Abstract
Even in the 21st century, studies aimed at characterizing the pathological paradigms associated with the development and progression of central nervous system diseases are primarily performed in laboratory animals. However, limited translational significance, high cost, and labor to develop the appropriate model (e.g., transgenic or inbred strains) have favored parallel in vitro approaches. In vitro models are of particular interest for cerebrovascular studies of the blood–brain barrier (BBB), which plays a critical role in maintaining the brain homeostasis and neuronal functions. Because the BBB dynamically responds to many events associated with rheological and systemic impairments (e.g., hypoperfusion), including the exposure of potentially harmful xenobiotics, the development of more sophisticated artificial systems capable of replicating the vascular properties of the brain microcapillaries are becoming a major focus in basic, translational, and pharmaceutical research. In vitro BBB models are valuable and easy to use supporting tools that can precede and complement animal and human studies. In this article, we provide a detailed review and analysis of currently available in vitro BBB models ranging from static culture systems to the most advanced flow-based and three-dimensional coculture apparatus. We also discuss recent and perspective developments in this ever expanding research field.
Keywords: in vitro models, blood–brain barrier, cell culture, CNS, endothelial, passive diffusion/transport, tight junction, alternative research
INTRODUCTION
The burgeoning field of cerebrovascular research has created new and exciting opportunities in both basic and clinical studies. Reproducing the physiological characteristics and response of the human brain microcapillaries in vitro represent a critical biotechnological breakthrough. Such a model may significantly help scientists to understand the mechanisms involved in the cerebrovascular response to a number of physiological and pathological stimuli. This, in turn, will provide new strategies to accelerate the development of novel central nervous system (CNS) drug therapies and reduce the burden of major neurological disorders. Many scientific evidences that the blood–brain barrier (BBB) dynamically responds to physiological and pathophysiological events (e.g., acute brain injury,1,2 rheological disturbances,3,4 and proinflammatory stimuli)4–6 leading to the pathogenesis and progression of many neurological disorders (such as epilepsy, multiple sclerosis, Alzheimer, and so on, mostly associated with increased BBB permeability)7–17 have shifted the current view of the BBB. This is now considered a dynamic and extremely complex vascular interface capable of exerting a vast array of specialized functions.18
The research community recognizes that mimicking the physiology and the functional response of the BBB in vitro is a challenging task. Initial in vivo studies provided critical insights on the pharmacodynamic and pharmacokinetic behavior of many different classes of drugs targeting the brain. These same studies also helped discover specific BBB transport systems by Oldendorf et al.19–22 in the early 1970s, which were then characterized in the late 1980s using a more sensitive in vivo method: the in situ brain perfusion technique.23,24
However, a new approach was necessary to simplify the overwhelming complexity posed by the in vivo experimental conditions and to expedite the characterization of the BBB at the cellular and molecular levels. The development of new cell culture techniques and improved in vitro technologies provided the necessary tools to overcome these complexities and advance our understanding of the physiology and functions of the BBB.25–35
STRUCTURE AND FUNCTIONS OF THE BBB
The BBB consists of microvascular endothelial cells (ECs) lining the brain microvessels. Closely associated astrocytic end-feet processes36 contribute to modulate ECs differentiation and the induction and maintenance of the BBB properties.32–34,37–40 The BBB ECs are characterized by scarce pinocytotic activity, lack of fenestrations (i.e., openings), and unique expression patterns of trans-membrane transport systems to regulate the traffic of substances into and out of the brain parenchyma.35 Interendothelial tight junctions (zonulae occludentes) between adjacent ECs form a physical barrier (gate function) that impedes blood-borne (including electrolytes and other water-soluble compounds)35,41 and xenobiotic substances in the systemic circulation from entering the brain through paracellular routes. Therefore, transit across the BBB involves translocation through the capillary endothelium by asymmetrically expressed carrier-mediated transport systems.35,42–44 These are responsible for the passage of certain water-soluble but biologically important substances (e.g., D-glucose, mono-carboxylic acids, amino acids, etc.)32,35,44 from the peripheral circulation to neurons in the parenchyma.
Specific efflux transport systems at the BBB [e.g., P-glycoprotein (P-gp),42 multidrug resistance-associate protein 4 (MRP4),44 and breast cancer resistance protein]45,46 protect the brain from potentially harmful amphipathic and hydrophobic substances.42,47 However, given their wide substrate specificity, these efflux transport systems may contribute to multiple drug resistance during pharmacological treatment of several CNS disorders such as epilepsy and brain tumors.31,48–53 Furthermore, specific cytochrome P450 enzymes (e.g., P450 3A4) expressed at the BBB endothelial level under pathological conditions (e.g., drug-resistant epilepsy)54,55 can catalyze the biotransformation of drugs targeting the brain (in addition to lipids and steroidal hormones),56 thus further reducing their bioavailability (see Fig. 1).
Figure 1. Schematic representation of a typical brain microcapillary.
Note that the passage of substances across the BBB endothelium is controlled by a multimodal barrier system: (1) gating barrier (tight junctions), which prevent paracellular diffusion of polar molecules; (2) transport barrier, which includes number of active efflux systems (P-gp, MRPs, etc.) with affinity for lipophilic substances; (3) metabolic/enzymatic barrier (cytochrome P450 enzymes, MAO, etc.), which catalyze the oxidation/metabolism of organic substrates including xenobiotic substances such as drugs and other potentially toxic chemicals.
IN VITRO BBBs: WHAT DO WE EXPECT FROM AN IDEAL MODEL?
The advancements occurred in the past two decades in the BBB modeling field were primarily dictated by two factors: (1) the need to advance our understanding of the complex molecular mechanisms regulating the brain environment and (2) facilitate the development of alternative and more effective pharmacological strategies aimed at CNS targets. Mimicking the functional properties of this very complex organ interfacing the brain with the systemic vascular environment is critical for the development of novel CNS drugs and is an extremely challenging task. Even though it is still beyond our ability to reproduce all the complex functions of the BBB ex situ, there are several major requirements that an ideal in vitro BBB model should meet (see Table 1). These include (1) enabling the expression of interendothelial tight junctions between adjacent ECs, which facilitate the formation of a very stringent and selective barrier (limited paracellular diffusion); (2) in vivo-like asymmetric distribution (basal vs. luminal) of relevant transporters (e.g., Pgp, glucose transporter 1/Glut-1, etc.),44 which confers polarization of the ECs35,57–60; (3) the expression of functional efflux mechanisms (e.g., P-gp, MRPs)61–63 and drug metabolism30,55,56,64; (4) the ability to discriminate the permeability of substances (in the absence of specific transport systems) according to their specific oil/water partition coefficient and molecular weight.65 Furthermore, an ideal BBB model should also be able to reproduce the effects of a large variety of hemodynamic (e.g., hypertension, flow cessation/stroke, etc.) and systemic/inflammatory insults on the BBB that have been observed in vivo and can lead to the pathogenesis and progression of major CNS disorders (epilepsy,12–14 Alzheimer’s disease,9,10,66 inflammation, brain edema, brain trauma, multiple sclerosis,2,5,67–71 etc.).
Table 1.
Functional and Structural Requirements for An Ideal In Vitro BBB Model to Mimic the In Vivo BBB Model
| Functional Features of An Ideal In Vitro BBB Model |
|---|
| Enable the expression of tight junctions between adjacent endothelial cells. |
| Negligible paracellular diffusion between endothelial cells. |
| Selective and asymmetric permeability to physiologically crucial ions (Na+, K+, CI−). |
| Functional expression of efflux systems and selective transport mechanisms (e.g., P-gp, MRPs, hexose, aminoacid, monocarboxylic acid, and other relevant transporters). |
| Expression of drug-metabolizing enzymes (P450s, MAO, etc.). |
| Exposure to laminal shear stress (apical membrane), glia (basal membrane), and other permissive factors that promotes growth inhibition and differentiation of endothelial cells. |
| Responsiveness to permeation modulators (e.g., hyperosmolar mannitol) as well as other stimuli (endogenous and exogenous) that can affect BBB integrity and function. |
| Ability to reproduce the effect of a wide range of physiological and pathophysiogical stimuli (hypertension, flow arrest, inflammation, etc.) that affect the BBB in vivo. |
| User friendly, scalable, and cost effective. |
Functional requirements for an ideal in vitro BBB model. The following table includes the in vitro functional and structural requirements essential to mimic the in vivo BBB.
MODULATORY ROLE OF SHEAR STRESS AND GLIA IN ENDOTHELIAL PHYSIOLOGY AT THE BBB
It is well accepted by the scientific community that at least two key factors must be available to the ECs to properly differentiate and express a barrier phenotype in vitro: (1) physiological shear stress (SS), which is a frictional force generated by the exposure of the apical membrane of the ECs to flow; (2) cell to cell interactions including the relative exposure to “permissive” or “promoting” factors released by the surrounding cellular environment (in particular, abluminal glia).3,33,34
The exposure to physiological SS plays a critical role in modulating ECs’ morphology. ECs grown in presence of flow are larger in volume than those grown under static conditions. They are also flattened and show an abundance of endocytic vesicles, microfilaments, and clathrin-coated pits,72 thus exhibiting a level of differentiation3,73,74 that more closely resembles that observed in BBB ECs in situ.75
Flow across the apical surface of the vascular endothelium activates a number of mechanosensors (e.g., integrins, caveolae, G proteins, and ion channels),4,76 which transduce physical stimuli into biochemical signals (see Fig. 2). Despite the variety of potential mechanosensors present on the luminal side of the EC membrane, one major common downstream effect is the activation of extracellular-signal-regulated kinases 1/2.4,77 These are pleiotropic modulators of the cell physiology and play an important role in the control of the expression of genes involved in the regulation of cell division, apoptosis, cell differentiation, and cell migration.3 They affect multiple endothelial functions including (1) the production of vasoactive substances78–80 and cell adhesion,72,73,81 (2) expression of tight junctions,3,82,83 (3) cell survival, (4) energy metabolism,3 and (5) the expression of asymmetrically localized enzymes and carrier-mediated transport systems that engender a truly “polarized” BBB endothelium phenotype.74,84 Turbulent, but not laminar, SS stimulates ECs’ turnover85 and accordingly, ECs’ DNA synthesis (cell division) in vivo occurs preferentially at branch orifices with low turbulent flow rates where atherosclerotic processes are generally initiated.
Figure 2. Effect of shear stress forces on BBB endothelial cells.
