Abstract
Introduction
Beyond documentation of high prevalence rates, research has not examined the qualities and characteristics of musculoskeletal symptoms in cancer survivors, possibly because measures have not been validated specifically for the assessment of these symptoms in survivors. We report here on a new measure of muscle and joint symptoms for survivors of hematologic malignancies and hematopoietic cell transplantation (HCT).
Methods
In a cross-sectional design, 130 adults, 5-20 years after HCT, completed patient-reported outcomes. Assessment included musculoskeletal symptoms on the Muscle and Joint Measure (MJM), as well as health-related quality of life and treatments.
Results
Principal components analysis using promax rotation revealed four subscales for the MJM with item factor loadings above 0.50: muscle aches or stiffness (myalgias), joint pain, stiffness or swelling (arthralgias), muscle cramps and muscle weakness. Variance explained by the total score was 77%. Internal consistency reliabilities of the subscales and total score ranged from 0.86 to 0.93. Validity was confirmed by correlations with the Short Form-36 bodily pain, physical function and vitality subscales, the Fatigue Symptom Inventory, and the Symptom Checklist-90-R depression (all P<.001).
Conclusions
Musculoskeletal symptoms in survivors who received HCT can be measured reliably and validly with the MJM. The measure requires testing to establish its psychometric properties with other diagnostic and treatment groups.
Implications for Cancer Survivors
The MJM has potential research and clinical value for addressing the musculoskeletal symptoms of survivors. The measure may assist with examining the mechanisms as well as treatments for these symptoms, which are among the most prevalent in long-term cancer survivors.
Keywords: Cancer survivors, musculoskeletal, symptoms, myalgias, arthralgias, muscles, joints, measurement
Introduction
Musculoskeletal symptoms are some of the most widely documented and persistent complications seen after a variety of cancer treatments[1]. High rates of musculoskeletal complaints and limitations in physical capacity are reported by breast cancer survivors[2,3] and adult survivors of childhood cancers[4,5]. Similarly, following high dose treatment and hematopoietic cell transplantation (HCT), these symptoms have been reported across most studies of long-term survivors[6-9]. However, understanding and treatment of these symptoms have been limited by lack of measures that characterize and evaluate their severity and impact.
Physical recovery occurs for most HCT survivors by one year after treatment and then remains fairly stable through five years[10]. Despite good physical function in at least 75% of long-term survivors, recent late follow-up case-control studies have documented an increased number of health problems and poorer physical function in these long-term survivors relative to controls[7,11,12]. Our own research has found that, among 10-year survivors of adult HCT, some of the most prevalent symptoms are musculoskeletal, with 35% having one or more symptoms versus 17% of matched controls, even after eliminating from calculations those survivors with avascular necrosis related to corticosteroid treatment for chronic graft versus host disease (GVHD)[7]. Survivors of childhood HCT report more muscle weakness (5.5% vs 1.6% for controls) and pain (21% vs 10% for controls), although specific locations or types of pain were not categorized[4]. Alkylating agents and total body irradiation (TBI), used regularly in HCT, have been associated with increased prevalence of musculoskeletal complications[7].
Limited literature has supported hypotheses about etiology or mechanisms for musculoskeletal symptoms in HCT survivors. Some research indicates that musculoskeletal problems existed prior to transplant and continued in the post-transplant period[13]. Treatment-related factors may also play a role. Patients who received allogeneic HCTs (transplant of stem cells from a donor) and had chronic GVHD were more likely to report muscle weakness than patients without chronic GVHD[4], indicating that long-term immunosuppression may be a key factor. In a separate study, patients with hematologic malignancy who received TBI with or without HCT were more likely to have decreased muscle strength than patients who did not receive TBI[14].
Inconsistencies and inadequacies in measurement of musculoskeletal symptoms restrict our ability to characterize these symptoms across diseases or treatments, model risk factors, understand the potentially varying mechanisms that cause these long-term deficits and, ultimately, determine options for preventing or treating these long-term complications. Despite the prevalence of reports of musculoskeletal problems, we are aware of no measures that permit description of the characteristics, intensity, or duration of these symptoms in oncology beyond general symptom measures[15,16]. The most commonly used and standardized cancer-specific measures of function and symptoms are designed for use during the acute treatment period. Generic, non-disease-specific measures of health-related quality of life with population-based norms, such as the widely used Short Form Health Survey-36 (SF-36)[17], are not designed to assess specific symptoms or functional deficits reported by cancer survivors[18]. Further, the similarities or distinctions between fatigue, which is relatively better described in cancer survivors, and musculoskeletal symptoms have not been delineated. Our own experience suggests that fatigue in long-term survivors is more associated with muscle weakness and loss of muscle mass (sarcopenia), and the related lack of stamina and strength, than with the tiredness that characterizes fatigue during treatment. Consequently, we have included weakness in our consideration of the characteristics and measurement of muscle and joint symptoms.
To address the deficit in knowledge about musculoskeletal complications in long-term HCT survivors, we determined that a measure was needed that would permit reliable and valid characterizing of musculoskeletal symptoms. Therefore the goal of the investigation presented here was to establish the psychometric properties of a new measure of muscle and joint symptoms for use with HCT patients, and potentially with other cancer survivors, including definition of subscales based on factor analysis, internal consistency reliability, and validity of the measure relative to established scales and diagnoses or treatments that would be expected to be associated with increased muscle or joint symptoms. For convergent validity, we hypothesized large size correlations (r>0.50) of Muscle and Joint Measure (MJM) scores with physical function and bodily pain as well as with depression, since depression is consistently associated with musculoskeletal and other chronic pain[19]. We predicted that patient reported vitality and fatigue also would have large size correlations with weakness on the MJM scores. For divergent validity, we thought it important to demonstrate that MJM symptoms are largely independent of general mental health and anxiety, thus we predicted small to medium correlations between these symptoms and the MJM scores.
