasing doses of HAP on the viability of HeLa cells after 24 hours or 48 hours of treatment (Fig. 2A). After staining the cells with Hoechst dye, we enumerated the number of apoptotic nuclei by fluorescence microscopy. No effects of HAP treatment were seen after 24 hours. After 48 hours of HAP treatment, we found that 35% of the cells were apoptotic at a dose of 1.32 mM, while 42% of the cells underwent apoptosis at 1.98 mM. This increase is significant (p<0.01) as compared to the 16% apoptosis observed at these doses after 24 hours of treatment. The two-fold increase in apoptotic cells observed after 48 hours of treatment suggests that HAP is cytotoxic to human cells, but cell divisions are necessary for HAP to exert its cytotoxic effects.
Figure 2. Effects of HAP treatment on apoptosis and ITPA levels in HeLa cells.
(A) HAP treatment causes apoptosis in HeLa cells after treatment for 24 or 48 hours. By two-way ANOVA, ptime = 0.001 and pconcentration = 0.0046. **p<0.01 by Bonferroni multiple comparison post test for column analysis comparing means for 24 hours vs. 48 hours. (B) HAP treatment leads to the increase of ITPA protein levels in HeLa extracts following treatment for both 24 hours and 48 hours. Western blots were performed as described in Materials and Methods. P- pure ITPA protein, C- untreated control, DMSO – solvent only. (C) HAP treatment does not increase levels of ITPA transcripts. The analysis was performed as described in Materials and Methods. HAP treatment was for 24 hours. Mr – 100 bp ladder.
By immunostaining of the whole cells as well as by immunoblot we have shown that HAP treatment causes an induction of the ITPA protein production in HCT116 cells [37]. We confirmed that this was the case for HeLa cells as well. We observed that ITPA protein levels were elevated after 24 hours as well as 48 hours after HAP treatment (Fig. 2B). The mechanism is unclear, but it does not occur at the level of transcription, as demonstrated by RT-PCR (Fig. 2C). The induction of the ITPA protein levels appeared to be more prominent after 48 hours. The increase of ITPA protein production in response to HAP treatment is consistent with the idea of a high demand for ITPA when the precursor pool is contaminated with dHAPTP.
HAP-induced apoptosis occurs through the intrinsic pathway
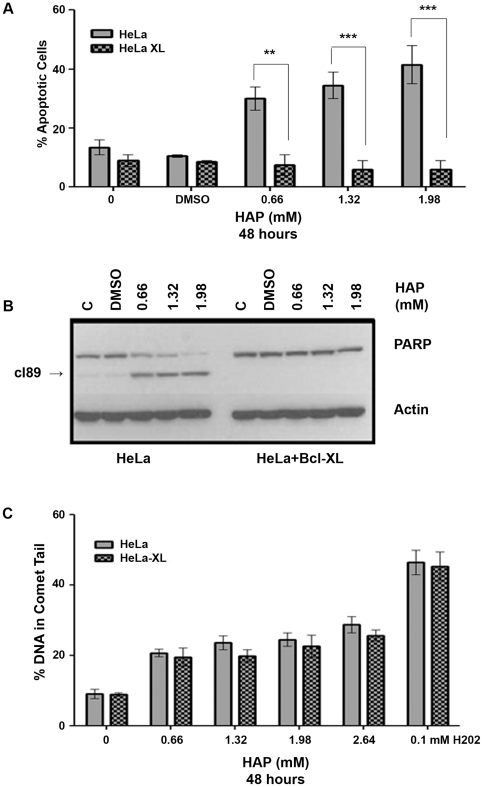
Apoptosis can occur by two pathways: either the extrinsic pathway that involves death receptors or the intrinsic pathway that occurs through the mitochondria. The intrinsic pathway can be blocked by the overexpression of the anti-apoptotic protein Bcl-xL [39]. To examine which pathway was involved in the case of HAP treatment, we assayed for apoptosis following a 48-hour HAP treatment of HeLa cells overexpressing Bcl-xL (henceforth referred to as HeLa-xL) [40]. As evident from Fig. 3A, the HeLa-xL cells were protected from HAP-induced apoptosis. We confirmed the protection of HeLa-xL cells from HAP-induced apoptosis by performing immunoblots for PARP cleavage, a hallmark of apoptosis [41]. No PARP cleavage (Fig. 3B) was observed in HeLa-xL cells, in a sharp contrast with HeLa cells.
Figure 3. HAP-induced apoptosis occurs through the intrinsic pathway.
(A) Protection from HAP-induced apoptosis by overexpression of Bcl-xL. Both HeLa and HeLa-xL cell lines were treated with increasing doses of HAP for 48 hours and the percentage of apoptotic cells was determined by Hoechst staining. By two-way ANOVA, pcell line<0.0001 and pconcentration = 0.009. **p<0.01,***p<0.001 by Bonferroni multiple comparison post test for column analysis comparing means for HeLa vs. HeLa-xL hours. (B) Confirmation of protection from HAP-induced apoptosis by Bcl-xL overexpression by immunoblot for PARP cleavage. HAP induced dose-dependent cleavage of PARP in regular HeLa cells but not in HeLa cells overexpressing Bcl-xL. PAPR cleavage product is marked as cl89. (C) HAP treatment results in similar levels of DNA breaks in HeLa and HeLa+Bcl-xL cell lines (p>0.05).
HAP treatment causes an accumulation of DNA strand breaks prior to the onset of apoptosis
Deletion of the E. coli ITPase, rdgB, results in the generation of DNA breaks and chromosome fragmentation due to the excision of hypoxanthine by Endo V [8], [9], [17]. We have previously shown that HAP induces DNA breaks in human cells [37]. Most likely, HAP in DNA is processed in a manner similar to the processing of hypoxanthine by either the human homolog of Endo V or some other yet to be identified enzymes. One possible candidate could be the AAG glycosylase, which can excise hypoxanthine [42]. The observation that HAP is capable of inducing apoptosis raised the possibility that the breaks we observe reflect the onset of apoptotic destruction of the nucleus. The HeLa-xL cells provided us with a good tool for distinguishing between the two scenarios. As Bcl-xL overexpression blocks apoptosis, we rationalized that DNA breaks occurring during the repair of HAP incorporated into DNA would not be affected by Bcl-xL overexpression and therefore could be distinguished from DNA breaks caused by the process of apoptosis itself. In the latter case, Bcl-xl overexpression would block the appearance of DNA breaks as apoptosis itself is suppressed. We studied the effect of increasing doses of HAP on the formation of DNA breaks by alkaline comet assay after 24 hours of HAP treatment in both cell lines. In prior experiments, this was a time point where HAP treatment did not cause more than 16% apoptosis. We observed that both cell lines accumulated similar levels of DNA breaks (Fig. 3C). These data suggest that HAP treatment does generate DNA breaks upstream to apoptosis.