Shear stress (SS) is a major pleiotropic modulator of the endothelial cell physiology by controlling gene involved in the regulation of cell division, differentiation, migration, and apoptosis. For example, the exposure to physiological SS promoted mitotic arrest by contact and the endothelium assumed the typical monolayer appearance observed in vivo.
Astrocytes–endothelial interaction is also of critical importance for the induction and maintenance of BBB properties.34,37 Astrocytes with their closely associated astrocytic end-feet processes share the basal lamina enveloping more than 99% of the BBB endothelium. Intercellular adhesion between astrocytes in the BBB have been observed in the form of gap junctions and adherens junctions.86
Initial observation by Davson and Oldendorf87 as well as subsequent studies by others have clearly shown that astrocytes’ interaction with the cerebral endothelium help modulate BBB function.34,35,88–90 This includes upregulation of tight junctional proteins and tightness91,92; specific transport systems including the glucose transporter 1 (GLUT1), the large neutral amino acid carrier (L-system), and the A-system amino acid carriers.32 Furthermore, astrocytes were shown to downregulate the expression of tissue plasminogen activator (tPA) and thrombomodulin in brain ECs in vitro.93 More recently, a novel “BBB protective” role of astrocytes has been reported. This includes the nitric oxide (NO)-mediated release of interleukin-6 (IL-6). IL-6 has been shown to trigger production of matrix metalloproteinase (MMP) inhibitors such as α2-macroglobulin, which is released by perivascular astrocytes.94–96 Astrocytes also serve as scaffolds, guiding neurons to their proper place during development and directing vessels of the BBB. Other studies also support the hypothesis of a neural induction of the cerebral microvasculature and BBB formation.97
The first try at reproducing in vitro the physiological characteristics and response of the human brain microcapillaries was attempted using isolated brain microvessels dissociated from the surrounding brain tissue.
ISOLATED BRAIN MICROVESSELS
Since the initial method was established by Ferenc Joó 40 years ago 182; several other methods have been developed to obtain purified, functionally intact cerebral microvessels free of contamination by other brain tissue. Brain capillaries from the cerebral cortical gray matter are initially separated from the remaining brain tissue (which is usually discarded). Purification of the isolated brain microvessels is then obtained by combinations of mechanical homogenization, enzymatic dissociation, filtration, density gradient centrifugation, and column filtration.98–100 Purity of microvessel preparation can be determined by visual inspection using light microscopy, scanning electron microscopy, and transmission electron microscopy98 in combination with the concurrent expression of multiple brain microvascular endothelial markers such us Glut-1, transferrin receptor (OX-26), ZO-1, claudin-5, and claudin-12,101–103 etc.). The lack of typical smooth muscle cells (such as calponin and desmin104,105) can be used to determine the absence of distal postcapillary venule fragments (and/or other larger vessels) in the preparation. However, this does not guarantee that BBB function and integrity are preserved. Isolated brain microvessels have been successfully used for the identification of mechanisms and biochemical signals that play a role in regulating BBB functions106–109 in health and diseased conditions (e.g., brain tumors).110 The major advantages of using isolated brain microvessels include the maintenance of the structural and cellular characteristics and properties (cell differentiation) in ex vivo experimentations. Additional advantages include the availability and an extensive number of well documented methods to obtain purified brain capillaries. On the contrary, there are a number of disadvantages that need to be taken into consideration: (1) the isolation procedures are quite difficult and labor intensive and despite the advancement in the purification techniques, the potential presence of neuronal and/or other contaminants is not negligible; (2) limited viability of the vascular endothelium possibly caused by metabolic deficiencies during vessel isolation. The use of alternative brain vessels such as pial microvessels have been considered but at the expense of lacking a number of peculiar characteristics proper of the BBB microcapillaries. These include (1) highly selective permeability to blood-borne and xenobiotic substances; (2) vascular polarization (the presence of asymmetrically distributed transporters including efflux systems such as P-gp and MRPs); (3) metabolic transformation of endogenous and exogenous substances, which has been shown in BBB endothelial and glial cells.54,111–113
Because of the technical and functional limitations related to the use of isolated brain microvessels, different approaches based on computational models, artificial membranes, and in vitro cell culture BBB systems have been pursued.
IN SILICO MODELS: ADVANTAGES AND LIMITATIONS
Methods based on computer simulation (computer-assisted structure-based drug design) also known as “in silico” modeling are gaining more and more interest in the research field as the incredible development of computer technology and advance algorithms are becoming available. The major appeal of in silico research in medicine is its potential to accelerate drug discovery by predicting the efficacy and bioavailability of novel drugs in relation to BBB permeability. This is achieved with respect to passive diffusion and identified active transport mechanisms114 using computational models that rely on known physicochemical parameters (e.g., solubility, lipophilicity, molecular size, hydrogen-bonding capacity, and charge). Using this technology, compounds are synthesized, prescreened, and virtually tested to predict how they will penetrate across the BBB into the brain. This allows for rapid and inexpensive screening of novel lead drugs, which limit the need for labor-intensive laboratory experiments and expensive clinical trials. Recently, this technique was used to discover potential inhibitors to an enzyme associated with cancer activity,115 which was then confirmed in vitro and in vivo.116 Several pharmaceutical companies (e.g., Roche) now use this technique as a routine part of the drug discovery process. However, this technology has limitations, which are related to the acquisition and reliability of the data sets necessary to elaborate the correct relationship between the three-dimensional (3D) structure of a molecule and its biological activity (structure–activity relationship, SAR). This is a necessary step to then determine the quantitative SAR.117–119 Progress in understanding the role of the physicochemical properties relevant to important processes in membrane permeability (e.g., BBB) brings the rational design of novel effective drugs more within reach. In this contest, the use of computational in silico models can help in initial screening and effectively accelerate the drug development process but they still need to be supported by in vitro and in vivo studies to gather a number of crucial physicochemical measurements (e.g., those necessary to assess the contributions of passive and active transport).120,121 Furthermore, at this stage, with respect to BBB, in silico models cannot be reliably used to predict brain distribution of a compound. In silico models still hold the promise for future breakthrough in the pharmaceutical drug development (at least for the foreseeable future) but cell-based and more importantly in vivo experimentations still will remain the “must to have” tools to support clinical studies and advancements in drug discovery.
IMMOBILIZED ARTIFICIAL MEMBRANE CHROMATOGRAPHY
Immobilized artificial membrane (IAM) chromatography phases attempt to mimic the membrane lipid environment found in living cells. This technology is more commonly used for purification of many biological substances but found other more specific applications in the study of drug–membrane interactions and drug permeability across the BBB.122 Originally developed at Purdue University by Charles Pidgeon, the IAM stationary phase consists of a monolayer of carboxyl phospholipids that bear interfacial functional groups identical to the membrane phospholipids forming the cell membranes (e.g., phosphatidylcholine, phosphatidylglycerol, phosphatidic acid, phosphatidylethanolamine, and phosphatidylserine) covalently immobilized on an inert silica support. The resulting IAM surface is a chemically stable chromatographic material, which mimics the lipid outer layer of a biological cell membrane. Because solute partitioning between the IAM bonded and aqueous mobile phases is similar to that observed between a liposome and the aqueous phase, IAM chromatography can be used for the non-covalent immobilization as well as analytical and preparative separation of membrane-associated proteins. IAM chromatography has also gained acceptance as a method to estimate membrane permeability of small drugs and to measure their specific phospholipophilicity.123–125 However, there are several limitations that hinder the validity of this technique as a method to assess drug permeability: (1) the IAM surface has limited ability to reproduce lateral membrane diffusion, which occurs at the cellular level in vivo; (2) the IAM surface is a lipid monolayer, thus using this model to extrapolate solute diffusion across a physiological membrane bilayer is extremely difficult; (3) IAM surface cannot mimic active efflux or drug metabolism, which normally occurs at the BBB level.30,55,61,62 Currently, IAM chromatography is primarily used as a single-step purification method for membrane proteins (e.g., aldolase B).126
PARALLEL ARTIFICIAL MEMBRANE PERMEABILITY ASSAYS
Parallel artificial membrane permeability assays (PAMPAs) have become a quite useful and versatile in vitro tool for predicting drug permeability across specific physiological membranes in vivo. The system is based on the use of specially designed artificial lipid-impregnated membrane established on a solid filter support (e.g., polycarbonate) between a donor and an acceptor compartment. In this system, multiwell microtiter plate is placed at the bottom for the donor compartment, whereas the artificial lipid membrane/acceptor compartment is placed on top in a “sandwich” like configuration. Initially, the test drug is added in the donor compartment and allowed to diffuse across the membrane. The drug diffused in the acceptor compartment is then measured and quantified (generally by ultraviolet–visible spectroscopy or liquid chromatography–mass spectrometry). PAMPAs have been developed to allow for a high degree of versatility, automation compatibility, and assay reproducibility. The components of the simplified model can be varied to mimic, with high accuracy and reproducibility, the in vivo biological membrane of interest (e.g., gastrointestinal tract,127,128 skin,129–131 and BBB).132,133 Furthermore, the system can be modified to assess the effect of a wide range of pH on the permeability of the test drug of interest. The effect of physiological pH or pH gradient134 to which the specific organ/membrane is exposed in vivo (e.g., gastric pH range 1.0–2.5, intestine pH range 6.6–7.0, etc.) can thus be tested. The donor and/or acceptor compartments can be modified to emulate in vivo conditions by incorporating solubilizing agents or additives that bind the compounds as they diffuse across the lipid membrane. PAMPAs rely on passive diffusion for permeability across membrane. This is one of the main entry routes for drugs absorption across the majority of physiological membranes and thus, PAMPAs are considered suitable early stage absorption screening tool alternatives to more demanding and expensive cell-based assays. However, a major limitation of PAMPA systems versus cell-based and in vivo studies is its the inability to reproduce active transport mechanisms (influx and/or efflux) or metabolic transformations that a compound may incur during transcellular routing across some physiological membranes (e.g., BBB endothelium51,54,55,135 etc.). This may significantly affect predictability of bioavailability of the drug on the targeted site and thus use of PAMPAs alone would be insufficient. Their combination with in silico (described above)51,55,135,136 and the development of more complex algorithms that better reflect the behavior of highly dynamic physiological membranes (e.g., BBB) can further extend the use of this broadly adopted technology beyond an early stage screening tool.
