Summary
The human adrenal cortex secretes mineralocorticoids, glucocorticoids and adrenal androgens. These steroids are produced from unique cell types located within the three distinct zones of the adrenal cortex. Disruption of adrenal steroid production results in a variety of diseases that can lead to hypertension, metabolic syndrome, infertility and androgen excess. The adrenal cortex is also a common site for the development of adenomas, and rarely the site for the development of carcinomas. The adenomas can lead to diseases associated with adrenal steroid excess, while the carcinomas are particularly aggressive and have a poor prognosis. In vitro cell culture models provide an important tool to examine molecular and cellular mechanisms controlling both the normal and pathologic function of the adrenal cortex. Herein we discuss the human adrenocortical cell lines and their use as model systems for adrenal studies.
Keywords: Adrenocortical cell line, Steroidogenesis, Cancer therapy, Adrenal cortex, model systems, cell lines
1. Introduction
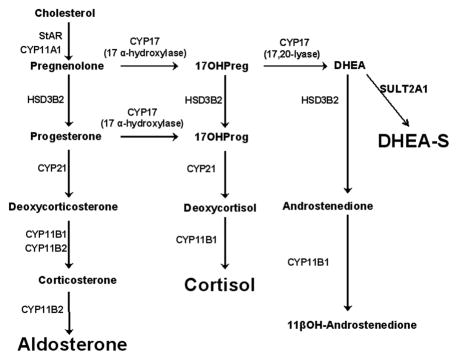
The adrenal cortex is composed of three functionally distinct regions, the zona glomerulosa (ZG), zona fasciculata (ZF), and zona reticularis (ZR). The ZG synthesizes mineralocorticoids; the ZF produces cortisol and the ZR secretes the so called adrenal androgens, DHEA and DHEA-sulfate. Each zone is preferentially regulated by different circulating factors that include angiotensin II (Ang II) and potassium (K+) for the ZG, adrenocorticotropic hormone (ACTH) for the ZF, and ACTH plus other yet to be determined factors for the ZR (Parker and Rainey, 2004)(Figure 1). It has been established that the reason each zone secretes a unique set of steroids is related to the selective expression of steroid-metabolizing enzymes within each zone (Rainey, 1999; Rainey et al., 2002; Vinson, 2003; Nguyen and Conley, 2008) (Figure 2). However, the molecular mechanisms that cause zone-specific expression patterns of enzymes are yet to be resolved.
Figure 1.
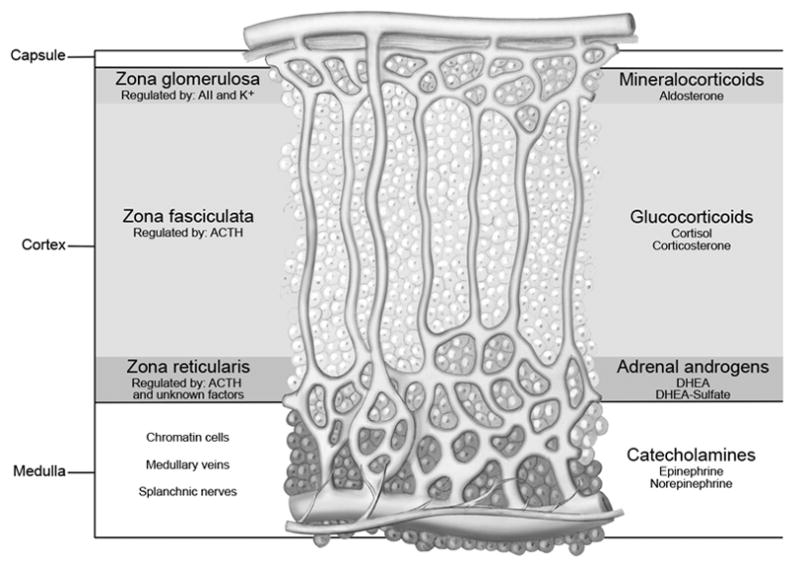
The adrenal cortex is divided into three histological and functionally distinct zones: the zona glomerulosa synthesizes mineralocorticoids; zona fasciculata produces cortisol and zona reticularis secrets the so called adrenal androgens, DHEA and DHEA-sulfate.
Figure 2.
Human adrenal steroid biosynthetic pathways illustrating the three main products of the human adrenal cortex: aldosterone, cortisol, and adrenal androgens (DHEA, DHEA-S) as well as the enzymes that synthesize these steroids. StAR = steroidogenic acute regulatory protein; CYP11A1 = cholesterol side-chain cleavage enzyme; HSD3B2 = 3 beta-hydroxysteroid dehydrogenase type II; CYP21 = 21-hydroxylase; CYP11B1 = 11-beta-hydroxylase; CYP11B2 = aldosterone synthase; CYP17 = 17-alpha-hydroxylase/17, 20 lyase; SULT2A1 =Steroid-sulfotransferase.
Adrenal steroid production remains an area of active research, which supports the need to develop appropriate cell models that can mimic adrenal physiology or pathology. Primary cultures of adrenocortical cells have proven to be useful for examining the mechanisms controlling many aspects of adrenal physiology (Chen and Hornsby, 2006; Kuulasmaa et al., 2008; Cardoso et al., 2009; Xing et al., 2010; Xing et al., 2011). However, several issues have limited the use of primary adrenal cells as in vitro models. The most common limitations are the constant requirement for fresh tissue and the difficulties associated with the isolation of adequate cortical cells. In addition, cells from different human donors are subject to considerable variability; whereas cells from rodents do not produce cortisol or adrenal androgens due to the lack of steroid 17α-hydroxylase (CYP17) expression. To overcome the problems with tissue accessibility and quality, many groups have attempted to establish cell lines from adrenocortical carcinomas. This approach has been somewhat successful leading to adrenal cell lines from several species and we have previously reviewed the overall development of these models (Rainey et al., 1994; Rainey et al., 2004). Herein, we focus only on the human adrenocortical cell lines and provide details with regard to their development and utility.
2. Human adrenocortical carcinoma cell lines
2.1 The NCI-H295 derived adrenocortical carcinoma cell lines
The NCI-H295 cell line was established from a female patient diagnosed with an adrenocortical carcinoma(Gazdar et al., 1990). A large invasive adrenocortical tumor was detected in this patient and was later reported to have metastasized to the lungs and liver. Following tumor extraction, the tissue was finely minced, defragmented and maintained in various serum-containing and serum-free culture media for a one year period. The most vigorous growing cells were selected and designated as the NCI-H295. Radioimmunoassay (RIA) and gas chromatography/mass spectroscopy (GCMS) analysis demonstrated that the selected cells could produce a variety of steroids (Gazdar et al., 1990).