ITPA knockdown cells are hypersensitive to HAP-induced apoptosis
To investigate the role of ITPA in protecting against HAP-induced cytotoxicity, we made stable knockdowns of ITPA by transfecting HeLa with plasmids expressing shRNA that targeted the ORF of ITPA. We obtained an efficient knockdown of ITPA (Fig. S1). The ITPA knockdown cells were viable, indicating that it is not an essential gene. Upon treatment with HAP for 24 hours, 30–50% of ITPA knockdown cells underwent apoptosis (p<0.001 for 0.66 mM, p<0.0001 for 1.32 mM and 1.98 mM) (Fig. 4). In control HeLa cells, comparable levels of apoptosis were observed only after 48 hours of treatment. No statistically significant differences were observed for the untransfected and non-targeting shRNA transfected controls. Thus, ITPA knockdown sensitizes cells to HAP-induced apoptosis. Hydrogen peroxide treatment is known to induce DNA breaks in HeLa cells and subsequently cause apoptosis [43], [44]. Therefore, we used this as a positive control for our apoptosis assays to determine if HAP-induced hypersensitivity to apoptosis was specific for ITPA knockdown. We found that hydrogen peroxide treatment caused the same level of apoptosis in all three cell lines. Taken together, our data suggest that ITPA plays an important role in protecting HeLa cells against HAP-induced apoptosis.
Figure 4. ITPA protects against HAP-induced apoptosis.
ITPA knockdown sensitizes cells to HAP-induced apoptosis. As compared to the control and non-targeting shRNA transfected cells, ITPA knockdown cells undergo approximately 30–50% apoptosis upon HAP treatment for 24 hours. Hydrogen peroxide treatment (0.1 mM, four hours) was used as a positive control. The difference between control cells and cells with the ITPA knockdown is highly significant (***p<0.001, ****p<0.0001). There was no difference between the control versus the non-targeting cell lines in all HAP doses tested. No significant difference in hydrogen peroxide-induced apoptosis was observed for all three cell lines.
Suppression of HAP-induced apoptosis by overexpression of ITPA or HAM1
In prior experiments we found that ITPA protein production was induced in response to HAP treatment (Fig. 2B). This suggested a putative role of ITPA in protecting against the harmful effects of HAP. Moreover, we found that ITPA knockdown made cells hypersensitive to HAP-induced apoptosis. To further establish the protective role against HAP-induced apoptosis, we determined whether overexpression of ITPA or its yeast ortholog, HAM1, protected HeLa cells from HAP-induced apoptosis. We transfected HeLa cells with two constructs where ITPase genes were under a strong constitutive promoter. One expresses ITPA-GFP, encoding for a fusion of ITPA with the GFP protein, another expresses HAM1-GFP, encoding for a fusion of Ham1 with the GFP protein. The production of both fusion proteins was confirmed by immunoblot (Fig. S2A). We compared the apoptotic response at a HAP dose of 1.98 mM for 48 hours. This dose of HAP caused 42% of the cells to undergo apoptosis (Fig. 3A). As compared to the cells transfected with vector alone, ITPA overexpressing cells were protected from HAP-induced apoptosis (Fig. 5A, p<0.01). A similar level of protection was also observed in the case of HAM1 overexpression (p<0.05).
Figure 5. ITPase overexpression suppresses HAP-induced cytotoxicity.
(A) Effect of overexpression of the ITPA and the gene encoding yeast ITPase, HAM1, on HAP-induced apoptosis in HeLa cells. Differences are significant (*p<0.05, **p<0.01). (B) Overexpression of the yeast HAM1 could rescue ITPA knockdown cells from hypersensitivity to HAP-induced apoptosis (****p<0.0001, n.s., not significant).
We have shown that the expression of wild-type ITPA as well as HAM1 could efficiently rescue cells from apoptosis caused by HAP treatment, suggesting that the yeast protein functions in the foreign environment (Fig. 5A). Therefore, we rationalized that the high expression of HAM1 should be able to complement the defects caused by ITPA knockdown in those cells as well. We anticipated that HAM1 would not be inhibited by shRNA targeting ITPA. We confirmed the expression of the HAM1 construct and consequent protein production in the knockdown cells by immunoblot (Fig. S2B). When we treated the transfectants with 1.98 mM HAP for 48 hours, we found that approximately 57% of cells transfected with the vector only underwent apoptosis. However, cells transfected with the vector expressing HAM1 were resistant, with only 13% undergoing apoptosis (Fig. 4B) (p<0.0001). Thus, HAM1 complemented the defect seen in the case of ITPA knockdown, proving that the differences in the HAP sensitivity of normal and ITPA knockdown cells are due to one gene, ITPA.
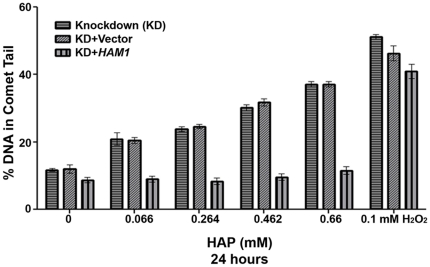
ITPA knockdown leads to elevated levels of HAP-induced DNA breaks
We had previously demonstrated that human dermal fibroblasts with endogenous ITPA-P32T (associated with null ITPase activity in erythrocytes, as discussed in the Introduction) accumulated more DNA breaks than the control fibroblast cell line with a wild-type ITPA [37]. The availability of ITPA knockdown HeLa cells transfected with control vector or vector expressing the yeast HAM1gene allowed us to study the effects of HAP in nearly isogenic cell lines. We assayed for levels of DNA breaks by alkaline comet assay at low doses of HAP. At these doses, we did not expect any HAP-induced apoptosis, which would otherwise confound the interpretation of the data since apoptosis itself causes DNA fragmentation. We found that ITPA knockdown cells and ITPA knockdown cells transfected with the empty vector accumulated more DNA breaks than the knockdown cells overexpressing HAM1 at low doses of HAP treatment (0.066–0.66 mM) (Fig. 6). At higher doses of HAP, the comet tails in the knockdown cells were too large to be precisely quantified. In the HAM1 overexpressing a knockdown cell comet tail at higher doses ranged from 13 to 20%, which is slightly less that in the original HeLa, Fig. 3C. No statistically significant difference in levels of DNA breaks was observed in response to hydrogen peroxide treatment in the three cell lines.
Figure 6. ITPA protects against HAP-induced DNA breaks.