IN VITRO CELL-BASED BBB MODELS
In vitro models of the BBB started to emerge in the early 1990s as potential new research tools complementary to in vivo and human studies in basic, translational, and clinical/pharmaceutical research bearing a number of desirable advantages: (1) cost effective—they are relatively inexpensive when compared with animal experimentation with a significantly higher throughput for drug permeability testing (a quality most desirable in the pharmaceutical industry); (2) simplified working environment—the artificial environment provided by these in vitro models allows studying and manipulating the BBB without the cumbersome number of additional variables to sort out when working with an entire organism; (3) versatility—they provide highly controllable and in some case quasi-physiological environments [cell-based and extracellular matrix (ECM) dynamic systems] outside of a living organism in which cells or tissue can be manipulated to study and dissect out the response of a specific tissue/organ to a broad range of physiological and experimental stimuli otherwise difficult to reproduce in vivo.
Noteworthy is the fact that cell-based BBB models can be established with any type of cell source (human, animal, or cell line derivative) including the availability of BBB ECs and astrocytes freshly isolated from human brain tissue (e.g., from patients undergoing temporal lobectomies for drug refractory epilepsy, brain tumor resections, aneurisms, etc.). This allows to some degree to reproduce in vitro pathological abnormalities (e.g., overexpression of drug efflux systems such as P-gp) that may facilitate the study of BBB dysfunctions in relation to the pathogenesis and progression of CNS disorders (e.g., drug-resistant epilepsy, brain tumor, etc.). However, the price for a simplified working environment and cost effectiveness is not something to take superficially. There are a number of serious drawbacks associated with the use of any in vitro systems including in vitro cell-based BBB models. Cells cultured ex situ in an artificially environment undergo dedifferentiation because of lack of exposure to physiological factors (whether diffusible from surrounding cells/tissue or dependent upon mechanical and physical stimuli such as SS and cell to cell contact). This can affect the expression (deregulation) of relevant cell biological features (e.g., transporters, ligands, enzymes, etc.), thus potentially altering the BBB physiology in vitro as well as its response to endogenous and exogenous stimuli. Therefore, validation of in vitro findings in in vivo conditions is necessary.
STATIC MODELS OF THE BBB: EC MONOCULTURES
The most common and widely utilized BBB model was developed based on a rather simplified view of the BBB, which was depicted as a monolayer of highly specialized brain microvascular ECs. This system, known as the Transwell apparatus (e.g., Corning, Lowell, MA), is a vertical side-by-side diffusion system across a microporous semipermeable membrane that separates the luminal (vascular) and the abluminal (parenchymal side) compartments. Brain vascular ECs (from various sources: bovine, rodent, porcine, non-human primate, and human) are grown to confluence on the upper (luminal) surface of the membrane immersed in their specific growth media. The advantages of cultured endothelium include the potential for using pure cell populations as well as their relative viability compared with isolated arterioles ex situ. The microporous membrane interface allows for nutrient exchange and the passage of cell-derived and exogenous substances but does not allow for cell movement across the two compartments.
Because of its intrinsic simplicity, this BBB model allows for high-throughput drug permeability testing and binding affinity (receptor–ligand) measurement.137 In particular, they are ideal systems to study Michaelis–Menten kinetics of transport because of fixed volumes in each compartment.137
However, this rather simplistic reconstruction of the BBB lacks a number of critical features that are necessary for the development of true BBB properties in vitro. The absence of natural physiological stimuli such as cell to cell interaction with perivascular astrocytes and other parenchymal cells (e.g., neurons), the exposure to intraluminal SS and circulating blood cells significantly limit the ability of the vascular endothelium to develop and/or maintain the intrinsic BBB properties and functions observed in vivo.138–140 Furthermore, this nonphysiological culture condition may accelerate endothelial dedifferentiation and further enhance the loss of the BBB characteristics with serial cell passage (e.g., cellular polarity determined by the functional specialization of the apical and basolateral membranes).57 The lack of antimitotic influences by laminin and flow shorten the life span of the endothelium and cause ECs to grow uncontrolled in a multilayer fashion141,142 when cells start dedifferentiating. ECs grown in Transwell tend to have irregular patterns of cell adhesion, which prompt the so-called “edge effect.” This term refers to a condition wherein the inability to form proper tight junctions between adjacent cells and between the endothelium alongside the perimeter of the membrane and the inner wall of the luminal chamber leads to artifactual paracellular diffusion, thus affecting the reliability of permeability measurements across the endothelial monolayer. This is of special concern for compounds that are highly hydrophilic and poorly cross the lipid membrane bilayer of the cells. In addition, EC monolayers are exposed to serum proteins on both sides (luminal and abluminal), whereas, in situ, this occurs only at luminal/vascular side and the abluminal side is either exposed to astrocytic/parenchymal released factors.
STATIC TWO-DIMENSIONAL MODELS OF THE BBB: COCULTURE OF ECS AND GLIA
The addition of abluminal astrocytes (in juxtaposition to the endothelial monolayer) have been shown to facilitate the formation of more stringent interendothelial tight junctions and the overall expression of proprietary BBB features,143 that more closely resemble that of the BBB in situ (see Fig. 3).
Figure 3. Schematic representation of a Transwell apparatus.
The Transwell (e.g., Corning) is a vertical side by side diffusion system across a semipermeable microporous membrane, which allows for free passage of nutrients and diffusible factors between the luminal and abluminal compartments. Depending on the pore size of the membrane, cell trafficking across the compartments (generally lumen to albumen) can be enabled. Note the cellular layout in monoculture (endothelial cells only) and coculture (luminal endothelial cells, which juxtapose perivascular/abluminal astrocytes) in vitro BBB configurations.
There is significant body of evidence, both in vitro and in vivo, to indicate that astrocyte interaction with the cerebral endothelium helps determine BBB function, morphology (i.e., tightness), and protein expression.89,90,144 Intercellular adhesion between astrocytes in the BBB has been observed in the form of gap and adherens junctions.86,102,103 The presence of glia and the establishment of glial–endothelial interactions have been shown to increase the expression of transporters (e.g., Glut-1 and MRPs such as P-gp) and tight junctions, and have also helped to induce a phenotype more closely mimicking that found in vivo. A limited cell polarity is inducible in ECs when cocultured with glia or in the presence of glial-conditioned medium.40,145 The exposure to glia promotes the expression of brain endothelial marker enzymes (e.g., OX-26, alkaline phosphatase, acetyle-holinesterase, Na+–K+-ATPase) as well as that of a number of specialized transport and efflux systems (e.g., facilitative Glut-1, γ-glutamyltransferase, and Pgp)32,34,57,59,146,147 typically observed in BBB ECs in vivo. Transendothelial electrical resistance (TEER), which measures the ionic conductance across the BBB, is typically higher in EC–glia coculture systems than EC monocultures. The difference in the measurement indicates formation of a more stringent and selective vascular bed than monocultures.
However, despite the clear advancement in BBB modeling provided by the use of abluminal endothelial–glial cocultures, static systems lack the ability to allow for endothelial exposure to physiological SS. Given the unique vascular modulatory role of shear forces as well as that of abluminal astrocytes (and perhaps pericytes),88 it is not surprising that attempts have been made to develop more sophisticated dynamic (flow-based) coculture BBB models.
DYNAMIC IN VITRO BBB MODELS
Cone–Plate Apparatus
The use of a purpose-built cone and plate viscometer was the first attempt to enable the endothelial exposure to flow in vitro.148 In this system, the ECs monolayer seeded on the bottom of the plate is exposed to SS generated by a rotating cone. The shear force is transmitted to the vascular endothelium through the culture media spinning in the direction of the rotating cone (see Fig. 4). The angular velocity and the cone angle determine the level of SS generated, which is not entirely uniform along the radius of the plate supporting the ECs monolayer. Reversing and pulsing the angular velocity of the cone can be used to achieve back-flow and pulsatile SS, respectively. The cone–plate apparatus described by Bussolari and coworkers148,149 was designed to study the effects of laminar or turbulent fluid SS on cells and the relative contribution of fluid viscosity, time of exposure, and other physiological variables. Laminar SS (as detailed above) plays a major role in BBB endothelial physiology such as promoting BBB tightness, the expression of drug resistance mechanisms, ion channels, and other specialized transport systems. SS can modulate even the bioenergetic behavior on the BBB endothelium.3 Previous studies have also shown that SS induces the production of vasoactive substances.150 However, turbulent SS has quite a different effect of BBB endothelial physiology including increased cell proliferation, loss of BBB tightness, and increased transendothelial permeability. Therefore, the cone–plate apparatus represents the first attempt to introduce laminar or turbulent flow in vitro to study its effect on BBB physiology
Figure 4. Schematic representation of a cone and plate viscometer.

The endothelial monolayer can be exposed to a quasi/uniform laminar or pulsatile shear stress (SS) by the use of a purpose-built cone and plate viscometer. Note that μ is the viscosity, ν is kinematic viscosity, ω is angular velocity, and &agr; is the cone angle. The cone angle and the angular velocity determine the level of SS to which the endothelium is exposed.
This system per se, however, was not designed to reproduce precisely the flow and milieu experienced by vascular endothelium in vivo. Furthermore, lacking the ability to expose ECs to perivascular modulatory factors (from glia and other cells) does not allow full differentiation of ECs into a BBB phenotype, thus further diminishing the significance and reliability of the results.
However, the importance of flow as a major modulator of BBB physiological properties and the need for BBB models better capable of mimicking the physiological function and response of the brain microcapillaries led researchers to develop new generations of dynamic in vitro systems. To date, two designs based on different technologies are available: (1) the so-called “dynamic in vitro BBB model” (DIV-BBB), which makes use of artificial capillary-like structural supports (hollow fibers) for coculturing ECs and astrocytes and also allows for the presence of intraluminal flow; (2) collagen 3D matrix supports (3D ECM-based BBB models).
3D Dynamic In Vitro Models of the BBB
The use of artificial capillary-like structures such as hollow fibers (generally made of thermoplastic polymers such as polypropylene, polysulfone, etc.) has been initially exploited for the construction of bioreactors for mass production of antibodies (or other biologically active substances) or for the expansion of hybridoma cells. More recently, the same technology has been adapted to model hollow organ-like structures such as the BBB.151 This newly developed in vitro system, which is referred to as the DIV-BBB model (see Fig. 5), allows brain microvascular ECs (animal or human, both primary cultures and cell lines) to be cocultured with abluminal astrocytes and under the exposure of quasi-physiological pulsatile laminar SS. This allows for the formation of a BBB that closely resembles the in situ both functionally and anatomically.75,141,152
Figure 5. Dynamic in vitro BBB model (DIV-BBB).

