Abstract
Guilt is a core emotion governing social behavior by promoting compliance with social norms or self-imposed standards. The goal of this study was to contrast guilty responses to actions that affect self versus others, since actions with social consequences are hypothesized to yield greater guilty feelings due to adopting the perspective and subjective emotional experience of others. Sixteen participants were presented with brief hypothetical scenarios in which the participant’s actions resulted in harmful consequences to self (guilt-self) or to others (guilt-other) during functional MRI. Participants felt more intense guilt for guilt-other than guilt-self and guilt-neutral scenarios. Guilt scenarios revealed distinct regions of activity correlated with intensity of guilt, social consequences of actions, and the interaction of guilt by social consequence. Guilt intensity was associated with activation of the dorsomedial PFC, superior frontal gyrus, supramarginal gyrus, and anterior inferior frontal gyrus. Guilt accompanied by social consequences was associated with greater activation than without social consequences in the ventromedial and dorsomedial PFC, precuneus, posterior cingulate, and posterior superior temporal sulcus. Finally, the interaction analysis highlighted select regions that were more strongly correlated with guilt intensity as a function of social consequence, including the left anterior inferior frontal gyrus, left ventromedial PFC, and left anterior inferior parietal cortex. Our results suggest these regions intensify guilt where harm to others may incur a greater social cost.
Keywords: guilt, empathy, perspective taking, social emotions, functional magnetic resonance imaging
INTRODUCTION
Guilt is a core emotion governing social behavior by promoting compliance with social norms or self-imposed standards (Hoffman, 1982). Guilt may be elicited when the affected individual bears personal responsibility for negative or harmful events that impact the same individual or others (Tangney and Dearing, 2002). Like other social emotions, guilt is predicated on self agency and when the harmful events affect another individual, guilt requires an inherent capacity for empathy in adopting the perspective of the affected individual(s) (Hoffman, 1982). Guilt concerns transgressions or failed behaviors that may have negative impacts on others (e.g. “I dropped my friend’s camera”), or on self (e.g. “I missed my workout today”), and in some instances negative impacts on self may also contain some component of embarrassment (e.g. “I stumbled and fell while getting on stage”). We sought to expand our understanding of the neural correlates of guilt by examining its interaction with social consequence (whether the action affected oneself or another individual), which has largely been ignored in the literature.
Over the past decade a growing literature has examined the neural correlates of pro-social emotions (Zahn et al., 2009c) and moral judgments (Moll et al., 2009). Within this broader area, a small number of studies have focused on guilt (Basile et al., 2010; Green et al., 2010; Kedia et al., 2008; Moll et al., 2011; Shin et al., 2000; Takahashi et al., 2004; Zahn et al., 2009C). Personal scripts eliciting guilt that were read during PET scanning recruited bilateral anterior temporal cortex, anterior cingulate gyrus, left anterior insula, and left inferior frontal gyrus (Shin et al., 2000). A comparison of positive and negative social emotions revealed that the region most frequently associated with guilt was in the anterior ventromedial PFC and the subgenual cingulate cortex (Zahn et al., 2009c). Use of action scripts found that prosocial emotions (guilt, embarrassment, compassion) elicited activation in the anterior medial PFC and the superior temporal sulcus (STS), whereas empathy also activated the mesolimbic pathway (Moll and de Oliveira-Souza, 2007). Patients with damage to anterior medial PFC together with lesions of more posterior ventromedial PFC display diminished guilt and compassion (Koenigs et al., 2007). Investigation of the evaluative processes comparing guilt and embarrassment showed that both conditions commonly activated the medial PFC and the left superior temporal sulcus (STS) (Takahashi et al., 2004).
In parallel to these studies of emotion, social cognition research has uncovered the neural systems associated with empathy or adopting the thoughts, feelings, and emotions of others (Amodio and Frith, 2006; Mason and Macrae, 2008). Empathy requires the subjective understanding and experiencing of others’ emotions while maintaining the distinct subjective experience of one’s own feelings (Batson, 2009). The experience of empathy highlights an important distinction between perspective taking of non-emotional information and perspective taking of emotional information. A number of regions can be hypothesized for empathy based on evidence from neuroimaging and lesion-based studies. These highlight a prominent role of the temporal poles (Ruby and Decety, 2004), believed to support conceptual representations of social information (Ross and Olson, 2010; Zahn et al., 2009B; Zahn et al., 2007), and activation of the inferior frontal gyrus (IFG) in emotional empathy, emotion recognition/evaluation (Jabbi et al., 2007; Schulte-Rüther et al., 2007), and correlation with emotional empathy scores (Kaplan and Iacoboni, 2006). However, empathy is not considered a purely emotional response but is linked with a cognitive component (Decety and Jackson, 2004; Preston and de Waal, 2002; Ruby and Decety, 2004), often considered in relation to mentalizing or Theory of Mind (Lamm et al., 2007) that are associated with the posterior superior temporal sulcus (Frith and Frith, 2003; Scholz et al., 2009), the temporoparietal junction (TPJ) (Samson et al., 2004; Saxe and Kanwisher, 2003), and posterior cingulate cortex (Lombardo et al., 2010). In addition, the medial frontal regions (de Waal, 2008) play a role, specifically frontopolar cortex (Ruby and Decety, 2004), and ventromedial PFC, which has been implicated by lesion data (Eslinger, 1998; Shamay-Tsoory et al., 2003) and fMRI data (Mitchell et al., 2006). Finally, the role of the precuneus for integration into a self-referential framework has been demonstrated in tasks such as making emotional judgments (Greene and Haidt, 2002; Maddock et al., 2003; Moll et al., 2005). We predicted that feelings for negative events that affect others compared to events affecting self would differentially activate regions linked to feelings of empathy as well as mentalizing and ToM. After controlling for overall intensity differences in the analyses, we predicted that guilt resulting from self-actions affecting others would differentially engage the ventromedial PFC (Mitchell et al., 2006), frontopolar cortex (Ruby and Decety, 2004), posterior superior temporal sulcus (Frith and Frith, 2003; Scholz et al., 2009), temporoparietal junction (Samson et al., 2004; Saxe and Kanwisher, 2003), IFG; Schulte-Rüther, 2007 #3498}, and the precuneus/posterior cingulate gyrus (Lombardo et al., 2010), consistent with predictions from prior literature.
There has been little research linking the findings in empathy and guilt, while research into other social emotions has expanded rapidly. Pro-social emotions, particularly guilt, clearly rely on neural systems that facilitate these processes seamlessly. However, because the patterns of neural activation in guilt and empathy overlap, it is important to determine the extent to which activation elicited by guilt scenarios is signaling affective functions or social cognition. We sought to examine the neural systems responsible for differentially modulating guilt and its interaction with systems engaged by empathy and perspective taking. We investigated activation, from event-related fMRI, evoked by reading guilt scenarios. These were scripted with the participant as agent, enacting scenarios resulting in harmful consequences to the participant (guilt-self) or someone other than the participant (guilt-other), whereas guilt-neutral (control) scenarios lacked negative consequences. Participants provided subjective ratings of guilt following each scenario. We hypothesized that the intensity of guilt associated with harm to others would be greater than for harm to self. Specifically, we predicted that guilt would be associated with regions implicated in prior research including the inferior frontal gyrus (Shin et al., 2000; Takahashi et al., 2004), posterior STS (Takahashi et al., 2004), frontopolar cortex (Basile et al., 2010; Moll et al., 2007; Moll et al., 2011; Kedia et al., 2008; Takahashi et al., 2004), septal and the nearby subgenual cingulate areas (Basile et al., 2010; Green et al., 2010; Moll et al., 2011; Zahn et al., 2009a; Zahn et al., 2009c), posterior cingulate/precuneus (Basile et al., 2010; Kedia et al., 2008), temporoparietal junction (Kedia et al., 2008; Moll et al., 2007), and the anterior temporal poles (Shin et al., 2000). Furthermore, we predicted that guilt feelings for negative events that affect others would activate guilt regions that are differentially responsive by social and interpersonal consequences.