Alkaline comet assay data reveals that as compared to the control and non-targeting shRNA-expressing cell lines, ITPA knockdown cells accumulated elevated levels of DNA breaks after 24 hours of treatment with HAP. At high doses of HAP, the sizes of the comet tails in the ITPA knockdown cells were too large to be quantified. The assay was performed at lower doses of HAP treatment in order to obtain measurable comet tails. As compared to the cells with vector, the overexpression of HAM1 suppressed the accumulation of HAP-induced DNA breaks in the ITPA knockdown cells. Statistical differences were measured by one-way ANOVA followed by Dunns multiple comparisons for column analysis. By two-way ANOVA, p = 0.008. Post-test analysis revealed differences (p<0.05) for knockdown versus knockdown+HAM1 and knockdown+vector versus knockdown+HAM1. No difference in levels of DNA breaks was observed for hydrogen peroxide treatment for all three cell lines.
ITPA knockdown in HeLa cells elevates HAP-induced mutagenesis
In addition to triggering apoptosis, we anticipated that contamination of the nucleotide precursor pools with non-canonical nucleotides could lead to elevated mutagenesis. HAP has been shown to be mutagenic and carcinogenic in mammalian cells [45], [46]. It is known that the disruption of the HAM1 gene in the budding yeast S. cerevisiae causes hypermutagenesis in response to HAP treatment [18]. We therefore investigated whether this phenotype would be seen in ITPA knockdown human cells as well. We determined that HAP-induced mutant frequencies at the HPRT locus (6-thioguanine resistance). The viability of HeLa cell lines with or without ITPA knockdown was not affected by HAP treatment. No statistically significant differences were observed between the untransfected and shRNA-transfected cells in the absence of HAP treatment. HAP was clearly mutagenic for HeLa cells, increasing mutant frequency three-fold at 0.1 mM and 13-fold at 1 mM (Suplementary Table 1). Next we compared HAP-induced mutant frequencies for HeLa and ITPA knockdown cells as described in Materials and Methods. We found that ITPA knockdown cells were more sensitive to HAP-induced mutagenesis at high doses as compared to the untransfected cells (p<0.01) (Fig. 7). This suggests that ITPA plays a role in the protection from HAP-induced mutations.
Figure 7. ITPA protects against HAP-induced mutagenesis.
The data represent the induced HPRT mutant frequency for the HAP-treated control and ITPA knockdown cells. At a low dose (0.1 mM) of HAP treatment for 24 hours the difference between cell lines was not significant but at dose 1 mM ITPA knockdown cells were more sensitive to HAP mutagenesis. (**p<0.01, n.s., not significant).
Discussion
An understanding of the role of ITPA in human cells is important because several alleles representing polymorphism in the ITPA gene are associated with the onset of thiopurine therapy-related diseases. We probed the function of ITPA by determining whether the enzyme protected HeLa cells against the harmful effects of the model purine analog, HAP.
Previous reports have shown that HAP treatment results in severe genotoxic stress to cells. HAP treatment causes chromosomal fragmentation and mutagenesis in human cells and in Syrian hamster embryo cells [31], [38]. We propose that HAP incorporated into the DNA of human cells (Fig. 1) is being repaired, intermediates of the repair cause breaks and these persisting breaks cause apoptosis.
We found that HAP treatment induced apoptosis in HeLa cells. The manifestation of apoptosis was prominent after 48 hours of treatment (Fig. 2A). This is in contrast to genotoxicants directly damaging DNA, like UV irradiation or hydrogen peroxide, which induce apoptosis within 4–6 hours of treatment [40], [43]. The delayed response suggests that HAP needs to be activated to dHAPTP and incorporated into DNA during replication in order to exert its cytotoxic effects. At present, the exact pathway of HAP activation in human cells is not known. We propose that it could be similar to the hypothetical pathways proposed for bacteria and yeast [26], [32]. As HAP treatment is indicative of the effects of nucleotide pool contamination, our data imply that elevated nucleotide pool contamination and the subsequent incorporation of base analogs into DNA causes apoptosis in human cells.
What is the mechanism underlying HAP cytotoxicity in human cells? Using an analogy to experiments with bacteria, one possibility is the generation of relatively long-lived single strand DNA breaks as intermediates during the repair of HAP in DNA. These breaks are converted to double strand breaks when the replication fork encounters the discontinuity in the template [9], [47], [48].
The incorporation of thiopurines into DNA causes DNA breaks and subsequent apoptosis due to incomplete mismatch repair [49], [50]. An elevated level of the incorporation of 8-oxoG into DNA triggers the accumulation of single-strand DNA breaks, which results in cell death in mouse cells [51], [52]. The situation with 8-OG in mammalian cells is thus different from bacteria, where no chromosomal DNA fragmentation occurs in the mutT mutants [53]. It is possible that clastogenicity of base analogs depends on the relative efficiency of the repair systems responsible for their removal from DNA. We propose that HAP-induced apoptosis is caused by persisting DNA breaks. We found that HAP treatment triggered the accumulation of DNA breaks, which later led to apoptosis in HeLa cells but not in HeLa-xL cells. Presumably, these DNA breaks could be caused by the inefficient excision of HAP from DNA by an uncharacterized glycosylase or nuclease. Endo V has been shown to play a key role in this process in E.coli lacking the ITPase gene, rdgB [9]. Orthologs of the nfi gene encoding for endonuclease V have been characterized in mice and found in the human genome [34], [35], [54]. The mouse enzyme was, however, was 50 times less active than the bacterial Endo V. The variants of human enzyme have been purified but no endonuclease activity was detected (Waisertreiger, unpublished, R. Dalhus, personal communication). The exact mechanism of HAP and hypoxanthine repair in humans remains to be determined.
We found that knocking down ITPA by shRNA sensitized cells to HAP-induced apoptosis (Fig. 4). The knockdown cells per se are viable, thereby indicating that ITPA is not an essential gene in human cells. Overexpression of the yeast HAM1 in the knockdown cells rescued them from the cytotoxic effects of HAP. The knockdown cells accumulated more DNA breaks, which were suppressed in knockdown cells that overexpressed HAM1 (Figs. 5B and 6). Thus, ITPA prevents the accumulation of HAP-induced DNA damage. In passages of mouse embryonic fibroblasts, a spontaneous increase in production of the NUDIX protein, NUDT16, suppressed the genome instability phenotypes associated with Itpa deletion, thus suggesting a functional redundancy between Itpa and NUDT16 [20]. Here, we did not observe any redundancy for ITPA function. Although NUDIX proteins are found in human cells, it is possible that they are activated under special conditions. It is also possible that ITPA knockdown by itself is insufficient to activate the expression of NUDIX genes in human cells in a relatively limited number of passages. Another possibility is that the functional redundancy between NUDIX genes and ITPA is specific to mice. Collectively, we have shown that ITPA plays a critical role in preventing HAP-induced apoptosis. This implies that ITPA could play a role in protecting against the incorporation of dITP/dXTP as well.