Methods
Participants
All regionally residing hematologic malignancy survivors who underwent a first HCT 5 to 20 years before evaluation at a major transplant center in Seattle were identified using the center’s research database. Since aims of the broader research included onsite tests, those who lived in the region defined by a 98XXX zip code (within about 3 hours driving distance) were contacted with a letter of approach if they were between the ages of 18 and 49 years old. The age limit of 49 at time of first contact was set to reduce confounding of results for the broader study aim of characterizing musculoskeletal complications associated with HCT by excluding those who have musculoskeletal problems potentially attributable to natural aging. Exclusion criteria for participation in the patient-reported outcomes (PRO) included having a recurrence or second cancer that was actively treated in the previous two years (other than basal or squamous cell skin cancer), and inability to read and understand English adequate to complete the assessments. This paper reports only on the PRO component of the study.
Procedure
The study design was cross-sectional. All procedures were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. Potentially eligible participants were sent up to three introductory letters for the study. Study staff called all survivors who did not actively opt-out to explain the study details, screen for eligibility and obtain oral consent. Survivors completed assessments online unless they requested paper and pen versions. Those requesting paper and pen forms were mailed the forms and information for survey completion, including a stamped return envelope for returning the materials. Participants who did not complete the assessment within two months, and after follow-up phone calls, were contacted for an abbreviated assessment by phone. Study staff were available in person when assessments were done on site or by phone to answer questions or address any concerns. Transplant-related medical information was abstracted from the center’s research medical records.
Measures
Muscle and Joint Measure (MJM)
The MJM was developed through a process of qualitative interviews followed by scale development. After IRB approval of the procedure, an initial group of 31 nationally dispersed 5 to 10 year HCT survivors who had been consented and enrolled in other longitudinal studies[7,20] agreed to participate in a qualitative, semi-structured phone interview. The interview was designed to determine the features of musculoskeletal symptoms, including characteristics, severity, temporal qualities, and impact on activities, in preparation for development of a PRO measure.
The MJM was prepared using qualitative responses from these interviews. The measure had four sections: muscle cramps (cramps), muscle weakness (weakness), muscle aches, pains, and stiffness (myalgias), and joint pain, swelling or stiffness (arthralgias). Each section repeated content about temporal qualities, severity, and impact. A temporal item asked about “How much of the time do you have <problem> in a usual month?” with responses from 1 = “it is completely unpredictable” to 9 = “all the time.” A severity item asked respondents to “rate how severe your <problems> are on the 0 to 10 scale below by circling the number that best fits how severe your problems are usually or most of the time,” with responses from 0 = “no problem at all” to 10 = “problem as bad as can possibly be.” Impact items asked respondents to rate whether problems wake the person when sleeping, or limit emotional well-being, physical activities, sitting or standing, walking, social activities or work, with response options from 1 = “not limited at all” to 4 = “yes, stop me from doing this activity.” Items asked only within specific sections included: 1. “When do you have muscle cramps or spasms (responses: 1 = “only when exercising” to 4 = “day and night”)? 2. “Does muscle weakness make you need to take naps or sleep longer” (responses: 1 = “rarely or not at all” to 4 = “3 to 5 times a week, or more”)? 3. “How much difficulty do you have, or how limited are you, when moving your joints” (responses: 1 = “not at all” to 4 = “severely”). The measure had a total of 38 items, plus descriptive questions about location of cramps, myalgias and arthralgias, whether the symptoms predated HCT or when they began, as well as diagnoses and types of treatment for these problems. These latter items were not included in the psychometric analyses for the MJM. Higher scores on the MJM indicated increased symptom severity.
Other outcomes
Medical records review provided cancer diagnosis, transplant regimen, date of transplant, occurrence of systemic chronic GHVD, and dates of relapse or second malignancy. Relapse and second malignancies were confirmed by PRO. Standard self-report items captured information on age, gender, race and ethnicity, education, income, marital status, treatment history and diagnosed medical problems. To distinguish major symptoms from infrequent aches and pains, survivors reported current medications taken at least weekly for chronic GVHD, or for any musculoskeletal pain problem.
Additional PRO measures reported here included the Short Form 36 Health Survey, version 2 (SF-36), the Fatigue Symptom Inventory (FSI), and the depression and anxiety subscales of the Symptom Checklist-90-R (SCL-90-R).
SF-36 (version 2)
The SF-36 has been widely used to assess health-related quality of life in studies of cancer survivors, and has age and gender-specific norms for the United States[17]. We utilized the standardized T scores for calculating the 8 subscale domains. This report focuses on the bodily pain, physical function, vitality and mental health subscales. The instrument’s internal consistency, validity among different medical groups, and test-retest reliability have been documented to be excellent[17,21,22]. Higher scores on the SF-36 indicate better function.
FSI
The FSI has been frequently used as a measure of fatigue in cancer survivors[23]. It has a total score based on 13 items that assess the duration, intensity and disruptiveness of fatigue and its impact on quality of life. It was designed for use in the cancer population and evidence supports its reliability and validity[23,24]. Higher scores on the FSI indicate greater fatigue.
SCL-90-R Depression and Anxiety
The SCL-90-R depression and anxiety scores were used to evaluate secondary aims of mood associations with musculoskeletal symptoms. The SCL-90-R measure is well standardized and widely used in medical studies, with strong reliability and validity with clinical populations, including cancer patients[25]. Higher scores on the SCL-90-R indicate increased depression or anxiety.
Statistical Analyses
Descriptive and inferential analyses were performed using Statistical Package for the Social Sciences version 17.0 (SPSS; SPSS Inc, Chicago, IL). We calculated descriptive statistics for the demographic and treatment characteristics of the participants. The sample size met criteria defined by Sapnas and Zeller[26] as sufficient to perform factor analysis. Since factors were predicted to be correlated, principal components analysis using promax rotation was performed to examine the factor structure of the MJM. To achieve the most parsimonious measure, items were deleted if they did not load on a single factor with loadings >0.50 and if they did not contribute to explained variance or internal consistency reliability. Internal consistency reliability of the items in the resulting subscales and the total score were calculated using Cronbach’s alpha. Pearson correlations, one-way analyses of variance, or t tests were used to examine differences in MJM subscales and total score across medical and demographic factors. For convergent and divergent validity testing, we used Cohen’s criterion[27] to interpret the magnitude of correlation coefficients (r < 0.3 = small, 0.3 ≤ r <0.5 = medium, r ≥ 0.5 = large). For convergent validity, using Pearson correlations we tested our predicted large associations for the MJM subscales and total score with the SF-36 physical function, bodily pain, and vitality subscales, FSI total score and SCL-90-R depression. For divergent validity testing, using Pearson correlations we tested our predicted small to moderate associations for the MJM subscales and total score with the SF-36 mental health and SCL-90-R anxiety subscales. Additional t tests were conducted to validate the MJM relationship to medication use that we expected would be related to musculoskeletal symptoms including chronic GVHD medications and pain medications.