METHODS
Participants
Behavioral and functional MRI data from sixteen men (mean age: 22.41 ± 2.69; 10 European American, 2 African American, 3 Asian American, and 1 Other/Multiracial) were analyzed. Nineteen right-handed men recruited from a university and community-based participant database provided written informed consent for study procedures approved by the Institutional Review Board at Duke University Medical Center. The study was limited to men as a lead-up to future research on guilt in combat-related posttraumatic stress disorder (PTSD). One participant was excluded for poor cooperation with study procedures (35% non-response rate), and two were excluded for technical difficulties with time locking fMRI acquisition with stimulus presentation. Participant screening with the Brief SCID revealed no Axis I psychiatric disorders or active substance use disorders.
Experimental Stimuli
Experimental stimuli consisted of brief hypothetical scenarios that were partitioned into three conditions: (i) the guilt-self condition depicted actions by the participant that resulted in negative consequences to the participant, (ii) the guilt-other condition depicted actions by the participant that resulted in negative consequences to someone other than the participant, and (iii) the guilt-neutral condition depicted actions that had no untoward consequences and therefore designed not to elicit guilt. A total of 180 stimuli, divided into 60 guilt-other, 60 guilt-self, and 60 guilt-neutral scenarios, were created in our lab that were modeled after stimuli developed by Takahashi and colleagues (2004). The 60 guilt-neutral stimuli were further subdivided into neutral-self and neutral-other conditions to investigate the main effect of social consequence (self versus other) as described later. Sample scenarios are provided in Table 1.
Table 1.
Sample guilt and perspective scenarios
| Guilt Self | Guilt Other | Guilt Neutral |
|---|---|---|
| You drink too much alcohol at a party and crash your car into a tree. | You injure two other people in an accident because you were drinking and driving. | You drive back home after spending time at a party with your friends. |
| You get fired from work because you forgot to submit an important report to your supervisor. | You forget to submit a group report at work resulting in two of your colleagues getting fired. | You collaborate with several colleagues to work on an important report at work. |
| You are fined for using a cell phone inside a hospital. | Your ringing cell phone disrupts a hospital patient’s heart monitoring device. | You return several calls on your cell phone while waiting at the park. |
| You get arrested for shoplifting a shirt at a store in the mall. | Two of your friends get arrested because of a shirt that you shoplifted. | After some deliberation, you purchase a shirt at a store you like. |
| You are in debt because you spent too much money on a car you couldn’t afford. | You spend your parents money on a car you can’t afford and they are now in debt. | Your neighbor who lives across the street washes his newly purchased car. |
| You drink more alcohol than you had planned to and feel sick the next day. | Your friend feels sick today because you forced them to drink more than they would have liked. | Several of your friends go out for drinks after work on a Thursday evening. |
To avoid presenting the same underlying scenario to participants for the guilt-self condition and the guilt-other condition, we created two stimulus sets (A and B). Each stimulus set consisted of 30 guilt-self and 30 guilt-other stimuli of unique scenarios. ‘Other’ scenarios in stimulus set A contained the corresponding ‘self’ scenarios in stimulus set B and vice versa (‘other’ scenarios in stimulus set B contained the corresponding ‘self’ scenarios in stimulus set A). Stimulus sets A and B were counterbalanced with random assignment across subjects, and both sets contained identical guilt-neutral stimuli.
To rule out differences in activation patterns due to syntactic complexity of sentences between our conditions of interest, a single trained rater conducted a content analysis using a formalized measure based on the number of subordinating conjunctions, pronouns, verb forms, noun phrases, and verb phrases (Szmrecsanyi, 2004). We assessed differences in syntactic complexity between the other- versus self-perspective scenarios and correlations with intensity of guilt level experienced (1=lowest through 4=highest). There were no differences in syntactic complexity between self- and other-based perspectives [t(152) = .004, p > .9] nor was there a correlation between syntactic complexity of scenarios and their mean guilt rating (r2 = .01, p = .15).
Behavioral Measures
During acquisition of fMRI, subjects were instructed to read the sentence presented and to “put yourself in the situation and imagine how you would feel if it were happening to you”. Subjects were instructed to respond to each stimulus with a right-handed button press to indicate their subjective intensity of guilt feelings. Each trial began with a stimulus from one of three conditions (guilt-self, guilt-other, and guilt-neutral) that appeared on the screen for 13 seconds, followed by two seconds to respond with a subjective guilt rating (1=no guilt, 4=highest guilt), and inter-stimulus instructions to “Clear your mind” that were jittered between 8 and 12 seconds. The fMRI session was partitioned into 8 runs each with 188 image volumes lasting 6′16″.
Outside the MRI environment, a separate sample of twelve subjects matched for age, gender, race, and recruited from the same subject pool as the fMRI participants, provided ratings for eleven different emotions elicited by the scenarios. Using a 1–4 rating scale participants rated feelings of anger, arousal, contentment, disgust, embarrassment, fear, guilt, pain, shame, tension, and valence. This assessment was conducted on a separate sample to characterize the emotions experienced upon encountering the scenarios for the first time. The goal of collecting these data was to assess how well our stimuli were tuned to guilt and the extent of overlap with other related emotions.
MRI Acquisition
Functional images were acquired on a 3-Tesla GE Signa EXCITE scanner using a spiral-in acquisition sequence that has superior coverage in the ventral frontal and anterior temporal regions as compared to spiral-out or EPI acquisition (see Supplementary data). A series of 34 axially oriented functional slices were acquired in an interleaved fashion for full-brain coverage with the following parameters: TR = 2000 ms; TE = 30 ms; FOV = 240 mm; matrix size = 642; voxel size = 3.75 × 3.75 × 3.8 mm. High-resolution three-dimensional spin-echo coplanar structural images were acquired in 68 axial slices (TR = 7.436; TE = 3.02 ms; FOV = 240 mm; matrix size = 2562; voxel size = 1.0 × 1.0 × 1.9 mm). High resolution T1-weighted images with 1 mm isometric voxels, later used for registration of functional images, were acquired using the Array Spatial Sensitivity Encoding Technique (ASSET) with fast spoiled gradient-recall (FSPGR; TR/TE/flip angle=7.484 ms/2.984 ms/12°, 256 mm FOV, 1 mm slice, 166 slices, 256×256 matrix, 1 Nex).
