Abstract
Background
3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) is a novel small molecule ribonucleotide reductase inhibitor. This study was designed to estimate the maximum-tolerated dose (MTD) and oral bioavailability of 3-AP in patients with advanced stage solid tumors.
Methods
Twenty patients received one dose of intravenous and subsequent cycles of oral 3-AP following a 3+3 patient dose-escalation. Intravenous 3-AP was administered to every patient at a fixed dose of 100 mg over a 2-hour infusion 1 week prior to the first oral cycle. Oral 3-AP was administered every 12 hours for 5 consecutive doses on days 1–3, days 8–10, and days 15–17 of every 28-day cycle. 3-AP was started at 50 mg with a planned dose escalation to 100, 150, and 200 mg. Dose-limiting toxicities (DLT) and bioavailability were evaluated.
Results
Twenty patients were enrolled. For dose level 1 (50mg), the second of three treated patients had a DLT of grade 3 hypertension. In the dose level 1 expansion cohort, three patients had no DLTs. No further DLTs were encountered during escalation until the 200 mg dose was reached. At the 200 mg 3-AP dose level, two treated patients had DLTs of grade 3 hypoxia. One additional DLT of grade 4 febrile neutropenia was subsequently observed at the de-escalated 150 mg dose. One DLT in 6 evaluable patients established the MTD as 150 mg per dose on this dosing schedule. Responses in the form of stable disease occurred in 5 (25%) of 20 patients. The oral bioavailability of 3-AP was 67 ± 29%, and was consistent with the finding that the MTD by the oral route was 33% higher than by the intravenous route.
Conclusions
Oral 3-AP is well-tolerated and has an MTD similar to its intravenous form after accounting for the oral bioavailability. Oral 3-AP is associated with a modest clinical benefit rate of 25% in our treated patient population with advanced solid tumors.
Keywords: 3-AP, phase I trial, oral Triapine, ribonucleotide reductase
Introduction
Ribonucleotide reductase (RR) is a highly regulated, omnipresent cellular enzyme in the deoxyribonucleotide de novo synthesis pathway [1]. RR reduces ribonucleotide diphosphates to corresponding deoxyribonucleotide diphosphates, an essential process for DNA synthesis and repair [1–2]. RR consists of two subunits: M1 (M for human and R for rodent) and M2 (R2). M1 (RRM1) protein is a MW 170 Kd dimer, containing a binding site for allosteric enzyme regulators [3]. M2 (RRM2) protein is a MW 88 Kd dimer harboring a tyrosine free radical stabilized by non-heme diferric iron centers crucial for enzyme activity [3]. RR is rate-limiting for DNA synthesis, indicating its important role in the regulation of cell proliferation [1–5]. A new functional RR protein family member has been cloned [6], designated p53R2 because it contains a p53-binding site. Ultraviolet (UV) light, gamma-irradiation, and doxorubicin treatment induce p53R2 expression by a p53-regulated mechanism [6–8], suggesting its role in repair of damaged DNA [6]. Cell DNA damage responses also have p53-independent means of increasing RR activity to facilitate timely repair of damaged DNA [7, 8].
Anticancer regimens incorporating RR inhibitors such as hydroxyurea have been successful [9]. However, RR-related leukopenia limits long-term treatment [9–11]. With the intent of lowering RR inhibitor toxicity through increases in drug class potency, investigators have initiated development of the 1000-fold more potent thiosemicarbazone therapeutic class of RR inhibitors. Novel to this drug class, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine®, NSC#663249) has seen single agent activity in phase 1 solid-cancer clinical trials, tolerated at doses of 96 to 100mg/m2 [12–14]. Moreover, 3-AP (25 mg/m2) given concomitantly with cisplatin (40 mg/m2) and daily radiation has resulted in significant complete response rates among women with advanced stage cervical cancer [15]. Pharmacokinetic data for 3-AP indicate that peak serum concentrations of 1–10 μM occur 1–2 hours after a 2-hour intravenous infusion. Because of the short-lived anticancer therapeutic benefit of RR inhibitors, there has been a clinical desire to develop an oral 3-AP formulation that permits daily dosing.
Here, we report the first phase 1 study evaluating the safety/tolerability of oral 3-AP capsules among patients with advanced stage solid-cancer patients. We also compare the pharmacologic bioavailability of oral 3-AP.
