Abstract
Purpose
Compare outcomes with vaginal gel versus intramuscular progesterone replacement in donor oocyte recipients.
Methods
A single-center retrospective analysis (January 2004–December 2006) evaluated pregnancy outcomes (serum human chorionic gonadotropin, implantation, clinical pregnancy, delivery, total pregnancy loss rates) for 225 recipients of embryos from donor (aged <32 years) oocytes. Vaginal progesterone gel (Crinone® 8%; 90 mg twice daily; n = 105) or intramuscular progesterone (50 mg once daily; n = 120) was started the afternoon of oocyte retrieval and continued until a negative pregnancy test or 10 weeks’ gestation.
Results
There were no statistically significant differences between groups for the five pregnancy outcomes; numerical results favored vaginal progesterone in all cases. Confidence intervals showed vaginal gel was within, or <1% from, a noninferiority limit of 10% versus intramuscular progesterone for four of five pregnancy outcomes.
Conclusions
Pregnancy outcomes were comparable for progesterone replacement with vaginal gel and intramuscular progesterone in an oocyte donation program.
Keywords: Oocyte donation, Progesterone replacement, Luteal phase, Crinone 8% (progesterone gel)
Introduction
The implantation process involves complex coordinated interactions between embryonic and endometrial cells. The ultimate goal of a viable pregnancy is achieved through assisted reproductive technology (ART) by maximizing embryo quality and coordinating blastocyst development with uterine receptivity [1–3]. These two critical factors, embryo quality and endometrial receptivity, must be synchronized precisely to achieve a viable pregnancy [4]. After the transfer of a high-quality embryo during ART, success depends on the histologic transformation of the endometrium into a receptive environment that allows for attachment, implantation, and continued development of the blastocyst [1–3]. This peri-implantation period defines the limited time in which there is a ‘window of endometrial receptivity to blastocyst implantation’ [1–4]. The mechanisms underlying this process of coordinated embryonic and endometrial growth and differentiation, although poorly understood, are synchronized, orchestrated, and regulated by fluctuating levels of the ovarian steroid hormones estrogen and progesterone [5].
Progesterone can be administered by oral, intramuscular (IM), or vaginal routes. Compared with IM and vaginal routes, oral progesterone has been associated with significantly lower rates of implantation and pregnancy during in vitro fertilization–embryo transfer (IVF-ET) [6, 7]. Crinone® (progesterone gel; Columbia Laboratories, Inc., Livingston, NJ, USA) is a vaginally administered gel containing 90 mg of micronized progesterone in an oil–water emulsion in a polycarbophil base. This unique delivery system provides a controlled and sustained release of progesterone through the vaginal wall over 24 h [8]. The vaginal route of progesterone administration afforded by the gel offers the advantage of targeted delivery and direct action on the endometrium (‘first uterine pass effect’) [9], convenience, tolerability, and patient preference compared with IM progesterone [10–12], vaginal suppositories [10], or capsules [13]. During the past decade, numerous studies have shown comparable efficacy between the vaginal progesterone gel and IM progesterone when used for luteal supplementation during IVF-ET [11, 12, 14, 15]. IM progesterone produces supraphysiologic serum progesterone levels that are significantly higher than those achieved with vaginal progesterone gel [9, 16, 17]. However, the progesterone levels at the target tissue—the endometrium—are significantly higher with vaginal gel than with IM progesterone [9]. Therefore, the vaginal progesterone gel affords comparable pregnancy outcomes without the high serum levels associated with systemic absorption [14, 15, 18, 19].
The implementation of the steroid hormone (estrogen and progesterone) replacement regimen has allowed for the successful transfer of embryos derived from donated oocytes to women with suppressed or nonfunctioning ovaries [20]. Although an abundance of published studies have documented comparable efficacy outcomes when the luteal phase is supplemented with vaginal progesterone gel versus IM progesterone during IVF-ET [11, 12, 14, 15], only one published efficacy study to date compares progesterone replacement regimens for oocyte donation [18]. Efficacy studies of recipients of embryos derived from donated oocytes provide an informative model for comparative studies of progesterone replacement. The effects of progesterone can be isolated to the exogenous progesterone administered for replacement because these women do not have functioning corpora lutea and thus endogenous progesterone is absent. Of note, once-daily (QD) dosing of the vaginal progesterone gel is recommended for supplementation and twice-daily (BID) dosing is recommended for replacement regimens [21]. Crinone® is the only treatment option approved by the Food and Drug Administration for progesterone replacement in ART.