Thiopurines like azathioprine are commonly used immunosuppressive or anti-cancer drugs that exert their cytotoxic effects by being converted into active nucleotides, which are then incorporated into DNA. ITPA is capable of destroying nucleoside triphosphate of 6-MP [55]. A number of reports link chronic immunosuppression/therapy with thiopurines like azathioprine and the onset of therapy-induced cancer. One of the mechanisms by which thiopurines bring about immunosuppression is by causing the death of cytotoxic T lymphocytes, which is elicited by the persistence of DNA breaks [50], [56]. It is plausible that the mechanism of cytotoxicity is similar for thiopurines and HAP. Increased DNA damage can force cells to undergo either apoptosis or senescence in order to prevent the passage of damaged DNA to progeny cells. While apoptosis is critical for tissue homeostasis, increased apoptosis is harmful because it can lead to organ damage and degeneration. Elevated levels of DNA breaks and apoptosis have been implicated as one of the causes of degenerative diseases such as diabetes, arthritis, and cardiac failure as well as neurodegenerative diseases like Alzheimer's disease, Huntington's disease and Parkinson's disease [57], [58]. The increased apoptosis observed in the ITPA knockdown cells in response to HAP treatment is clinically relevant because it raises the possibility that individuals with ITPase deficiency could be at risk of developing therapy-induced organ damage and degeneration.
Therapy-induced cancer is a serious side effect of long-term thiopurine-mediated immunosuppression [50], [56]. In transplant recipients, a decade long exposure to thiopurines like azathioprine is associated with the onset of cancers such as leukemia and squamous cell carcinomas. Moreover, there is a putative association between P32T ITPA, prolonged azathioprine therapy and the onset of carcinoma [59]. We have shown that ITPA knockdown results in increased HAP-induced mutagenesis. This is in line with our previous findings in yeast wherein HAP treatment in yeast with a HAM1 mutation resulted in two orders of magnitude higher mutagenesis as compared to their wild-type counterparts [18], [29]. In human cells, however, ITPA knockdown did not result in such a dramatic phenotype as was observed with yeast. The situation resembles the discrepancies in magnitude of the effect of the absence of 8-oxoguanine triphosphatase in bacteria (mutT is a very strong, up to 1000-fold, mutator [60]) and mice (Mth−/− cells possess a very weak two-fold mutator phenotype [61]). In spite of this relatively small effect on the mutation rates, the deletion of Mth1caused an accumulation of 8-oxoguanine in DNA and resulted in an increase in tumors in mice. In our study, ITPA plays a more prominent role in prevention of apoptosis that mutagenesis caused by HAP, suggesting that different pathways lead to these events. It is also possible that the yeast ham 1 mutant hypermutability maybe a very special case, because yeast possess neither a molybdenum cofactor-dependent pathway of HAP destruction [62], [63], nor Endo V. Elevated levels of DNA breaks and the increase in HAP-induced mutant frequencies observed in the case of ITPA knockdown provide a possible link between ITPA deficiency and a predisposition to therapy-related and spontaneous cancer caused by intrinsic base analogs.
We have demonstrated that the elevated nucleotide pool contamination by nucleotides containing HAP, and by extrapolation, endogenous hypoxanthine and xanthine, causes high levels of apoptosis in human cells which, if unchecked, could lead to the onset of degenerative diseases. We have uncovered the critical role of ITPA in maintaining the stability of the genome and apoptosis in human cells. Based on the data obtained in this study and concepts generated with model systems, we propose the following model for the role of ITPA in human cells (Fig. 8). In the presence of functional ITPA, the accumulation of non-canonical nucleotides like dITP, dXTP or dHAPTP is prevented due to the ITPase activity. This precludes the accumulation of base analogs hypoxanthine, xanthine or HAP into DNA and helps maintain the genome stability. In the absence of functional ITPase, non-canonical nucleotides accumulate in the precursor pool and base analogs are incorporated into DNA by the replicative DNA polymerases. Repair of base analogs results in the accumulation of DNA single-strand breaks, which are converted to double-strand breaks during replication. This triggers apoptosis and increased levels of apoptosis contribute to the onset of degenerative diseases. In the absence of repair, base analogs persist in DNA, causing errors in replication. This leads to the accumulation of mutations, which predisposes individuals to cancer development. Overall, the findings from this study suggest that ITPA is an important factor in the maintenance of genome stability and protection from the onset of therapy-induced degenerative diseases and cancer.
Figure 8. Model for the protective role of ITPA against HAP-induced genotoxicity and mutagenesis.
In the presence of functional ITPA, the accumulation of non-canonical nucleotides like dHAPTP is abrogated by the ITPase, thereby preventing their incorporation into DNA. In the absence of functional ITPase, dHAPTP accumulates in the precursor pool and is incorporated into DNA by the replicative DNA polymerases. Grey circles represent HAP accumulation in DNA. Slow excision of base analogs by an unknown nuclease/glycosylase results in the accumulation of single-strand DNA breaks, which triggers apoptosis. Increased levels of apoptosis contribute to the onset of degenerative diseases. In the absence of repair, HAP persists in DNA causing incorrect pairing with T or C, thus leading to the accumulation of mutations, which predisposes individuals to the development of cancer.
It is also worth mentioning that, in some cases, the defect of ITPA could be a benefit in special situations, because the condition prevents hemolytic anemia in hepatitis C patients treated by ribavirin [64]. This might partially explain why ITPA-P32T allele is retained in population and demands further studies of the role of ITPA and its variants in humans.
Materials and Methods
Cells and cell culture
HeLa cervical carcinoma cell line and its derivative overexpressing Bcl-xL (HeLa-xL) were described previously [40]. Both cell lines were cultivated in DMEM (Invitrogen, U.S.A.) containing 10% fetal bovine serum (FBS, Gibco) at 37°C in a 5% CO2 atmosphere.
Chemicals
The base analog 6N-hydroxylaminopurine (HAP) was obtained from MP Biologicals Inc. U.S.A. Stocks of HAP were prepared in DMSO and heated slightly to facilitate dissolution of HAP powder. Hydrogen peroxide (H1009) was obtained from Sigma Co (St. Louis, MO). Doses of HAP used in the experiments ranged from 0.066 mM to 1.98 mM, which corresponded to concentrations of 10 µg/mL to 300 µg/mL.