Because the original NCI-H295 cells grow very slowly as loosely attached cell clusters, alternative growth conditions were sought to segregate a population of cells with better monolayer attachment and more rapid growth. To achieve this goal, cells were continuously flushed with growth medium to remove the floating suspended cells and retain the attached subtype. Based on the serum supplement used for growth, three strains were developed and have been termed H295R-S1, H295R-S2 and H295R-S3 (Rainey et al., 2004). All three strains grow as adherent monolayer cultures (Table 1). However, the responses of these strains and their growth characteristics vary significantly, which appears related to the different growth medium. As a result the functional aspects of the H295R cells vary in individual laboratory where tissue culture conditions are different that those used for their isolation.
Table 1.
Summary of Human Adrenocortical Cell Lines
| Cell Line Name | Ang II | K+ | ACTH | cAMP | Steroids produced | Reference |
|---|---|---|---|---|---|---|
| NCI-H295 | ND | ND | − | + | Mineralocorticoids Glucocorticoids Adrenal Androgens |
(Gazdar et al., 1990; Staels et al., 1993; Rainey et al., 1994) |
| NCI-H295A | − | ND | − | + | Mineralocorticoid Glucocorticoids Adrenal Androgens |
(Rodriguez et al., 1997; Huang et al., 2005) |
| NCI-H295R Strains | + | + | −/+ | + | Mineralocorticoids Glucocorticoids Adrenal Androgens |
(Bird et al., 1993;Rainey et al., 1994; Bird et al., 1995; Clark et al., 1995; Denner et al., 1996) |
| L-251 | ND | ND | − | ND | None reported | (Schteingart et al., 2001) |
| ACT-1 | ND | ND | ND | ND | None reported | (Ueno et al., 2001) |
| SW13 | ND | ND | ND | ND | None reported | (Leibovitz et al., 1973) |
| Pediatric adrenocortical adenoma derived cell line | ND | ND | ND | ND | Mineralocorticoids Glucocorticoids |
(Almeida et al., 2008) |
| HAC15 | + | + | + | + | Mineralocorticoids Glucocorticoids Adrenal Androgens |
(Parmar et al., 2008) |
| PRKAR1A inactivated cell line | ND | ND | − | − | None reported | (Nesterova et al., 2008) |
Abbreviation: ND, Not determined
H295R-S1 grows in a commercially available Nu-Serum type I (5%, Collaborative Biomedical Products, Bedford, MA) supplemented medium. This strain is available from the American Type Culture Collection as ATCC CRL-2128. Strain 2 (H295R-S2) grows in the medium with the serum substitute called Ultroser-G (2.5%, Pall Corporation, Port Washington, NY). Previous assessments reported increased adrenal cell growth and steroidogenic function with this media supplement (McAllister and Hornsby, 1987; Hornsby and McAllister, 1991; McAllister et al., 1994). Indeed these cells remain highly differentiated and respond to Ang II and K+ treatment by increased steroid production. Strain 3 (H295R-S3) grows in a serum called Cosmic Calf (10%, Invitrogen, Grand Island, NY).
Walter Miller and colleagues used the parental NCI-H295 cell line to select another monolayer strain called H295A (Rodriguez et al., 1997). The method for isolation of this strain was similar to that described for H295R, relying on the removal of non-attached cells with medium changes, to select a population of cells that grew as a monolayer. Interestingly, H295A cells have limited response to Ang II (Table 1).
In an attempt to develop a new human adrenocortical carcinoma (HAC) cell line with ACTH responsiveness, Parmer et al. isolated clonal populations of cells from what was thought to be a “novel” adrenal tumor (Parmar et al., 2008). However, subsequent single-nucleotide polymorphism (SNP) array analysis indicated that the clones were isolated from contaminated H295R cells. Two of the clones (HAC13 and HAC15) responded well to Ang II and K+ treatment, with increased aldosterone production. HAC15 cells also exhibited modest response to ACTH through significant increases in cortisol production and steroidogenic enzyme expression (Table 1). Compared to the NCI-H295 cell strains, isolated from a mixed population of tumor cells, the HAC cell clones are monoclonal which may provide a more stable steroidogenic phenotypes with time in culture.
2.2 SW13 human adrenal carcinoma derived cell line
SW13 cells were derived from a small cell carcinoma in the adrenal cortex of a 55-year-old female (Table 1). Although the cell line was taken from a surgically removed adrenal, SW13 cells produce no steroids and it is unclear whether the cell line was derived from a primary cancer arising from the adrenal cortex or from metastasis to the adrenal cortex (Leibovitz et al., 1973). SW13 cells are available from the American Type Culture Collection (CCL-105). These cells have a mosaic pattern of vimentin expression and are deficient in the mammalian homologues of Brahma genes, Brm and BRG1 (Hedberg and Chen, 1986; Butler et al., 2000; Yamamichi-Nishina et al., 2003). Due to the lack of steroidogenic phenotype, the usefulness of these cells as an adrenocortical model system is limited.
2.3 PRKAR1A inactivated adrenocortical cells
This cell line was derived from the adrenal glands of a patient diagnosed with primary pigmented nodular adrenocortical disease (PPNAD) and Carney complex (Table 1). The PRKAR1A gene that encodes the regulatory subunit type 1A (RIα) of cAMP-dependent protein kinase (PKA) was inactivated via gene mutation after the introduction of this cell line. PRKAR1A cells originally produced cortisol in the early passages, but lost this capacity while in culture (Nesterova et al., 2008). Other than steroid production, this cell line was mainly used for cAMP/PKA signal pathway studies, which involved adrenal tumorogenesis (Almeida and Stratakis, 2011).
2.4 ACT-1 human adrenal carcinoma derived cell line
ACT-1 cells were isolated from a 62 year old male patient who was initially diagnosed with a left adrenocortical carcinoma. ACT-1 cells were only shown to express HSD3B2 enzymes, and were devoid of any adrenocortical steroid production (Ueno et al., 2001). Thus, ACT-1 cells have limited adrenocortical function and therefore serve in a restricted role for adrenal steroidogenic studies (Table 1). However, these cells may prove useful to screen for adrenocortical carcinoma therapies (Kiiveri et al., 2004).