Results
Participants
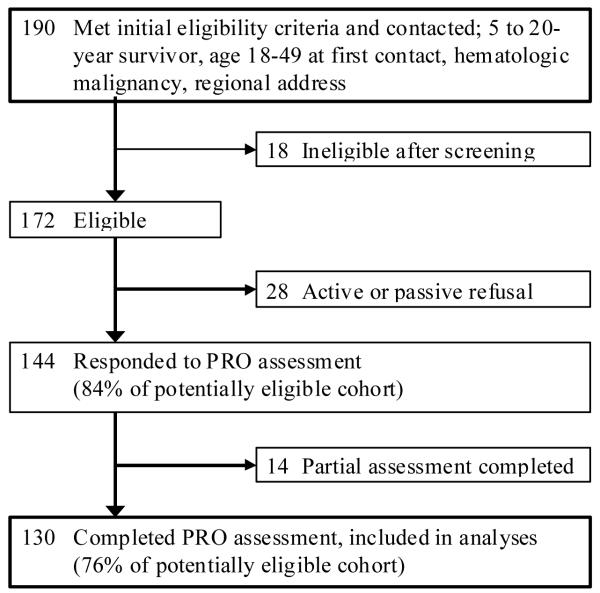
Of the 190 potentially eligible participants identified in the institutional medical record database, 172 could be contacted for screening. Of these, 144 participants were eligible and agreed to participate (Figure 1). Of the 144 participants, 130 (90%) completed the PRO that included the MJM items. Of these 130 participants, 28 (22%) completed an abbreviated phone assessment that did not include the FSI or the SCL-90-R, so are not included in analyses reporting those measures.
Figure 1.
Flow diagram for study participation.
Table 1 presents demographic and medical characteristics of the N=130 study participants. Approximately half of the sample was male (52%). Mean age at the time of transplant was 28.2 years (SD 9.9) and mean age at the time of assessment was 39.5 years (SD 8.8). Time between HCT and the date of assessment ranged from 5.0 to 20.9 years, and the mean time since transplant was 11.3 years (SD 4.6). No participants had current evidence of active disease (as required for eligibility), or had received a second transplant, though 7 (5%) had evidence of relapse after their transplant, treated with immune-modulators, but not within the 2 years before assessment. Nine survivors (7%) indicated they had been diagnosed with a second cancer since their transplant, 5 of these were localized skin cancers, and none occurred in the 2 years before assessment as required for study eligibility. The majority self-identified as Caucasian (N=117, 90%) and non-Hispanic, non-Latino (N=123, 95%), without a college degree (68%), and married (61%). In addition, a majority reported working full time for pay (63%) and had a total household income of $40,000 or greater (67%).
Table 1.
Demographic and medical characteristics
| N = 130 | |
|---|---|
| Age at Assessment, Mean (SD) | 39.5 (8.8) |
| Range | 18-50 |
|
| |
| Age at Treatment, Mean (SD) | 28.2 (9.9) |
| Range | 2-45 |
|
| |
| Years since Transplant, Mean, Median (SD) | 11.3, 10.13 (4.6) |
| 5.0 - 9.9, N (%) | 64 (49) |
| 10.0 - 14.9, N (%) | 30 (23) |
| 15.0 – 20.9, N (%) | 36 (28) |
|
| |
| Gender, N (%) | |
| Male | 68 (52) |
| Female | 62 (48) |
|
| |
| Race, N (%) | |
| Caucasian | 117 (90) |
| Native American, Alaska Native | 6 (5) |
| Asian | 3 (2) |
| Pacific Islander | 1 (1) |
| African-American | 1 (1) |
| Mixed | 2 (1) |
|
| |
| Ethnicity, N (%) | |
| Hispanic, Latino | 7 (5) |
| Non-Hispanic and Non-Latino | 123 (95) |
|
| |
| Education, N (%) | |
| High School or Less | 22 (17) |
| Vocational School or Some College | 66 (51) |
| College Degree or Greater | 42 (32) |
|
| |
| Income, N (%) | |
| < $40,000 | 37 (28) |
| $40,000 - $79,999 | 29 (22) |
| > $80,000 | 58 (45) |
| Not Reporting | 6 (5) |
|
| |
| Marital Status, N (%) | |
| Married, Living with partner | 83 (64) |
| Single, Divorced | 47 (36) |
|
| |
| Diagnosis, N (%) | |
| Acute Leukemia | 43 (33) |
| Chronic Myeloid Leukemia | 35 (27) |
| Non-Hodgkin Lymphoma | 23 (17) |
| Hodgkin Disease | 15 (12) |
| Myelodysplastic Syndromes | 7 (5) |
| Other | 7 (6) |
|
| |
| Source of Stem Cells, N (%) | |
| Bone Marrow | 91 (70) |
| Peripheral Blood | 39 (30) |
|
| |
| Donor Type, N (%) | |
| Autologous | 37 (28) |
| Allogeneic related | 50 (39) |
| Allogeneic unrelated | 43 (33) |
|
| |
| Total Body Irradiation, N (%) | |
| 0-200 cGY | 42 (32) |
| 800-1200 cGY | 52 (40) |
| 1320 cGY | 18 (14) |
| 1440-1575 cGY | 18 (14) |
|
| |
| Chronic Graft versus Host Disease History: Yes, N (%) | 54 (42) |
HCT = hematopoietic cell transplantation
Analyses revealed no significant differences between phone interview responders and complete assessment responders on the demographic or medical characteristics in Table 1, or for any of the outcomes tested including MJM subscale or total scores (all P>.10).