Transfection of human cells and generation of stable cell lines
Transfection experiments were carried out using Lipofectamine LTX reagent (Invitrogen, U.S.A.) according to the manufacturer's instructions. Plasmids for the expression of shRNA against ITPA (Cat # KH0744P) were purchased from SABiosciences (MD). The shRNA encoded by these plasmids target the ORF of ITPA. HeLa cells were transfected by the four plasmids individually and assayed for knockdown efficiency by immunoblot and HAP cytotoxicity by Hoechst staining. As per the instructions provided by the manufacturer, the two plasmids with the highest knockdown efficiency were selected for creating cell lines with stable ITPA knockdown. In this case, 1 µg each of plasmids 1 and 2 were used for co-transfection and stable maintenance of plasmids was achieved by selection for puromycin resistance. Stable transfectants were cultivated in DMEM+10% FBS+3 µg/mL puromycin for at least one week before proceeding with further experimentation. ITPA stable knockdown typically lasted for about three weeks.
Plasmid construction
DNA fragments with ITPA and HAM1 were PCR-amplified from previously constructed bacterial expression plasmids [65], [66], [67]. Both ORFs were cloned into the EcoRI-BamHI sites of the mammalian expression vector pEGFP-C1 in frame with GFP.
Antibodies
Antibody against PARP (#9542) was purchased from Cell Signaling. Antibody against β-Actin (A5441) was purchased from Sigma Co (St. Louis, MO). Antibody against GFP (SC9996) was purchased from Santa Cruz Biotechnology Inc. The polyclonal antibody against ITPA was developed in–house [67].
Immunoblotting
HeLa cells were harvested by scraping with plastic scrapers and pelleted at 1000 g at 4°C. The cell pellets were washed twice to remove traces of medium. Lysates were prepared by resuspending the harvested cell pellets in NP-40 lysis buffer containing 0.1 mM PMSF, 1× HALT protease inhibitor cocktail (Fisher Scientific) and 1 mM sodium orthovanadate. Protein concentrations in the lysates were determined by a Bradford assay (Biorad). Separation of proteins was done by SDS PAGE. The amount of lysate corresponding to 50 µg of protein was boiled in Laemmli's buffer containing 0.1 M DTT and then loaded onto a 4–20% Tris-Glycine gel (Invitrogen, U.S.A.). Resolved proteins were transferred onto a PVDF (Millipore) membrane and blocked in 5% non-fat dry milk/1× PBST for 20 min. Thereafter, membranes were incubated with the appropriate dilution of the primary antibody with shaking overnight at 4°C. Membranes were washed three times in 1× PBST for 10 min each and incubated with appropriately diluted HRP-linked secondary antibody (Fisher Scientific) for one hour at room temperature. The membrane was washed three times in 1× PBST for 15 min each, and signals were detected by Immobilion Western chemiluminescent HRP substrate (ECL, Millipore), according to the manufacturer's instructions.
RT-PCR
Total RNA was extracted using RNeasy Kit (Qiagen). The cDNA was synthesized from 2 µg of RNA using the Superscript III first strand cDNA synthesis kit (Invitrogen), according to the manufacturer's instructions. The part of the ITPA transcript was amplified using the primers 5′-TCATTGGTGGGGAAGAAGATC-3′ and 5′AAGCTGCCAAACTGCCAAA-3′. PCR amplification using these primers gives 550 bp of product. The primers 5′-TCCACCACCCTGTTGCTGTA-3′ and 5′-ACCACAGTCCATGCCATCAC-3′ were used for amplification of the part of the housekeeping gene GAPDH transcript to give a product of 108 bp.
Quantification of the levels of apoptosis
Apoptosis was quantified according to nuclear morphology by using Hoechst 33342 at 1 µg/mL (Molecular Probes). Three different viewing areas were randomly chosen for each experiment. Pictures containing approximately 300 cells were taken for each Hoechst stained viewing area. The percentage of cells undergoing nuclear condensation was calculated for each viewing area. At least three independent experiments were quantified for each data point.
Alkaline comet assay for assessing accumulation of DNA breaks
Comet assay with HAP was described previously. All of the required chemicals were purchased from Trevigen, Inc. (U.S.A.) [37].
Determination of the frequency of HAP-induced HPRT mutants
Parent HeLa cells and ITPA knockdown cells were plated at a density of 10 million cells per 10 cm dish. Cells were allowed to attach overnight, following which the growth medium was replaced with fresh medium or by medium with the appropriate dose of HAP. Cells were incubated for 24 hours and then the treatment medium was replaced with normal growth medium for 24 hours to allow for phenotypic expression of 6-TG resistant mutants. Cells were then trypsinized and 107 cells were plated onto twenty 10 cm dishes containing growth medium supplemented with 6 µg/mL 6-thioguanine (Sigma, St. Louis, MO) at a density of 5×105 cells per plate. For plating efficiency, cells were plated in three plates with normal growth medium at a density of 5×102 per plate. After two weeks, cells were fixed with 70% ethanol and stained with 5% Giemsa solution in PBS. Colonies were counted macroscopically for plating efficiency. For 6-TG resistance, colonies were counted using an inverted light microscope at 10× magnification. Aggregates of 50 cells or more were scored as a colony. Mutant frequency was calculated using the formula of Glaab and Tyndall (49):
This experiment was repeated three times with three consecutive passages of cells. In every experiment we determined the induced mutant frequency, where the background mutant frequency of untreated cells was subtracted from the frequency of mutants in the HAP treated cultures. Spontaneous mutant frequencies in these experiments ranged from 100 to 500×10−7, which is consistent with the published data for HeLa and it derivatives [68].
Statistical analyses
Each experiment was repeated independently at least twice. All data are expressed as mean +/−SEM of several experiments. Statistical analysis was performed using Graphpad PRISM software (PRISM, CA). Unless otherwise indicated, statistically significant differences between means were estimated using two-way ANOVA to compare concentrations and cell lines. The Bonferroni multiple comparison post-test was used to analyze differences between groups. Values were considered statistically different if the probability of the difference by random fluctuation was less than 0.05.
Supporting Information
ITPA knockdown in HeLa cells. HeLa cells stably expressing shRNA against ITPA were immunoblotted for the level of ITPA protein. Cells expressing shRNA against ITPA showed an almost complete inhibition of ITPA protein production.
(TIF)
ITPases overproduction in HeLa cells. Immunoblot for eGFP in cells transfected with constructs expressing either ITPA or HAM1 as GFP fusion proteins. Cells transfected with vector showed a lower molecular weight band of 27 kDa corresponding to the molecular weight of eGFP. Cells transfected with the ITPA-GFP and HAM1-GFP showed higher molecular weight bands of approximately 50 kDa, corresponding to the weights of the two GFP fusions with ITPases. We show the immunoblot for eGFP in ITPA knockdown cells. HeLa cells with ITPA knockdown were transfected with constructs encoding for Ham1 as a GFP fusion protein.