2.5 RL-251 human adrenal carcinoma derived cell line
A right adrenal mass was removed and placed in cell suspension from a 75 year old male who presented with high blood pressure and fever. As with H295R cell lines, the isolated RL-251 cultures exhibited an abnormal karyotypic profile, consisting of numerous deletions and translocations (Table 1). Assessments of adrenocortical function showed atypical steroidogenesis, and lack of response upon ACTH stimulation (Schteingart et al., 2001). To date, steroid production has not been observed in the RL-251 cell line. Intriguingly, in the initial report, RL-251 cells expressed ample amounts of interleukin-8, epithelial cell-derived neutrophil-activating peptide 78, growth regulated oncogene-α and growth regulated oncogene-y. These molecules are CXC chemokine family cytokines that have potent angiogenic activity essential for tumor growth. It is speculated that these chemokines may play a role in the enhancement of carcinoma growth and proliferation in an autocrine or paracrine manner (Schteingart et al., 2010). While a lack of reported steroidogenesis and hormonal responses make these cells an inappropriate model to study steroidogenesis, these cells may prove useful in identifying the role of chemokines in adrenocortical carcinomas.
2.7 Pediatric adrenocortical adenoma derived cell line
Primary adrenocortical adenoma cells were isolated from a 1 year old female that presented with virilization and Cushing’s syndrome (Almeida et al., 2008). The cell line initially grew at a slow rate with a spindle-like morphology. Melan-A, a melanocytic differentiation marker, which is expressed in steroid hormone producing adrenal adenomas and carcinomas, was detected in the cells (Ghorab et al., 2003). Biosynthesis of cortisol, aldosterone, androstenedione, and 17-hydroxyprogesterone was observed along with the expression of steroidogenic enzymes that included HSD3B2, CYP11B1 and CYP21 (Table 1). Taking into consideration that this newly developed pediatric cell line was last reported to have only reached eight passages, the likelihood of the cell line’s wide-spread use remains uncertain.
2.8 SV40 transformed adrenal cell lines
Simian virus 40 (SV40) T-antigen is a viral oncogene which is capable of transforming many cell types (Hornsby et al., 1989). The use of a SV40 T-antigen transformation strategy resulted in the production of human fetal adrenal cell clones that responded to cAMP with an increase in both CYP17 and CYP11A1, but no change in CYP21 and CYP11B1 (Table 1). These transformed cells were maintained in culture for 30 to 40 population doublings after isolation, but then entered a “crisis” stage and stopped dividing (Cheng et al., 1992). The inability to maintain these cell cultures for extended periods limits their widespread use as a steroidogenic or carcinoma model.
3. Steroidogenic enzyme expression and steroid production
SW13, PRKAR1A, ACT-1, and RL-251 cells appear to lack steroidogenic potential. The previously described pediatric adrenocortical adenoma derived cell line and the SV40 transformed adrenal cell lines showed some steroidogenic capacity, but both stopped growing after several passages. In this section, we will limit our discussion to the continuous growing NCI-H295 cell strains and clonal HAC15 cells.
After establishment of NCI-H295 cells, primary assessment of steroidogenic capacity was performed using GCMS and radioimmunoassay. Of a total of 30 different steroids reportedly synthesized and secreted by parental NCI-H295, approximately 20 were formally identified as known steroid hormones. The production of these steroids suggested the original NCI-H295 cells expressed all of the enzymes participating in normal human adrenal steroidogenesis (Gazdar et al., 1990). Along with the parental NCI-H295, the H295R and H295A cell strains have also been used as models for studying steroidogenic enzyme gene expression. Transcripts encoding StAR, HSD3B2, as well as the five forms of cytochrome P450 known to be involved in normal adrenal steroidogenesis (CYP11A1, CYP17, CYP21, CYP11B2 and CYP11B1), are detectable in the H295 cell strains, and HAC15 clonal cell line (Bird et al., 1993b; Bird et al., 1993a; Bird et al., 1995a; Bird et al., 1995b; Bird et al., 1996; Denner et al., 1996; Samandari et al., 2007; Parmar et al., 2008).
The ability to produce steroids that span the multiple zones of the adrenal cortex, suggests that the NCI-H295 derived cell lines remain pluripotent with regard to adrenocortical differentiation. These cells produce an array of steroids even under basal conditions (Rainey et al., 1994; Xing et al., 2011). It is noteworthy that treatment with agonists appears to selectively promote the synthesis of certain zone-specific steroid hormone groups in H295R. For instance, Ang II and K+ stimulation drastically increased aldosterone production (Bird et al., 1993b; Bird et al., 1993a; Rainey et al., 1994; Clark et al., 1995), while treatment with forskolin induced cortisol, 11β-hydroxyandrostenedione, DHEA, DHEA-sulfate, corticosterone, 11-deoxycortisol, and androstenedione production (Rainey et al., 1993; Xing et al., 2011). As stated above, with a wide variety of effective agonists, a mosaic of unique steroid expression profiles are possible in H295A, H295R and HAC15 cells, thus making these cell lines potential models for steroid hormone biosynthesis in all adrenocortical zones.
4. Receptors and responsiveness to agonist
Although Ang II, K+ and ACTH are the primary regulators of adrenal steroid hormone production, there was no indication of hormonal responsiveness in the original description of the NCI-H295 cells (Gazdar et al., 1990). However, subsequent to original report, the responses to Ang II, K+, and ACTH treatment, as well as expression of corresponding receptors, were characterized for the H295A, H295R, and HAC15 cell models. Ang II is the primary hormonal regulator within the renin-angiotensin-aldosterone system (RAAS), and it acts on the adrenal glomerulosa by binding to type 1 Ang II (AT1) receptors to increase the production of aldosterone. The H295R cell has proven to be a useful model to study Ang II regulated aldosterone production(Nogueira et al., 2007; Otani et al., 2008; Nogueira et al., 2009). Only AT1 receptor antagonists have a significant inhibitory effect on these cells, while type 2 AngII (AT2) receptor antagonists have little impact on Ang II stimulation, demonstrating that H295R cells respond almost exclusively to the AT1 receptors(Bird et al., 1993a; Bird et al., 1994). Subsequent studies also revealed AT1 receptor coupled with the expression of phosphoinositidase C increased inositol phosphates in H295R cells(Bird et al., 1993b). The HAC15 cell line also resulted in an increase of aldosterone production when stimulated by Ang II(Parmar et al., 2008). In contrast, the H295A cell strain did not show a significant increase of steroid production when stimulated by Ang II, which is consistent with the known low level of AT1 receptor expression in this cell strain(Samandari et al., 2007).