Muscle and Joint Measure Psychometrics
Before conducting psychometric analyses of the MJM, item response patterns and content of the item responses and written comments were reviewed. No unusual patterns were identified in responses. Descriptive statistics for items tested in the original principal components analysis are listed in Table 2.
Table 2.
Original Muscle and Joint Measure item descriptive statistics
| Content of item |
Item | Mean | Standard Deviation |
Possible Response Range |
Actual Response Range |
In Final Measure? (Yes/No) |
|---|---|---|---|---|---|---|
| Arthralgias | How much of the time (daily vs. weekly vs. monthly)? |
2.03 | 2.53 | 0-9 | 0-9 | Yes |
| Arthralgias | How much difficulty moving your joints? |
.50 | .79 | 0-3 | 0-3 | No* |
| Arthralgias | Severity usually or most of the time? |
2.14 | 2.93 | 0-10 | 0-10 | Yes |
| Arthralgias | Wake you when are sleeping? |
.46 | .94 | 0-3 | 0-3 | Yes |
| Arthralgias | Impact your emotional wellbeing? | .31 | .67 | 0-3 | 0-3 | Yes |
| Arthralgias | Limit or prevent physical activities? |
.57 | .86 | 0-3 | 0-3 | Yes |
| Arthralgias | Limit or prevent sitting or standing? |
.30 | .59 | 0-2 | 0-3 | Yes |
| Arthralgias | Limit or prevent walking? | .26 | .57 | 0-2 | 0-3 | No* |
| Arthralgias | Limit or prevent work activity? |
.31 | .65 | 0-3 | 0-3 | No |
| Arthralgias | Limit or prevent social activity? |
.16 | .48 | 0-3 | 0-3 | No* |
| Myalgias | How much of the time (daily vs. weekly vs. monthly)? |
2.32 | 2.74 | 0-9 | 0-9 | Yes |
| Myalgias | Severity, usually or most of the time? |
2.81 | 3.41 | 0-10 | 0-9 | Yes |
| Myalgias | Wake you or when you are sleeping? |
.52 | .97 | 0-3 | 0-3 | Yes |
| Myalgias | Impact your emotional well-being? |
.38 | .66 | 0-3 | 0-3 | Yes |
| Myalgias | Limit or prevent physical activities? |
.47 | .74 | 0-3 | 0-3 | Yes |
| Myalgias | Limit or prevent sitting or standing? |
.31 | .60 | 0-3 | 0-2 | Yes |
| Myalgias | Limit or prevent walking? | .28 | .56 | 0-3 | 0-2 | No* |
| Myalgias | Limit or prevent work activity? |
.31 | .66 | 0-3 | 0-3 | No |
| Myalgias | Limit or prevent social activity? |
.18 | .48 | 0-3 | 0-2 | No |
| Cramps | Severity, usually when you have them? |
3.91 | 3.51 | 0-10 | 0-10 | Yes |
| Cramps | Impact your emotional wellbeing? | .37 | .73 | 0-3 | 0-3 | Yes |
| Cramps | When during the day? | 1.91 | 1.70 | 0-4 | 0-4 | Yes |
| Cramps | How much of the time (daily vs. weekly vs. monthly)? |
2.67 | 2.74 | 0-9 | 0-9 | Yes |
| Cramps | Limit or prevent physical activities? |
.38 | .72 | 0-3 | 0-3 | Yes |
| Cramps | Limit or prevent sitting or standing? |
.28 | .60 | 0-3 | 0-3 | Yes |
| Cramps | Limit or prevent walking? | .25 | .53 | 0-3 | 0-2 | No* |
| Cramps | Limit or prevent work activity? |
.24 | .54 | 0-3 | 0-3 | No* |
| Cramps | Limit or prevent social activity? |
.13 | .42 | 0-3 | 0-3 | No* |
| Weakness | How much of the time (daily vs. weekly vs. monthly)? |
2.09 | 3.17 | 0-9 | 0-9 | Yes |
| Weakness | Severity usually or most of the time? |
1.80 | 2.50 | 0-9 | 0-10 | Yes |
| Weakness | Make you need to take naps or sleep longer? |
.28 | .73 | 0-3 | 0-3 | Yes |
| Weakness | Impact your emotional wellbeing? | .35 | .62 | 0-3 | 0-3 | Yes |
| Weakness | Limit or prevent physical activities? |
.55 | .54 | 0-3 | 0-3 | Yes |
| Weakness | Limit or prevent sitting or standing? |
.20 | .49 | 0-3 | 0-3 | Yes |
| Weakness | Limit or prevent walking? | .22 | .50 | 0-3 | 0-3 | No |
| Weakness | Limit or prevent work activity? |
.30 | .65 | 0-3 | 0-3 | No* |
| Weakness | Limit or prevent social activity? |
.18 | .42 | 0-3 | 0-2 | No* |
Item loaded strongly on one subscale but was not included in the final measure because it did not increase the explained variance or contribute to improved reliability of the subscale.
The final principal components analysis of the MJM items revealed the presence of five main components with eigenvalues exceeding 1 (Table 3). Based on Catell’s[28] scree test, we retained these five factors. Items that did not load on one of the five factors or did not contribute additional explained variance or reliability were deleted from the measure. In total, 25 items remained in the final factor structure, with one factor each comprising weakness, myalgias and arthralgias. The cramps subscale included two factors; intensity and impact loaded separately. The five factor solution with 25 items explained a total of 76.8% of the variance (Table 4). Overall internal consistency reliability for the 25 items was α = 0.93. The communalities for each of the items were above 0.5, ranging from 0.58 to 0.87.
Table 3.