(TIF)
Frequencies of spontaneous and HAP-induced HRPT mutants in HeLa cells.
(DOC)
Acknowledgments
We are grateful to Dr. Murat Saparbaev (Université Paris-Sud, Institut de Cancérologie Gustave Roussy, Villejuif, France) for his suggestion on the method to demonstrate HAP incorporation into DNA, Dr. Polina Shcherbakova for critical reading and Kristi Berger for expert editing of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by an Nebraska Department of Health and Human Services LB506 Grant, by a University of Nebraska Medical Center Eppley Cancer Center 010107 Pilot Grant, and in part by the National Institute of Cancer Grant R01 CA129925 to Youri I. Pavlov. Miriam R. Menezes was supported by a University of Nebraska Medical Center graduate student fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Loeb LA. Mutator phenotype in cancer: origin and consequences. Seminars in Cancer Biology. 2010;20:279–280. doi: 10.1016/j.semcancer.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galperin MY, Moroz OV, Wilson KS, Murzin AG. House cleaning, a part of good housekeeping. Molecular Microbiology. 2006;59:5–19. doi: 10.1111/j.1365-2958.2005.04950.x. [DOI] [PubMed] [Google Scholar]
- 4.Mathews CK. DNA precursor metabolism and genomic stability. FASEB J. 2006;20:1300–1314. doi: 10.1096/fj.06-5730rev. [DOI] [PubMed] [Google Scholar]
- 5.Freese E. The difference between spontaneous and base analog-induced mutations of phage T4. Proc Natl Acad Sci U S A. 1959;45:622–633. doi: 10.1073/pnas.45.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedberg EC, McDaniel LD, Schultz RA. The role of endogenous and exogenous DNA damage and mutagenesis. Current Opinion in Genetics & Development. 2004;14:5–10. doi: 10.1016/j.gde.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya H. Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: approaches using synthetic oligonucleotides and nucleotides: survey and summary. Nucleic Acids Research. 2003;31:517–531. doi: 10.1093/nar/gkg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradshaw JS, Kuzminov A. RdgB acts to avoid chromosome fragmentation in Escherichia coli. . Molecular Microbiology. 2003;48:1711–1725. doi: 10.1046/j.1365-2958.2003.03540.x. [DOI] [PubMed] [Google Scholar]
- 9.Burgis NE, Brucker JJ, Cunningham RP. Repair system for noncanonical purines in Escherichia coli. Journal of Bacteriology. 2003;185:3101–3110. doi: 10.1128/JB.185.10.3101-3110.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutation Research. 1991;250:3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- 11.Sekiguchi M, Mo JY, Maki H. Molecular mechanisms for controlling spontaneous and induced mutagenesis. Nucleic Acids Symposium Series. 1992;(27):101–102. [PubMed] [Google Scholar]
- 12.Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 13.Kozmin SG, Schaaper RM, Shcherbakova PV, Kulikov VN, Noskov VN, et al. Multiple antimutagenesis mechanisms affect mutagenic activity and specificity of the base analog 6-N-hydroxylaminopurine in bacteria and yeast. Mutation Research. 1998;402:41–50. doi: 10.1016/s0027-5107(97)00280-7. [DOI] [PubMed] [Google Scholar]
- 14.Sakumi K, Abolhassani N, Behmanesh M, Iyama T, Tsuchimoto D, et al. ITPA protein, an enzyme that eliminates deaminated purine nucleoside triphosphates in cells. Mutation Research. 2010;703:43–50. doi: 10.1016/j.mrgentox.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Lin S, McLennan AG, Ying K, Wang Z, Gu S, et al. Cloning, expression, and characterization of a human inosine triphosphate pyrophosphatase encoded by the ITPA gene. The Journal of Biological Chemistry. 2001;276:18695–18701. doi: 10.1074/jbc.M011084200. [DOI] [PubMed] [Google Scholar]
- 16.Verhoef VL, Fuller SA, Morris AJ. Individual variation of nucleoside triphosphate pyrophosphohydrolase activity in human erythrocytes, granulocytes, lymphocytes, and platelets. Biochemical Genetics. 1980;18:235–245. doi: 10.1007/BF00484239. [DOI] [PubMed] [Google Scholar]
- 17.Kouzminova EA, Rotman E, Macomber L, Zhang J, Kuzminov A. RecA-dependent mutants in Escherichia coli reveal strategies to avoid chromosomal fragmentation. Proceedings of the National Academy of Sciences of the U S A. 2004;101:16262–16267. doi: 10.1073/pnas.0405943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noskov VN, Staak K, Shcherbakova PV, Kozmin SG, Negishi K, et al. HAM1, the gene controlling 6-N-hydroxylaminopurine sensitivity and mutagenesis in the yeast Saccharomyces cerevisiae. . Yeast (Chichester, England) 1996;12:17–29. doi: 10.1002/(SICI)1097-0061(199601)12:1%3C17::AID-YEA875%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Behmanesh M, Sakumi K, Abolhassani N, Toyokuni S, Oka S, et al. ITPase-deficient mice show growth retardation and die before weaning. Cell Death and Differentiation. 2009;16:1315–1322. doi: 10.1038/cdd.2009.53. [DOI] [PubMed] [Google Scholar]
- 20.Abolhassani N, Iyama T, Tsuchimoto D, Sakumi K, Ohno M, et al. NUDT16 and ITPA play a dual protective role in maintaining chromosome stability and cell growth by eliminating dIDP/IDP and dITP/ITP from nucleotide pools in mammals. Nucleic Acids Research. 2010;38:2891–2903. doi: 10.1093/nar/gkp1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierau J, Lindhout M, Bakker JA. Pharmacogenetic significance of inosine triphosphatase. Pharmacogenomics. 2007;8:1221–1228. doi: 10.2217/14622416.8.9.1221. [DOI] [PubMed] [Google Scholar]
- 22.Marsh S, King CR, Ahluwalia R, McLeod HL. Distribution of ITPA P32T alleles in multiple world populations. Journal of Human Genetics. 2004;49:579–581. doi: 10.1007/s10038-004-0183-y. [DOI] [PubMed] [Google Scholar]
- 23.Shipkova M, Lorenz K, Oellerich M, Wieland E, von Ahsen N. Measurement of erythrocyte inosine triphosphate pyrophosphohydrolase (ITPA) activity by HPLC and correlation of ITPA genotype-phenotype in a Caucasian population. Clinical Chemistry. 2006;52:240–247. doi: 10.1373/clinchem.2005.059501. [DOI] [PubMed] [Google Scholar]