In 1998, Gibbons et al. [18] published the first progesterone replacement comparative study in oocyte donation. Their prospective randomized trial showed that the vaginal progesterone gel was as effective as IM progesterone when used for progesterone replacement in recipients of embryos from anonymously donated oocytes. In the study, 72 women undergoing oocyte donation received Crinone® (90 mg BID) or IM progesterone (100 mg QD) in a 7:3 ratio, respectively. The vaginal progesterone gel (n = 54) was administered from the evening of cycle day 14 until a negative pregnancy test or up to 10 weeks’ estimated gestational age (EGA) if pregnant. IM progesterone (n = 18) was administered similarly starting on cycle day 15 at 100 mg QD, with the dosage reduced to 50 mg QD at 8 weeks’ EGA if pregnant. The ongoing pregnancy rates were 17 of 54 (31%) for the vaginal progesterone gel and 4 of 18 (22%) for IM progesterone, supporting the authors’ conclusion of equal effectiveness.
Since the publication of this landmark ART study [18] showing comparable efficacy between vaginal progesterone gel and IM progesterone during hormone replacement cycles, no other trial has further compared vaginal progesterone to IM progesterone in support of oocyte donation cycles. We have access to a large database of recipients of embryos from anonymously donated oocytes who were treated with vaginal progesterone gel or IM progesterone. For this reason, we conducted a retrospective analysis of these data to compare the pregnancy outcomes between the two types of progesterone replacement regimens in a real-world setting. To our knowledge, this study is the largest ever performed to evaluate the outcomes of different progesterone replacement regimens used for oocyte donation cycles.
Materials and methods
This is a retrospective analysis of oocyte donation outcomes at a single, large, active ART center. Pregnancy outcomes (positive serum human chorionic gonadotropin [hCG], implantation, clinical pregnancy, delivery, and total pregnancy loss rates) were evaluated for 225 recipients of embryos from anonymously donated oocytes in an active oocyte donation program from January 2004 to December 2006, when vaginal progesterone gel and IM progesterone replacement regimens were widely used in the clinic. By definition, the age of the anonymous donors was <32 years and all donors were required to have ≥10 normal antral follicles. Recipients who received oocytes from known donors were not included in this study. Anonymous donors underwent standard pretreatment medication with oral contraceptive pill and monitoring followed by a gonadotropin-releasing hormone (GnRH) antagonist (Cetrotide® [cetrorelix acetate for injection; EMD Serono, Inc., Rockland, MA, USA] 0.25 mg or Ganirelix Acetate Injection [Organon USA Inc., Roseland, NJ, USA] 250 μg) with gonadotropins (Gonal-f™ [follitropin alfa injection; EMD Serono, Inc.] or Follistim® [follitropin beta injection; Organon USA Inc.] 150 to 300 IU) and luteinizing hormone (Luveris® [lutropin alfa for injection; EMD Serono, Inc.] 75 IU QD) added on day 2 of menses. Recipients on continuous oral contraceptive pills received 10 units of Lupron® (leuprolide acetate; TAP Pharmaceuticals, Inc., Lake Forest, IL, USA) on cycle day 15, or Lupron was started on cycle day 21. Estrogen was started 4 days before the donor expected menses (Estrace® [estradiol tablets, USP; Warner Chilcott US, LLC, Rockaway, NJ, USA] 2 mg orally BID plus Vivelle-Dot® [estradiol transdermal delivery system; Noven Pharmaceuticals, Inc., Miami, FL, USA] 0.1-mg patch changed every 3 days). Endometrial transvaginal ultrasound was performed on day 7 of estrogen, and, if the endometrial thickness was <6 mm, the estrogen dose was increased and remonitoring was performed 3 days later. Progesterone (Crinone® [90 mg BID] or IM progesterone [50 mg QD]) was started the afternoon of donor oocyte retrieval. Conventional in vitro fertilization or, if male factor was present, intracytoplasmic sperm injection was used. All embryo transfers were performed under ultrasound guidance using a SureView® Catheter (SureView Wallace Embryo Replacement Catheter; Smiths Medical, Hythe, Kent, UK). The cervix was cleansed using three saline-soaked sponges, and excess mucus was removed using a tuberculin syringe. A trial catheter placement was performed before all embryo transfers per usual protocol. Patients using the vaginal gel were instructed to hold the morning dose until after the transfer was performed. Embryo transfer was performed on day 4 of progesterone therapy and blastocyst transfer on day 6. (During the study period, a protocol shift from embryo transfer to blastocyst transfer occurred in our center.) All women underwent pregnancy testing 14 days after oocyte retrieval using serum hCG levels. If the recipient was pregnant, progesterone and estrogen were continued at the same dosages until 10 weeks’ EGA. Clinical pregnancy was confirmed by the presence of an intra-uterine gestational sac with a positive fetal heart beat visualized on transvaginal ultrasound examination at 6 to 7 weeks’ EGA.