Principal components analysis pattern matrix factor loadings of the final promax rotation for the Muscle and Joint Measure
| Item content by subscale | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 |
|---|---|---|---|---|---|
| Arthralgias | |||||
| How much of the time (during the day/week/month)? |
0.96 | −0.03 | −0.05 | 0.13 | −0.22 |
| Severity usually or most of the time? | 1.01 | −0.06 | −0.01 | 0.01 | −0.07 |
| Wake you when are sleeping? | 0.75 | 0.12 | 0.06 | 0.02 | −0.05 |
| Impact your emotional well-being? | 0.71 | −0.04 | −0.06 | −0.09 | 0.36 |
| Limit or prevent physical activities? | 0.88 | 0.11 | −0.07 | 0.01 | −0.01 |
| Limit or prevent sitting or standing? | 0.88 | −0.12 | 0.08 | −0.02 | 0.10 |
| Myalgias | |||||
| How much of the time (during the day/week/month)? |
−0.14 | 0.00 | 1.03 | 0.04 | −0.17 |
| Severe usually or most of the time? | 0.02 | −0.04 | 0.94 | 0.03 | −0.08 |
| Wake you or when you are sleeping? | −0.05 | 0.03 | 0.77 | 0.14 | 0.07 |
| Impact your emotional well-being? | −0.06 | −0.05 | 0.69 | −0.14 | 0.44 |
| Limit or prevent physical activities? | 0.22 | 0.18 | 0.72 | −0.09 | −0.05 |
| Limit or prevent sitting or standing? | 0.29 | −0.03 | 0.59 | −0.09 | 0.15 |
| Cramps | |||||
| When (during the day)? | −0.01 | −0.07 | 0.01 | 0.90 | 0.03 |
| How much of the time (during the day/week/month)? |
0.10 | 0.04 | 0.01 | 0.89 | −0.13 |
| Severity usually when you have them? | 0.02 | −0.01 | −0.06 | 0.82 | 0.18 |
| Wake you when you are sleeping? | −0.01 | 0.08 | 0.20 | 0.54 | 0.21 |
| Impact your emotional well-being? | −0.09 | −0.04 | 0.06 | 0.14 | 0.86 |
| Limit or prevent physical activities? | 0.08 | 0.16 | −0.06 | 0.07 | 0.72 |
| Limit or prevent sitting or standing? | −0.05 | −0.11 | −0.04 | −0.02 | 0.98 |
| Weakness | |||||
| How much of the time (during the day/week/month)? |
−0.13 | 1.02 | 0.03 | 0.05 | −.28 |
| Severity usually or most of the time? | 0.00 | 0.94 | −0.02 | −0.05 | 0.01 |
| Make you need to take naps or sleep longer? |
−0.02 | 0.69 | 0.12 | 0.06 | 0.00 |
| Impact your emotional well-being? | −0.02 | 0.77 | −0.16 | −0.03 | 0.37 |
| Limit or prevent physical activities? | 0.12 | 0.82 | 0.09 | −0.06 | −0.09 |
| Limit or prevent sitting or standing? | 0.09 | 0.72 | −0.06 | 0.00 | 0.19 |
Table 4.
Muscle and Joint Measure subscale and total score psychometric properties
| Subscale | Number of Items |
Scale Mean (SD) |
Percent of Cohort with No Symptoms (Score = 0) |
Variance Explained by Subscale Alone or Total Score Principal Components Analysis |
Internal Consistency Reliability |
|---|---|---|---|---|---|
| Arthralgias | 6 | 0.78 (1.03) |
55 | 74.9% | 0.90 |
| Myalgias | 6 | 0.89 (1.06) |
52 | 73.5% | 0.89 |
| Cramps | 7 | 1.07 (0.95) |
38 | 77.2% | 0.86 |
| Weakness | 6 | 0.67 (0.93) |
61 | 71.9% | 0.89 |
| Total score | 25 | 0.85 (0.76) |
15 | 76.8% | 0.93 |
Each factor was then examined in principal components analysis with promax rotation to determine the psychometric support for each subscale to be used as a stand alone scale (Table 4). Items for arthralgias, myalgias, and weakness subscales each loaded on a single factor, while cramps again loaded on 2 factors. Each subscale independently explained more than 70% of the variance when examined alone, each had factor loadings for the items above 0.60, and each had internal consistency reliability of α > 0.85 (Table 4).
The four content-based subscales consisted of six or seven items each, with intensity including two items or four items for cramps, and impact including four items for each subscale, except three items for cramps. To equally weight the intensity and impact contributions to subscales and the total score, means of the intensity and impact items were calculated separately. We then computed an overall mean for each subscale from these two component means. For the total MJM score, we computed an overall mean using each of the four subscale means. Intercorrelations between the MJM total score and subscales, as well as between subscales were medium to large in size, with all r > 0.30 (Table 5).
Table 5.
Intercorrelations within the Muscle and Joint Measure (MJM)
| MJM Total Score and Subscales* | ||||
|---|---|---|---|---|
| Measure | Total Score |
Arthralgias | Myalgias | Cramps |
| Arthralgias | .82 | -- | ||
| Myalgias | .80 | .53 | -- | |
| Cramps | .72 | .47 | .47 | -- |
| Weakness | .72 | .50 | .44 | .30 |
All p ≤.001
Items measuring spine and neck pain and stiffness were included in the original assessment items, with parallel structure to joints/arthralgias. However, these items did not add further unique information to the factor structure, explained variance, or reliability of the MJM. Each subscale included descriptive items such as where in the body the symptoms were located. For example, joints included options for knees, hips, hands or finger, shoulders, other places. Spine and neck included specifying location options for neck, upper spine, middle spine and/or lower spine. In examination of the responses and descriptive information, 69% of respondents reported no spine or neck symptoms (subscale score = 0) and over half of those reporting problems indicated that spine and neck symptoms pre-dated their HCT.