Data Analyses
Functional data sets were analyzed using FSL version 4.1.4 [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), University of Oxford, U.K.] (Smith, 2004). Paradigm timing files were converted to FSL compatible format and NIFTI image data files were generated. Preprocessing was applied to individual subjects’ data using the following steps: (i) motion correction with Motion Correction FMRIB Linear Image Registration Tool (MCFLIRT) (Jenkinson et al., 2002) to correct for motion within each experimental run using the middle volume of the run as reference, (ii) slice timing correction to shift each time-series by an appropriate fraction of a TR relative to the middle of the TR period based on an interleaved slice acquisition sequence, (iii) brain extraction using the Brain Extraction Tool (BET) to remove the skull prior to analysis (Smith, 2002), (iv) spatial smoothing using a Gaussian kernel of FWHM 5 mm to reduce noise and improve sensitivity, (v) intensity normalization whereby the entire 4D data set was normalized by a single scaling factor or grand mean scaling so higher-level analyses remain valid, and (vi) highpass temporal filtering (100 s) to remove low frequency artifacts (Jenkinson et al., 2002). Functional images for each subject were co-registered to structural images in native space, and structural images were normalized to structural standard images, defined by the standard brain supplied in FSL (MNI152). The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. All registrations were carried out using FMRIB Linear Image Registration Tool (FLIRT) for a linear (affine with 12 degrees of freedom) global optimization method of intermodal registration (Jenkinson and Smith, 2001).
Statistical Modeling
Rather than examine neural activation associated with guilt by contrasting guilt scenarios (guilt-other and guilt-self) with guilt-neutral scenarios, we used the guilt ratings provided by scanned subjects to increase statistical power available with a parametric regression approach. This provided modeling of main effects of guilt, main effects of perspective taking, and interaction of guilt by perspective taking. A whole-brain ANOVA was used to model activity specific to guilt, activity specific to perspective taking, and the interaction between guilt and perspective. A 2 × 4 ANOVA modeled the within-subjects factor guilt (four levels; 1 – 4 guilt rating; 1 = low guilt, 4 = high guilt) and the within-subjects factor social consequence (two levels; other, self). The first level design in FSL was set up with eight explanatory variables (EVs) corresponding to all possible combinations of social consequence and guilt rating that included guilt and neutral scenarios (O1, O2, O3, O4, S1, S2, S3, S4) to interrogate activation related to each EV. Guilt-neutral scenarios were included in this regression because they comprised most of the ‘1’ ratings with guilt scenarios generally receiving ratings of ‘2’ and above. We used first level contrasts in FSL from all the runs to set up the following contrasts at the second level: (i) main effect of guilt was modeled to identify voxels with activity that was positively correlated with participants’ guilt ratings without regard to perspective, (ii) main effect of guilt was modeled to identify voxels with activity that was negatively correlated with participants’ guilt ratings without regard to social consequence, (iii) main effect of other > self without regard to guilt rating was obtained by contrasting all other scenarios (GO1, GO2, GO3, GO4, NO1, NO2, NO3, NO4) with all self scenarios (S1, S2, S3, S4, NS1, NS2, NS3, NS4), (iv) main effect of self > other without regard to guilt rating, and (v) interaction of guilt * social consequence identified voxels with a greater positive correlation of activation with guilt ratings arising from scenarios that affected others relative to those that just affected self.
The third level analyses averaged the second level contrasts of parameter estimates (copes) across 16 subjects with automatic outlier de-weighting. Average maps were calculated with a mixed effects higher-level analysis FMRIB Local Analysis of Mixed Effects (FLAME). Protection against false positive detection of active voxels resulting from multiple comparison testing was based on Gaussian Random Field Theory by imposing a conservative mean threshold of Z > 2.3 for cluster formation and a corrected significance threshold of P < 0.05 (Forman et al., 1995). This method for controlling the family wise error, based on the chance probability of obtaining a specified number of clusters that exceed a specified number of voxels above a specified threshold, is implemented in FSL.
RESULTS
Emotion Ratings
The guilt scenarios were rated for the intensity for each of eleven emotions by a second group of participants outside the scanner. These subjects rated scenarios as eliciting significantly higher levels of guilt than each of the other ten emotions (p < .02 for all), but not significantly greater than the level of embarrassment [t(24) = 1.5, p = .15]. Further behavioral results for embarrassment are reported in the Supplementary data. Mean ratings, and statistics comparing the emotions are reported in detail in Table 2.
Table 2.
Emotions Elicited by Scenarios *
| Emotion Elicited | Mean Rating | t-stat | p-value |
|---|---|---|---|
| Anger | 2.78 (.095) | 2.4 | .02 |
| Arousal | 2.53 (.136) | 3.6 | .001 |
| Contentment | 1.46 (.040) | 17.5 | .0001 |
| Disgust | 2.82 (.080) | 2.6 | .02 |
| Embarrassment | 2.91 (.116) | 1.5 | .15 |
| Fear | 2.49 (.107) | 5.2 | .0001 |
| Guilt | 3.08 (.078) | - | - |
| Pain | 2.26 (.140) | 5.6 | .0001 |
| Shame | 2.85 (.076) | 3.0 | .007 |
| Tension | 2.76 (.092) | 3.3 | .003 |
| Valence | 1.43 (.049) | 18.2 | .0001 |
Using a 1 – 4 rating scale, a second group of participants (n=12), responding outside the scanner, rated feelings of anger, arousal, contentment, disgust, embarrassment, fear, guilt, pain, shame, tension, and valence elicited by each guilt scenario.
The SEM is reported in parentheses below the mean.
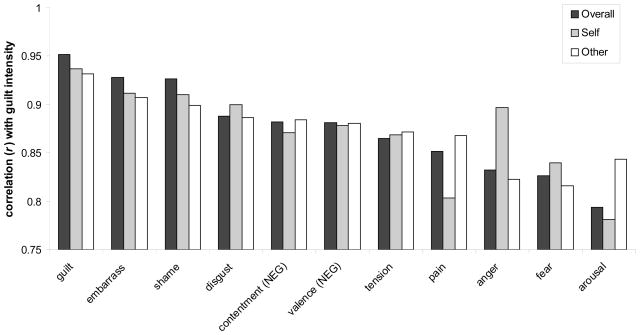
Using the guilt intensity for each scenario provided by subjects in the scanner (n=16) and the emotional intensity for each scenario provided by the second group outside the scanner (n=12), correlations between the guilt rating and the rating for each of the 11 emotions are presented in Figure 1. The strongest correlations were present for guilt (replicating ratings between the scanner group and the non-scanner group) with progressively decreasing correlation strengths for embarrassment, shame, disgust, contentment, valence, tension, pain, anger, fear, and arousal (see Supplementary data).
Figure 1.
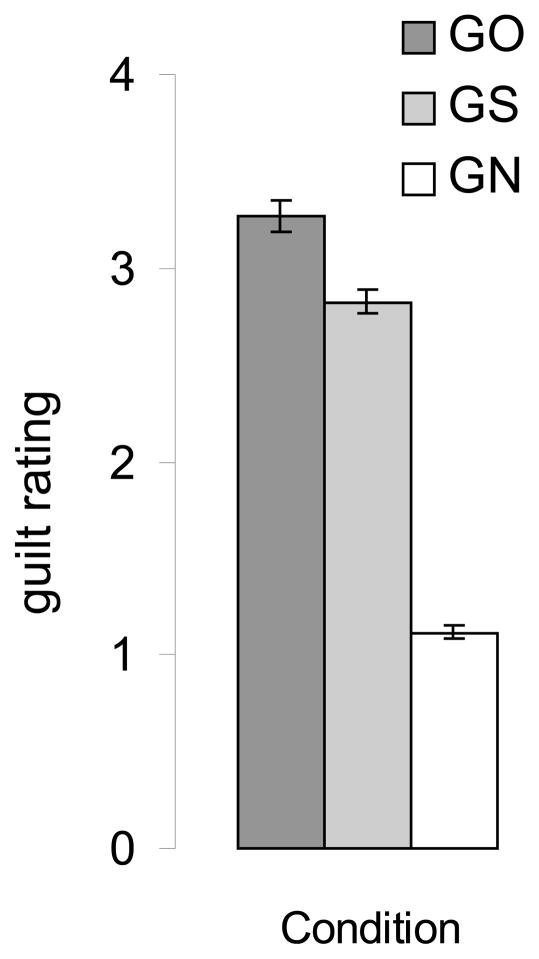
The participant ratings for the intensity of guilt experienced in response to scenarios differed for guilt-other (GO), guilt-self (GS), and guilt-neutral (GN) conditions. Greater intensity of guilt was reported for guilt-other than guilt-self and guilt-neutral conditions, as well as greater intensity of guilt for guilt-self than the guilt-neutral condition.