The top panel shows a schematic representation of a DIV-BBB module. In this system, cerebral endothelial cells are cultured in the lumen of fibronectin-coated polypropylene microporous hollow fibers, in the presence of astrocytes cultured on poly-D-lysine-coated outer surface of the same. The bundle of hollow fibers is suspended inside a sealed chamber. The artificial capillaries are in continuity with a medium source through a flow path consisting of gas-permeable silicon tubing. Ports positioned on either side of the module allow access to the luminal and abluminal compartments. The capillaries are exposed to luminal pulsatile flow generated by a pumping mechanism. Note the pressure waveforms changes (pre-versus postcapillaries) mimicking the rheological changes observed in vivo.
The functional core of the DIV-BBB apparatus is represented by a bundle of microporous pronectin-coated polypropylene hollow fibers, which enable co-culturing of brain and non-brain vascular ECs from various origins [such as human umbilical vein endothelial cells (HUVECs)] with glial cells. These glial cells are seeded in juxtaposition to the ECs on the outer surface (perivascular/parenchymal side of the brain microcapillaries) of the same hollow fibers. The artificial capillaries are connected to a media reservoir through a gas-permeable (e.g., silicon) tubing system for the exchange of O2 and CO2. A servo-controlled variable-speed pulsatile pump generates the intraluminal flow, which can be regulated to produce (based on the diameter of the hollow fibers and the viscosity of the medium) SS levels and intraluminal pressure physiologically comparable to that observed in capillaries in vivo (generally between 5 and 23 dynes/cm2).149,153 The same computer controlled pumping mechanism can be used to reproduce the rheological features of other vascular districts (e.g. venules and large arteries), thus further expanding the usability of this system in vascular research. The DIV-BBB allows reproduction of multiple functional properties and physiological responses observed at the BBB in situ. These include low permeability to intraluminal potassium and polar molecules (e.g., sucrose and other paracellular markers), high TEER,154 negligible extravasation of proteins, the expression of specialized transporters, ion channels,141,155 and efflux systems (e.g., Glut-1 and Pgp,).155,156 In addition, this system allows for concomitant circulation of blood cells,157 which enables to study a number of cerebrovascular/systemic pathologies related to rheological impairments and/or inflammation158 including hypoperfusion, cerebral ischemia,68 and brain edema.71 On par with previously described BBB models, the DIV-BBB can be established using primary (commercially available or freshly isolated from brain and non-brain tissues)75,156 or immortalized cell lines.154 Furthermore, the structure and design of a variant of the original DIV-BBB model allows for the inclusion of other relevant cell types,141 which might even include pericytes and neurons.
Recently revised DIV-BBB systems enable cell trafficking across the BBB, which further extend the usability of this model to study and characterize the role of the BBB in the pathogenesis of major neuroinflammatory diseases (e.g., meningitis,159 multiple sclerosis,160 Alzheimer, etc.). The DIV-BBB provides a number of appealing advantages over conventional static systems. However, considering recent trends in the pharmaceutical field and clinical interests in the study of the CNS, there are a number of limitations that need to be addressed to expand the adoption of this BBB model. More specifically, its design does not allow for visualization of the intraluminal compartment to assess morphological and/or phenotypic changes of the vascular endothelium. Also, in contrast to conventional static BBB models (e.g., Transwell), this apparatus is not designed for a high-throughput pharmaceutical study, and the technical skills/time required to establish the system is significantly higher. Cell characterization is possible but limited because it requires harvesting the cells from either compartment. This is an invasive procedure that can alter (to various degrees) a number of morphological and physiological characteristics of the cells. Additionally, the initial cell load requirement to establish the DIV-BBB model is quite significant (>1 × 106 cells), especially if primary cultures are to be used. Limited availability of manufacturers could be considered relatively inconvenient. However, increased awareness about the DIV-BBB and larger adoption of this model can help solve this issue in the near future.
3D ECM-Based BBB Models
Despite the low awareness of 3D tissue culturing systems in several research fields (including neurobiology and cerebrovascular research), the potential benefits of culturing cells using 3D artificial supports (even though more expensive and perhaps less convenient than current static two-dimensional models) is self-evident when one considers that the in situ microenvironment plays a critical role in modulating/regulating the functional properties of that specific organ (e.g., the BBB).161,162 3D in vitro tissue models are already available for drug discovery and transport studies related to a variety of organs (e.g., liver) and tissues (e.g., cardiac, muscle, bone, etc.) as well as malignancy in solid tumors.163 Similar advancements in tissue culture technology are now making the appearance in cerebrovascular and BBB research.
In vitro BBB models grown on self-polymerizing ECM protein scaffolds provide an alternative to the use of artificial capillary-like structures supports (e.g., DIV-BBB).
Three-dimensional ECM-based BBB culture systems enable close interactions between cells (and the exposure to the corresponding trophic and differentiating released factors) and the formation of quasi-physiological biochemical gradient exposure is necessary for cell-to-cell communication and cross-signaling (see Fig. 6). With recent advancement in the development of microfluidic systems, it is now possible to monitor cell migration in a 3D environment164,165 with a direct application for cancer metastasis and angiogenesis studies. These models can thus study the effect of physiological and pathological stimuli in 3D BBB microenvironments. Furthermore, several high-resolution 3D imaging techniques based on the use of confocal microscopy, multiphoton microscopy, and optical coherence tomography available today, which can allow repeated assessment of dynamic changes to cells cultured in the 3D ECM microenvironments.
Figure 6. Schematic representation of a 3D ECM-based in vitro BBB model.
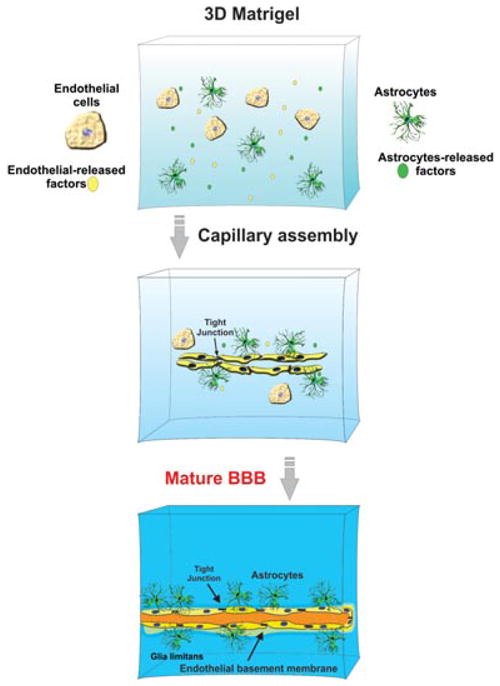
Note the assembly of a microcapillary-like structure.
However, the development of an in vivo-like matrix architecture is a complex issue to address. Although being convenient to study specific roles of various ECM proteins (e.g., laminin, collagen, fibronectin, etc.) in cell differentiation, self-polymerizing ECM models (cross-linked ECM) can only provide a uniform matrix lacking the discontinuities and gaps that may normally be present in vivo and allow for cell migration. This problem was partially addressed by using cell-derived matrices, which maintain some of the original gaps that accommodate the cells.164,166 The omission of minor ECM constituents potentially significant in the architectural assembly and matrix property is another concern until more sophisticated 3D matrix models become available in the future.
CELL LINES OR PRIMARY CULTURES?
Several cell lines (both animal and human derived) have been developed as biological surrogates for BBB modeling. These cell lines bear many advantages over primary cultures, which include being easy to grow and retain their differentiating properties even after repetitive passages, reducing cost and labor. Only a few immortalized human endothelial cell lines (HMEC-1,167 HCMEC/D3,154,168 and TY08169) have been developed and reportedly used for the establishment of in vitro BBB models. Animal-derived immortalized cell lines are more commonly used. Immortalized rat brain endothelial 4 cells are probably the most extensively animal-derived cell line used for BBB modeling,170–173 although their use is only limited to static coculture conditions. These cells functionally express a variety of BBB transporters, drug-metabolizing enzyme activities, and mechanisms of active extrusion including Pgp and multidrug resistance-associated protein (Mrp1).111,147,174–178 The use of intestine-derived cell line Caco-2 in the establishment of an in vitro BBB has also been taken into consideration.179 Differences in drug permeability between the intestine and brain vascular-derived cells are too significant and limit the use of Caco-2 cells for BBB modeling.180,181 However, genetic manipulation by the use of retroviruses and the introduction of immortalizing genes can affect a wide variety of cellular functions, potentially altering the physiological response to endogenous and exogenous stimuli, to which BBB ECs in vivo are normally subjected.
Highly purified populations of cultured human brain (human brain microvascular endothelial cells, HBMECs) and non-brain (e.g., HUVECs) vascular cells exhibiting excellent characteristics for the study of the developmental and pathophysiological processes of the BBB became available in the early 1980s. Unfortunately, the high level of technical skill necessary to isolate these cells from the native tissue (especially brain tissue) as well as their rapid senescence, evident just after few cycles of cell division, made their use quite costly and time consuming. Today, they are more easily accessible through a number of companies that have made them commercially available, although still for a premium price. Among primary BBB cells, those of human origin provide the most accurate representation for studying neurological diseases involving BBB dysfunction (e.g., drug-resistant epilepsy).55,156 Human specimens offer a variety of neurological etiologies that otherwise would be near impossible to recapitulate in cell lines or animal-brain-derived (rodent, porcine, or bovine) primary cultures. Even though HBMECs are not yet a realistic (cost-effective) alternative to the use of cell lines for industrial (pharmaceutical) screening (such as testing permeability, toxicity, etc.) and testing of novel drugs, they have unmatched value for basic and translational research.
PERSPECTIVE DEVELOPMENT IN BBB MODELING: CONCLUSIONS
The need to limit the increasing costs of experimental studies, prescreening, and validating novel CNS therapeutics is continuously pushing researchers to develop BBB in vitro models capable of high-throughput testing to accelerate drug design. On the contrary, basic and translational researchers are instead pushing toward more realistic BBB models wherein multiple determining factors (e.g., cell–cell interactions, cell migration, flow, microenvironment influences, etc.) can be reproduced, dissected out, and characterized. Requirements such as “higher predictability,” “fully scalable,” “customizable,” and so on are becoming a frequent conundrum in pharmaceutical and biotech industries that researchers/developers will have to tackle. New artificial BBB systems will be required to incorporate a number of automatisms (e.g., control/adjust oxygen and CO2 levels in the culture medium, real-time monitoring of BBB integrity, medium sampling, etc.) including an array of computer-controlled sensors to monitor a variety of physiological parameters (e.g. NO, glucose, lactate, etc.). This automatism will greatly minimize time-consuming routine maintenance and monitoring and facilitate operator efforts. Additional advancements will come with the introduction of new cell culture support materials. For example, incorporating the desired anchoring molecules (for cell adhesion or to stimulate specific cellular responses) in their matrix construction would allow for a higher degree of control over cell differentiation, cell interaction, and cell response. However, despite all the advancements in this field, it is important to understand that in vitro BBB models are not a replacement for in vivo and human studies but rather a complementary set of tools to facilitate drug discovery and to aid in the acquisition of additional knowledge (see Table 2).