Methods
Patient Selection
Eligibility criteria included histological- or cytological-confirmation of solid cancer tumors not amenable to curative surgery, chemotherapy or radiation. Patients had tumors that were measurable by Response Evaluation Criteria in Solid Tumors (RECIST, v1.0). Patients must have had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, be 18 years of age or older, and be able to provide written informed consent. Adequate bone marrow function (neutrophils ≥ 1500/uL, platelets ≥ 100,000/uL, hemoglobin ≥ 10 gm/dL with transfusions permitted) and kidney function (creatinine ≤ 1.5 or calculated creatinine clearance ≥ 50 mL/min) must have been recorded prior to enrollment. Patients had ≤ institutional upper limits of normal bilirubin and ALT, AST, and alkaline phosphatase ≤ 2.5 × upper limit of normal. Patients were excluded if they were pregnant or breastfeeding women, had glucose-6-phosphate dehydrogenase deficiency (due to the risk of methemoglobinemia associated with 3-AP [14]), brain metastases, another malignancy (except early stage squamous cell carcinoma of skin or cervix), or an uncontrolled intercurrent illness (e.g., infection, congestive heart failure, unstable angina, cardiac arrhythmia, congenital or acquired immune deficiency, or psychiatric illness that could potentially impact compliance).
Treatment Regimen and Strata
All patients received intravenous 3-AP 7 days prior to the first oral cycle at a dose of 100 mg with blood samples drawn over 8 hours to determine pharmacokinetics. Oral 3-AP was administered every 12 hours for 5 consecutive doses on days 1 to 3, days 8 to 10, and days 15 to 17 of every 28-day cycle. Oral 3-AP started from 50 mg every 12 hours and was increased to dose levels of 100 mg, 150 mg, and 200 mg every 12 hours in cohorts of 3 patients, expanded to 6 patients if 1 of 3 patients experienced a DLT in the first cycle. There were no intra-patient dose escalations. The initial dosing of 50 mg every 12 hours was based on a small, exploratory clinical evaluation of the bioavailability of oral triapine by Vion Pharmaceuticals, and this dose was found to be well below the MTD of 96 mg/m2/day established by the phase I study of IV daily dosing for 5 days [13]. Patients were asked to fast (except for water) for 2 hours prior to dosing and for 1 hour after ingesting the 3-AP capsule. All patients were observed clinically for 3 to 4 hours after oral 3-AP administration during the first week of the first oral treatment cycle. Treatment was continued until progression of disease, unacceptable toxicity, intercurrent illness, declining performance status preventing further treatment, or patient withdrawal. Patients developing emesis with the initial or a subsequent treatment received prophylactic antiemetic treatment prior to every subsequent dose.
3-AP was held if the neutrophil count was < 1000/uL and platelets < 50,000/uL or for any ≥ grade 2 non-hematologic toxicity except for grade 2 fatigue and anorexia. The dose of 3-AP in the next cycle was permanently reduced one dose level for the following: (a) grade 2 neutropenia and thrombocytopenia, (b) ≥ grade 3 neutropenia, or (c) ≥ grade 3 thrombocytopenia. CSF use was allowed if cycles were held for neutropenia. Treatment was discontinued permanently for grade 4 non-hematologic adverse events, adverse events clinically necessitating treatment cycle delay for more than 2 weeks or need of more than 2 dose reductions.
Pharmacokinetics and Bioavailability
Intravenous 3-AP pharmacokinetics were determined from the single 100 mg 2-hour infusion administered 7 days before oral therapy. Serum samples (5 mL in red top Vacutainer® tubes) were collected from a site contralateral to the site of infusion at the following times: pre-dose, then during the infusion at approximately 0.5, 1, and 2 hours (just prior to the end of infusion), and 0.25, 0.5, 1, 2, 4, and 8 hours after the end of infusion. Oral 3-AP pharmacokinetics was determined with 5 mL serum samples collected at the following times around the first oral dose: pre-dose, and every 15 minutes until 2 hours, and then at 3, 4, 6, and 8 hours following the dose. Serum samples were analyzed for 3-AP levels in the California Cancer Consortium’s Analytical Pharmacology Core Facility (APCF) using a validated HPLC/UV assay [12,13]. Pharmacokinetic analyses were performed with both compartmental and non-compartmental methods using the data from individual patients. Non-compartmental methods were carried out using statistical moment theory and the rule of linear trapezoids. Summary statistics of the pharmacokinetic parameters following either an intravenous of oral triapine dose for the population, including the bioavailability (AUCoral/AUCiv) were derived from the parameters obtained in the individual patients.
Response and Toxicity Evaluation
Computed tomography or magnetic resonance imaging scans of measurable lesions were obtained at baseline and every 8 weeks. Responses were classified according to RECIST (v1.0, [16]). National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE (v3.0) was used to grade adverse events. Dose limiting toxicity (DLT) was defined as ≥ grade 3 non-hematologic toxicity (excluding alopecia, controllable nausea and vomiting, and hypertriglyceridemia recovering within 1 week), grade 4 thrombocytopenia, grade 4 febrile neutropenia requiring hospitalization, or treatment delay of > 2 weeks as a result of unresolved toxicity. The toxicity must have been definitely, probably, or possibly attributed to the oral 3-AP and have occurred during the first cycle of treatment to be a DLT. Patients removed from study due to symptomatic hypoxia, methemoglobinemia, or hypotension (systolic BP < 85 mmHg) were also considered to have experienced a DLT.