The other major physiologic regulator of adrenal aldosterone production is extracellular K+. An increase in intracellular calcium levels, in response to elevated K+, mediates an increase in aldosterone biosynthesis. In addition, there is evidence of an intra-adrenal renin/angiotensin system, in which K+ stimulation increases the production of both Ang I and Ang II(Hilbers et al., 1999). The H295R cell line is used by many laboratories as a model to study the mechanisms of K+ regulation of adrenal steroid production(Romero et al., 2006; Bandulik et al., 2010; Nogueira et al., 2010). HAC15 cells also show a significant increase of aldosterone production in response to the K+ treatment. However, response of the H295A cell strain to K+ is currently unknown.
ACTH, along with its receptor (melanocortin 2 receptor, MC2R), is the primary hormonal regulator of adrenal cortisol production. Interestingly, the H295R cell line is only mildly responsive to ACTH, while most other adrenocortical cell lines are completely unresponsive(Parmar et al., 2008). However, ACTH treatment of H295R cells results in an acute increase in aldosterone biosynthesis, though the cell line lacks long term responsiveness to ACTH(Staels et al., 1993; Janes et al., 2008; Parmar et al., 2008). The H295A strain exhibited similar ACTH receptor expression to H295R according to a comparison study between these two cell strains(Samandari et al., 2007). Since ACTH primarily regulates cortisol production through cAMP signaling, the addition of either forskolin (to activate adenylyl cyclase) or cAMP analogues is often used to overcome this lack of effect of ACTH in H295A and H295R strains. (Rainey et al., 1993; Rainey et al., 1994; Bird et al., 1996; Samandari et al., 2007). Another alternate strategy could involve the use of transgenic technology to reinstate MC2R expression in the cell lines. However, attempts to do so have resulted in an increase in receptor expression, but not in a detectable response to ligand in H295R cells (unpublished observation). The MC2R gene is expressed in HAC15 cells, with mRNA levels that are higher than those found in the H295R cell line(Parmar et al., 2008). However, the expression of the MC2R gene and the response to ACTH of the HAC15 clones is lower compared to primary culture of adrenal cells. At present, investigations of ACTH responsiveness rely heavily on the primary culture of human adrenal cells and mouse Y1 adrenal cell line (Schimmer et al., 2006; Xing et al., 2011).
5. Cell lines as adrenal cancer therapy tools
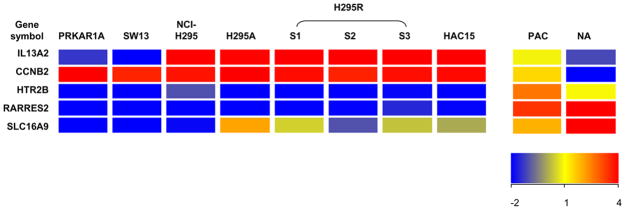
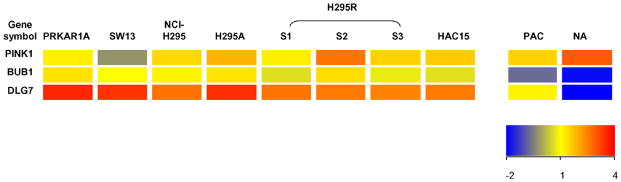
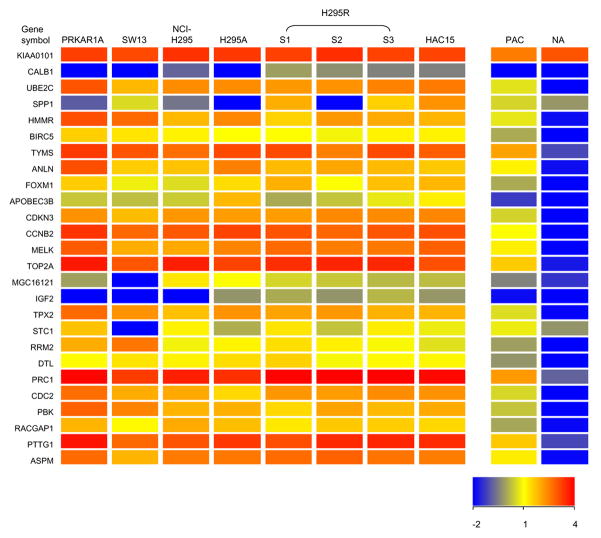
Adrenocortical carcinoma (ACC) is a rare malignant neoplasm with a poor prognosis. Previous studies showed that several genes are associated with ACC. Fernandez-Ranvier et al. compared the gene profiles between 11 malignant adrenocortical carcinomas and 78 benign adrenocortical adenoma, and showed that the overexpression of CCNB2 (cyclin B2) and IL13RA2 (interleukin 13 receptor, alpha 2); and decreased expression of SLC16A9 (solute carrier family 16, member 9), HTR2B (5-hydroxytryptamine receptor 2B) and RARRES2 (retinoic acid receptor responder ) are related to the risk of malignancy of adrenocortical tumors(Fernandez-Ranvier et al., 2008). Interestingly, H295 cell strains had higher CCNB2, IL13A2 and lower SLC16A9, HTR2B and RARRES2 mRNA levels compared with normal adrenal tissue (Figure 3). de Reynies et al. found that DLG7 (Discs large homolog 7), PINK1 (PTEN induced putative kinase 1) and BUB1B (budding uninhibited by benzimidazoles 1 homolog beta) were related to the outcome prognosis of adrenocortical carcinoma(de Reynies et al., 2009). These genes were also differentially expressed between H295 cell strains and normal adrenal tissues in a manner similar to that seen for ACC versus normal adrenal tissue (Figure 4). Giordano et al compared ACC with adrenocortical adenoma and normal adrenal tissue by using cDNA microarray, and showed that more than 20 genes significantly highly expressed in ACC(Giordano et al., 2009). Most of the genes in their analysis list are also expressed higher in SW13, PRKAR1A, and H295 cell strains when compared to normal adrenal tissue according to our microarray analysis (Figure 5). These data suggest that H295 derived cell lines are likely appropriate models for certain aspects of ACC study.
Figure 3.