Muscle and Joint Measure Validity Testing
Table 6 displays convergent validity testing, which confirms hypothesized correlations between MJM scores and the SF-36 physical function, bodily pain and SCL-90-R depression (all r > 0.40 and all P < .001). SF-36 vitality and FSI subscales also correlated with MJM scores, but vitality was not selectively more strongly correlated with the MJM weakness subscale as we had predicted. Fatigue and weakness shared 40% of their variance (r = .63, P < .001), about the same as fatigue and muscle aches (r = .62). In general, correlations with cramps were weakest in validity testing. As predicted, physical function, bodily pain and depression were more strongly correlated with MJM scores than were mental health and anxiety. Ongoing use of systemic chronic GVHD medications was related most strongly to greater MJM weakness (t = −3.3, P < .001), and also to MJM arthralgias (t = −2.4, P = .02) and total score (t = −2.3, P = .02), but not to cramps (t = −0.13, P = .90) and only trended toward an association with myalgias (t = −1.8, P = .08). On the other hand, a history of GVHD was unrelated to all MJM scores (all P > .35). Use of pain medication at least weekly was associated with higher MJM pain-related subscale scores (cramps: t = −2.1, P = .04, arthralgias: t = −4.2, P < .001, myalgias: t = −3.2, P = .002). However, weakness scores did not differ between those who did or did not use pain medications at least weekly (P =.30), nor, for these long-term survivors, were any MJM scores related to receipt of TBI during conditioning for HCT (all P >.20). For divergent validity testing, as predicted, MJM scores generally had small to moderate size correlations with SF-36 mental health and SCL-90-R anxiety (Table 6). Although still significant these correlations were lower than for physical function, bodily pain and depression.
Table 6.
Validation of the Muscle and Joint Measure (MJM) with other patient reported outcomes
| MJM Total Score and Subscales | |||||
|---|---|---|---|---|---|
| Predicted Convergent Validity Measure |
Total Score |
Arthralgias | Myalgias | Cramps | Weakness |
| SF-36: Physical Function T score |
−.58 | −.47 | −.41 | −.38 | −.52 |
| SF-36: Bodily Pain T score |
−.80 | −.67 | −.68 | −.59 | −.53 |
| SCL-90-R Depression | .65 | .47 | .58 | .45 | .52 |
| SF-36: Vitality T score | −.57 | −.40 | −.53 | −.38 | −.49 |
| Fatigue Symptom Inventory Total |
.72 | .47 | .62 | .51 | .63 |
|
Predicted Divergent Validity Measures |
|||||
| SCL-90-R Anxiety | .50 | .39 | .43 | .37 | .35 |
| SF-36: Mental Health T score |
−.49 | −.29 (.002) | −.52 | −.29 (.004) | −.41 |
All P<.001 unless otherwise noted.
Of note, for these survivors all aged 18-50, age was not consistently related to MJM scores: total score r = 0.19 (P = .03), cramps r = 0.24 (P = .01), arthralgias r = 0.14, myalgias r = 0.08 (ns), weakness r = 0.09, (ns).
Discussion
This study provides evidence that the Muscle and Joint Measure (MJM) can be a useful new tool to assess musculoskeletal symptoms in HCT survivors, and potentially in other cancer survivors. All of the factor loadings were above 0.50 for the overall scale and within the individual subscales, with explained variances above 70% for each. Internal reliabilities for the four subscales and total score were above 0.85.
Validity testing confirmed that the strongest associations occurred between bodily pain and arthralgias and myalgias, and between fatigue and both weakness and myalgias. Similarly, use of pain medication was related to higher subscale scores for arthralgias, myalgias and cramps, but not greater weakness. As predicted, the subscales of the MJM were least associated with general mental health and anxiety, while associations with depression were stronger, especially for myalgias and weakness. These results suggest that the MJM is capturing physical symptoms without major interference from emotional factors. Further, these results confirmed previously noted associations between weakness and current use of chronic GVHD medications[4], but did not replicate previous findings of higher musculoskeletal complaints for those who had a past history of chronic GVHD or who received TBI in their conditioning before HCT[14]. This latter lack of association with treatment type may result from the many years (5-20) since treatment and the mediating events since then. It is also possible that with physiologic tests measuring strength and flexibility, deficits would be found that are not captured in patient-reported symptom measures.
Still lagging in the field of survivorship research are longitudinal studies that explore the trajectory of musculoskeletal symptoms after cancer treatment. In part, this gap is explained by the diversity and complexity of these symptoms, and the lack of identified symptom clusters that would move forward the understanding of both measurement and mechanisms as has occurred with the science and treatment of symptoms during active anti-cancer therapy. The current results on the measurement of muscle and joint symptoms long after treatment, including their associations with pain, depression and fatigue, may offer opportunities to examine underlying, linking mechanisms in long-term HCT survivors as have been identified for these symptoms during acute treatment or in non-HCT cancer survivors including inflammatory cytokines, muscle-related growth factors and hormonal changes [29-34]. Accurate measurement of muscle and joint symptoms in survivors, beginning with a clear understanding of prevalence and characteristics of these symptoms, similarly may help advance the understanding of mechanisms for these long-term complications.
Of note, we found that spine and neck aches, pain and stiffness did not explain further variance or improve the reliability of the MJM measure, nor did they occur independently of other MJM symptoms. Furthermore, on closer examination of the location and other descriptive item responses about these symptoms, we found that spine and neck symptoms were reported by 31% of survivors, with over half of the onset events predating transplants. Thus it seems that spine and neck symptoms are not a prevalent consequence of HCT. Nonetheless, these symptoms are reminders that survivors experience both post-treatment and premorbid pain syndromes that need to be addressed when evaluating and providing integrated treatment recommendations for survivors.
Development of adequate assessments for musculoskeletal symptoms in HCT survivors as well as other cancer survivors is important also because many of these symptoms may be treatable. Anti-inflammatory or other pain medications or even antidepressants may be helpful in treating some arthralgias or myalgias[35]. Behavioral interventions which include exercise and strength training can also alleviate pain, stiffness and weakness[36-40]. However, further intervention studies focused specifically on methods to decrease musculoskeletal syndromes in HCT and other cancer survivors are needed.
The current study focused on HCT survivors. The items of the MJM are not cancer-specific so the measure could be used with general population control groups or patients with other chronic illnesses, but requires confirmatory psychometric testing with non-HCT cohorts. Next steps are underway to test the MJM with other cancer populations and with general populations to allow comparison of symptoms with normative rates, severity and characteristics. Also of value would be to examine MJM findings in cancer survivors relative to other chronic disease populations with prevalent musculoskeletal symptoms.