Statistical Considerations
The primary objective of this phase I trial was to determine the maximum tolerated dose (MTD) of oral 3-AP. To be evaluable for toxicity, a patient must have received at least 1 complete cycle of treatment and be observed for at least 4 weeks after the start of the first cycle or have experienced a DLT. The maximum tolerated dose (MTD) was defined as the highest dose tested in which no more than 1 of 6 patients evaluable for toxicity experienced a DLT attributable to the oral 3-AP. Dose escalations proceeded according to a standard 3 + 3 design. The phase I trial was closed when 6 patients had been treated at a dose level and evaluated with no more than 1 DLT attributable to 3-AP. For the secondary objective of the study to describe the serum pharmacokinetics and bioavailability of oral 3-AP, the relationship of the AUC to the dose was assessed by least-square regression analysis.
Informed Consent and Regulatory Approval
The study was reviewed and approved by the Cancer Therapy Evaluation Program of the National Cancer Institute, and by the Institutional Review Board at each participating institution. All patients provided written informed consent.
RESULTS
Patient Characteristics
Twenty patients were enrolled and treated between February 2007 and May 2009. Baseline patient characteristics are shown in Table 1, including age, gender, performance, primary sites of solid cancer. On this study, 20 patients had received prior chemotherapy, 13 had received prior radiation, and 12 had received prior cancer-related surgery.
Table 1.
Patient characteristics
| Patient Demographics | ||
|---|---|---|
| Number of patients | 20 | |
| Age (years) | Median (range) | 60 (26–81) |
| Gender | Male | 9 |
| Female | 11 | |
| Race | Caucasian | 15 |
| Asian | 4 | |
| African-American | 1 | |
| ECOG Performance | 0 | 6 |
| Status | 1 | 14 |
| Prior chemotherapy regimens | Median (range) | 3 (1–9) |
| Primary Site | Colorectal | 4 |
| Pancreas | 4 | |
| Stomach | 2 | |
| Breast | 1 | |
| Cervix | 1 | |
| Gallbladder | 1 | |
| Liver | 1 | |
| Uterus | 1 | |
| Oropharynx | 1 | |
| Parotid gland | 1 | |
| Thyroid | 1 | |
| Skin | 1 | |
| Nervous System | 1 | |
Treatment Administered
Patients were enrolled to four dose levels: 6 patients at dose level 1 (50 mg), one of whom did not complete the first cycle; 4 patients at dose level 2 (100 mg), one of whom did not complete the first cycle; 8 patients at dose level 3 (150 mg), two of whom did not complete the first cycle; and 2 patients at dose level 4 (200 mg). The median number of treatment cycles given was 2 (1 cycle equals 28-days of 3-AP). Therapy was discontinued for disease progression in 10 patients (50%) after a median of 2 cycles (range 2–15) and adverse events in 8 patients (40%) after a median of 1 cycle (range 0–2). There was failure to complete the first cycle in 2 patients (10%), one due to constipation from pain medications and one due to pain and bowel obstruction prior to receiving oral 3-AP. The 8 patients stopping therapy for adverse events included one who received only the initial IV dose.
Adverse Events
All graded adverse events are shown in Table 2. Grades 3 and 4 adverse events for all patients and by dose level are shown in Table 3. Among the 20 patients enrolled 27 hematological and infectious adverse events were observed, the majority (19 of 27 [70%]) were reversible grade 1 or 2. Neutropenia was the most frequent treatment-related hematological toxicity, with initially 2 patients with grade 3–4 adverse events observed at the 150 mg dose level though not dose-limiting given no fever requiring hospitalization. One patient had grade 4 neutropenia at the 200 mg dose level though also not dose-limiting given no fever requiring hospitalization. Grade 4 thrombocytopenia was also noted at the 200 mg dose level. Dose-limiting grade 4 febrile neutropenia in one patient was not observed until enrollment on the de-escalated 150 mg dose expansion cohort.
Table 2.
All Grades Adverse Events attributed to treatment
| All Grades
|
||
|---|---|---|
| Toxicity | No. of Patients | % |
| Hematologic & Infectious | ||
| Hemoglobin (anemia) | 7 | 35 |
| Neutropenia | 11 | 55 |
| Thrombocytopenia | 6 | 30 |
| Infection | 3 | 15 |
| Constitutional | ||
| Fatigue | 5 | 25 |
| Hemorrhage/Thrombosis | 1/0 | 5/0 |
| Hypertension/Hypotension | 1/1 | 5/5 |
| Hepatic | ||
| Alkaline phosphatase | 2 | 10 |
| (AST/SGOT) | 2 | 10 |
| Gastrointestinal | ||
| Diarrhea | 1 | 5 |
| Anorexia | 4 | 20 |
| Vomiting/Nausea | 3/5 | 15/25 |
| Pain | ||
| Headache | 4 | 20 |
| Pulmonary | ||
| Hypoxia | 2 | 10 |
| Renal/Metabolic | ||
| Hypokalemia | 1 | 5 |
| Hyperglycemia | 3 | 15 |
Table 3.