- 24.Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, et al. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics. 2004;14:181–187. doi: 10.1097/00008571-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Marinaki AM, Duley JA, Arenas M, Ansari A, Sumi S, et al. Mutation in the ITPA gene predicts intolerance to azathioprine. Nucleosides, Nucleotides & Nucleic Acids. 2004;23:1393–1397. doi: 10.1081/NCN-200027639. [DOI] [PubMed] [Google Scholar]
- 26.Burgis NE, Cunningham RP. Substrate specificity of RdgB protein, a deoxyribonucleoside triphosphate pyrophosphohydrolase. The Journal of Biological Chemistry. 2007;282:3531–3538. doi: 10.1074/jbc.M608708200. [DOI] [PubMed] [Google Scholar]
- 27.Pavlov YI, Suslov VV, Shcherbakova PV, Kunkel TA, Ono A, et al. Base analog N6-hydroxylaminopurine mutagenesis in Escherichia coli: genetic control and molecular specificity. Mutation Research. 1996;357:1–15. doi: 10.1016/0027-5107(96)00060-7. [DOI] [PubMed] [Google Scholar]
- 28.Shcherbakova PV, Pavlov YI. Mutagenic specificity of the base analog 6-N-hydroxylaminopurine in the URA3 gene of the yeast Saccharomyces cerevisiae. Mutagenesis. 1993;8:417–421. doi: 10.1093/mutage/8.5.417. [DOI] [PubMed] [Google Scholar]
- 29.Pavlov Iu I. Mutants of Saccharomyces cerevisiae supersensitive to the mutagenic effect of 6-N-hydroxylaminopurine. Genetika. 1986;22:2235–2243. [PubMed] [Google Scholar]
- 30.Shcherbakova PV, Noskov VN, Pshenichnov MR, Pavlov YI. Base analog 6-N-hydroxylaminopurine mutagenesis in the yeast Saccharomyces cerevisiae is controlled by replicative DNA polymerases. Mutation Research. 1996;369:33–44. doi: 10.1016/s0165-1218(96)90045-2. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsui T, Maizumi H, Barrett JC. Induction by modified purines (2-aminopurine and 6-N-hydroxylaminopurine) of chromosome aberrations and aneuploidy in Syrian hamster embryo cells. Mutation Research. 1985;148:107–112. doi: 10.1016/0027-5107(85)90213-1. [DOI] [PubMed] [Google Scholar]
- 32.Stepchenkova EI, Koz'min SG, Alenin VV, Pavlov I. Genetic control of metabolism of mutagenic purine base analogs 6-hydroxylaminopurine and 2-amino-6-hydroxylaminopurine in yeast Saccharomyces cerevisiae. Genetika. 2009;45:471–477. [PMC free article] [PubMed] [Google Scholar]
- 33.Abdul-Masih MT, Bessman MJ. Biochemical studies on the mutagen, 6-N-hydroxylaminopurine. Synthesis of the deoxynucleoside triphosphate and its incorporation into DNA in vitro. The Journal of Biological Chemistry. 1986;261:2020–2026. [PubMed] [Google Scholar]
- 34.Kow YW. Repair of deaminated bases in DNA. Free Radic Biol Med. 2002;33:886–893. doi: 10.1016/s0891-5849(02)00902-4. [DOI] [PubMed] [Google Scholar]
- 35.Dalhus B, Arvai AS, Rosnes I, Olsen OE, Backe PH, et al. Structures of endonuclease V with DNA reveal initiation of deaminated adenine repair. Nature Structural & Molecular Biology. 2009;16:138–143. doi: 10.1038/nsmb.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demple B, Johnson A, Fung D. Exonuclease III and endonuclease IV remove 3′ blocks from DNA synthesis primers in H2O2-damaged Escherichia coli. Proc Natl Acad Sci U S A. 1986;83:7731–7735. doi: 10.1073/pnas.83.20.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waisertreiger IS, Menezes MR, Randazzo J, Pavlov YI. Elevated levels of dna strand breaks induced by a base analog in the human cell line with the P32T itpa Variant. Journal of Nucleic Acids. 2010;2010:872180. doi: 10.4061/2010/872180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biesele JJ. Some Morphological Effects of Alkylating Agents. Experimental Cell Research. 1963;24:(Suppl 9):525–534. doi: 10.1016/0014-4827(63)90293-3. [DOI] [PubMed] [Google Scholar]
- 39.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Reviews Molecular Cell Biology. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 40.Lopez H, Zhang L, George NM, Liu X, Pang X, et al. Perturbation of the Bcl-2 network and an induced Noxa/Bcl-xL interaction trigger mitochondrial dysfunction after DNA damage. The Journal of Biological Chemistry. 2010;285:15016–15026. doi: 10.1074/jbc.M109.086231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullen P. PARP cleavage as a means of assessing apoptosis. Methods in Molecular Medicine. 2004;88:171–181. doi: 10.1385/1-59259-406-9:171. [DOI] [PubMed] [Google Scholar]
- 42.Vallur AC, Maher RL, Bloom LB. The efficiency of hypoxanthine excision by alkyladenine DNA glycosylase is altered by changes in nearest neighbor bases. DNA Repair (Amst) 2005;4:1088–1098. doi: 10.1016/j.dnarep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura J, Purvis ER, Swenberg JA. Micromolar concentrations of hydrogen peroxide induce oxidative DNA lesions more efficiently than millimolar concentrations in mammalian cells. Nucleic Acids Research. 2003;31:1790–1795. doi: 10.1093/nar/gkg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szmigiero L, Studzian K. H2O2 as a DNA fragmenting agent in the alkaline elution interstrand crosslinking and DNA-protein crosslinking assays. Analytical Biochemistry. 1988;168:88–93. doi: 10.1016/0003-2697(88)90014-0. [DOI] [PubMed] [Google Scholar]
- 45.Barrett JC. Induction of gene mutation in and cell transformation of mammalian cells by modified purines: 2-aminopurine and 6-N-hydroxylaminopurine. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:5685–5689. doi: 10.1073/pnas.78.9.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmore E, Kakunaga T, Barrett JC. Comparison of spontaneous mutation rates of normal and chemically transformed human skin fibroblasts. Cancer Research. 1983;43:1650–1655. [PubMed] [Google Scholar]
- 47.Budke B, Kuzminov A. Production of clastogenic DNA precursors by the nucleotide metabolism in Escherichia coli. Molecular Microbiology. 2010;75:230–245. doi: 10.1111/j.1365-2958.2009.06994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukas L, Kuzminov A. Chromosomal fragmentation is the major consequence of the rdgB defect in Escherichia coli. Genetics. 2006;172:1359–1362. doi: 10.1534/genetics.105.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brem R, Li F, Montaner B, Reelfs O, Karran P. DNA breakage and cell cycle checkpoint abrogation induced by a therapeutic thiopurine and UVA radiation. Oncogene. 2010;29:3953–3963. doi: 10.1038/onc.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nature Reviews Cancer. 2008;8:24–36. doi: 10.1038/nrc2292. [DOI] [PubMed] [Google Scholar]