Guilt Ratings
Ratings for intensity of guilt experienced in response to scenarios (see Figure 2) showed a main effect of condition (3 levels; guilt-other, guilt-self, guilt-neutral) using a one-way ANOVA [F(2,48) = 352.7, p < .0001]. Planned comparisons higher guilt ratings in response to guilt-other (mean=3.2 SE=.06) than guilt-self (mean=2.8 SE=.08) [t(15) = 9.2, p < .0001] and guilt-neutral conditions (mean=1.1 SE=.03) [t(15) = 35.9, p < .0001], as well as greater intensity of guilt for guilt-self than for the guilt-neutral condition [t(15) = 22.0, p < .0001].
Figure 2.
A separate correlation between guilt and each of the 11 emotions was computed from the guilt intensity (1=low, 4=high) for each scenario provided by subjects in the scanner (n=16) and the emotional intensity experienced (1=low, 4=high) for each scenario provided by a separate group of subjects outside the scanner (n=12). The correlation is represented by the height of the bar, separately for self- and other-perspective scenarios and for all scenarios. The strongest correlations were present for guilt and progressively decreasing correlation strengths for embarrassment, shame, disgust, contentment, valence, tension, pain, anger, fear, and arousal.
Guilt Processes
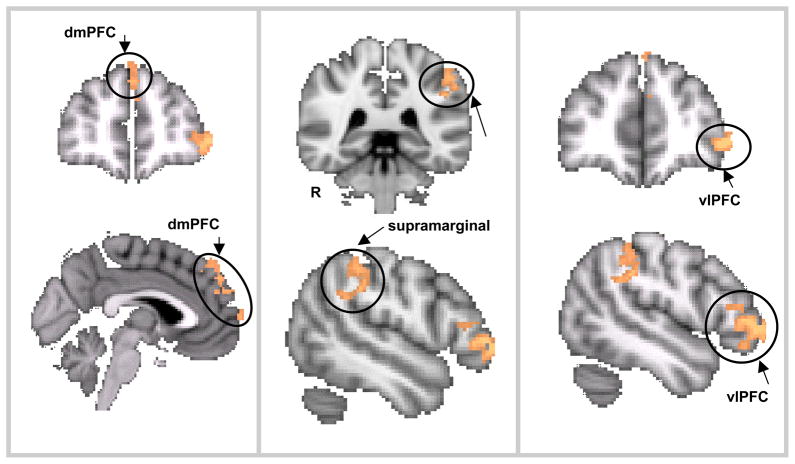
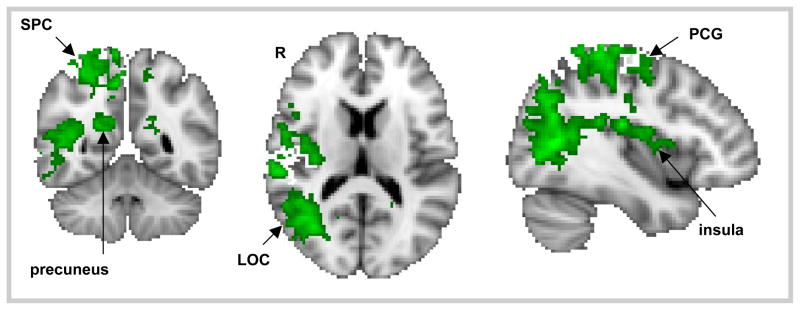
Activation was positively correlated with guilt ratings, regardless of social consequence (main effect of guilt), in the left ventrolateral PFC extending into the left orbitofrontal cortex, left supramarginal gyrus, left dorsal medial prefrontal cortex, and frontopolar cortex (see Figure 3; Table 3). Activation was negatively correlated with guilt ratings mainly in the right lateral occipital cortex, the right superior parietal cortex, the right and left precuneus, the right posterior middle temporal gyrus, right postcentral gyrus, right superior frontal gyrus, and right insular cortex (see Figure 4; Table 4). The septal/subgenual area was active at a more permissive statistical threshold (p < .01; uncorrected).
Figure 3.
The main effect of guilt, where activation was positively correlated with guilt ratings, included the dorsomedial PFC (left), the supramarginal gyrus (center), and the ventrolateral PFC (right)
Table 3.
Guilt Linear Positive
| Neural system | Num. of Voxels | Max. Intensity | Std. Dev. Intensity | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| ventrolateral PFC | 690 | 3.8 | .51 | −50 | 46 | −2 |
|
| ||||||
| superior frontal gyrus | 84 | 2.9 | .23 | 0 | 50 | 38 |
|
| ||||||
| paracingulate gyrus | 13 | 2.7 | .13 | −2 | 48 | 26 |
|
| ||||||
| supramarginal gyrus | 608 | 3.1 | .67 | −48 | −38 | 34 |
|
| ||||||
| dorsomedial PFC | 212 | 3.3 | .58 | −6 | 68 | 16 |
|
| ||||||
| orbitofrontal cortex | 32 | 3.0 | .20 | −46 | 38 | −12 |
The Z statistic images were thresholded using clusters determined by Z > 2.3 and a family wise error corrected cluster significance of P<0.05 was applied to the suprathreshold clusters.
Figure 4.
The main effect of guilt, where activation was negatively correlated with guilt ratings, included the right lateral occipital cortex (LOC), the right superior parietal cortex (SPC), the right and left precuneus, the right posterior middle temporal gyrus, right postcentral gyrus (PCG), right superior frontal gyrus, and right insular cortex
Table 4.
Guilt Linear Negative
| Neural system | Num. of Voxels | Max Intensity | Std Dev Intensity | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| middle frontal gyrus | 496 | 3.6 | .61 | 30 | 22 | 44 |
|
| ||||||
| supplementary cortex | 170 | 3.1 | .56 | 12 | −12 | 44 |
|
| ||||||
| cingulate gyrus | 246 | 3.8 | .59 | 6 | −44 | 50 |
|
| ||||||
| postcentral gyrus | 2629 | 3.9 | 1.36 | 36 | −34 | 52 |
|
| ||||||
| precuneus cortex | 2411 | 4.1 | 1.22 | 8 | −48 | 50 |
|
| ||||||
| superior parietal lobe | 2077 | 3.9 | 1.36 | 36 | −34 | 52 |
|
| ||||||
| cuneus cortex | 834 | 3.6 | 1.13 | −20 | −68 | 24 |
|
| ||||||
| inferior frontal gyrus | 237 | 3.3 | 1.18 | 50 | 20 | 18 |
|
| ||||||
| parietal operculum cortex | 776 | 3.8 | 1.18 | 40 | −22 | 20 |
|
| ||||||
| orbitofrontal cortex | 32 | 3.0 | .20 | −46 | 38 | −12 |
|
| ||||||
| lateral occipital cortex, anterior division | 918 | 4.3 | .96 | 42 | −62 | 8 |
|
| ||||||
| lateral occipital cortex, superior division | 3053 | 4.0 | 1.19 | 24 | −70 | 48 |
The Z statistic images were thresholded using clusters determined by Z > 2.3 and a family wise error corrected cluster significance of P<0.05 was applied to the suprathreshold clusters.