Table 2.
Side by Side Comparison Between In Vitro, In Vivo, and Clinical Studies to Elucidate Differences, Overlaps, and Comlementaries Between Them
| Measurable Endpoints | In Vivo | Clinical | In Vivo |
|---|---|---|---|
| Animal | Human | Static and Dynamic | |
| Brain imaging | Yes | Yes | No |
| Histology | Yes | Yes, but limited to tissue reactions from patients or post-mortem donors | No |
| Molecular biomarkers | Yes | Yes, but limited (e.g., serum urine) | Limited |
| Molecular mechanisms | Yes | No | Yes |
| Intravital microscopy | Yes | No | No |
| Monitoring clinical outcome (e.g., progression/regression) | Yes but correlation with patients can be difficult | Yes | No |
| Longitudinal BBB response | No | No | Yes |
| Cell isolation | Yes, but labour intensive | Mostly Limited to tissue reactions and blood cells | Yes |
| Cell manipulation | Limited | No | Yes |
| Control over physiological and experimental variables to dissect out specific effects | Limited | No | Yes |
| Drug permeability | Yes, but labour intensive | No | Yes |
| Drug distribution | Yes | No (limited to constrast enhancement for CT Scan and MRI) | No |
| Drug efficacy and safety | Yes | Limited to retrospective data analysis | No |
| Time course (acute BBB experimentation) | Limited and labour intensive | No | Yes |
| Clinical significance | Vary | Yes (with large data seta and mostly retrospective | Limited (require validation |
| Best suited for | Translational and Functional | Functional and Clinical studies | Basic and Translational |
Side by side comparison between in vitro, in vivo and clinical studies. The table reports relevant examples to elucidate differences, overlaps, and complementarities between them.
Predictability of drug permeability across the BBB based on physicochemical parameters is not accurate because it lacks the contribution of active transport systems and metabolic transformation on drug bioavailability. Advanced cell-based models can to some extent reproduce the physiological behavior of the BBB in situ, thus providing more accurate results. However, validation in vivo is still required. Furthermore, in vitro cell-based models cannot provide information on drug distribution into the brain once the drug passes the BBB. It is possible that future and more advanced in silico models can partially address this issue. It is also important to understand that the goodness/reliability of a BBB model is not absolute but strongly depends on its specific use. This is a major determinant for the accuracy of the results as well as their translational/clinical significance.
Whether in vitro, in vivo, or clinical, each set of research tools and related technologies bring something unique on the table. The challenge for future breakthrough in CNS drug discovery is bringing together all these technologies as a team to facilitate the understanding of the pathogenesis of a disease, provide useful insights to modulate counteractive physiological responses, and accelerate the development of novel clinical strategies and pharmacological treatments to reduce the burden of many CNS diseases.
Acknowledgments
This work was supported by the National Institute of Health/National Institute on Drug Abuse grant number R01-DA029121-01A1 and Alternative Research Development Foundation grant to Dr. Luca Cucullo.
Footnotes
Disclosure/conflict of interest: Dr. Luca Cucullo owns stocks in Flocel Inc.
References
- 1.Nag S, Kapadia A, Stewart DJ. Review: Molecular pathogenesis of blood–brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol. 2011;37:3–23. doi: 10.1111/j.1365-2990.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 2.Shlosberg D, Benifla M, Kaufer D, Friedman A, Medscape Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in blood–brain barrier endothelial physiology. BMC Neurosci. 2011;12:40. doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traub O, Berk BC. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 5.Holman DW, Klein RS, Ransohoff RM. The blood–brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812:220–230. doi: 10.1016/j.bbadis.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: Leucocyte–endothelial cell crosstalk at the blood–brain barrier: A prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 7.Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood–brain barrier model. J Biol Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrall AJ, Wardlaw JM. Blood–brain barrier: Ageing and microvascular disease—Systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood–brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 10.Poduslo JF, Curran GL, Wengenack TM, Malester B, Duff K. Permeability of proteins at the blood–brain barrier in the normal adult mouse and double transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2001;8:555–567. doi: 10.1006/nbdi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 11.Friedman A. Blood–brain barrier dysfunction, status epilepticus, seizures, and epilepsy: A puzzle of a chicken and egg? Epilepsia. 2011;52(Suppl 8):19–20. doi: 10.1111/j.1528-1167.2011.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DW, Moon Y, Gee NH, Choi JW, Oh J. Blood–brain barrier disruption is involved in seizure and hemianopsia in nonketotic hyperglycemia. Neurologist. 2011;17:164–166. doi: 10.1097/NRL.0b013e3182173528. [DOI] [PubMed] [Google Scholar]
- 13.Friedman A, Kaufer D, Heinemann U. Blood–brain barrier breakdown-inducing astrocytic transformation: Novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomkins O, Shelef I, Kaizerman I, Eliushin A, Afawi Z, Misk A, Gidon M, Cohen A, Zumsteg D, Friedman A. Blood–brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psychiatry. 2008;79:774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood–brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–614. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett J, Basivireddy J, Kollar A, Biron KE, Reickmann P, Jefferies WA, McQuaid S. Blood–brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J Neuroimmunol. 2010;229:180–191. doi: 10.1016/j.jneuroim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 17.McQuaid S, Cunnea P, McMahon J, Fitzgerald U. The effects of blood–brain barrier disruption on glial cell function in multiple sclerosis. Biochem Soc Trans. 2009;37:329–331. doi: 10.1042/BST0370329. [DOI] [PubMed] [Google Scholar]
- 18.Banks WA. Blood–brain barrier as a regulatory interface. Forum Nutr. 2010;63:102–110. doi: 10.1159/000264398. [DOI] [PubMed] [Google Scholar]
- 19.Oldendorf WH. Measurement of brain uptake of radio-labeled substances using a tritiated water internal standard. Brain Res. 1970;24:372–376. doi: 10.1016/0006-8993(70)90123-x. [DOI] [PubMed] [Google Scholar]
- 20.Oldendorf WH. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971;221:1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- 21.Oldendorf WH. Blood–brain barrier permeability to lactate. Eur Neurol. 1971;6:49–55. doi: 10.1159/000114465. [DOI] [PubMed] [Google Scholar]
- 22.Oldendorf WH, Hyman S, Braun L, Oldendorf SZ. Blood–brain barrier: Penetration of morphine, codeine, heroin, and methadone after carotid injection. Science. 1972;178:984–986. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- 23.Smith QR, Takasato Y, Sweeney DJ, Rapoport SI. Regional cerebrovascular transport of leucine as measured by the in situ brain perfusion technique. J Cereb Blood Flow Metab. 1985;5:300–311. doi: 10.1038/jcbfm.1985.39. [DOI] [PubMed] [Google Scholar]
- 24.Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol. 1984;247:H484–H493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- 25.Bradbury MW. The blood–brain barrier. Exp Physiol. 1993;78:453–472. doi: 10.1113/expphysiol.1993.sp003698. [DOI] [PubMed] [Google Scholar]
- 26.Dermietzel R, Krause D. Molecular anatomy of the blood–brain barrier as defined by immunocytochemistry. Int Rev Cytol. 1991;127:57–109. doi: 10.1016/s0074-7696(08)60692-0. [DOI] [PubMed] [Google Scholar]
- 27.Rubin LL, Staddon JM. The cell biology of the blood–brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 28.Engelhardt B. Development of the blood–brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- 29.Grant GA, Janigro D. The blood–brain barrier. In: Winn HR, editor. Youmans neurological surgery. Philadelphia, Pennsylvania: Saunders; 2004. pp. 153–174. [Google Scholar]
- 30.Ghosh C, Gonzalez-Martinez J, Hossain M, Cucullo L, Fazio V, Janigro D, Marchi N. Pattern of P450 expression at the human blood–brain barrier: Roles of epileptic condition and laminar flow. Epilepsia. 2010;51:1408–1417. doi: 10.1111/j.1528-1167.2009.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott NJ, Khan EU, Rollinson CM, Reichel A, Janigro D, Dombrowski SM, Dobbie MS, Begley DJ. Drug resistance in epilepsy: The role of the blood–brain barrier. Novartis Found Symp. 2002;243:38–47. [PubMed] [Google Scholar]
- 32.Abbott NJ. Astrocyte–endothelial interactions and blood–brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott NJ. Dynamics of CNS barriers: Evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbott NJ, Ronnback L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 35.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Emmi A, Wenzel HJ, Schwartzkroin PA, Taglialatela M, Castaldo P, Bianchi L, Nerbonne J, Robertson GA, Janigro D. Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes. J Neurosci. 2000;20:3915–3925. doi: 10.1523/JNEUROSCI.20-10-03915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 38.Hamm S, Dehouck B, Kraus J, Wolburg-Buchholz K, Wolburg H, Risau W, Cecchelli R, Engelhardt B, Dehouck MP. Astrocyte mediated modulation of blood–brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004;315:157–166. doi: 10.1007/s00441-003-0825-y. [DOI] [PubMed] [Google Scholar]