Baseline recipient characteristics included recipient age, the number of cycles, and cycle length. Oocyte and embryo characteristics that were evaluated included the number of oocytes retrieved, number of mature oocytes, fertilization rate, number of embryos transferred, number of embryos frozen, and number of embryos with high implantation potential (HIP) transferred (including blastocysts from HIP embryos) [22]. A HIP embryo was identified as having >4 cells on day 2, >7 cells on day 3, <20% fragmentation, and the absence of multinucleated blastomeres [22].
Implantation rate was defined as the percentage of embryos that implanted successfully relative to the total number of embryos transferred. The total pregnancy loss rate was calculated as the difference between the number of recipients with a positive serum hCG and the number of recipients who had a delivery as a percentage of the recipients with a positive serum hCG. Positive serum hCG, implantation, clinical pregnancy, delivery, and total pregnancy loss rates were compared between recipients treated with vaginal progesterone gel (n = 105) and those treated with IM progesterone (n = 120).
Statistical analyses
Baseline recipient characteristics were summarized between the two groups using descriptive statistics (n, mean, standard deviation). Pregnancy outcome rates were calculated as the number and percentage of recipients with positive serum hCG, clinical pregnancy, and delivery. The implantation rate and total pregnancy loss rate were calculated as described above. Statistical analysis was performed using a two-sided Fisher’s exact test. The differences between the pregnancy outcome rates for vaginal progesterone gel versus IM progesterone were calculated and 95% confidence intervals (CIs) with correction for continuity were constructed.
Results
Both groups were clinically homogeneous in all clinical and laboratory parameters evaluated, including the number of cycles, recipient age, cycle length, number of oocytes, number of mature oocytes, fertilization rate, number of embryos transferred, number of embryos frozen, and number of embryos with HIP transferred (Table 1).
Table 1.
Data from recipients treated with vaginal progesterone gel versus intramuscular progesterone
| All anonymous oocyte donation cycles | Vaginal progesterone gel | Intramuscular progesterone |
|---|---|---|
| Number of cycles | 105 | 120 |
| Recipient age, yearsa | 41.5 ± 4.73 | 41.8 ± 4.12 |
| Cycle length, daysa | 19.7 ± 6.33 | 18.2 ± 5.55 |
| Number of oocytesb | 17.97 | 17.90 |
| Number of mature oocytesb | 15.01 | 15.23 |
| Fertilization rate, %a | 66.26 ± 21.50 | 67.52 ± 18.98 |
| Number of embryos transferreda | 1.93 ± 0.37 | 2.06 ± 0.33 |
| Number of embryos frozenb | 4.42 | 4.43 |
| Number of embryos with HIP transferreda | 1.55 ± 0.74 | 1.67 ± 0.64 |
HIP high implantation potential.
aMean±standard deviation.
bMean.