All Grade 3–4 Adverse Events attributed to treatment and by dose
| All Gr 3–4 | 50 mg | 100 mg | 150 mg | 200 mg | |
|---|---|---|---|---|---|
| Toxicity | No. of Patients (%) | No. of Patients (%) | No. of Patients (%) | No. of Patients (%) | No. of Patients (%) |
| Hematologic & Infectious | |||||
| Hemoglobin (anemia) | 2 (10) | 0 | 1 (25) | 0 | 1 (50) |
| Neutropenia | 4 (20) | 0 | 0 | 3 (38)** | 1 (50)* |
| Thrombocytopenia | 1 (5) | 0 | 0 | 0 | 1 (50)* |
| Infection | 1 (5) | 0 | 0 | 0 | 1 (50) |
| Constitutional | |||||
| Fatigue | 1 (5) | 0 | 0 | 0 | 1 (50) |
| Hypertension | 1 (5) | 1 (17) | 0 | 0 | 0 |
| Hepatic | |||||
| (AST/SGOT) | 1 (5) | 0 | 0 | 0 | 1 (50)* |
| Gastrointestinal | |||||
| Anorexia | 1 (5) | 0 | 0 | 0 | 1 (50) |
| Pulmonary | |||||
| Hypoxia | 2 (10) | 0 | 0 | 0 | 2 (100) |
represented grade 4 toxicity
one patient with grade 4 febrile neutropenia occurring after de-escalation from 200 mg dose
Graded non-hematological adverse events exceeding an incidence of 15% included fatigue, nausea and emesis, hypoxia, headache, and hyperglycemia. Significant DLT occurring in the first cycle was observed in 2 patients at the 200 mg dose exhibiting grade 3 hypoxia with decreased O2 saturation at rest requiring continuous supplemental oxygen. Grade 4 AST elevation was also observed in the second patient at the 200 mg dose level also considered to be a DLT.
Dose Escalation Summary and MTD
Table 4 summarizes the number of patients evaluable for toxicity and DLTs observed on study. The second patient at the starting 50 mg dose level experienced a DLT resulting in the expansion of the dose level to 3 additional patients. All 3 patients on the expanded level were evaluable for cycle one toxicity and did not experience a DLT. At the 100 mg dose level, 4 patients were accrued, of whom 3 were evaluable for toxicity. No patient experienced a DLT at this dose level, and the decision was made to escalate to 150 mg. All 3 patients accrued at this dose level were evaluable for toxicity and no patient experienced a DLT. At the 200 mg dose level, the first 2 patients accrued each experienced a DLT. Five additional patients were accrued at the 150 mg dose level, of whom 2 were not evaluable for toxicity. One DLT was observed in the 6 evaluable patients establishing 150 mg as the MTD.
Table 4.
Treatment Summary
| Oral 3-AP (mg every 12 Hrs.) | No.Pts. Treated | No. Pts. excluded from cycle one toxicity evaluation | No. Pts. excluded from Response Evaluationd | No. Completed Cycles Median (range) (excluding ineligible pts for response) | No. Pts. w/DLTs | DLT Description | Best Responses During Therapy (all pts for response) |
|---|---|---|---|---|---|---|---|
| 50 | 6 | 0 | 1a | 2 (0–16) | 1 | 2nd Pt: Grd. 3 Hypertension | SD – 2 PD – 3 N\A – |
| 100 | 4 | 1b | 1b | 2 (0–3) | 0 | --- | SD – 1 PD – 2 N\A - 1 |
| 150 | 8 | 2 | 4 | 1 (0–9) | 1 | 8th Pt: Grd. 4 Febrile Neutropenia | SD – 2 PD – 2 N\A – |
| 200 | 2 | 0 | 2c | 0 (0–0) | 2 | 1st Pt: Grd. 3 Hypoxia 2nd Pt. Grd. 3 Hypoxia 2nd Pt: Grd. 4 AST |
N\A - 2 |
Second patient discontinued treatment prior to completing cycle 1 of oral 3-AP (Patient experienced a DLT then declined further treatment).
First patient was admitted to hospital prior to completion of first cycle of oral 3-AP.
First two patients experienced DLTs and did not complete the first cycle of oral 3-AP.
Patients were not eligible for evaluation due to not completing the initial 2 cycles either due to toxicity or declining further therapy.
Treatment Response
Twelve of the 20 patients with measurable disease were evaluable for response (Table 4). Stable disease as the best response was documented in 5 patients (25%) of the entire enrolled study population. The median duration of stable disease response was 2 months, with the longest progression-free interval of 15 months seen in a patient with pancreatic adenocarcinoma. All twenty (100%) have died in long-term follow-up, with progressive disease confirmed in 10 patients while on study.