This investigation offers several strengths and limitations. First, this is the first report that we know of that provides a measure developed specifically to collect information on musculoskeletal symptoms in a cancer survivor population. In addition, we utilized a comprehensive sample of HCT survivors from our center, with a wide range of survivorship years and balanced representation of genders, and were able to achieve a high response rate.
A limitation in the current validation the MJM is that the measure was not compared with established and validated rheumatology scales to determine if this scale is correlated with them or to compare sensitivity and specificity of them in the HCT population. The Western Ontario and MacMaster Universities Osteoarthritis Index (WOMAC) is the most widely used and similar measure, not specific to an anatomical location, that we found in publication searches[41]. Although we administered this measure, the scale developers do not permit the WOMAC to be used in validation research for other measures, therefore we are not able to include those results here. Future research should look at the MJM relative to arthralgia and myalgia scales developed and tested in non-cancer populations. Examples include the Health Assessment Questionnaire (HAQ and modified MHAQ and MDHAQ) and the Arthritis Impact Measurement Scales (AIMS) designed for rheumatic diseases[42-44]. Other measures specific to the upper extremity are also available and tested primarily with rheumatology patients, such as the Disabilities of the Arm, Shoulder and Hand Outcomes (DASH) Questionnaire and the Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands (SACRAH or M-SACRAH)[45,46]. These would also be of value to test relative to the MJM for sensitivity and specificity in detecting disabilities related to cancer and HCT survivorship. Of interest, in testing many of these scales, investigators have used the SF-36 to validate the measures, as was done with the current research, or to compare their relative sensitivity and specificity[45,47,48].
Other limitations include the relatively small sample size and the limited generalizability based on use of survivors from one transplant center, with a history of hematologic malignancies, who received HCT as part of their treatment and lived in the Western Washington region. Further testing is needed to assure that results and psychometrics apply equally to survivors from a broader geographic region, from other transplant centers, or who received other types of treatment for other malignancy diagnoses. Another limitation is the dependence on PRO for establishing validity. Physiologic and functional measures would extend the validity testing to include the association of PRO to these outcomes. Future research needs to validate the MJM against quantitative measures of function in multiple anatomic areas. Further, our dependence on remote contacts through mail, phone or internet responses may increase the potential for unknown enrollment biases.
In summary, the psychometrics for the MJM suggest that this measure may be a promising tool for further research into the understanding musculoskeletal symptoms commonly reported by long-term survivors of cancer. As more patients survive cancer, and specifically HCT, increasing attention must be paid to not only curing the underlying disease, but also to recognizing and improving long-term physical, psychological, and social sequelae of treatment. Multi-dimensional assessment that includes, but is not restricted to, musculoskeletal symptoms will support progress in health outcomes research on survivors.
Acknowledgements
We are grateful to the survivors who extended themselves to participate in this study. Support for this research was provided by grants CA103728 from the National Cancer Institute and National Institute on Aging, CA112631 from the National Cancer Institute, and a private donation from Robert E. Frey.
Reference List
- 1.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112:2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. Journal of Clinical Oncology. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 3.Nieboer P, Buijs C, Rodenhuis S, Seynaeve C, Beex LVAM, van der Wall E, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. Journal of Clinical Oncology. 2005;23:8296–8304. doi: 10.1200/JCO.2005.10.167. [DOI] [PubMed] [Google Scholar]
- 4.Gurney JG, Ness KK, Rosenthal J, Forman SJ, Bhatia S, Baker KS. Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood: results from the Bone Marrow Transplant Survivor study. Cancer. 2006;106:1402–1408. doi: 10.1002/cncr.21752. [DOI] [PubMed] [Google Scholar]
- 5.Ness KK, Baker KS, Dengel DR, Youngren N, Sibley S, Mertens AC, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2007;49:975–981. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 6.Adams C, August CS, Maguire H, Sladky JT. Neuromuscular complications of bone marrow transplantation. Pediatric Neurology. 1995;12:58–61. doi: 10.1016/0887-8994(94)00097-l. [DOI] [PubMed] [Google Scholar]
- 7.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. Journal of Clinical Oncology. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 8.Bush NE, Haberman M, Donaldson G, Sullivan KM. Quality of life of 125 adults surviving 6-18 years after bone marrow transplantation. Social Science & Medicine. 1995;40:479–490. doi: 10.1016/0277-9536(94)00153-k. [DOI] [PubMed] [Google Scholar]
- 9.Kovalszki A, Schumaker GL, Klein A, Terrin N, White AC. Reduced respiratory and skeletal muscle strength in survivors of sibling or unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2008;41:965–969. doi: 10.1038/bmt.2008.15. [DOI] [PubMed] [Google Scholar]
- 10.Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers MED, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 11.Baker KS, Gurney JG, Ness KK, Bhatia R, Forman SJ, Francisco L, et al. Late effects in survivors of chronic myeloid leukemia treated with hematopoietic cell transplantation: results from the Bone Marrow Transplant Survivor Study. Blood. 2004;104:1898–1906. doi: 10.1182/blood-2004-03-1010. [DOI] [PubMed] [Google Scholar]
- 12.Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. Journal of Clinical Oncology. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 13.White AC, Terrin N, Miller KB, Ryan HF. Impaired respiratory and skeletal muscle strength in patients prior to hematopoietic stem-cell transplantation. Chest. 2005;128:145–152. doi: 10.1378/chest.128.1.145. [DOI] [PubMed] [Google Scholar]