Heatmap of CCNB2, IL13A2, SLC16A9, HTR2B and RARRES2 genes in human PRKAR1A, SW13, H295 cell strains, HAC15 cells, primary adrenal cells and normal adrenal tissue based on microarray analysis. CCNB2, IL13A2 were expressed at higher level while SLC16A9, HTR2B and RARRES2 were expressed at lower level in most of the cell lines compared with the human normal adrenal tissue or primary culture of adrenal cells. The expression of these genes in cell lines showed a consistency with its expression in malignant adrenocortical tumor according to Fernandez-Ranvier et al. study(Fernandez-Ranvier et al., 2008). The color of heatmap indicates the expression value of each gene in the corresponding sample, and is based on the bar in the lower right-hand corner. PAC=primary adrenal cells; NA= normal adrenal tissue.
Figure 4.
Heatmap of DLG7, PINK1 and BUB1B genes, which were able to predict adrenocortical tumor prognosis(de Reynies et al., 2009), in human PRKAR1A, SW13, H295 cell strains, HAC15 cells, primary adrenal cells and normal adrenal tissue based on microarray analysis. Expression in these cancer prognostic genes varied between the cell lines and normal adrenal tissue. The color of heatmap indicates the expression value based on the bar in the lower right-hand corner. PAC=primary adrenal cells; NA= normal adrenal tissue.
Figure 5.
Heatmap of genes found to be elevated in ACC by Giordano et al. study(Giordano et al., 2009). These genes also showed a higher expression level in most cell lines compared with normal adrenal tissue. Primary cultures of adrenal cells had a phenotype between normal adrenal tissue and the cell lines. The expression level of each gene was indicated by bar in the lower right-hand corner. PAC=primary adrenal cells; NA= normal adrenal tissue.
In vitro cancer therapy screening represents an important preclinical assessment of anti-cancer compounds before any clinical evaluation (Weiss et al., 1980; Fisher et al., 1981; Bodrogi, 1989; Shoemaker, 2006). SW13 and NCI-H295 cell strains have been widely used as tools for screening anti-cancer drugs. Suramin, one of the first anti-cancer drugs tested in the NCI-H295 cells, reduced the production of glucocorticoids, mineralocorticoids and adrenal androgens(La Rocca et al., 1990). Another proposed anti-cancer drug, mitotane, strongly suppressed cell growth of NCI-H295 cells(Schteingart et al., 1993), and later adopted as an anti-adrenocortical carcinoma drug(Veytsman et al., 2009; Bertagna, 2010; Fassnacht et al.). Recently, a study showed that mitotane increased the effect of radiotherapy on both H295R and SW13 cell models(Cerquetti et al., 2010). Fallo et al. tested the cytotoxic/anti-proliferation drugs, taxol and paclitaxel, which effectively exhibited dose-dependent inhibition of cellular growth and steroidogenesis in NCI-H295 cells (Fallo et al., 1996; Fallo et al., 1998). The adrenostatic compounds aminoglutethimide (AG), metyrapone (MTP) and etomidate (ETO) were also tested in NCI-H295 cells for their anti-proliferative properties. AG and ETO inhibited cell proliferation and ETO was much more potent than AG(Fassnacht et al., 2000). Thiazolidinediones (TZDs) are specific peroxisome proliferator-activated receptor (PPAR)-gamma ligands. Examination in NCI-H295 cells suggested that TZDs might have favorable effects in the treatment of a variety of tumors and appear to act as differentiation-inducing agents(Betz et al., 2005). Rosiglitazone, a member of TZDs, showed autophagy in H295R cells and cell cycle deregulation in SW13 cells, which suggested a potential therapy role for rosiglitazone in ACC therapy(Cerquetti et al., 2011) Interestingly, TZD also showed suppression in CYP11B2 expression and aldosterone production when tested in H295R cells(Uruno et al., 2011).
Human adrenocortical carcinoma cell lines have also been utilized to assess the role of certain interferons, chemokines and growth factors in adrenocortical cancers.(Schteingart et al., 2001; van Koetsveld et al., 2006; Almeida et al., 2008; Schteingart et al., 2010) T-cell factor/β-catenin antagonists, PKF115-584 and type I insulin-like growth factor receptor inhibitors have been recently tested in the NCI-H295 cells(Doghman et al., 2008; Barlaskar et al., 2009). These biochemicals are targeted to a limited number of known genetic mutations in adrenocortical carcinomas which are also present in the NCI-H295 cells. Finally, NCI-H295 cells were also tumorigenic when inoculated subcutaneously into nude mice lacking a thymus, a species model frequently used for preclinical anti-carcinoma drug screening(Gazdar et al., 1990).
6. Conclusion
Human adrenocortical cell lines represent a crucial tool for molecular and cellular studies that cannot practically be done in animal models. Currently available human cell lines can produce mineralocorticoids, glucocorticoids and adrenal androgens, respond to AngII, ACTH and K+, and act as a screening tool for cancer therapies. The reliance on one adrenal carcinoma (the NCI-H295) as a source for most of the models is a concern and a limitation that supports the need for the development of additional human adrenal cell lines.
Acknowledgments
Grant Support: This work was supported by National Institute of Health grant DK43140 to WER.