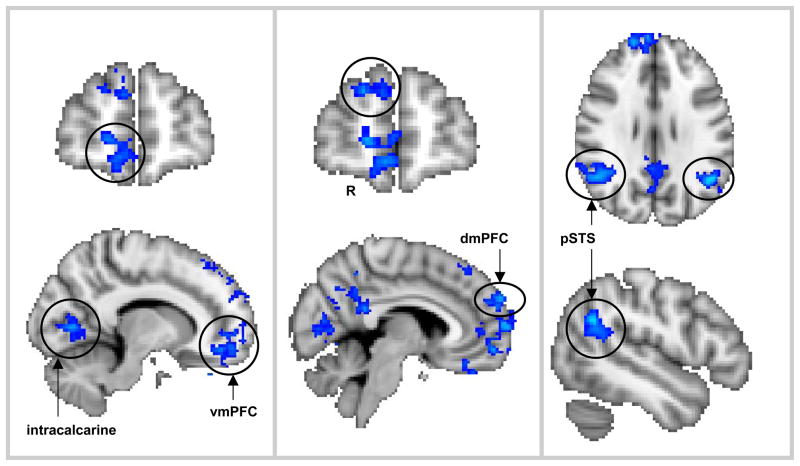
Social Consequences of Guilty Actions
Activation for the contrast comparing guilt-other greater than guilt-self, regardless of intensity ratings (main effect of social consequence), was found in the right dorsomedial PFC, right ventromedial PFC, frontopolar cortex, right and left posterior cingulate gyrus, right and left posterior superior temporal sulcus, right and left precuneus, right intracalcarine, lingual, and fusiform gyri (see Figure 5; Table 5). No significant differences in activation emerged in the guilt-self < guilt-other contrast.
Figure 5.
The main effect of social consequence, where activation was greater for other- than self-consequences, included the ventromedial PFC, intracalcarine (left panel), dorsomedial PFC (center panel), the posterior superior temporal sulcus (pSTS), precuneus (right panel), and the posterior cingulate cortex.
Table 5.
Other > Self
| Neural system | Num. of Voxels | Max. Intensity | Std. Dev. Intensity | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| ventromedial PFC | 35 | 2.9 | .11 | 26 | −6 | 36 |
|
| ||||||
| supracalcarine cortex | 565 | 3.6 | 1.01 | −20 | −68 | 24 |
|
| ||||||
| intracalcarine cortex | 23 | 2.8 | .20 | 26 | −72 | 8 |
|
| ||||||
| posterior superior temporal sulcus | 612 | 6.4 | .80 | 44 | −62 | 24 |
|
| ||||||
| cingulate gyrus, poserior division | 246 | 3.8 | .59 | 6 | −44 | 50 |
|
| ||||||
| precuneus cortex | 2411 | 4.1 | 1.13 | 8 | −48 | 50 |
|
| ||||||
| dorsomedial PFC | 515 | 3.6 | .61 | 24 | 14 | 50 |
The Z statistic images were thresholded using clusters determined by Z > 2.3 and a family wise error corrected cluster significance of P<0.05 was applied to the suprathreshold clusters.
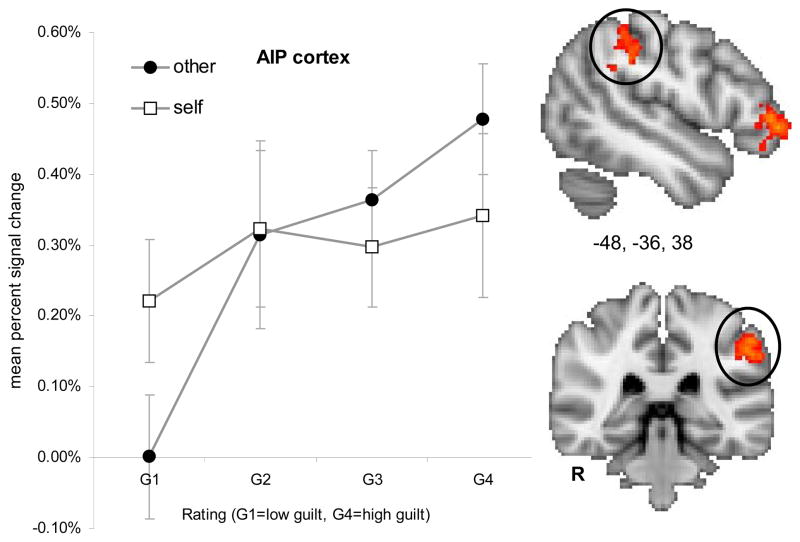
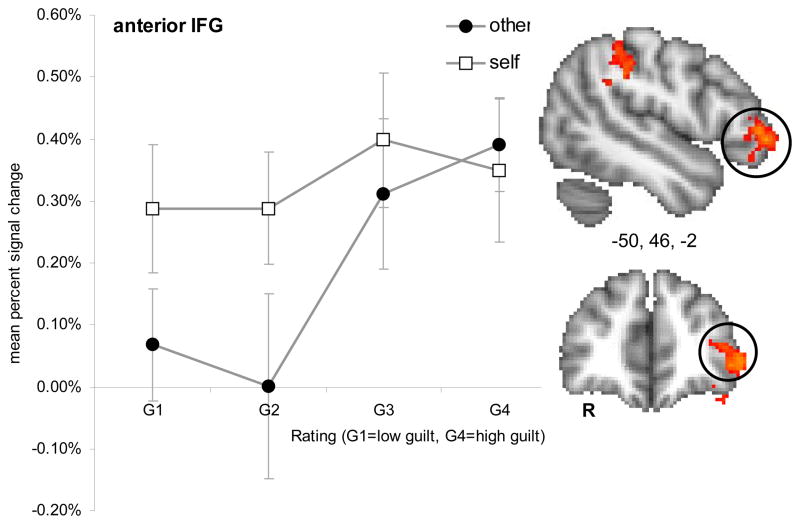
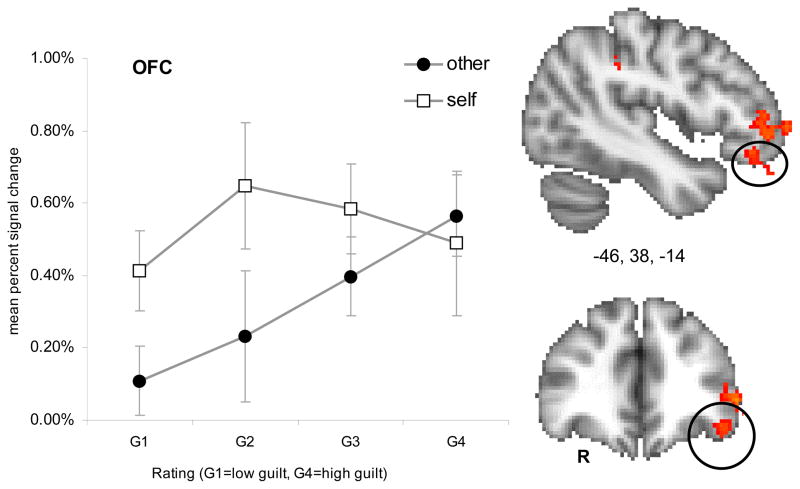
Interaction of Guilt and Social Consequence
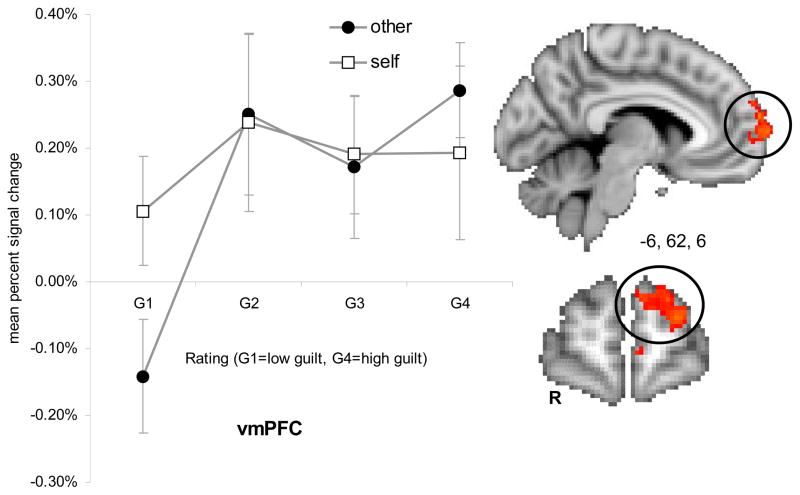
The interaction of guilt * social consequence modeled voxels showing greater correlation with guilt ratings when actions affected others as compared to the self. Active regions for this interaction included the left anterior inferior parietal (AIP) cortex, left anterior inferior frontal gyrus, left orbitofrontal gyrus, and left ventromedial PFC (frontopolar cortex). Thresholded activation maps (p < .05; corrected) of the interaction effect and mean percent signal change are shown for each region in Figure 6a–d and Table 6.