- 39.Kramer SD, Schutz YB, Wunderli-Allenspach H, Abbott NJ, Begley DJ. Lipids in blood–brain barrier models in vitro II: Influence of glial cells on lipid classes and lipid fatty acids. In Vitro Cell Dev Biol Anim. 2002;38:566–571. doi: 10.1290/1543-706X(2002)38<566:LIBBMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90:526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- 41.Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier: Development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 42.Schinkel AH. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv Drug Deliv Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 43.O’Kane RL, Hawkins RA. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood–brain barrier. Am J Physiol Endocrinol Metab. 2003;285:E1167–E1173. doi: 10.1152/ajpendo.00193.2003. [DOI] [PubMed] [Google Scholar]
- 44.Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. Subcellular localization of transporters along the rat blood–brain barrier and blood–cerebral–spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Tournier N, Chevillard L, Megarbane B, Pirnay S, Scherrmann JM, Decleves X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) Int J Neuropsychopharmacol. 2010;13:905–915. doi: 10.1017/S1461145709990848. [DOI] [PubMed] [Google Scholar]
- 46.Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: A perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagenbuch B, Gao B, Meier PJ. Transport of xenobiotics across the blood–brain barrier. News Physiol Sci. 2002;17:231–234. doi: 10.1152/nips.01402.2002. [DOI] [PubMed] [Google Scholar]
- 48.Dombrowski SM, Desai SY, Marroni M, Cucullo L, Goodrich K, Bingaman W, Mayberg MR, Bengez L, Janigro D. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia. 2001;42:1501–1506. doi: 10.1046/j.1528-1157.2001.12301.x. [DOI] [PubMed] [Google Scholar]
- 49.Marroni M, Marchi N, Cucullo L, Abbott NJ, Signorelli K, Janigro D. Vascular and parenchymal mechanisms in multiple drug resistance: a lesson from human epilepsy. Curr Drug Targets. 2003;4:297–304. doi: 10.2174/1389450033491109. [DOI] [PubMed] [Google Scholar]
- 50.Oby E, Janigro D. The blood–brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 51.Ueno M, Nakagawa T, Wu B, Onodera M, Huang CL, Kusaka T, Araki N, Sakamoto H. Transporters in the brain endothelial barrier. Curr Med Chem. 2010;17:1125–1138. doi: 10.2174/092986710790827816. [DOI] [PubMed] [Google Scholar]
- 52.Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 53.Cox DS, Scott KR, Gao H, Raje S, Eddington ND. Influence of multidrug resistance (MDR) proteins at the blood–brain barrier on the transport and brain distribution of enaminone anticonvulsants. J Pharm Sci. 2001;90:1540–1552. doi: 10.1002/jps.1104. [DOI] [PubMed] [Google Scholar]
- 54.Ghosh C, Marchi N, Desai NK, Puvenna V, Hossain M, Gonzalez-Martinez J, Alexopoulos AV, Janigro D. Cellular localization and functional significance of CYP3A4 in the human epileptic brain. Epilepsia. 2011;52:562–571. doi: 10.1111/j.1528-1167.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh C, Puvenna V, Gonzalez-Martinez J, Janigro D, Marchi N. Blood–brain barrier P450 enzymes and multidrug transporters in drug resistance: A synergistic role in neurological diseases. Curr Drug Metab. 2011;12:742–749. doi: 10.2174/138920011798357051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutheil F, Jacob A, Dauchy S, Beaune P, Scherrmann JM, Decleves X, Loriot MA. ABC transporters and cytochromes P450 in the human central nervous system: Influence on brain pharmacokinetics and contribution to neurodegenerative disorders. Expert Opin Drug Metab Toxicol. 2010;6:1161–1174. doi: 10.1517/17425255.2010.510832. [DOI] [PubMed] [Google Scholar]
- 57.Betz AL, Goldstein GW. Polarity of the blood–brain barrier: Neutral amino acid transport into isolated brain capillaries. Science. 1978;202:225–227. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- 58.Betz AL, Goldstein GW. The basis for active transport at the blood–brain barrier. Adv Exp Med Biol. 1980;131:5–16. doi: 10.1007/978-1-4684-3752-2_1. [DOI] [PubMed] [Google Scholar]
- 59.Betz AL, Firth JA, Goldstein GW. Polarity of the blood–brain barrier: Distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 1980;192:17–28. doi: 10.1016/0006-8993(80)91004-5. [DOI] [PubMed] [Google Scholar]
- 60.Betz AL, Goldstein GW. Specialized properties and solute transport in brain capillaries. Annu Rev Physiol. 1986;48:241–250. doi: 10.1146/annurev.ph.48.030186.001325. [DOI] [PubMed] [Google Scholar]
- 61.Shen S, Zhang W. ABC transporters and drug efflux at the blood–brain barrier. Rev Neurosci. 2010;21:29–53. doi: 10.1515/revneuro.2010.21.1.29. [DOI] [PubMed] [Google Scholar]
- 62.Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood–brain barrier. Trends Pharmacol Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kusuhara H, Sugiyama Y. Active efflux across the blood–brain barrier: Role of the solute carrier family. Neurorx. 2005;2:73–85. doi: 10.1602/neurorx.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, De WI, Decleves X. ABC transporters, cytochromes P450 and their main transcription factors: Expression at the human blood–brain barrier. J Neurochem. 2008;107:1518–1528. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- 65.Abbott NJ, Romero IA. Transporting therapeutics across the blood–brain barrier. Mol Med Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- 66.Kalaria RN. The blood–brain barrier and cerebral microcirculation in Alzheimer disease. Cerebrovasc Brain Metab Rev. 1992;4:226–260. [PubMed] [Google Scholar]
- 67.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood–brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143–155. [PubMed] [Google Scholar]
- 68.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 69.Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, Janigro D. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pachter JS, de Vries HE, Fabry Z. The blood–brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62:593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- 71.Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Inflammation and brain edema: New insights into the role of chemokines and their receptors. Acta Neurochir Suppl. 2006;96:444–450. doi: 10.1007/3-211-30714-1_91. [DOI] [PubMed] [Google Scholar]
- 72.Ballermann BJ, Ott MJ. Adhesion and differentiation of endothelial cells by exposure to chronic shear stress: A vascular graft model. Blood Purif. 1995;13:125–134. doi: 10.1159/000170195. [DOI] [PubMed] [Google Scholar]
- 73.Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 74.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87:320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santaguida S, Janigro D, Hossain M, Oby E, Rapp E, Cucullo L. Side by side comparison between dynamic versus static models of blood–brain barrier in vitro: A permeability study. Brain Res. 2006;1109:1–13. doi: 10.1016/j.brainres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 76.Ando J, Yamamoto K. Vascular mechanobiology: Endothelial cell responses to fluid shear stress. Circ J. 2009;73:1983–1992. doi: 10.1253/circj.cj-09-0583. [DOI] [PubMed] [Google Scholar]
- 77.Chretien ML, Zhang M, Jackson MR, Kapus A, Langille BL. Mechanotransduction by endothelial cells is locally generated, direction-dependent, and ligand-specific. J Cell Physiol. 2010;224:352–361. doi: 10.1002/jcp.22125. [DOI] [PubMed] [Google Scholar]
- 78.Grabowski EF, Jaffe EA, Weksler BB. Prostacyclin production by cultured endothelial cell monolayers exposed to step increases in shear stress. J Lab Clin Med. 1985;105:36–43. [PubMed] [Google Scholar]
- 79.Moore JP, Weber M, Searles CD. Laminar shear stress modulates phosphorylation and localization of RNA polymerase II on the endothelial nitric oxide synthase gene. Arterioscler Thromb Vasc Biol. 2010;30:561–567. doi: 10.1161/ATVBAHA.109.199554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17:187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 81.Ott MJ, Ballermann BJ. Shear stress-conditioned, endothelial cell-seeded vascular grafts: Improved cell adherence in response to in vitro shear stress. Surgery. 1995;117:334–339. doi: 10.1016/s0039-6060(05)80210-7. [DOI] [PubMed] [Google Scholar]
- 82.Walsh TG, Murphy RP, Fitzpatrick P, Rochfort KD, Guinan AF, Murphy A, Cummins PM. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J Cell Physiol. 2011 doi: 10.1002/jcp.22655. [DOI] [PubMed] [Google Scholar]
- 83.Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol. 2007;292:H3190–H3197. doi: 10.1152/ajpheart.01177.2006. [DOI] [PubMed] [Google Scholar]
- 84.Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 85.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone MA., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davson H, Oldendorf WH. Symposium on membrane transport. Transport in the central nervous system. Proc R Soc Med. 1967;60:326–329. doi: 10.1177/003591576706000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood–brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2011;31:693–705. doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: An efficient in vitro model. Brain Res. 1987;433:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 90.Beck DW, Vinters HV, Hart MN, Cancilla PA. Glial cells influence polarity of the blood–brain barrier. J Neuropathol Exp Neurol. 1984;43:219–224. doi: 10.1097/00005072-198405000-00001. [DOI] [PubMed] [Google Scholar]
- 91.Rist RJ, Romero IA, Chan MW, Couraud PO, Roux F, Abbott NJ. F-actin cytoskeleton and sucrose permeability of immortalised rat brain microvascular endothelial cell monolayers: Effects of cyclic AMP and astrocytic factors. Brain Res. 1997;768:10–18. doi: 10.1016/s0006-8993(97)00586-6. [DOI] [PubMed] [Google Scholar]
- 92.Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, Kato T. Induction of blood–brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 93.Tran ND, Correale J, Schreiber SS, Fisher M. Transforming growth factor-beta mediates astrocyte-specific regulation of brain endothelial anticoagulant factors. Stroke. 1999;30:1671–1678. doi: 10.1161/01.str.30.8.1671. [DOI] [PubMed] [Google Scholar]
- 94.Aschner M. Immune and inflammatory responses in the CNS: Modulation by astrocytes. Toxicol Lett. 1998;102–103:283–287. doi: 10.1016/s0378-4274(98)00324-5. [DOI] [PubMed] [Google Scholar]