Footnotes
Disclosure statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida MQ, Stratakis CA. How does cAMP/protein kinase A signaling lead to tumors in the adrenal cortex and other tissues? Molecular and Cellular Endocrinology. 2011;336:162–168. doi: 10.1016/j.mce.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, Lerario AM, Maciel CC, Mattos GE, Jorge AA, Mendonca BB, Latronico AC. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab. 2008;93:3524–31. doi: 10.1210/jc.2008-0065. [DOI] [PubMed] [Google Scholar]
- Bandulik S, Penton D, Barhanin J, Warth R. TASK1 and TASK3 Potassium Channels: Determinants of Aldosterone Secretion and Adrenocortical Zonation. Hormone and Metabolic Research. 2010;42:450–457. doi: 10.1055/s-0029-1243601. [DOI] [PubMed] [Google Scholar]
- Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94:204–12. doi: 10.1210/jc.2008-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagna X. Adrenal Cortical Carcinoma: Recent Advances in Diagnosis and Clinical Management. Acta Endocrinologica-Bucharest. 2010;6:237–250. [Google Scholar]
- Betz MJ, Shapiro I, Fassnacht M, Hahner S, Reincke M, Beuschlein F. Peroxisome proliferator-activated receptor-gamma agonists suppress adrenocortical tumor cell proliferation and induce differentiation. J Clin Endocrinol Metab. 2005;90:3886–96. doi: 10.1210/jc.2004-1267. [DOI] [PubMed] [Google Scholar]
- Bird IM, Mason JI, Rainey WE. Regulation of type 1 angiotensin II receptor messenger ribonucleic acid expression in human adrenocortical carcinoma H295 cells. Endocrinology. 1994;134:2468–74. doi: 10.1210/endo.134.6.8194473. [DOI] [PubMed] [Google Scholar]
- Bird IM, Mason JI, Rainey WE. Hormonal regulation of angiotensin II type 1 receptor expression and AT1-R mRNA levels in human adrenocortical cells. Endocr Res. 1995a;21:169–82. doi: 10.3109/07435809509030432. [DOI] [PubMed] [Google Scholar]
- Bird IM, Mason JI, Oka K, Rainey WE. Angiotensin-II stimulates an increase in cAMP and expression of 17 alpha-hydroxylase cytochrome P450 in fetal bovine adrenocortical cells. Endocrinology. 1993a;132:932–4. doi: 10.1210/endo.132.2.8381079. [DOI] [PubMed] [Google Scholar]
- Bird IM, Pasquarette MM, Rainey WE, Mason JI. Differential control of 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase expression in human adrenocortical H295R cells. J Clin Endocrinol Metab. 1996;81:2171–8. doi: 10.1210/jcem.81.6.8964847. [DOI] [PubMed] [Google Scholar]
- Bird IM, Word RA, Clyne C, Mason JI, Rainey WE. Potassium negatively regulates angiotensin II type 1 receptor expression in human adrenocortical H295R cells. Hypertension. 1995b;25:1129–34. doi: 10.1161/01.hyp.25.6.1129. [DOI] [PubMed] [Google Scholar]
- Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993b;133:1555–61. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- Bodrogi I. Third-line chemotherapy of resistant advanced testicular cancer. Prog Clin Biol Res. 1989;303:749–58. [PubMed] [Google Scholar]
- Butler R, Robertson J, Gallo JM. Mutually exclusive expression of beta(III)-tubulin and vimentin in adrenal cortex carcinoma SW13 cells. FEBS Lett. 2000;470:198–202. doi: 10.1016/s0014-5793(00)01316-8. [DOI] [PubMed] [Google Scholar]
- Cardoso CC, Bornstein SR, Hornsby PJ. New methods for investigating experimental human adrenal tumorigenesis. Mol Cell Endocrinol. 2009;300:175–9. doi: 10.1016/j.mce.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerquetti L, Sampaoli C, Amendola D, Bucci B, Misiti S, Raza G, De Paula U, Marchese R, Brunetti E, Toscano V, Stigliano A. Mitotane sensitizes adrenocortical cancer cells to ionizing radiations by involvement of the cyclin B1/CDK complex in G(2) arrest and mismatch repair enzymes modulation. International Journal of Oncology. 2010;37:493–501. doi: 10.3892/ijo_00000698. [DOI] [PubMed] [Google Scholar]
- Cerquetti L, Sampaoli C, Amendola D, Bucci B, Masuelli L, Marchese R, Misiti S, De Venanzi A, Poggi M, Toscano V, Stigliano A. Rosiglitazone induces autophagy in H295R and cell cycle deregulation in SW13 adrenocortical cancer cells. Exp Cell Res. 2011 doi: 10.1016/j.yexcr.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Chen M, Hornsby PJ. Adenovirus-delivered DKK3/WNT4 and steroidogenesis in primary cultures of adrenocortical cells. Horm Metab Res. 2006;38:549–55. doi: 10.1055/s-2006-950500. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Flasch MV, Hornsby PJ. Expression of 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in fetal human adrenocortical cells transfected with SV40 T antigen. J Mol Endocrinol. 1992;9:7–17. doi: 10.1677/jme.0.0090007. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol. 1995;9:1346–55. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- de Reynies A, Assie G, Rickman DS, Tissier F, Groussin L, Rene-Corail F, Dousset B, Bertagna X, Clauser E, Bertherat J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27:1108–15. doi: 10.1200/JCO.2008.18.5678. [DOI] [PubMed] [Google Scholar]
- Denner K, Rainey WE, Pezzi V, Bird IM, Bernhardt R, Mathis JM. Differential regulation of 11 beta-hydroxylase and aldosterone synthase in human adrenocortical H295R cells. Mol Cell Endocrinol. 1996;121:87–91. doi: 10.1016/0303-7207(96)03853-1. [DOI] [PubMed] [Google Scholar]
- Doghman M, Cazareth J, Lalli E. The T cell factor/beta-catenin antagonist PKF115-584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab. 2008;93:3222–5. doi: 10.1210/jc.2008-0247. [DOI] [PubMed] [Google Scholar]
- Fallo F, Pilon C, Barzon L, Pistorello M, Pagotto U, Altavilla G, Boscaro M, Sonino N. Effects of taxol on the human NCI-H295 adrenocortical carcinoma cell line. Endocr Res. 1996;22:709–15. doi: 10.1080/07435809609043766. [DOI] [PubMed] [Google Scholar]