- 51.Nakabeppu Y, Oka S, Sheng Z, Tsuchimoto D, Sakumi K. Programmed cell death triggered by nucleotide pool damage and its prevention by MutT homolog-1 (MTH1) with oxidized purine nucleoside triphosphatase. Mutation Research. 2010;703:51–58. doi: 10.1016/j.mrgentox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Oka S, Nakabeppu Y. DNA glycosylase encoded by MUTYH functions as a molecular switch for programmed cell death under oxidative stress to suppress tumorigenesis. Cancer Science. 2011;102:677–682. doi: 10.1111/j.1349-7006.2011.01869.x. [DOI] [PubMed] [Google Scholar]
- 53.Rotman E, Kuzminov A. The mutT defect does not elevate chromosomal fragmentation in Escherichia coli because of the surprisingly low levels of MutM/MutY-recognized DNA modifications. Journal of Bacteriology. 2007;189:6976–6988. doi: 10.1128/JB.00776-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moe A, Ringvoll J, Nordstrand LM, Eide L, Bjoras M, et al. Incision at hypoxanthine residues in DNA by a mammalian homologue of the Escherichia coli antimutator enzyme endonuclease V. Nucleic Acids Research. 2003;31:3893–3900. doi: 10.1093/nar/gkg472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakker JA, Lindhout M, Habets DD, van den Wijngaard A, Paulussen AD, et al. The Effect of ITPA Polymorphisms on the Enzyme Kinetic Properties of Human Erythrocyte Inosine Triphosphatase Toward its Substrates ITP and 6-Thio-ITP. Nucleosides, Nucleotides & Nucleic Acids. 2011;30:839–849. doi: 10.1080/15257770.2011.606789. [DOI] [PubMed] [Google Scholar]
- 56.Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. British Medical Bulletin. 2006;79–80:153–170. doi: 10.1093/bmb/ldl020. [DOI] [PubMed] [Google Scholar]
- 57.Caldecott KW. Single-strand break repair and genetic disease. Nature Reviews Genetics. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 58.Oka S, Ohno M, Tsuchimoto D, Sakumi K, Furuichi M, et al. Two distinct pathways of cell death triggered by oxidative damage to nuclear and mitochondrial DNAs. The EMBO Journal. 2008;27:421–432. doi: 10.1038/sj.emboj.7601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong H, Xin HW, Wu XC, Li Q, Xiong L, et al. Association between inosine triphosphate pyrophosphohydrolase deficiency and azathioprine-related adverse drug reactions in the Chinese kidney transplant recipients. Fundamental & Clinical Pharmacology. 2010;24:393–400. doi: 10.1111/j.1472-8206.2009.00770.x. [DOI] [PubMed] [Google Scholar]
- 60.Fowler RG, Schaaper RM. The role of the mutT gene of Escherichia coli in maintaining replication fidelity. FEMS Microbiology Reviews. 1997;21:43–54. doi: 10.1111/j.1574-6976.1997.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 61.Tsuzuki T, Egashira A, Igarashi H, Iwakuma T, Nakatsuru Y, et al. Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8-oxo-dGTPase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11456–11461. doi: 10.1073/pnas.191086798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kozmin SG, Leroy P, Pavlov YI, Schaaper RM. YcbX and yiiM, two novel determinants for resistance of Escherichia coli to N-hydroxylated base analogues. Molecular Microbiology. 2008;68:51–65. doi: 10.1111/j.1365-2958.2008.06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kozmin SG, Pavlov YI, Dunn RL, Schaaper RM. Hypersensitivity of Escherichia coli Delta(uvrB-bio) mutants to 6-hydroxylaminopurine and other base analogs is due to a defect in molybdenum cofactor biosynthesis. Journal of Bacteriology. 2000;182:3361–3367. doi: 10.1128/jb.182.12.3361-3367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cariani E, Villa E, Rota C, Critelli R, Trenti T. Review: Translating pharmacogenetics into clinical practice: interleukin (IL)28B and inosine triphosphatase (ITPA) polymophisms in hepatitis C virus (HCV) infection. Clinical Chemistry and Laboratory Medicine. 2011;49:1247–1256. doi: 10.1515/CCLM.2011.618. [DOI] [PubMed] [Google Scholar]
- 65.Kozmin SG, Leroy P, Pavlov YI. Overexpression of the yeast HAM1 gene prevents 6-N-hydroxylaminopurine mutagenesis in Escherichia coli. Acta Biochimica Polonica. 1998;45:645–652. [PubMed] [Google Scholar]
- 66.Porta J, Kolar C, Kozmin SG, Pavlov YI, Borgstahl GE. Structure of the orthorhombic form of human inosine triphosphate pyrophosphatase. Acta crystallographica Section F, Structural Biology and Crystallization Communications. 2006;62:1076–1081. doi: 10.1107/S1744309106041790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stepchenkova EI, Tarakhovskaya ER, Spitler K, Frahm C, Menezes MR, et al. Functional study of the P32T ITPA variant associated with drug sensitivity in humans. Journal of Molecular Biology. 2009;392:602–613. doi: 10.1016/j.jmb.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian Y, Yu Y, Cheng X, Luo J, Xie H, et al. Molecular events after antisense inhibition of hMSH2 in a HeLa cell line. Mutation Research. 1998;418:61–71. doi: 10.1016/s1383-5718(98)00108-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ITPA knockdown in HeLa cells. HeLa cells stably expressing shRNA against ITPA were immunoblotted for the level of ITPA protein. Cells expressing shRNA against ITPA showed an almost complete inhibition of ITPA protein production.
(TIF)
ITPases overproduction in HeLa cells. Immunoblot for eGFP in cells transfected with constructs expressing either ITPA or HAM1 as GFP fusion proteins. Cells transfected with vector showed a lower molecular weight band of 27 kDa corresponding to the molecular weight of eGFP. Cells transfected with the ITPA-GFP and HAM1-GFP showed higher molecular weight bands of approximately 50 kDa, corresponding to the weights of the two GFP fusions with ITPases. We show the immunoblot for eGFP in ITPA knockdown cells. HeLa cells with ITPA knockdown were transfected with constructs encoding for Ham1 as a GFP fusion protein.
(TIF)
Frequencies of spontaneous and HAP-induced HRPT mutants in HeLa cells.
(DOC)