Figure 6.
Figure 6a. Interaction effect of guilt (G1–G4) and social consequence (self or other) in the left anterior inferior parietal (AIP) cortex. Error bars represent standard error of the mean (SEM).
Figure 6b. Interaction effect of guilt (G1–G4) and social consequence (self or other) in the left anterior inferior frontal gyrus (IFG). Error bars represent standard error of the mean (SEM).
Figure 6c. Interaction effect of guilt (G1–G4) and perspective (self or other) in the left orbitofrontal cortex (OFC). Error bars represent standard error of the mean (SEM).
Figure 6d. Interaction effect of guilt (G1–G4) and perspective in the left ventromedial prefrontal cortex (vmPFC). Error bars represent standard error of the mean (SEM).
Table 6.
Interaction of Guilt * Perspective
| region | hemisphere | #voxels | z-score | std. dev. | MNI coordinates (mm) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| inferior frontal gyrus | L | 746 | 3.5 | .52 | −50 | 46 | −2 |
| ventromedial- PFC, frontal pole | L | 450 | 3.1 | .12 | −22 | 60 | 14 |
| orbitofrontal cortex | L | 50 | 2.8 | .24 | −44 | 36 | −12 |
| anterior inferior parietal cortex | L | 403 | 3.3 | .75 | −54 | −36 | 40 |
DISCUSSION
Comparison of neural activation evoked by guilt scenarios in which the participants’ actions resulted in negative consequences to self or to others revealed distinct regions correlated with intensity of guilt, regions recruited by social consequence, and regions where social consequence modulated the guilt response (interaction effect). Regions associated with guilt intensity included the dorsomedial PFC, frontopolar cortex, supramarginal gyrus, and the ventrolateral PFC. Guilty actions leading to harm to others relative to oneself elicited greater activation of the ventromedial and dorsomedial PFC, frontopolar cortex, precuneus, posterior cingulate, and bilateral posterior STS. Finally, select regions were differentially engaged by the intensity of guilt feelings when accompanied by social consequences that included the anterior part of the left IFG, the left ventromedial PFC, and the left anterior inferior parietal cortex.
Ratings of Guilt Feelings
Participants reported feeling the most intense guilt for guilt-other scenarios. A host of features may modulate the intensity of guilt for events affecting self versus others, such as the perceived agent of responsibility, locus of control over negative events, perceived moral transgression, damage to social or interpersonal relationships, perceived level of harm to the affected individual(s), among others (Tangney, 1996; Tangney and Dearing, 2002). It is also conceivable that in specific circumstances one may feel more guilt for self consequences (e.g. overindulging in food might elicit subjectively more guilt than offering food to a friend who overindulges). Thus, it is possible that this finding may be specific to the cognitive and affective features captured by the scenarios we selected. Importantly, the differences in overall intensity ratings were controlled in the ANOVA when considering the other factors of interest.
Neural Mapping of Guilt
Elevated feelings of guilt (main effect of guilt) selectively engaged the dorsomedial PFC, frontopolar cortex, supramarginal gyrus, and the ventrolateral PFC. These regions were largely consistent with the regions about which we hypothesized based on prior work, particularly with respect to inferior frontal gyrus (Shin et al., 2000; Takahashi et al., 2004), frontopolar cortex (Basile et al., 2010; Moll et al., 2007; Moll et al., 2011), dorsomedial PFC (Kedia et al., 2008; Takahashi et al., 2004), supramarginal gyrus (Kedia et al., 2008; Moll et al., 2007), and at a reduced significance threshold also septal/subgenual cingulate areas (Basile et al., 2010; Green et al., 2010; Moll et al., 2011; Zahn et al., 2009a; Zahn et al., 2009c). However, other hypothesized areas including the posterior STS (Takahashi et al., 2004), posterior cingulate/precuneus (Basile et al., 2010; Kedia et al., 2008) and the anterior temporal poles (Shin et al., 2000) were not observed in our results.
While null results are challenging to interpret, it is instructive to consider possible reasons for the lack of findings for these three regions. An important distinction when relating to prior work is that previous studies examined guilt broadly, whereas our study was designed to additionally investigate the unique contribution of social consequence and assess the interaction of these variables. Therefore, variance associated with this variable, which likely includes processes related to perspective-taking and empathy, were controlled in the present study when assessing the neural circuitry related to guilt. Some prior studies appear to include these related processes in their main contrasts of guilt. Consistent with the logic outlined above, converging findings in all three regions have been implicated in a range of tasks involving mentalizing, ToM, and complex aspects of person perception (Amodio and Frith, 2006; Frith and Frith, 1999; Saxe et al., 2004). These tasks generally elicit activity in the posterior STS, an area that has been linked to attention shifting and the perception of biological motion, and the temporal poles, believed to support conceptual representations of social information (Frith and Frith, 1999; Scholz et al., 2009). Finally, the role of the precuneus for integration into a self-referential framework has been demonstrated in tasks including making emotional judgment (Greene and Haidt, 2002; Maddock et al., 2003; Moll et al., 2005).
Furthermore, it is important to consider the broader issue that guilt scenarios may elicit myriad emotions that are difficult to study in isolation. In the present study, several other emotions were experienced by subjects while reading the guilt scenarios, although guilt was the most intense emotion experienced (see Figure 1). In the present study, we cross-validated the guilt ratings of the participants with an independent sample. The strongest correlations across samples were present for guilt, with progressively decreasing correlation strengths for embarrassment, shame, disgust, contentment, valence, tension, pain, anger, fear, and arousal. This issue has largely been overlooked by previous guilt studies. For instance, Shin and colleagues (2000) also examined a similar range of emotions and compared the magnitude of these emotions elicited by guilt scripts as compared to neutral scripts. However, Shin et al and most prior studies did not actually compare the magnitude of these emotions to the magnitude of guilt elicited specifically by the guilt scripts (Basile et al., 2010; Kedia et al., 2008; Ruby and Decety, 2004; Shin et al., 2000; Takahashi et al., 2004; Zahn et al., 2009c), while some assessed this relationship in a few select emotions (Green et al., 2010; Kedia et al., 2008; Zahn et al., 2009c) or none at all (Basile et al., 2010; Takahashi et al., 2004). One notable exception by Moll et al (2007) found that the emotional scripts elicited the following emotions in order of intensity: indignation-other, guilt, compassion, embarrassment, indignation-self, and disgust.