Pharmacokinetics of Oral 3-AP
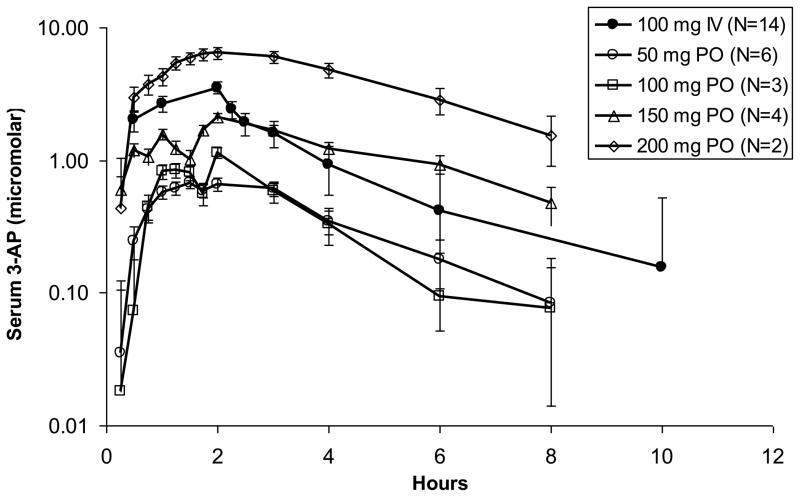
Pharmacokinetic data were obtained from 15 patients. A total of 14 of these subjects had data available following both an intravenously and orally administered dose for determination of oral bioavailability. The pharmacokinetic results are summarized in Table 5 and illustrated in Figure 1. As shown in the figure, the exposure of orally administered 3-AP increased in a dose dependent manner. Peak serum concentrations occurred at approximately the same time when the drug was administered orally as when it was given as a 2 hour intravenous infusion. The terminal elimination half-lives and mean residence times were also roughly equivalent with the two routes of administration.
Table 5.
Summary of 3-AP Pharmacokinetic Results
| Intravenous Route | Oral Route | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | IV Dose | Oral Dose | Cmax (uM) | Tmax (hr) | MRT^ (hr) | AUC (uM* hr) | CLsys (L/hr) | Cmax (uM) | Tmax (hr) | MRT (hr) | AUC (uM* hr) | CL/F (L/hr) | F (Oral/IV)# |
| 001 | 100 | 50 | 3.5 | 2.0 | 3.0 | 14.5 | 0.028 | 1.5 | 3.0 | 4.2 | 5.2 | 0.020 | 0.72 |
| 002 | 100 | 50 | 2.7 | 2.0 | 2.7 | 8.3 | 0.016 | 0.9 | 0.8 | 2.6 | 1.9 | 0.007 | 0.46 |
| 003 | 100 | 50 | 3.2 | 2.0 | 3.0 | 11.3 | 0.022 | 1.5 | 1.0 | 2.5 | 3.5 | 0.014 | 0.62 |
| 004 | 100 | 50 | 2.9 | 2.0 | 2.3 | 7.2 | 0.014 | 0.5 | 1.5 | 3.0 | 1.6 | 0.006 | 0.45 |
| 005 | 100 | 50 | 2.1 | 2.0 | 2.5 | 5.7 | 0.011 | 0.5 | 1.3 | 2.6 | 1.4 | 0.006 | 0.50 |
| 006 | 100 | 50 | 2.5 | 2.0 | 3.1 | 10.0 | 0.020 | 1.1 | 1.5 | 3.4 | 4.1 | 0.016 | 0.82 |
| 007 | 100 | 100 | 2.8 | 2.0 | 2.5 | 8.8 | 0.017 | 2.4 | 2.0 | 2.8 | 6.5 | 0.025 | 0.74 |
| 008 | 100 | 100 | 5.0 | 2.0 | 2.4 | 4.8 | 0.009 | 2.5 | 1.0 | 3.0 | 2.3 | 0.009 | 0.48 |
| 009 | 100 | 150 | 5.4 | 2.0 | 2.5 | 12.1 | 0.024 | 5.9 | 2.0 | 3.5 | 8.0 | 0.010 | 0.44 |
| 010 | 100 | 150 | 2.4 | 2.0 | 2.6 | 14.1 | 0.027 | 2.4 | 0.8 | 1.7 | 21.7 | 0.028 | 1.03 |
| 011 | 100 | 150 | 4.7 | 2.0 | 3.0 | 7.0 | 0.014 | 1.6 | 6.0 | 5.4 | 4.3 | 0.006 | 0.41 |
| 012 | 100 | 150 | 6.7 | 2.0 | 3.5 | 16.1 | 0.031 | 10.1 | 1.5 | 3.3 | 8.5 | 0.011 | 0.35 |
| 013 | 100 | 200 | 1.9 | 2.0 | 2.3 | 25.6 | 0.050 | 0.8 | 1.5 | 2.5 | 56.0 | 0.055 | 1.09 |
| 014 | 100 | 200 | 3.7 | 2.0 | 2.3 | 10.2 | 0.020 | 4.6 | 3.0 | 3.9 | 19.5 | 0.019 | 1.28 |
| Avg. | 2.8 | 2.0 | 2.8 | 9.5 | 0.022 | 5.0* | 1.9 | 3.2 | 10.6* | 0.017 | 0.67 | ||
| Std Dev. | 0.5 | 0.0 | 0.3 | 3.1 | 0.01 | 3.8 | 1.4 | 0.9 | 7.6 | 0.01 | 0.29 | ||
Mean residence time
F normalized to a total oral dose of 100 mg
Average values for patients receiving an oral dose of 150 mg
Figure 1.