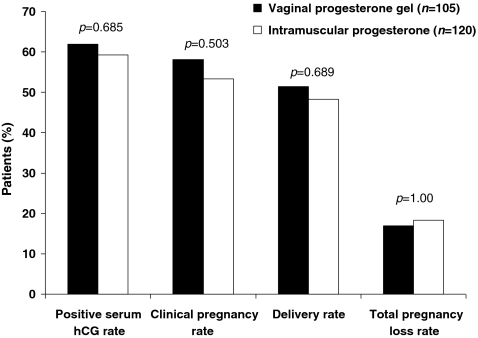
No statistically significant differences were observed between the two groups for any of the pregnancy outcome rates (Table 2, Fig. 1). Four of the five outcomes showed lower bounds of the CIs within or <1% from a noninferiority limit of 10%. The upper bound of the CI for difference in total pregnancy loss rate (12.9%) was also near this threshold for noninferiority. All five outcomes showed a numerical advantage for vaginal progesterone gel.
Table 2.
Pregnancy outcomes in recipients treated with vaginal progesterone gel versus intramuscular progesterone
| Pregnancy outcome | Descriptive statistica | Vaginal progesterone gel (n = 105) | Intramuscular progesterone (n = 120) |
|---|---|---|---|
| Positive serum hCG rate | n (%) | 65 (61.9) | 71 (59.2) |
| p value | 0.685 | ||
| Difference | 2.7 | ||
| 95% CI | −10.9, 16.4 | ||
| Implantation rate | n (%) | 89/203 (43.8) | 91/245 (37.1) |
| p value | 0.175 | ||
| Difference | 6.7 | ||
| 95% CI | −2.9, 16.3 | ||
| Clinical pregnancy rate | n (%) | 61 (58.1) | 64 (53.3) |
| p value | 0.503 | ||
| Difference | 4.8 | ||
| 95% CI | −9.1, 18.6 | ||
| Delivery rate | n (%) | 54 (51.4) | 58 (48.3) |
| p value | 0.689 | ||
| Difference | 3.1 | ||
| 95% CI | −10.9, 17.1 | ||
| Total pregnancy loss rate | n (%) | 11/65 (16.9) | 13/71 (18.3) |
| p value | 1.000 | ||
| Difference | −1.4 | ||
| 95% CI | −15.7, 12.9 |
CI confidence interval.
ap value based on Fisher’s exact test.
Fig. 1.
Pregnancy outcomes in recipients treated with vaginal progesterone gel versus intramuscular progesterone. (The total pregnancy loss rate is based on the number of patients with positive serum human chorionic gonadotropin [hCG], not the total number of patients in the treatment group)
Discussion
This current analysis represents the largest analysis of pregnancy outcomes comparing vaginal progesterone gel and IM progesterone replacement regimens in recipients of embryos from anonymously donated oocytes. An advantage of this single-center ART study is the consistency of the institutional protocol, patient selection criteria, laboratory performance, and procedural techniques. However, potential limitations of a retrospective single center also are recognized, and the aim for these results to be replicated is acknowledged. Donor-oocyte recipients have nonfunctioning, absent, or suppressed ovaries and therefore do not have functioning corpus lutea or endogenous progesterone production. Use of this recipient patient population allows direct evaluation of the contribution of exogenously administered progesterone to pregnancy outcomes. The progesterone administered as replacement progesterone is the only available progesterone in the oocyte donation model as opposed to supplementation in an IVF-ET model, where there are endogenous and exogenous progesterone sources.
The study design only included recipients who received oocytes obtained from anonymous donors aged <32 years. Because oocytes donated to the recipients in this analysis were from young donors, the oocyte quality was optimal in both groups and comparable between the groups. The recipient age and oocyte/embryo parameters, including the number of embryos with HIP transferred, were comparable between the two groups. Because the oocyte/embryo quality was graded as good and evenly matched in both groups, the endometrial effect of progesterone on pregnancy outcomes can be separated from the contribution made by oocyte or embryo quality.