Negative correlations with subjective ratings of guilt were visualized in the superior parietal cortex, lateral occipital cortex, and precuneus (see Figure 4). These regions generally showed activation above baseline or at baseline activation for low guilt and progressive deactivation of increasing magnitude with increasing levels of guilt. This phenomenon has some features in common with resting activity; however, this pattern of activity cannot be attributed to spontaneous free-flowing thoughts typical of rest. Rather, we interpret this activity as Moll and colleagues (2007; 2005) have done, by attributing it to “emotionally neutral but motivationally relevant action sequence and behavioral contingencies that take place during social navigation”. This pattern is consistent with activation associated with social behaviors of emotionally neutral scripts that share neural substrates with the “default mode”. Indeed the free-flowing spontaneous thoughts that predominate during rest are congruent with the non-emotional guilt-neutral scenarios used in our study.
Neural Mapping of Social Consequence
Our results show activation associated with social consequence of one’s actions (harm to others caused by self) in the ventromedial PFC, frontopolar cortex, dorsomedial PFC, precuneus, posterior cingulate, and bilateral posterior STS. These finding were consistent with our predictions in the ventromedial PFC (Mitchell et al., 2006), frontopolar cortex (Ruby and Decety, 2004), posterior superior temporal sulcus (Frith and Frith, 2003; Scholz et al., 2009), and the precuneus/posterior cingulate gyrus (Lombardo et al., 2010). However, two regions we predicted – the temporoparietal junction (Samson et al., 2004; Saxe and Kanwisher, 2003) and the IFG (Schulte-Rüther et al., 2007) – were not observed. The lack of IFG activation was surprising given its putative role in empathy (Kaplan and Iacoboni, 2006) and emotion recognition/evaluation (Jabbi et al., 2007; Schulte-Rüther et al., 2007). Likewise the temporoparietal junction has been consistently reported in relation to empathy (Jabbi et al., 2007; Schulte-Rüther et al., 2007), mentalizing and ToM (Samson et al., 2004; Saxe and Kanwisher, 2003). One possible explanation is the role of self as agent in causing harm to the other. It is possible that for some participants, the guilt-other scenarios may lead to greater preoccupation with self actions rather than thoughts about the harm caused to another. This possibility requires further investigation to confirm. The self-perspective compared to other-perspective has been linked to the ventromedial PFC (Ames et al., 2008) and the precuneus (Saxe and Kanwisher, 2003; Saxe et al., 2006), whereas when subjects are asked to consider the perspective of another individual, the temporoparietal junction comes online (Ruby and Decety, 2004; Saxe et al., 2006).
Guilt and Social Consequence
Whereas guilt and constructs related to social consequence, such as empathy and perspective-taking, have both been investigated independent of one another, to our knowledge, this is the first study to explicitly incorporate the role of social consequence in examining the neural correlates of guilt. Our findings show that guilt feelings resulting from harm to others leads to distinct experiences and differentially modulates activation in a left lateralized network comprised of the anterior IFG, anterior inferior parietal cortex, ventromedial PFC, and lateral orbitofrontal cortex. Specifically, activity in the anterior IFG and the orbitofrontal cortex (see Figure 6b and 6c) associated with actions resulting in harm to self was independent of the intensity of guilt feelings, whereas activity in these regions was associated with actions resulting in harm to others only with high intensity guilt feelings but not for low intensity guilt feelings. On the other hand, the interaction effect in the anterior inferior parietal cortex was qualitatively different (see Figure 6a). Here the response was associated with moderate activation at all levels for guilt-self, whereas the guilt-other condition resulted in comparatively greater activation for highly intense feelings of guilt and comparatively low activation for mild feelings of guilt. Our results suggest that activity in these regions is differentially modulated for guilt-other as compared to guilt-self, consistent with the idea that harm to others may incur a greater cost to one’s social standing. In particular, the inferior parietal cortex also plays an important role, according to Chiao and colleagues (2009), in evaluating the social status of another individual, which is an important component in calculating the social cost of harming another individual.
The orbitofrontal cortex is another region that is associated with negative emotional states (Moll et al., 2007; Zahn et al., 2009c), most notably with the social emotions of regret (Camille et al., 2004) and embarrassment (Takahashi et al., 2004). Damage to the orbitofrontal cortex may manifest in impaired social perception (Moll et al., 2005) and an impaired ability to incorporate emotions into decision making (Bechara et al., 2000). Our findings in the orbitofrontal cortex are consistent with earlier predictions of guilt induced activation of this region (Shin et al., 2000), however most prior reports have failed to demonstrate orbitofrontal cortex activation in response to guilt (Basile et al., 2010; Moll et al., 2007; Ruby and Decety, 2004; Shin et al., 2000; Takahashi et al., 2004; Zahn et al., 2009c). Given that the stimuli in the present study elicited feelings of guilt along with a secondary component of embarrassment, a possible is that orbitofrontal activation may in part be linked to embarrassment which has a higher affinity to the violation of social conventions (choices of clothing, etiquette and hygiene, etc.) (Haidt, 2003; Tangney, 1996).
Limitations
A key limitation that is difficult to address in any design, and consequently was shared by other studies in this field, was the challenge in eliciting guilt without also eliciting a complex array of other emotions such as shame, anger, disgust, and negative valence more generally. Our behavioral data provide some insight into the complex landscape of emotional responses that are experienced concomitantly with guilt. Relatedly, one limitation to interpreting our social consequence manipulation was that no explicit measure of perspective-taking or empathy was collected.
The present behavioral differences of greater guilt feelings for events harming others may be explained by the neural processes outlined in this paper. However, it is possible to construct scenarios where more guilt is experienced for events affecting self and less for others. To the extent that the behavioral findings are specific to the chosen scenarios, the fMRI findings would be similarly construed. Furthermore, guilt feelings elicited by guilt-self scenarios might be qualitatively different from guilt elicited by guilt-other scenarios. While we sometimes experience guilt for our own wrong deed, guilt is frequently associated with harm to others. Harm to self (e.g. “you are in debt because you spent too much money on a car you couldn’t afford”) may often induce regret rather than guilt. Conversely, a guilt-self scenario (e.g. “you get arrested for shoplifting a shirt at a store”) resulting in potential harm to a storekeeper may also be construed as guilt-other. Therefore, the present results must be interpreted with the understanding that the scenarios are not valid in all settings or the classifications may change depending on social and personal context. Relatedly, while guilt was rated higher than other emotions, there was also a contribution from embarrassment. Finally, it is always difficult to extrapolate from hypothesized scenarios to real-world situations involving guilty actions that are known to affect others directly.
The study was conducted in a sample of men as a prelude to studying guilt in combat-related PTSD. The exclusion of women may influence the behavioral and neural findings. Substantial evidence shows that when reasoning about others, women are more inclined to empathize than men (Toussaint and Webb, 2005), with a corresponding gender difference in neural activation (Singer et al., 2006). Therefore, the present findings ought to be viewed in this context.