Mean Concentration Versus Time Plots for IV (closed circles) and Oral (open symbols) 3-AP. Peak serum concentrations occurred at the same time when the drug was administered orally or intravenously. The terminal elimination half-life was also roughly equivalent with the two routes of administration.
The mean oral bioavailability (Foral/iv) across all dose levels was 0.69 ± 0.29. At an oral MTD dose of 150 mg, the mean AUC was 10.6 ± 7.6 μM·hr, and was similar to the mean AUC with an intravenous dose of 100 mg of 9.5·3.1 μM hr. Mean peak serum concentrations with an oral dose of 150 and an intravenous dose of 100 mg were 5.0 ± 3.8 μM and 3.5 ± 1.4 μM, respectively.
DISCUSSION
Twice daily oral 3-AP of 150 mg was safely administered to patients with advanced stage solid cancers. Oral bioavailability was 67% of the administered dose, with 150 mg every 12 hours on days 1–3, 8–10, and 15–17 of each 28-day cycle being the MTD. Dose-limiting hypoxia occurred at the 200 mg 3-AP dose level as well as grade 4 neutropenia, thrombocytopenia, and elevated liver enzymes. Stable disease was documented in 5 (25%) of 20 patients, with a median duration of 2 months.
Pharmacologic inhibition of RR has become an attractive ‘pathbreaking’ means of anti-cancer treatment. Two phase 1 clinical trials of single agent intravenous 3-AP have shown administration of 3-AP achieves stable disease (30% and 16%) in pre-treated advanced stage patients [12–13]. An additional phase I trial of intravenous 3-AP in combination with gemcitabine also showed 42% of patients had at least stable disease as a best response after 4 months of treatment [14]. Likewise, a phase 1 clinical trial of radiation, cisplatin, and three-times weekly intravenous 3-AP (25mg/m2) showed a durable 18-month complete pelvic tumor response rate in 100% (10 of 10) women treated with advanced stage cervical cancer [15]. Given the rapid metabolism of 3-AP (T½ ≈ 2.5 hours), the most convenient formulation of 3-AP for further phase 3 clinical development would be an oral tablet. In that our study showed satisfactory bioavailability of oral 3-AP tablets in direct comparison to intravenous delivery further strengthens an argument for oral dosing of 3-AP in future clinical trials. For these reasons, there is interest in investigating daily dosing of oral 3-AP co-administered with radiation in international clinical trials for treatment of cervical cancer because (a) it permits optimal radiation-drug timed effect [8, 18] and (b) it removes the impediment of refrigeration and intravenous tubing seldom realized in underdeveloped nations where cervical cancer is common.
In this study, intravenous and oral 3-AP pharmacokinetics were compared to determine oral bioavailability. Our results demonstrate that the time course of drug appearance and disappearance from serum is very similar when 3-AP is administered orally or intravenously as a 2 hour infusion (Fig. 1). Furthermore, the finding of an average oral bioavailability of 67% is consistent with our finding that the MTD of 3-AP by the oral route is 33% higher than by the intravenous route. 3-AP treatment was scheduled twice daily to provide repeated drug-induced RR inhibition, and thereby, prolonged inhibition of on-demand deoxyribonucleotide synthesis during attempted cell proliferation. From our adverse event and pharmacological data, frequent oral 3-AP dosing at its MTD appears safe, and provides precedent for future 3-AP mediated trials of chemotherapy or radiatherapy sensitization. Perhaps the most outstanding anticancer benefit of an RR inhibitor like 3-AP is its stalling of DNA damage repair mechanisms and this will ultimately enhance tumor-directed cytotoxicity [8, 18].