- 95.Aschner M. Astrocytes as mediators of immune and inflammatory responses in the CNS. Neurotoxicology. 1998;19:269–281. [PubMed] [Google Scholar]
- 96.Cucullo L, Marchi N, Marroni M, Fazio V, Namura S, Janigro D. Blood–brain barrier damage induces release of {alpha}2-macroglobulin. Mol Cell Proteomics. 2003;2:234–241. doi: 10.1074/mcp.M200077-MCP200. [DOI] [PubMed] [Google Scholar]
- 97.Bauer HC, Bauer H. Neural induction of the blood–brain barrier: Still an enigma. Cell Mol Neurobiol. 2000;20:13–28. doi: 10.1023/A:1006939825857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silbergeld DL, Ali-Osman F. Isolation and characterization of microvessels from normal brain and brain tumors. J Neurooncol. 1991;11:49–55. doi: 10.1007/BF00166997. [DOI] [PubMed] [Google Scholar]
- 99.Catalan RE, Martinez AM, Aragones MD, Fernandez I. Substance P stimulates translocation of protein kinase C in brain microvessels. Biochem Biophys Res Commun. 1989;164:595–600. doi: 10.1016/0006-291x(89)91501-5. [DOI] [PubMed] [Google Scholar]
- 100.Markovac J, Goldstein GW. Transforming growth factor beta activates protein kinase C in microvessels isolated from immature rat brain. Biochem Biophys Res Commun. 1988;150:575–582. doi: 10.1016/0006-291x(88)90432-9. [DOI] [PubMed] [Google Scholar]
- 101.Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;210:81–86. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
- 102.Orte C, Lawrenson JG, Finn TM, Reid AR, Allt G. A comparison of blood–brain barrier and blood–nerve barrier endothelial cell markers. Anat Embryol (Berl) 1999;199:509–517. doi: 10.1007/s004290050248. [DOI] [PubMed] [Google Scholar]
- 103.Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: Delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 104.Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De SS, Allt G. Contractile proteins in pericytes at the blood–brain and blood–retinal barriers. J Neurocytol. 2001;30:35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- 105.Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: Spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55:1–11. doi: 10.1111/j.1432-0436.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 106.Collado MP, Latorre E, Fernandez I, Aragones MD, Catalan RE. Brain microvessel endothelin type A receptors are coupled to ceramide production. Biochem Biophys Res Commun. 2003;306:282–285. doi: 10.1016/s0006-291x(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 107.Catalan RE, Martinez AM, Aragones MD, Martinez A, Diaz G. Endothelin stimulates phosphoinositide hydrolysis and PAF synthesis in brain microvessels. J Cereb Blood Flow Metab. 1996;16:1325–1334. doi: 10.1097/00004647-199611000-00030. [DOI] [PubMed] [Google Scholar]
- 108.Catalan RE, Martinez AM, Aragones MD, Hernandez F, Diaz G. Endothelin stimulates protein phosphorylation in blood–brain barrier. Biochem Biophys Res Commun. 1996;219:366–369. doi: 10.1006/bbrc.1996.0239. [DOI] [PubMed] [Google Scholar]
- 109.Catalan RE, Martinez AM, Aragones MD, Garde E, Diaz G. Platelet-activating factor stimulates protein kinase C translocation in cerebral microvessels. Biochem Biophys Res Commun. 1993;192:446–451. doi: 10.1006/bbrc.1993.1435. [DOI] [PubMed] [Google Scholar]
- 110.Guerin C, Laterra J, Hruban RH, Brem H, Drewes LR, Goldstein GW. The glucose transporter and blood–brain barrier of human brain tumors. Ann Neurol. 1990;28:758–765. doi: 10.1002/ana.410280606. [DOI] [PubMed] [Google Scholar]
- 111.Chat M, Bayol-Denizot C, Suleman G, Roux F, Minn A. Drug metabolizing enzyme activities and superoxide formation in primary and immortalized rat brain endothelial cells. Life Sci. 1998;62:151–163. doi: 10.1016/s0024-3205(97)01061-8. [DOI] [PubMed] [Google Scholar]
- 112.Meyer RP, Gehlhaus M, Knoth R, Volk B. Expression and function of cytochrome p450 in brain drug metabolism. Curr Drug Metab. 2007;8:297–306. doi: 10.2174/138920007780655478. [DOI] [PubMed] [Google Scholar]
- 113.Strolin BM, Tipton KF, Whomsley R. Amine oxidases and monooxygenases in the in vivo metabolism of xenobiotic amines in humans: Has the involvement of amine oxidases been neglected? Fundam Clin Pharmacol. 2007;21:467–480. doi: 10.1111/j.1472-8206.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 114.Zhang EY, Phelps MA, Cheng C, Ekins S, Swaan PW. Modeling of active transport systems. Adv Drug Deliv Rev. 2002;54:329–354. doi: 10.1016/s0169-409x(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 115.Rohrig UF, Awad L, Grosdidier A, Larrieu P, Stroobant V, Colau D, Cerundolo V, Simpson AJ, Vogel P, Van den Eynde BJ, Zoete V, Michielin O. Rational design of indoleamine 2,3-dioxygenase inhibitors. J Med Chem. 2010;53:1172–1189. doi: 10.1021/jm9014718. [DOI] [PubMed] [Google Scholar]
- 116.Dolusic E, Larrieu P, Blanc S, Sapunaric F, Norberg B, Moineaux L, Colette D, Stroobant V, Pilotte L, Colau D, Ferain T, Fraser G, Galeni M, Frere JM, Masereel B, Van den EB, Wouters J, Frederick R. Indol-2-yl ethanones as novel indoleamine 2,3-dioxygenase (IDO) inhibitors. Bioorg Med Chem. 2011;19:1550–1561. doi: 10.1016/j.bmc.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 117.Cheng A, Diller DJ, Dixon SL, Egan WJ, Lauri G, Merz KM., Jr Computation of the physiochemical properties and data mining of large molecular collections. J Comput Chem. 2002;23:172–183. doi: 10.1002/jcc.1164. [DOI] [PubMed] [Google Scholar]
- 118.Feng MR. Assessment of blood–brain barrier penetration: In silico, in vitro and in vivo. Curr Drug Metab. 2002;3:647–657. doi: 10.2174/1389200023337063. [DOI] [PubMed] [Google Scholar]
- 119.Podlogar BL, Muegge I. “Holistic” in silico methods to estimate the systemic and CNS bioavailabilities of potential chemotherapeutic agents. Curr Top Med Chem. 2001;1:257–275. doi: 10.2174/1568026013395146. [DOI] [PubMed] [Google Scholar]
- 120.Ecker GF, Noe CR. In silico prediction models for blood–brain barrier permeation. Curr Med Chem. 2004;11:1617–1628. doi: 10.2174/0929867043365071. [DOI] [PubMed] [Google Scholar]
- 121.Rebitzer S, Annibali D, Kopp S, Eder M, Langer T, Chiba P, Ecker GF, Noe CR. In silico screening with benzofurane- and benzopyrane-type MDR-modulators. Farmaco. 2003;58:185–191. doi: 10.1016/S0014-827X(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 122.Reichel A, Begley DJ. Potential of immobilized artificial membranes for predicting drug penetration across the blood–brain barrier. Pharm Res. 1998;15:1270–1274. doi: 10.1023/a:1011904311149. [DOI] [PubMed] [Google Scholar]
- 123.Ong S, Liu H, Pidgeon C. Immobilized-artificial-membrane chromatography: Measurements of membrane partition coefficient and predicting drug membrane permeability. J Chromatogr A. 1996;728:113–128. doi: 10.1016/0021-9673(95)00837-3. [DOI] [PubMed] [Google Scholar]
- 124.Pidgeon C, Ong S, Liu H, Qiu X, Pidgeon M, Dantzig AH, Munroe J, Hornback WJ, Kasher JS, Glunz L. IAM chromatography: An in vitro screen for predicting drug membrane permeability. J Med Chem. 1995;38:590–594. doi: 10.1021/jm00004a004. [DOI] [PubMed] [Google Scholar]
- 125.Giaginis C, Tsantili-Kakoulidou A. Alternative measures of lipophilicity: From octanol–water partitioning to IAM retention. J Pharm Sci. 2008;97:2984–3004. doi: 10.1002/jps.21244. [DOI] [PubMed] [Google Scholar]
- 126.Liu H, Cohen DE, Pidgeon C. Single step purification of rat liver aldolase using immobilized artificial membrane chromatography. J Chromatogr B Biomed Sci Appl. 1997;703:53–62. doi: 10.1016/s0378-4347(97)00411-8. [DOI] [PubMed] [Google Scholar]
- 127.Fortuna A, Alves G, Soares-da-Silva P, Falcao A. Optimization of a parallel artificial membrane permeability assay for the fast and simultaneous prediction of human intestinal absorption and plasma protein binding of drug candidates: Application to dibenz[b,f]azepine-5-carboxamide derivatives. J Pharm Sci. 2011 doi: 10.1002/jps.22796. [DOI] [PubMed] [Google Scholar]
- 128.Nishimuta H, Sato K, Yabuki M, Komuro S. Prediction of the intestinal first-pass metabolism of CYP3A and UGT substrates in humans from in vitro data. Drug Metab Pharmacokinet. 2011 doi: 10.2133/dmpk.DMPK-11-RG-034. [DOI] [PubMed] [Google Scholar]
- 129.Sinko B, Kokosi J, Avdeef A, Takacs-Novak K. A PAMPA study of the permeability-enhancing effect of new ceramide analogues. Chem Biodivers. 2009;6:1867–1874. doi: 10.1002/cbdv.200900149. [DOI] [PubMed] [Google Scholar]
- 130.Ottaviani G, Martel S, Carrupt PA. Parallel artificial membrane permeability assay: A new membrane for the fast prediction of passive human skin permeability. J Med Chem. 2006;49:3948–3954. doi: 10.1021/jm060230+. [DOI] [PubMed] [Google Scholar]
- 131.Nagahara N, Tavelin S, Artursson P. Contribution of the paracellular route to the pH-dependent epithelial permeability to cationic drugs. J Pharm Sci. 2004;93:2972–2984. doi: 10.1002/jps.20206. [DOI] [PubMed] [Google Scholar]
- 132.Mensch J, LJL, Sanderson W, Melis A, Mackie C, Verreck G, Brewster ME, Augustijns P. Application of PAMPA-models to predict BBB permeability including efflux ratio, plasma protein binding and physicochemical parameters. Int J Pharm. 2010;395:182–197. [PubMed] [Google Scholar]
- 133.Dagenais C, Avdeef A, Tsinman O, Dudley A, Beliveau R. P-glycoprotein deficient mouse in situ blood–brain barrier permeability and its prediction using an in combo PAMPA model. Eur J Pharm Sci. 2009;38:121–137. doi: 10.1016/j.ejps.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Velicky M, Bradley DF, Tam KY, Dryfe RA. In situ artificial membrane permeation assay under hydrodynamic control: Permeability–pH profiles of warfarin and verapamil. Pharm Res. 2010;27:1644–1658. doi: 10.1007/s11095-010-0150-6. [DOI] [PubMed] [Google Scholar]
- 135.Ueno M. Molecular anatomy of the brain endothelial barrier: An overview of the distributional features. Curr Med Chem. 2007;14:1199–1206. doi: 10.2174/092986707780597943. [DOI] [PubMed] [Google Scholar]
- 136.Akamatsu M, Fujikawa M, Nakao K, Shimizu R. In silico prediction of human oral absorption based on QSAR analyses of PAMPA permeability. Chem Biodivers. 2009;6:1845–1866. doi: 10.1002/cbdv.200900112. [DOI] [PubMed] [Google Scholar]