- Fallo F, Pilon C, Barzon L, Pistorello M, Pagotto U, Altavilla G, Boscaro M, Sonino N. Paclitaxel is an effective antiproliferative agent on the human NCI-H295 adrenocortical carcinoma cell line. Chemotherapy. 1998;44:129–34. doi: 10.1159/000007104. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Libe R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011 doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Hahner S, Beuschlein F, Klink A, Reincke M, Allolio B. New mechanisms of adrenostatic compounds in a human adrenocortical cancer cell line. Eur J Clin Invest. 2000;30(Suppl 3):76–82. doi: 10.1046/j.1365-2362.2000.0300s3076.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ranvier GG, Weng J, Yeh RF, Khanafshar E, Suh I, Barker C, Duh QY, Clark OH, Kebebew E. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch Surg. 2008;143:841–6. doi: 10.1001/archsurg.143.9.841. discussion 846. [DOI] [PubMed] [Google Scholar]
- Fisher RI, Terry WD, Hodes RJ, Rosenberg SA, Makuch R, Gordon HG, Fisher SG. Adjuvant immunotherapy or chemotherapy for malignant melanoma. Preliminary report of the National Cancer Institute randomized clinical trial. Surg Clin North Am. 1981;61:1267–77. doi: 10.1016/s0039-6109(16)42582-x. [DOI] [PubMed] [Google Scholar]
- Gazdar AF, Oie HK, Shackleton CH, Chen TR, Triche TJ, Myers CE, Chrousos GP, Brennan MF, Stein CA, La Rocca RV. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990;50:5488–96. [PubMed] [Google Scholar]
- Ghorab Z, Jorda M, Ganjei P, Nadji M. Melan A (A103) is expressed in adrenocortical neoplasms but not in renal cell and hepatocellular carcinomas. Appl Immunohistochem Mol Morphol. 2003;11:330–3. doi: 10.1097/00129039-200312000-00009. [DOI] [PubMed] [Google Scholar]
- Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15:668–76. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg KK, Chen LB. Absence of intermediate filaments in a human adrenal cortex carcinoma-derived cell line. Exp Cell Res. 1986;163:509–17. doi: 10.1016/0014-4827(86)90081-9. [DOI] [PubMed] [Google Scholar]
- Hilbers U, Peters J, Bornstein SR, Correa FM, Johren O, Saavedra JM, Ehrhart-Bornstein M. Local renin-angiotensin system is involved in K+-induced aldosterone secretion from human adrenocortical NCI-H295 cells. Hypertension. 1999;33:1025–30. doi: 10.1161/01.hyp.33.4.1025. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ, McAllister JM. Culturing steroidogenic cells. Methods Enzymol. 1991;206:371–80. doi: 10.1016/0076-6879(91)06107-e. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ, Ryan RF, Cheng CY. Replicative senescence and differentiated gene expression in cultured adrenocortical cells. Exp Gerontol. 1989;24:539–58. doi: 10.1016/0531-5565(89)90059-4. [DOI] [PubMed] [Google Scholar]
- Janes ME, Chu KM, Clark AJ, King PJ. Mechanisms of adrenocorticotropin-induced activation of extracellularly regulated kinase 1/2 mitogen-activated protein kinase in the human H295R adrenal cell line. Endocrinology. 2008;149:1898–905. doi: 10.1210/en.2007-0949. [DOI] [PubMed] [Google Scholar]
- Kiiveri S, Liu J, Heikkila P, Arola J, Lehtonen E, Voutilainen R, Heikinheimo M. Transcription factors GATA-4 and GATA-6 in human adrenocortical tumors. Endocr Res. 2004;30:919–23. doi: 10.1081/erc-200044149. [DOI] [PubMed] [Google Scholar]
- Kuulasmaa T, Jaaskelainen J, Suppola S, Pietilainen T, Heikkila P, Aaltomaa S, Kosma VM, Voutilainen R. WNT-4 mRNA expression in human adrenocortical tumors and cultured adrenal cells. Horm Metab Res. 2008;40:668–73. doi: 10.1055/s-2008-1078716. [DOI] [PubMed] [Google Scholar]
- La Rocca RV, Stein CA, Danesi R, Jamis-Dow CA, Weiss GH, Myers CE. Suramin in adrenal cancer: modulation of steroid hormone production, cytotoxicity in vitro, and clinical antitumor effect. J Clin Endocrinol Metab. 1990;71:497–504. doi: 10.1210/jcem-71-2-497. [DOI] [PubMed] [Google Scholar]
- Leibovitz A, McCombs WM, 3rd, Johnston D, McCoy CE, Stinson JC. New human cancer cell culture lines. I. SW-13, small-cell carcinoma of the adrenal cortex. J Natl Cancer Inst. 1973;51:691–7. [PubMed] [Google Scholar]
- McAllister JM, Hornsby PJ. Improved clonal and nonclonal growth of human, rat and bovine adrenocortical cells in culture. In Vitro Cell Dev Biol. 1987;23:677–85. doi: 10.1007/BF02620980. [DOI] [PubMed] [Google Scholar]
- McAllister JM, Byrd W, Simpson ER. The effects of growth factors and phorbol esters on steroid biosynthesis in isolated human theca interna and granulosa-lutein cells in long term culture. J Clin Endocrinol Metab. 1994;79:106–12. doi: 10.1210/jcem.79.1.8027214. [DOI] [PubMed] [Google Scholar]
- Nesterova M, Bossis I, Wen F, Horvath A, Matyakhina L, Stratakis CA. An immortalized human cell line bearing a PRKAR1A-inactivating mutation: effects of overexpression of the wild-type Allele and other protein kinase A subunits. J Clin Endocrinol Metab. 2008;93:565–71. doi: 10.1210/jc.2007-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- Nogueira EF, Bollag WB, Rainey WE. Angiotensin II regulation of adrenocortical gene transcription. Mol Cell Endocrinol. 2009;302:230–6. doi: 10.1016/j.mce.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira EF, Gerry D, Mantero F, Mariniello B, Rainey WE. The role of TASK1 in aldosterone production and its expression in normal adrenal and aldosterone-producing adenomas. Clinical Endocrinology. 2010;73:22–29. doi: 10.1111/j.1365-2265.2009.03738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira EF, Vargas CA, Otis M, Gallo-Payet N, Bollag WB, Rainey WE. Angiotensin-II acute regulation of rapid response genes in human, bovine, and rat adrenocortical cells. J Mol Endocrinol. 2007;39:365–74. doi: 10.1677/JME-07-0094. [DOI] [PubMed] [Google Scholar]
- Otani H, Otsuka F, Inagaki K, Suzuki J, Miyoshi T, Kano Y, Goto J, Ogura T, Makino H. Aldosterone breakthrough caused by chronic blockage of angiotensin II type 1 receptors in human adrenocortical cells: possible involvement of bone morphogenetic protein-6 actions. Endocrinology. 2008;149:2816–25. doi: 10.1210/en.2007-1476. [DOI] [PubMed] [Google Scholar]
- Parker KL, Rainey WE. The Adrenal Gland (Text book of Endocrine Physiology. Vol. 14. Oxford university press; New York: 2004. pp. 319–348. [Google Scholar]
