Abstract
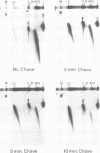
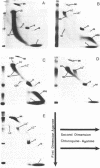
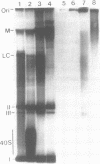
Highly compacted (40S) SV40 DNA replication intermediates formed in vivo during aphidicolin exposure and immediately broke down in two stages. In the rapid initial stage, single strand DNA breaks caused loss of superhelicity in the 40S replication intermediates. This DNA breakage was accompanied by the formation of strong, permanent protein-DNA crosslinks which reached a maximum as nicking of the aberrant DNA replication intermediates was completed. These protein-associated DNA strand breaks were not repaired. In the slower second stage of breakdown, the aberrant DNA replication intermediates remained nicked and strongly associated with protein as they underwent DNA replication fork breakage and recombinational changes to produce high molecular weight forms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. H., Sørensen B. S., Christiansen K., Svejstrup J. Q., Lund K., Westergaard O. Studies of the topoisomerase II-mediated cleavage and religation reactions by use of a suicidal double-stranded DNA substrate. J Biol Chem. 1991 May 15;266(14):9203–9210. [PubMed] [Google Scholar]
- Champoux J. J., McCoubrey W. K., Jr, Been M. D. DNA structural features that lead to strand breakage by eukaryotic type-I topoisomerase. Cold Spring Harb Symp Quant Biol. 1984;49:435–442. doi: 10.1101/sqb.1984.049.01.049. [DOI] [PubMed] [Google Scholar]
- Dinter-Gottlieb G., Kaufmann G. Aphidicolin arrest irreversibly impairs replicating simian virus 40 chromosomes. J Biol Chem. 1983 Mar 25;258(6):3809–3812. [PubMed] [Google Scholar]
- Dröge P., Sogo J. M., Stahl H. Inhibition of DNA synthesis by aphidicolin induces supercoiling in simian virus 40 replicative intermediates. EMBO J. 1985 Dec 1;4(12):3241–3246. doi: 10.1002/j.1460-2075.1985.tb04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover T. W., Berger C., Coyle J., Echo B. DNA polymerase alpha inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum Genet. 1984;67(2):136–142. doi: 10.1007/BF00272988. [DOI] [PubMed] [Google Scholar]
- Goscin L. P., Byrnes J. J. DNA polymerase delta: one polypeptide, two activities. Biochemistry. 1982 May 11;21(10):2513–2518. doi: 10.1021/bi00539a034. [DOI] [PubMed] [Google Scholar]
- Hecht F., Glover T. W. Cancer chromosome breakpoints and common fragile sites induced by aphidicolin. Cancer Genet Cytogenet. 1984 Oct;13(2):185–188. doi: 10.1016/0165-4608(84)90060-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang Y., Chang C. C., Trosko J. E. Aphidicolin-induced endoreduplication in Chinese hamster cells. Cancer Res. 1983 Mar;43(3):1361–1364. [PubMed] [Google Scholar]
- Jaenisch R., Mayer A., Levine A. Replicating SV40 molecules containing closed circular template DNA strands. Nat New Biol. 1971 Sep 15;233(37):72–75. doi: 10.1038/newbio233072a0. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Structural and functional properties of calf thymus DNA polymerase delta. Prog Nucleic Acid Res Mol Biol. 1981;26:83–96. doi: 10.1016/s0079-6603(08)60396-7. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Nethanel T., Reisfeld S., Dinter-Gottlieb G., Kaufmann G. An Okazaki piece of simian virus 40 may be synthesized by ligation of shorter precursor chains. J Virol. 1988 Aug;62(8):2867–2873. doi: 10.1128/jvi.62.8.2867-2873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainaldi G., Sessa M. R., Mariani T. Inhibitors of DNA synthesis induce sister chromatid exchanges at the early S phase of the cell cycle. Chromosoma. 1984;90(1):46–49. doi: 10.1007/BF00352277. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Garon C. F., Salzman N. P. Superhelix density of replicating simian virus 40 DNA molecules. J Mol Biol. 1974 Dec 5;90(2):371–379. doi: 10.1016/0022-2836(74)90380-5. [DOI] [PubMed] [Google Scholar]
- Sherwood S. W., Schumacher R. I., Schimke R. T. Effect of cycloheximide on development of methotrexate resistance of Chinese hamster ovary cells treated with inhibitors of DNA synthesis. Mol Cell Biol. 1988 Jul;8(7):2822–2827. doi: 10.1128/mcb.8.7.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C. G., Snapka R. M. Exposure to camptothecin breaks leading and lagging strand simian virus 40 DNA replication forks. Biochem Biophys Res Commun. 1990 Apr 16;168(1):135–140. doi: 10.1016/0006-291x(90)91684-k. [DOI] [PubMed] [Google Scholar]
- Shin C. G., Snapka R. M. Patterns of strongly protein-associated simian virus 40 DNA replication intermediates resulting from exposures to specific topoisomerase poisons. Biochemistry. 1990 Dec 11;29(49):10934–10939. doi: 10.1021/bi00501a011. [DOI] [PubMed] [Google Scholar]
- Shin C. G., Strayer J. M., Wani M. A., Snapka R. M. Rapid evaluation of topoisomerase inhibitors: caffeine inhibition of topoisomerases in vivo. Teratog Carcinog Mutagen. 1990;10(1):41–52. doi: 10.1002/tcm.1770100106. [DOI] [PubMed] [Google Scholar]
- Shure M., Pulleyblank D. E., Vinograd J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 1977;4(5):1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapka R. M. Topoisomerase inhibitors can selectively interfere with different stages of simian virus 40 DNA replication. Mol Cell Biol. 1986 Dec;6(12):4221–4227. doi: 10.1128/mcb.6.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S., Sala F., Pedrali-Noy G. Aphidicolin and eukaryotic DNA synthesis. Adv Exp Med Biol. 1984;179:169–181. doi: 10.1007/978-1-4684-8730-5_17. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Talanian R. V., Wright G. E. The roles of DNA polymerases alpha and delta in DNA replication. Pharmacol Ther. 1990;47(1):105–115. doi: 10.1016/0163-7258(90)90047-6. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]