- 137.Berezowski V, Landry C, Lundquist S, Dehouck L, Cecchelli R, Dehouck MP, Fenart L. Transport screening of drug cocktails through an in vitro blood–brain barrier: Is it a good strategy for increasing the throughput of the discovery pipeline? Pharm Res. 2004;21:756–760. doi: 10.1023/b:pham.0000026424.78528.11. [DOI] [PubMed] [Google Scholar]
- 138.Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 139.Hamm S, Dehouck B, Kraus J, Wolburg-Buchholz K, Wolburg H, Risau W, Cecchelli R, Engelhardt B, Dehouck MP. Astrocyte mediated modulation of blood–brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004;315:157–166. doi: 10.1007/s00441-003-0825-y. [DOI] [PubMed] [Google Scholar]
- 140.Toimela T, Maenpaa H, Mannerstrom M, Tahti H. Development of an in vitro blood–brain barrier model—cytotoxicity of mercury and aluminum. Toxicol Appl Pharmacol. 2004;195:73–82. doi: 10.1016/j.taap.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 141.Cucullo L, McAllister MS, Kight K, Krizanac-Bengez L, Marroni M, Mayberg MR, Stanness KA, Janigro D. A new dynamic in vitro model for the multidimensional study of astrocyte–endothelial cell interactions at the blood–brain barrier. Brain Res. 2002;951:243–254. doi: 10.1016/s0006-8993(02)03167-0. [DOI] [PubMed] [Google Scholar]
- 142.Ziegler T, Nerem RM. Effect of flow on the process of endothelial cell division. Arterioscler Thromb. 1994;14:636–643. doi: 10.1161/01.atv.14.4.636. [DOI] [PubMed] [Google Scholar]
- 143.Goldstein GW. Endothelial cell–astrocyte interactions. A cellular model of the blood–brain barrier. Ann N Y Acad Sci. 1988;529:31–39. doi: 10.1111/j.1749-6632.1988.tb51417.x. [DOI] [PubMed] [Google Scholar]
- 144.Cancilla PA, DeBault LE. Neutral amino acid transport properties of cerebral endothelial cells in vitro. J Neuropathol Exp Neurol. 1983;42:191–199. doi: 10.1097/00005072-198303000-00008. [DOI] [PubMed] [Google Scholar]
- 145.Cucullo L, Marchi N, Marroni M, Fazio V, Namura S, Janigro D. Blood–brain barrier damage induces release of {alpha}2-macroglobulin. Mol Cell Proteomics. 2003;2:234–241. doi: 10.1074/mcp.M200077-MCP200. [DOI] [PubMed] [Google Scholar]
- 146.Grant GA, Abbott NJ, Janigro D. Understanding the physiology of the blood–brain barrier: In vitro models. News Physiol Sci. 1998;13:287–293. doi: 10.1152/physiologyonline.1998.13.6.287. [DOI] [PubMed] [Google Scholar]
- 147.Bendayan R, Lee G, Bendayan M. Functional expression and localization of P-glycoprotein at the blood–brain barrier. Microsc Res Tech. 2002;57:365–380. doi: 10.1002/jemt.10090. [DOI] [PubMed] [Google Scholar]
- 148.Bussolari SR, Dewey CF, Jr, Gimbrone MA., Jr Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum. 1982;53:1851–1854. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- 149.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 150.Ando J, Kamiya A. Flow-dependent regulation of gene expression in vascular endothelial cells. Jpn Heart J. 1996;37:19–32. doi: 10.1536/ihj.37.19. [DOI] [PubMed] [Google Scholar]
- 151.Stanness KA, Guatteo E, Janigro D. A dynamic model of the blood–brain barrier “in vitro. Neurotoxicology. 1996;17:481–496. [PubMed] [Google Scholar]
- 152.Stanness KA, Westrum LE, Fornaciari E, Mascagni P, Nelson JA, Stenglein SG, Myers T, Janigro D. Morphological and functional characterization of an in vitro blood–brain barrier model. Brain Res. 1997;771:329–342. doi: 10.1016/s0006-8993(97)00829-9. [DOI] [PubMed] [Google Scholar]
- 153.Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, Chatzoulis DZ. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- 154.Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: A marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- 155.McAllister MS, Krizanac-Bengez L, Macchia F, Naftalin RJ, Pedley KC, Mayberg MR, Marroni M, Leaman S, Stanness KA, Janigro D. Mechanisms of glucose transport at the blood–brain barrier: An in vitro study. Brain Res. 2001;904:20–30. doi: 10.1016/s0006-8993(01)02418-0. [DOI] [PubMed] [Google Scholar]
- 156.Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood–brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48:505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 157.Krizanac-Bengez L, Mayberg MR, Cunningham E, Hossain M, Ponnampalam S, Parkinson FE, Janigro D. Loss of shear stress induces leukocyte-mediated cytokine release and blood–brain barrier failure in dynamic in vitro blood–brain barrier model. J Cell Physiol. 2006;206:68–77. doi: 10.1002/jcp.20429. [DOI] [PubMed] [Google Scholar]
- 158.Banks WA, Erickson MA. The blood–brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 159.van der FM, Hoppenreijs S, van Rensburg AJ, Ruyken M, Kolk AH, Springer P, Hoepelman AI, Geelen SP, Kimpen JL, Schoeman JF. Vascular endothelial growth factor and blood–brain barrier disruption in tuberculous meningitis. Pediatr Infect Dis J. 2004;23:608–613. doi: 10.1097/01.inf.0000131634.57368.45. [DOI] [PubMed] [Google Scholar]
- 160.Minagar A, Alexander JS. Blood–brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 161.Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21:107–112. doi: 10.1016/j.semcancer.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 162.Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- 163.Elliott NT, Yuan F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci. 2011;100:59–74. doi: 10.1002/jps.22257. [DOI] [PubMed] [Google Scholar]
- 164.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 165.Toh YC, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, van Noort D, Hutmacher DW, Yu H. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip. 2007;7:302–309. doi: 10.1039/b614872g. [DOI] [PubMed] [Google Scholar]
- 166.Camos S, Mallolas J. Experimental models for assaying microvascular endothelial cell pathophysiology in stroke. Molecules. 2010;15:9104–9134. doi: 10.3390/molecules15129104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 168.Vu K, Weksler B, Romero I, Couraud PO, Gelli A. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood–brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryot Cell. 2009;8:1803–1807. doi: 10.1128/EC.00240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sano Y, Shimizu F, Abe M, Maeda T, Kashiwamura Y, Ohtsuki S, Terasaki T, Obinata M, Kajiwara K, Fujii M, Suzuki M, Kanda T. Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood–brain barrier function. J Cell Physiol. 2010;225:519–528. doi: 10.1002/jcp.22232. [DOI] [PubMed] [Google Scholar]
- 170.Reichel A, Abbott NJ, Begley DJ. Evaluation of the RBE4 cell line to explore carrier-mediated drug delivery to the CNS via the L-system amino acid transporter at the blood–brain barrier. J Drug Target. 2002;10:277–283. doi: 10.1080/10611860290031930. [DOI] [PubMed] [Google Scholar]
- 171.Faria A, Pestana D, Teixeira D, Azevedo J, De FV, Mateus N, Calhau C. Flavonoid transport across RBE4 cells: A blood–brain barrier model. Cell Mol Biol Lett. 2010;15:234–241. doi: 10.2478/s11658-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fischer W, Praetor K, Metzner L, Neubert RH, Brandsch M. Transport of valproate at intestinal epithelial (Caco-2) and brain endothelial (RBE4) cells: Mechanism and substrate specificity. Eur J Pharm Biopharm. 2008;70:486–492. doi: 10.1016/j.ejpb.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 173.Aschner M, Fitsanakis VA, dos Santos AP, Olivi L, Bressler JP. Blood–brain barrier and cell–cell interactions: Methods for establishing in vitro models of the blood–brain barrier and transport measurements. Methods Mol Biol. 2006;341:1–15. doi: 10.1385/1-59745-113-4:1. [DOI] [PubMed] [Google Scholar]
- 174.Begley DJ, Lechardeur D, Chen ZD, Rollinson C, Bardoul M, Roux F, Scherman D, Abbott NJ. Functional expression of P-glycoprotein in an immortalised cell line of rat brain endothelial cells, RBE4. J Neurochem. 1996;67:988–995. doi: 10.1046/j.1471-4159.1996.67030988.x. [DOI] [PubMed] [Google Scholar]
- 175.Chishty M, Begley DJ, Abbott NJ, Reichel A. Functional characterisation of nucleoside transport in rat brain endothelial cells. Neuroreport. 2003;14:1087–1090. doi: 10.1097/01.wnr.0000072843.93264.ff. [DOI] [PubMed] [Google Scholar]
- 176.Cestelli A, Catania C, D’Agostino S, Di LI, Licata L, Schiera G, Pitarresi GL, Savettieri G, De CV, Giandalia G, Giannola LI. Functional feature of a novel model of blood brain barrier: Studies on permeation of test compounds. J Control Release. 2001;76:139–147. doi: 10.1016/s0168-3659(01)00431-x. [DOI] [PubMed] [Google Scholar]
- 177.Regina A, Koman A, Piciotti M, El Hafny B, Center MS, Bergmann R, Couraud PO, Roux F. Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J Neurochem. 1998;71:705–715. doi: 10.1046/j.1471-4159.1998.71020705.x. [DOI] [PubMed] [Google Scholar]
- 178.El Hafny B, Cano N, Piciotti M, Regina A, Scherrmann JM, Roux F. Role of P-glycoprotein in colchicine and vinblastine cellular kinetics in an immortalized rat brain microvessel endothelial cell line. Biochem Pharmacol. 1997;53:1735–1742. doi: 10.1016/s0006-2952(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 179.Zhu ZB, Makhija SK, Lu B, Wang M, Rivera AA, Preuss M, Zhou F, Siegal GP, Alvarez RD, Curiel DT. Transport across a polarized monolayer of Caco-2 cells by transferrin receptor-mediated adenovirus transcytosis. Virology. 2004;325:116–128. doi: 10.1016/j.virol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 180.Lohmann C, Huwel S, Galla HJ. Predicting blood–brain barrier permeability of drugs: Evaluation of different in vitro assays. J Drug Target. 2002;10:263–276. doi: 10.1080/10611860290031903. [DOI] [PubMed] [Google Scholar]
- 181.Lundquist S, Renftel M, Brillault J, Fenart L, Cecchelli R, Dehouck MP. Prediction of drug transport through the blood–brain barrier in vivo: A comparison between two in vitro cell models. Pharm Res. 2002;19:976–981. doi: 10.1023/a:1016462205267. [DOI] [PubMed] [Google Scholar]
- 182.Joo F. The blood–brain barrier in in vitro: ten years of reasearch on microvessels isolated from the brain. Neurochem Int. 1985;7:1–25. doi: 10.1016/0197-0186(85)90002-6. [DOI] [PubMed] [Google Scholar]