- Parmar J, Key RE, Rainey WE. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J Clin Endocrinol Metab. 2008;93:4542–6. doi: 10.1210/jc.2008-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE. Adrenal zonation: clues from 11beta-hydroxylase and aldosterone synthase. Mol Cell Endocrinol. 1999;151:151–60. doi: 10.1016/s0303-7207(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Mason JI. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol. 1994;100:45–50. doi: 10.1016/0303-7207(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Saner K, Schimmer BP. Adrenocortical cell lines. Mol Cell Endocrinol. 2004;228:23–38. doi: 10.1016/j.mce.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–9. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Sawetawan C, Hanley NA, McCarthy JL, McGee EA, Wester R, Mason JI. Regulation of human adrenal carcinoma cell (NCI-H295) production of C19 steroids. J Clin Endocrinol Metab. 1993;77:731–7. doi: 10.1210/jcem.77.3.8396576. [DOI] [PubMed] [Google Scholar]
- Rodriguez H, Hum DW, Staels B, Miller WL. Transcription of the human genes for cytochrome P450scc and P450c17 is regulated differently in human adrenal NCI-H295 cells than in mouse adrenal Y1 cells. J Clin Endocrinol Metab. 1997;82:365–71. doi: 10.1210/jcem.82.2.3721. [DOI] [PubMed] [Google Scholar]
- Romero DG, Welsh BL, Gomez-Sanchez EP, Yanes LL, Rilli S, Gomez-Sanchez CE. Angiotensin II-mediated protein kinase D activation stimulates aldosterone and cortisol secretion in H295R human adrenocortical cells. Endocrinology. 2006;147:6046–55. doi: 10.1210/en.2006-0794. [DOI] [PubMed] [Google Scholar]
- Samandari E, Kempna P, Nuoffer JM, Hofer G, Mullis PE, Fluck CE. Human adrenal corticocarcinoma NCI-H295R cells produce more androgens than NCI-H295A cells and differ in 3beta-hydroxysteroid dehydrogenase type 2 and 17,20 lyase activities. J Endocrinol. 2007;195:459–72. doi: 10.1677/JOE-07-0166. [DOI] [PubMed] [Google Scholar]
- Schimmer BP, Cordova M, Cheng H, Tsao A, Goryachev AB, Schimmer AD, Morris Q. Global profiles of gene expression induced by adrenocorticotropin in Y1 mouse adrenal cells. Endocrinology. 2006;147:2357–67. doi: 10.1210/en.2005-1526. [DOI] [PubMed] [Google Scholar]
- Schteingart DE, Benitez R, Bradford C, Narayan A, Wang SM. Expression of Anti-apoptosis Genes Determines the Response of Adrenal Cancer to Apoptosis-inducing Chemotherapy. Anticancer Research. 2010;30:4805–4809. [PubMed] [Google Scholar]
- Schteingart DE, Giordano TJ, Benitez RS, Burdick MD, Starkman MN, Arenberg DA, Strieter RM. Overexpression of CXC chemokines by an adrenocortical carcinoma: a novel clinical syndrome. J Clin Endocrinol Metab. 2001;86:3968–74. doi: 10.1210/jcem.86.8.7780. [DOI] [PubMed] [Google Scholar]
- Schteingart DE, Sinsheimer JE, Counsell RE, Abrams GD, Mcclellan N, Djanegara T, Hines J, Ruangwises N, Benitez R, Wotring LL. Comparison of the Adrenalytic Activity of Mitotane and a Methylated Homolog on Normal Adrenal-Cortex and Adrenal-Cortical Carcinoma. Cancer Chemotherapy and Pharmacology. 1993;31:459–466. doi: 10.1007/BF00685036. [DOI] [PubMed] [Google Scholar]
- Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nature Reviews Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- Staels B, Hum DW, Miller WL. Regulation of steroidogenesis in NCI-H295 cells: a cellular model of the human fetal adrenal. Mol Endocrinol. 1993;7:423–33. doi: 10.1210/mend.7.3.8387159. [DOI] [PubMed] [Google Scholar]
- Ueno M, Nakashima J, Akita M, Ban SI, Nakanoma T, Iida M, Deguchi N. Characterization of a newly established cell line derived from human adrenocortical carcinoma. Int J Urol. 2001;8:17–22. doi: 10.1046/j.1442-2042.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- Uruno A, Matsuda K, Noguchi N, Yoshikawa T, Kudo M, Satoh F, Rainey WE, Hui XG, Akahira J, Nakamura Y, Sasano H, Okamoto H, Ito S, Sugawara A. Peroxisome proliferator-activated receptor-gamma suppresses CYP11B2 expression and aldosterone production. Journal of Molecular Endocrinology. 2011;46:37–49. doi: 10.1677/JME-10-0088. [DOI] [PubMed] [Google Scholar]
- van Koetsveld PM, Vitale G, de Herder WW, Feelders RA, van der Wansem K, Waaijers M, van Eijck CHJ, Speel EJM, Croze E, van der Lely AJ, Lamberts SWJ, Hofland LJ. Potent inhibitory effects of type I interferons on human adrenocortical carcinoma cell growth. Journal of Clinical Endocrinology & Metabolism. 2006;91:4537–4543. doi: 10.1210/jc.2006-0620. [DOI] [PubMed] [Google Scholar]
- Veytsman I, Nieman L, Fojo T. Management of Endocrine Manifestations and the Use of Mitotane As a Chemotherapeutic Agent for Adrenocortical Carcinoma. Journal of Clinical Oncology. 2009;27:4619–4629. doi: 10.1200/JCO.2008.17.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson GP. Adrenocortical zonation and ACTH. Microsc Res Tech. 2003;61:227–39. doi: 10.1002/jemt.10331. [DOI] [PubMed] [Google Scholar]
- Weiss RB, Charles LM, Macdonald JS. M-Amsa - an Exciting New Drug in the National-Cancer-Institute Drug Development Program. Cancer Clinical Trials. 1980;3:203–209. [PubMed] [Google Scholar]
- Xing Y, Edwards MA, Ahlem C, Kennedy M, Cohen A, Gomez-Sanchez CE, Rainey WE. The effects of ACTH on steroid metabolomic profiles in human adrenal cells. J Endocrinol. 2011;209:327–35. doi: 10.1530/JOE-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing YW, Parker CR, Edwards M, Rainey WE. ACTH is a potent regulator of gene expression in human adrenal cells. Journal of Molecular Endocrinology. 2010;45:59–68. doi: 10.1677/JME-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamichi-Nishina M, Ito T, Mizutani T, Yamamichi N, Watanabe H, Iba H. SW13 cells can transition between two distinct subtypes by switching expression of BRG1 and Brm genes at the post-transcriptional level. J Biol Chem. 2003;278:7422–30. doi: 10.1074/jbc.M208458200. [DOI] [PubMed] [Google Scholar]