The current results are consistent with the previous results by Gibbons et al. [18] that showed similar pregnancy outcome(s) with vaginal progesterone gel compared with IM progesterone when used as part of hormonal replacement in recipients of embryos derived from donated oocytes. Thus, taken together, the results reported in Gibbons et al. [18] and those reported in the current study demonstrate that the use of Crinone® 90 mg BID for replacement cycles is comparable to IM progesterone regardless of the dose of IM progesterone used (50 mg QD or 100 mg QD). Gibbons et al. [18] also showed ‘in phase’ endometrial biopsies during mock cycles for all subjects in both treatment groups and comparable pregnancy outcomes between the two groups, but without high levels of systemic absorption in the vaginal progesterone gel group. Significantly lower serum progesterone levels were observed at all time points beyond cycle day 15 (days 15, 20, 24, and 26) in the vaginal progesterone gel group versus the IM progesterone group. The mean progesterone level during vaginal gel use was 19.0 ng/ml versus a mean level of 89.3 ng/ml for the last three time points with IM progesterone use. Implantation, clinical pregnancy, and ongoing pregnancy rates were favorable for vaginal progesterone gel versus IM progesterone (23% vs 18%, 48% vs 28%, and 31% vs 22%, respectively) in recipients of embryos from donated oocytes [10], with similar findings of comparability in our present study (43.8% vs 37.1%, 58.1% vs 53.3% ,and 51.4% vs 48.3%, respectively), including the numerical advantage for vaginal progesterone gel. The current study was not designed for detecting differences between the treatment modalities or for establishing prespecified limits of noninferiority or equivalence. However, the lower limits of the CIs for four of the five pregnancy outcomes were within or <1% from a noninferiority limit of 10% to IM progesterone (i.e., −10.9%, −2.9%, −9.1%, and −10.9%) and near this limit for the fifth outcome (upper bound for difference in pregnancy loss 12.9%).
Studies show that patients prefer vaginal progesterone gel to IM progesterone [10–12]. IM injection of progesterone in oil requires laborious and painful daily administration. In addition to patient discomfort and pain at the injection site, IM progesterone also can lead to potentially serious adverse events [23], including injection-site reactions [24] and sterile abscesses [25, 26], allergic reactions to the oil vehicle [27], sciatic nerve injury causing impairment of sensory and motor function of the lower extremity [28], and rare reports of allergic pneumonitis [29, 30]. In contrast, vaginal progesterone gel is easy to use and associated with minimal side effects. Studies show that patients prefer vaginal administration of the progesterone gel compared with the other vaginal formulations that use capsules and suppositories [10, 13].
Future prospectively designed studies should be performed given the comparable results between vaginal progesterone gel and IM progesterone replacement regimens in this oocyte donation study. There also has been a paucity of studies comparing progesterone replacement regimens in frozen embryo transfer procedures. Future studies comparing progesterone replacement regimens for ART should focus on frozen embryo transfer in addition to oocyte donation protocols.
In conclusion, progesterone replacement with vaginal progesterone gel was comparable to IM progesterone in terms of pregnancy outcomes within a single oocyte donation program. Additionally, the vaginal progesterone gel has the advantage of avoiding painful IM injections.
Acknowledgements
The authors thank Lisa Schwartz, MD, Consultant, Marianne B. Zajdel (ReSearch Pharmaceutical Services, Inc., Fort Washington, PA, USA), Jinling Wu, PhD, Marsha Hall, and Colleen Hedge (Scientific Connexions, Newtown, PA, USA) for editorial support.
Conflicts of Interest and Financial Support B.M.B. is a consultant to and speaker for Columbia Laboratories, Inc. J.A.P. is a consultant to Columbia Laboratories, Inc. This study was supported by funding from Columbia Laboratories, Inc. Watson Pharmaceuticals, Inc. provided funding for editorial support in the preparation of this manuscript.
Footnotes
Capsule Pregnancy outcomes in donor oocyte recipients are similar for progesterone replacement with vaginal progesterone gel and with intramuscular injections of progesterone.