- 14.Hovi L, Era P, Rautonen J, Siimes MA. Impaired muscle strength in female adolescents and young adults surviving leukemia in childhood. Cancer. 1993;72:276–281. doi: 10.1002/1097-0142(19930701)72:1<276::aid-cncr2820720148>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Velikova G, Weis J, Hjermstad MJ, Kopp M, Morris P, Watson M, et al. The EORTC QLQ-HDC29: a supplementary module assessing the quality of life during and after high-dose chemotherapy and stem cell transplantation. European Journal of Cancer. 2007;43:87–94. doi: 10.1016/j.ejca.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36 Health Survey. QualityMetric Incorporated; Lincoln, RI: 2000. [Google Scholar]
- 18.Ware JE, Snow KK, Kosinski M. SF-36 health survey: manual and interpretation guide. The Health Institute, New England Medical Center; Boston: 1997. [Google Scholar]
- 19.Laird BJ, Boyd AC, Colvin LA, Fallon MT. Are cancer pain and depression interdependent? A systematic review. Psycho-Oncology. 2009;18:459–464. doi: 10.1002/pon.1431. [DOI] [PubMed] [Google Scholar]
- 20.Syrjala KL, Kurland BF, Abrams JR, Sanders JE, Heiman JR. Sexual function changes during the 5 years after high-dose treatment and hematopoietic cell transplantation for malignancy, with case-matched controls at 5 years. Blood. 2008;111:989–996. doi: 10.1182/blood-2007-06-096594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland HJ, Fyles GM, Adams G, Hao Y, Lipton JH, Minden MD, et al. Quality of life following bone marrow transplantation: a comparison of patient reports with population norms. Bone Marrow Transplantation. 1997;19:1129–1136. doi: 10.1038/sj.bmt.1700806. [DOI] [PubMed] [Google Scholar]
- 22.McHorney CA, Ware JE, Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Quality of Life Research. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 24.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Quality of Life Research. 2000;9:847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 25.Mulrow CD, Williams JW, Gerety MB, Ramirez G, Montiel OM, Kerber C. Case-finding instruments for depression in primary care settings. Annals of Internal Medicine. 1995;122:913–921. doi: 10.7326/0003-4819-122-12-199506150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Sapnas KG, Zeller RA. Minimizing sample size when using exploratory factor analysis for measurement. Journal of Nursing Measurement. 2002;10:135–154. doi: 10.1891/jnum.10.2.135.52552. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 28.Catell RB. The scree test for number of factors. Multivariate Behavioral Research. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- 29.Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, et al. Journal of the National Cancer Institute; National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue; July 15-17, 2002; 2004. pp. 9–16. Monographs. [DOI] [PubMed] [Google Scholar]
- 30.Wang XS. Pathophysiology of cancer-related fatigue. Clinical Journal of Oncology Nursing. 2008;12:11–20. doi: 10.1188/08.CJON.S2.11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism: a cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 32.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosomatic Medicine. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW. T-cell homeostatis in breast cancer survivors with persistent fatigue. Journal of the National Cancer Institute. 2003;95:1165–1168. doi: 10.1093/jnci/djg0019. [DOI] [PubMed] [Google Scholar]
- 34.Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis & Rheumatism. 2005;52:2594–2598. doi: 10.1002/art.21364. [DOI] [PubMed] [Google Scholar]
- 35.Deininger MW, O’Brien SG, Ford JM, Druker BJ. Practical management of patients with chronic myeloid leukemia receiving imatinib. Journal of Clinical Oncology. 2003;21:1637–1647. doi: 10.1200/JCO.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 36.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Cancer treatment-induced alterations in muscular fitness and quality of life: the role of exercise training. Annals of Oncology. 2007;18:1957–1962. doi: 10.1093/annonc/mdm364. [DOI] [PubMed] [Google Scholar]
- 37.van Weert E, Hoekstra-Weebers J, Grol B, Otter R, Arendzen HJ, Postema K, et al. A multidimensional cancer rehabilitation program for cancer survivors: effectiveness on health-related quality of life. Journal of Psychosomatic Research. 2005;58:485–496. doi: 10.1016/j.jpsychores.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Journal of Clinical Oncology. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 39.Winett RA, Carpinelli RN. Potential health-related benefits of resistance training. Preventive Medicine. 2001;33:503–513. doi: 10.1006/pmed.2001.0909. [DOI] [PubMed] [Google Scholar]
- 40.van Weert E, Hoekstra-Weebers JE, May AM, Korstjens I, Ros WJ, van der Schans CP. The development of an evidence-based physical self-management rehabilitation programme for cancer survivors. Patient Education & Counseling. 2008;71:169–190. doi: 10.1016/j.pec.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index. Pain. 2005;100:55–64. doi: 10.1016/s0304-3959(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 42.Fransen J, van Riel PL. Outcome measures in inflammatory rheumatic diseases. Arthritis Research & Therapy. 2009;11:244. doi: 10.1186/ar2745. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pincus T, Yazici Y, Bergman M. Development of a multi-dimensional health assessment questionnaire (MDHAQ) for the infrastructure of standard clinical care. Clinical & Experimental Rheumatology. 2005;23:S19–S28. [PubMed] [Google Scholar]
- 44.Pincus T, Askanase AD, Swearingen CJ. A multi-dimensional health assessment questionnaire (MDHAQ) and routine assessment of patient index data (RAPID3) scores are informative in patients with all rheumatic diseases. Rheumatic Diseases Clinics of North America. 2009;35:819–827. doi: 10.1016/j.rdc.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Chiari-Grisar C, Koller U, Stamm TA, Wanivenhaus A, Trieb K. Performance of the disabilities of the arm, shoulder and hand outcome questionnaire and the Moberg picking up test in patients with finger joint arthroplasty. Archives of Physical Medicine & Rehabilitation. 2006;87:203–206. doi: 10.1016/j.apmr.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Sautner J, Andel I, Rintelen B, Leeb BF. Development of the M-SACRAH, a modified, shortened version of SACRAH (Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands) Rheumatology. 2004;43:1409–1413. doi: 10.1093/rheumatology/keh360. [DOI] [PubMed] [Google Scholar]
- 47.Uhlig T, Haavardsholm EA, Kvien TK. Comparison of the Health Assessment Questionnaire (HAQ) and the modified HAQ (MHAQ) in patients with rheumatoid arthritis. Rheumatology. 2006;45:454–458. doi: 10.1093/rheumatology/kei181. [DOI] [PubMed] [Google Scholar]
- 48.Stamm T, Mathis M, Aletaha D, Kloppenburg M, Machold K, Smolen J. Mapping hand functioning in hand osteoarthritis: comparing self-report instruments with a comprehensive hand function test. Arthritis & Rheumatism. 2007;57:1230–1237. doi: 10.1002/art.22989. [DOI] [PubMed] [Google Scholar]