Future work might better address some of the limitations we highlighted and further examine the parameter space relevant to guilt processing. This work might incorporate not just how much guilt the subject experienced, but other experiences such as level of embarrassment, level of regret, intentional versus accidental harm to others, level of perceived suffering of the individual that is harmed, level of negative consequences resulting from the action, level of empathy, and the extent of the participant’s role in events that resulted in negative consequences for the participant or for others. In behavioral research on the role of agency on emotions, guilt was selected as the most frequent emotion in the negative self-agency condition out of a choice of shame/embarrassment, guilt, indignation/anger, pride, gratitude whereas anger was selected the most frequent negative emotion elicited by other-agency (Zahn et al., 2009c). These investigations as well as those in clinical disorders of PTSD and depression are certain to be important to this understudied area of social neuroscience.
Conclusions
The intensity of guilt associated with harm to others is greater than for harm to self, and differential activation in the anterior IFG, ventromedial PFC, and the anterior inferior parietal cortex forms the neural basis of these varied guilt experiences. The neural response to guilt intensity can be distinguished from the response to social consequence, as well as regions where social consequence modulates the response to guilt. Our results suggest specific regions intensify guilt when harm to others may incur greater social cost. These findings may also have important implications for understanding psychiatric conditions like PTSD and depression, where alterations in guilt and empathic capacity may constitute core symptoms.
Supplementary Material
Highlights.
The goal was to investigate the neural processes linking perspective taking and guilt.
Select regions show stronger correlation with feelings of guilt for the other > self perspective.
These regions include the anterior IFG, ventromedial PFC, and anterior inferior parietal cortex.
These regions intensify guilt associated with harm to others which may incur greater social cost.
Acknowledgments
Support: This research was supported by the National Institutes of Health Grant K23 MH073091 (RAM), the Department of Veterans Affairs, Mental Illness Research Education and Clinical Center Grant for Post-Deployment Mental Health (RAM, SS, JDN, ESS, GM)
We thank Courtney C. Haswell for helping analyze functional MRI and behavioral data, and Michele Diaz and Vanessa M. Brown for assessing syntactic complexity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ames DL, Jenkins AC, Banaji MR, Mitchell JP. Taking another person’s perspective increases self-referential neural processing. Psychological Science. 2008;19:642–644. doi: 10.1111/j.1467-9280.2008.02135.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Basile B, Mancini F, Macaluso E, Caltagirone C, Frackowiak RS, Bozzali M. Deontological and Altruistic Guilt: Evidence for Distinct Neurobiological Substrates. Human Brain Mapping. 2010 doi: 10.1002/hbm.21009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD. Two forms of perspective taking: Imagining how another feels and imagining how you would feel. In: Markman KD, Klein WMP, Suhr JA, editors. Handbook of imagination and mental simulation. New York, NY US: Psychology Press; 2009. pp. 267–279. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Oby ER, Li Z, Parrish T, Bridge DJ. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 2009;47:354–363. doi: 10.1016/j.neuropsychologia.2008.09.023. [DOI] [PubMed] [Google Scholar]
- de Waal FBM. Putting the altruism back into altruism: The evolution of empathy. Annual Review of Psychology. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ. Neurological and neuropsychological bases of empathy. Eur Neurol. 1998;39:193–199. doi: 10.1159/000007933. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. MagnResonMed. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Ralph MAL, Moll J, Stamatakis EA, Grafman J, Zahn R. Selective functional integration between anterior temporal and distinct fronto-mesolimbic regions during guilt and indignation. Neuroimage. 2010;52:1720–1726. doi: 10.1016/j.neuroimage.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J, Haidt J. How (and where) does moral judgment work? Trends in Cognitive Sciences. 2002;6:517–523. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Haidt J. The moral emotions. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences. New York, NY US: Oxford University Press; 2003. pp. 852–870. [Google Scholar]
- Hoffman M. Development of prosocial motivation: Empathy and guilt. In: Eisenberg N, editor. The development of prosocial behavior. New York: Academic Press; 1982. pp. 281–313. [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaplan JT, Iacoboni M. Getting a grip on other minds: mirror neurons, intention understanding, and cognitive empathy. Soc Neurosci. 2006;1:175–183. doi: 10.1080/17470910600985605. [DOI] [PubMed] [Google Scholar]
- Kedia G, Berthoz S, Wessa M, Hilton D, Martinot JL. An agent harms a victim: A functional magnetic resonance imaging study on specific moral emotions. Journal of Cognitive Neuroscience. 2008;20:1788–1798. doi: 10.1162/jocn.2008.20070. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The Neural Substrate of Human Empathy: Effects of Perspective-taking and Cognitive Appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Baron-Cohen S, Consortium MA. Shared Neural Circuits for Mentalizing about the Self and Others. Journal of Cognitive Neuroscience. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence detection task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Macrae CN. Perspective-taking from a social neuroscience standpoint. Group Processes & Intergroup Relations. 2008;11:215–232. [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R. Moral judgments, emotions and the utilitarian brain. Trends in Cognitive Sciences. 2007;11:319–321. doi: 10.1016/j.tics.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Garrido GJ, Bramati IE, Caparelli-Daquer EMA, Paiva MLMF, Zahn R, Grafman J. The self as a moral agent: linking the neural bases of social agency and moral sensitivity. Soc. 2007;2:336–352. doi: 10.1080/17470910701392024. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Zahn R. Neuroscience and morality: Moral judgments, sentiments, and values. In: Narvaez D, Lapsley DK, editors. Personality, identity, and character: Explorations in moral psychology. New York, NY US: Cambridge University Press; 2009. pp. 106–135. [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Bramati IE, Krueger F, Tura B, Cavanagh AL, Grafman J. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. Neuroimage. 2011;54:1735–1742. doi: 10.1016/j.neuroimage.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. Neuroimage. 2010;49:3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: Linking developmental psychology and functional neuroimaging. Annual Review of Psychology. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive & Affective Neuroscience. 2006;1:229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS ONE [Electronic Resource] 2009;4:e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy. Journal of Cognitive Neuroscience. 2007;19:1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15:324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, Alpert NM, Fischman AJ, Rauch SL. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biological Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Overview of fMRI analysis. British Journal of Radiology. 2004;77:S167–S175. doi: 10.1259/bjr/33553595. [DOI] [PubMed] [Google Scholar]
- Szmrecsanyi B. Operationalizing Syntactic Complexity. International Conference on the Statistical Analysis of Textual Data; Louvain, BELGIUM, JDAT. 2004. [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004;23:967–974. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Tangney JP. Conceptual and methodological issues in the assessment of shame and guilt. Behaviour Research & Therapy. 1996;34:741–754. doi: 10.1016/0005-7967(96)00034-4. [DOI] [PubMed] [Google Scholar]
- Tangney JP, Dearing RL. Shame and Guilt. New York: The Guilford Press; 2002. [Google Scholar]
- Toussaint L, Webb JR. Gender differences in the relationship between empathy and forgiveness. Journal of Social Psychology. 2005;145:673–685. doi: 10.3200/SOCP.145.6.673-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, de Oliveira-Souza R, Bramati I, Garrido G, Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neurosci Lett. 2009a;457:107–110. doi: 10.1016/j.neulet.2009.03.090. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009b;132:604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, Grafman J. The neural basis of human social values: Evidence from functional MRI. Cerebral Cortex. 2009c;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.