The therapeutic efficacy of single agent 3-AP was low in that only 25% of treated patients attained stable disease as the best response on this study. This finding is consistent with other human anticancer phase 1 and 2 testing of single agent RR inhibitors (e.g., hydroxyurea, gemcitabine, and 3-AP). Despite our observation of a progression-free interval of 15 months in a single patient with pancreatic cancer, phase II trials of intravenous 3-AP in pancreatic and kidney cancer were not found to exhibit meaningful clinical activity [19–20]. Combining RR inhibitors, such as a phase II trial of intravenous 3-AP and gemcitabine in relapsed nonsmall cell lung cancer, also only yielded a 20% stable disease rate [21]. However, substantial therapeutic response gains have been realized when RR inhibitors have been co-administered with cytotoxic chemotherapy and radiation [9, 15, 22]. This discordant finding is most likely attributed to the impeded supply of deoxyribonucleotides demanded by cells for repair of damaged DNA. While deoxyribonucleotide numbers needed to fix damaged DNA vary from a few for double-strand break repair to hundreds for base damage and single-strand gaps, the rate-limiting step in supply of deoxyribonucleotides is catalyzed by RR. Blockade of RR by 3-AP or other RR inhbitors considerably reduces deoxyribonucleotides furnished de novo on-demand in conditions of DNA damage. Since cells avoid genotoxic stress resulting from large fluctuations in deoxyribonucleotide reserves [23], de novo synthesis of deoxyribonucleotides by RR is a critical early response to DNA damage. Indeed, the two isoforms of the RR small subunit M2 or p53R2 are tuned to cell demands of deoxyribonucleotides. The RR M2 protein is tightly restricted to S-phase replication of DNA by a KEN-box promoting degradation in late mitosis. The RR p53R2 protein is constitutively active throughout the cell cycle, but regulated in its activity by a reversible protein-protein interaction with p53 [24] and in its expression level by a p53-induced transcription mechanism [6]. Pre-clinical and clinical data collected thusfar suggest that the greatest gains in clinical benefit from RR inhibitors occur when cellular DNA is damaged and cell deoxyribonucleotides demands are high. If oral 3-AP proceeds to further clinical testing, it is recommended that 3-AP dosing follow DNA-damaging therapies.
In summary, oral 3-AP dosing at the oral MTD provides drug exposure equivalent to its intravenous form. The tolerable adverse event profile of 3-AP alone at its MTD makes it an attractive drug partner for anticancer trials in combination with chemotherapy and radiation treatments. A phase II trial of intravenous triapine added to days 1, 3, 5, 8, 10, 12, 15, 17, 19, 22, 24, 26, 29, 31, and 33 of cisplatin and radiation therapy in cervical and vaginal malignancies is ongoing [ClinicalTrials.gov identifier NCT00941070]. Based on the data of Kunos et al. cervical cancer would be the most attractive disease to utilize oral 3-AP in combination with cisplatin and radiation [15].
Acknowledgments
Sources of Support: This study was supported by the National Institutes of Health, National Cancer Institute under Cooperative Agreements with the Cancer Therapy Evaluation Program (U01 CA62505, City of Hope Medical Center and U01 CA099168, University of Pittsburgh Cancer Institute) and a Cancer Center Support Grant (P30 CA033572, City of Hope Medical Center).
Footnotes
Conflicts of Interest Notification: Each author certifies that he or she has no commercial associations (DISCLOSURES: NONE, e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article. Each author certifies that his or her institution has approved this retrospective investigation and that all investigations were conducted in conformity with ethical principles of research. The corresponding author certifies that all authors provided substantial conceptual or analytic contributions during manuscript preparation, and thus, satisfactorily qualify for authorship under the “Uniform Requirements.” All authors grant approval for publication.
References
- 1.Jordan A, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 1998;67:71–98. doi: 10.1146/annurev.biochem.67.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Thelander L, Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986;6:3433–42. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eklund H, Uhlin U, Farnegardh M, Logan DT, Nordlund P. Structure and function of the radical enzyme ribonucleotide reductase. Prog Biophys Mol Biol. 2001;77:177–268. doi: 10.1016/s0079-6107(01)00014-1. [DOI] [PubMed] [Google Scholar]
- 4.Thelander M, Graslund A, Thelander L. Subunit M2 of mammalian ribonucleotide reductase. Characterization of a homogeneous protein isolated from M2-overproducing mouse cells. J Biol Chem. 1985;260:2737–41. [PubMed] [Google Scholar]
- 5.Yen Y, Grill SP, Dutschman GE, Chang CN, Zhou BS, Cheng YC. Characterization of a hydroxyurea-resistant human KB cell line with supersensitivity to 6-thioguanine. Cancer Res. 1994;54:3686–91. [PubMed] [Google Scholar]