References
- 1.Dey SK, Johnson DC. Embryo-uterine interaction in implantation. Life Sci. 1980;27:2381–2384. doi: 10.1016/0024-3205(80)90508-1. [DOI] [PubMed] [Google Scholar]
- 2.Dey SK, Davis DL, Hersey RM, Weisz J, Johnson DC, Pakrasi PL. Physiological aspects of blastocyst uterine interaction. J Biosci. 1984;6(Suppl 2):23–31. doi: 10.1007/BF02716713. [DOI] [Google Scholar]
- 3.Emiliani S, Delbaere A, Devreker F, Englert Y. Embryo-maternal interactive factors regulating the implantation process: implications in assisted reproduction. Reprod Biomed. 2005;Online 10:527–40. [DOI] [PubMed]
- 4.Ziegler D, Fanchin R, Massonneau M, Bergeron C, Frydman R, Bouchard P. Hormonal control of endometrial receptivity. The egg donation model and controlled ovarian hyperstimulation. Ann NY Acad Sci. 1994;734:209–220. doi: 10.1111/j.1749-6632.1994.tb21749.x. [DOI] [PubMed] [Google Scholar]
- 5.Punyadeera C, Verbost P, Groothuis P. Oestrogen and progestin responses in human endometrium. J Steroid Biochem Mol Biol. 2003;84:393–410. doi: 10.1016/S0960-0760(03)00061-X. [DOI] [PubMed] [Google Scholar]
- 6.Friedler S, Raziel A, Schachter M, Strassburger D, Bukovsky I, Ron-El R. Luteal support with micronized progesterone following in-vitro fertilization using a down-regulation protocol with gonadotropin-releasing hormone agonist: a comparative study between vaginal and oral administration. Hum Reprod. 1999;14:1944–1948. doi: 10.1093/humrep/14.8.1944. [DOI] [PubMed] [Google Scholar]
- 7.Licciardi FL, Kwiatkowski A, Noyes NL, Berkley AS, Krey LL, Grifo JA. Oral versus intramuscular progesterone for in vitro fertilization: a prospective randomized study. Fertil Steril. 1999;71:614–618. doi: 10.1016/S0015-0282(98)00515-9. [DOI] [PubMed] [Google Scholar]
- 8.Bulletti C, Ziegler D, Flamigni C, Giacomucci E, Polli V, Bolelli G, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073–1079. doi: 10.1093/humrep/12.5.1073. [DOI] [PubMed] [Google Scholar]
- 9.Ciccinelli E, Ziegler D, Bulletti C, Matteo MG, Schronauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol. 2000;95:403–406. doi: 10.1016/S0029-7844(99)00542-6. [DOI] [PubMed] [Google Scholar]
- 10.Levine H. Luteal support from the vaginal progesterone (P) gel Crinone 8%: preliminary results of multicenter trial show higher pregnancy rates than historical controls. J Soc Gynecol Investig. 2000;7(Suppl 571):203A. [Google Scholar]
- 11.Schoolcraft WB, Hesla JS, Gee MJ. Experience with progesterone gel for luteal support in a highly successful IVF programme. Hum Reprod. 2000;15:1284–1288. doi: 10.1093/humrep/15.6.1284. [DOI] [PubMed] [Google Scholar]
- 12.Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein MD. Comparison of Crinone 8% intravaginal gel and intramuscular progesterone supplementation for in vitro fertilization/embryo transfer in women under age 40: interim analysis of a prospective randomized trial. Fertil Steril. 2008;89:485–487. doi: 10.1016/j.fertnstert.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Simunic V, Tomic V, Tomic J, Nizic D. Comparative study of the efficacy and tolerability of two vaginal progesterone formulations, Crinone 8% gel and Utrogestan capsules, used for luteal support. Fertil Steril. 2007;87:83–87. doi: 10.1016/j.fertnstert.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 14.Anserini M, Costa M, Remorgida V, Sarli R, Guglielminetti E, Ragni R. Luteal phase support in assisted reproduction cycles using either vaginal (Crinone 8) or systemic (Prontogest) progesterone: results of a prospective, randomized study. Minerva Ginecol. 2001;53:297–301. [PubMed] [Google Scholar]