- 6.Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, Takei Y, Nakamura Y. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404:42–9. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- 7.Guittet O, Hakansson P, Voevodskaya N, Fridd S, Graslund A, Arakawa H, Nakamura Y, Thelander L. Mammalian p53R2 protein forms an active ribonucleotide reductase in vitro with the R1 protein, which is expressed both in resting cells in response to DNA damage and in proliferating cells. J Biol Chem. 2001;276:40647–51. doi: 10.1074/jbc.M106088200. [DOI] [PubMed] [Google Scholar]
- 8.Kunos CA, Chiu SM, Pink J, Kinsella TJ. Modulating radiation resistance by inhibiting ribonucleotide reductase in cancers with virally or mutationally silenced p53 protein. Radiat Res. 2009 Dec;172(6):666–76. doi: 10.1667/RR1858.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hreshchyshyn MM, Aron BS, Boronow RC, Franklin EW, III, Shingleton HM, Blessing JA. Hydroxyurea or placebo combined with radiation to treat stages IIIB and IV cervical cancer confined to the pelvis. Int J Radiat Oncol Biol Phys. 1979;5:317–22. doi: 10.1016/0360-3016(79)91209-4. [DOI] [PubMed] [Google Scholar]
- 10.Stehman FB, Bundy BN, Kucera PR, Deppe G, Reddy S, O’Connor DM. Hydroxyurea, 5-fluorouracil infusion, and cisplatin adjunct to radiation therapy in cervical carcinoma: phase I-II trial of the Gynecologic Oncology Group. Gyn Oncol. 1997;66:262–7. doi: 10.1006/gyno.1997.4761. [DOI] [PubMed] [Google Scholar]
- 11.Rose P, Ali S, Watkins E, Thigpen J, Deppe G, Clark-Pearson D, Insalaco S. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 25(19):2804–2810. doi: 10.1200/JCO.2006.09.4532. [DOI] [PubMed] [Google Scholar]
- 12.Feun L, Modiano M, Lee K, Mao J, Marini A, Savaraj N, Plezia P, Almassian B, Colacio E, Fischer J, MacDonald S. Phase 1 and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chem Pharma. 2002;50(3):223–9. doi: 10.1007/s00280-002-0480-0. [DOI] [PubMed] [Google Scholar]
- 13.Murren J, Modiano M, Clairmont C, Lambert P, Savaraj N, Doyle T, Sznol M. Phase 1 and pharmacokinetic study of Triapine, a potent ribonucleotide reductase inhibitor, administered for five days in patients with advanced solid tumors. Clin Cancer Res. 2003;9(11):4092–100. [PubMed] [Google Scholar]
- 14.Yen Y, Margolin K, Doroshow J, Fishman M, Johnson B, Clairmont C, Sullivan D, Sznol M. A phase I trial of 3-minopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother Pharmacol. 2004;54:331–342. doi: 10.1007/s00280-004-0821-2. [DOI] [PubMed] [Google Scholar]
- 15.Kunos CA, Waggoner S, von Gruenigen V, Eldermire E, Pink J, Dowlati A, Kinsella TJ. Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clin Cancer Res. 2010 Feb 15;16(4):1298–306. doi: 10.1158/1078-0432.CCR-09-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 18.Kunos CA, Radivoyevitch T, Pink J, Chiu SM, Stefan T, Jacobberger J, Kinsella TJ. Ribonucleotide reductase inhibition enhances chemoradiosensitivity of human cervical cancers. Radiat Res. 2010;174(5):574–81. doi: 10.1667/RR2273.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attia S, Kolesar J, Mahoney MR, Pitot HC, Laheru D, Heun J, Huang W, Eickhoff J, Erlichman C, Holen KD. A phase 2 consortium (P2C) trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) for advanced adenocarcinoma of the pancreas. Invest New Drugs. 2008 Aug;26(4):369–79. doi: 10.1007/s10637-008-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knox JJ, Hotte SJ, Kollmannsberger C, Winquist E, Fisher B, Eisenhauer EA. Phase II study of Triapine in patients with metastatic renal cell carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC IND.161) Invest New Drugs. 2007 Oct;25(5):471–7. doi: 10.1007/s10637-007-9044-9. [DOI] [PubMed] [Google Scholar]
- 21.Traynor AM, Lee JW, Bayer GK, Tate JM, Thomas SP, Mazurczak M, Graham DL, Kolesar JM, Schiller JH. A phase II trial of triapine (NSC# 663249) and gemcitabine as second line treatment of advanced non-small cell lung cancer: Eastern Cooperative Oncology Group Study 1503. Invest New Drugs. 2010 Feb;28(1):91–7. doi: 10.1007/s10637-009-9230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duenas-Gonzalez A, Cetina-Perez L, Lopez-Graniel C, Gonzalez-Enciso A, Gomez-Gonzalez E, Rivera-Ruby L, Montalvo-Esquicel G, Munoz-Gonzalez D, Robles-Flores J, Vazquez-Govea E, De La Garza J, Mohar A. Pathologic response and toxicity assessment of chemoradiotherapy with cisplatin versus cisplatin plus gemcitabine in cervical cancer: a randomized phase II study. Int J Radiat Oncol Biol Phys. 2005;61(3):817–23. doi: 10.1016/j.ijrobp.2004.07.676. [DOI] [PubMed] [Google Scholar]
- 23.Hakansson P, Hofer A, Thelander L. Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J Biol Chem. 2006;281:7834–41. doi: 10.1074/jbc.M512894200. [DOI] [PubMed] [Google Scholar]
- 24.Xue L, Zhou B, Liu X, Qiu W, Jin Z, Yen Y. Wild-type p53 regulates human ribonucleotide reductase by protein-protein interaction with p53R2 as well as hRRM2 subunits. Cancer Res. 2003;63(5):980–6. [PubMed] [Google Scholar]