- 15.Chantilis SJ, Zeitoun KM, Patel SI, Johns DA, Madziar VA, McIntire DD. Use of Crinone vaginal progesterone gel for luteal support in in vitro fertilization cycles. Fertil Steril. 1999;72:823–829. doi: 10.1016/S0015-0282(99)00362-3. [DOI] [PubMed] [Google Scholar]
- 16.Ottoson UB, Carlstrom K, Damber JE, Schoultz B. Serum levels of progesterone and some of its metabolites including deoxycorticosterone after oral and parenteral administration. Br J Obstet Gynaecol. 1984;91:1111–1119. doi: 10.1111/j.1471-0528.1984.tb15086.x. [DOI] [PubMed] [Google Scholar]
- 17.Stanczyk FZ. Pharmacokinetics of progesterone administered by the oral and parenteral routes. J Reprod Med. 1999;44:141–147. [PubMed] [Google Scholar]
- 18.Gibbons WE, Toner JP, Hammacher P, Kolm P. Experience with a novel vaginal progesterone preparation in a donor oocyte program. Fertil Steril. 1998;69:96–101. doi: 10.1016/S0015-0282(97)00457-3. [DOI] [PubMed] [Google Scholar]
- 19.Williams SC, Donahue J, Muasher SJ. Vaginal progesterone therapy during programmed cycles for frozen embryo transfer: an analysis of serum progesterone levels and pregnancy rates [Abstract P-363] Fertil Steril. 2000;74(Suppl 1):S209. doi: 10.1016/S0015-0282(00)01336-4. [DOI] [Google Scholar]
- 20.Lutjen P, Trounson A, Leeton J, Findlay J, Wood C, Renou P. The establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failure. Nature. 1984;307:174–175. doi: 10.1038/307174a0. [DOI] [PubMed] [Google Scholar]
- 21.Crinone® 4% and Crinone® 8% (progesterone gel) [prescribing information], Livingston, NJ: Columbia Laboratories, Inc. 2009.
- 22.Witmyer J, Wang S, Alper M, Powers R. HIP embryos: effective criteria for high pregnancy rates with low high order multiples. Fertil Steril. 2003;80:S139–S140. doi: 10.1016/S0015-0282(03)01248-2. [DOI] [Google Scholar]
- 23.Greenblatt DJ, Allen MD. Intramuscular injection site complications. JAMA. 1978;49:536. [PubMed] [Google Scholar]
- 24.Al-Shawaf T, Zosmer A, Dirnfeld M, Grudzinkas G. Safety of drugs used in assisted reproduction techniques. Drug Saf. 2005;28:513–528. doi: 10.2165/00002018-200528060-00004. [DOI] [PubMed] [Google Scholar]
- 25.Penzias A, Alper M. Luteal support with vaginal micronized progesterone gel in assisted reproduction. Reprod Biomed. 2003;Online 6:187–295. [DOI] [PubMed]
- 26.Phipps WR, Benson CB, McShane PM. Severe thigh myositis following intramuscular progesterone injection in an in vitro fertilization patient. Fertil Steril. 1988;49:536–537. doi: 10.1016/s0015-0282(16)59787-8. [DOI] [PubMed] [Google Scholar]
- 27.Phy J. Hypersensitivity to progesterone-in-oil after in vitro fertilization and embryo transfer. Fertil Steril. 2003;80:1272–1275. doi: 10.1016/S0015-0282(03)01170-1. [DOI] [PubMed] [Google Scholar]
- 28.Kline DG, Kim D, Midha R, Harsh C, Tiel R. Management and results of sciatic nerve injuries: a 24-year experience. J Neurosurg. 1998;89:13–23. doi: 10.3171/jns.1998.89.1.0013. [DOI] [PubMed] [Google Scholar]
- 29.Bouckaert Y, Robert F, Englert Y, Backer D, Vuyst P, Delbaere A. Acute eosinophilic pneumonia associated with intramuscular injection of progesterone as luteal phase support after IVF: case report. Hum Reprod. 2004;19:1806–1810. doi: 10.1093/humrep/deh316. [DOI] [PubMed] [Google Scholar]
- 30.Veysman B, Vlahos I, Oshva L. Pneumonitis and eosinophilia after in vitro fertilization. Ann Emerg Med. 2006;47:472–475. doi: 10.1016/j.annemergmed.2005.12.023. [DOI] [PubMed] [Google Scholar]