Abstract
Inhibitory receptors on immune cells are pivotal regulators of immune escape in cancer. Among these inhibitory receptors, CTLA-4 (targeted clinically by ipilimumab) serves as a dominant off-switch while other receptors such as PD-1 and LAG-3 seem to serve more subtle rheostat functions. However, the extent of synergy and cooperative interactions between inhibitory pathways in cancer remain largely unexplored. Here we reveal extensive co-expression of PD-1 and LAG-3 on tumor-infiltrating CD4+ and CD8+ T cells in three distinct transplantable tumors. Dual anti-LAG-3/anti-PD-1 antibody treatment cured most mice of established tumors that were largely resistant to single antibody treatment. Despite minimal immunopathological sequelae in PD-1 and LAG-3 single knockout mice, dual knockout mice abrogated self-tolerance with resultant autoimmune infiltrates in multiple organs, leading to eventual lethality. However, Lag3−/−Pdcd1−/− mice demonstrated markedly increased survival from and clearance of multiple transplantable tumors. Together, these results define a strong synergy between the PD-1 and LAG-3 inhibitory pathways in tolerance to both self and tumor antigens. Additionally, they argue strongly that dual blockade of these molecules represents a promising combinatorial strategy for cancer.
Keywords: LAG-3, PD-1, tumor, T cells, immunotherapy
Introduction
T cell-mediated anti-tumor immune responses are essential for effective deletion of primary tumor lesions and for protection against metastases (1). While the immune system can detect and destroy malignant cells, tumors escape surveillance by a variety of cell intrinsic and extrinsic mechanisms (1-3). As with chronic viral infection (4), tumor antigen-specific CD4+ and CD8+ T cells display impaired effector function and an exhausted phenotype characterized by decreased production of proinflammatory cytokines and hyporesponsiveness to antigenic restimulation (5). This is mediated by cell-extrinsic mechanisms, such as regulatory T cells (Treg), and cell-intrinsic mechanisms, such as inhibitory molecules that are upregulated on exhausted, tumor infiltrating lymphocytes (TILs). In combination, these inhibitory mechanisms represent a formidable barrier to effective antitumor immunity (6-10).
Inhibitory receptors such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, CD152), lymphocyte-activation gene 3 (LAG-3, CD223), and programmed cell death 1 (PD-1, CD279) function at multiple levels to ensure appropriate T cell homeostasis, activation and differentiation (7, 11-17). Furthermore, all three inhibitory molecules also contribute to cell-extrinsic regulation by controlling Treg homeostasis and function, mediating induced Treg development, and mitigating dendritic cell differentiation and function (13-16, 18, 19). Data from genetically-manipulated mice indicate that CTLA-4 represents a basic and indispensible “off switch”, while PD-1 and LAG-3 play more subtle roles in immune regulation. Whereas Ctla4−/− mice develop a severe lymphoproliferative disease and are usually moribund by 3-4 weeks of age (20), Pdcd1−/− (which encodes PD-1) mice live beyond one year while developing subtle and variable immune-based disease manifestations depending on genetic background; Pdcd1−/− BALB/c mice develop dilated cardiomyopathy 5-30 weeks of age, while Pdcd1−/− C57BL/6 mice develop a protracted lupus-like condition that takes over 6 months to develop (21, 22). Unmanipulated Lag3−/− C57BL/6 mice do not develop any disease manifestations within the first year of life (23).
Recent studies have revealed that LAG-3 and PD-1 are co-expressed on tolerized TILs suggesting that they may contribute to tumor-mediated immune suppression (5, 24). Preclinical models using antibody treatment to block LAG-3 for cancer treatment demonstrate enhanced activation of antigen-specific T cells at the tumor site and disruption of tumor growth (25). Abrogation of PD1 signaling in mice leads to enhanced CTL killing, cytokine production, and tumor-bearing animal survival over several different tumor models (26). Based on their roles in T cell inhibition and anti-tumor immune regulation, individual antibody blockade of both CTLA-4 and PD-1 have been reported to demonstrate clinical utility (27, 28). Given this information, LAG-3 and PD-1 represent a potentially beneficial pairing for dual pathway blockade in cancer therapy. However, little is known about the extent of cooperative interaction between these regulatory pathways, information critical to the development of combinatorial immunotherapy based on simultaneous blockade of multiple receptors or ligands. In this study we investigate whether there is synergy between LAG-3 and PD-1 by analysis of tumor growth and clearance in blocking antibody treated mice and Lag3−/−Pdcd1−/− mice.
Materials and Methods
Mouse strains and cell lines
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Lag3−/− mice were provided by Y. H. Chien (Stanford University, PaloAlto, CA) with permission from C. Benoist and D. Mathis (Joslin Diabetes Center, Boston, MA) (23, 29). Pdcd1−/− mice were provided by Lieping Chen (Johns Hopkins University, Baltimore, MA) with permission from T. Honjo (Kyoto University, Kyoto, Japan) (30). At St. Jude Children’s Research Hospital, the Lag3−/−, Pdcd1−/− and Lag3−/−Pdcd1−/− mice were backcrossed onto a C57BL/6 background an additional five, nine and five generations respectively, and a genome wide SNP analysis indicated that 100% of the markers were C57BL/6 for Lag3−/− and Pdcd1−/− mice and 90% for the Lag3−/−Pdcd1−/− mice. At Johns Hopkins, the Lag3−/−Pdcd1−/− were backcrossed five generations onto a B10.D2 background and crossed with Clone 4 (CL4) TCR transgenic mice. Animal experiments were performed in specific pathogen-free facilities accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC), at St. Jude Children’s Research Hospital and Johns Hopkins Kimmel Cancer Center and approved by the respective Animal Care and Use Committees. The mice at St. Jude Children’s Research Hospital are also Helicobacter- and MNV-free. B16 melanoma cells were obtained from MJ Turk (Dartmouth College, Hanover, NH). This line has been authenticated by the RADIL at the University of Missouri (9/18/2008) and maintained in continuous culture for no more than six months post-testing. It was also tested by IMPACT I PCR Profile at the RADIL at the University of Missouri (10/10/2008). MC38 cells were obtained from JP Allison (Memorial Sloan-Kettering Cancer Center, New York, NY), authenticated by the RADIL (3/10/2003), and tested by IMPACT I at the RADIL at the University of Missouri (3/18/2010). Sa1N cells were originally obtained from JP Allison (Memorial Sloan-Kettering Cancer Center, New York, USA). While these cells have not been authenticated, they perform as described in the literature in syngeneic A/J mice (14) and tested by IMPACT I at the RADIL at the University of Missouri (1/29/2011).
Flow cytometry, intracellular cytokine staining and cytokine analysis
Single-cell suspensions were prepared from spleens, inguinal, brachial, and axillary lymph nodes, and tumors. Cells were stained with fluorescent-labeled antibodies (BioLegend, San Diego, CA; BD-Bioscience Pharmingen, San Diego, CA; or eBiosciences, San Diego, CA) and analyzed by either FACSCalibur or LSR II flow cytometer (BD, San Diego, CA).). The following clones were used: CD4 (GK1.5), CD8α (53-6.7), CD11c (N418), CD25 (PC61), CD44 (IM7), CD45R/B220 (RA3-6B2), CD69 (H1.2F3), PD-1 (RMP1-30), TCR-β (H57-597), Thy1.1 (HIS51), IFN-γ (XMG1.2), TNF-α (MP6-XT22), IL-17 (TC11-18H10), Foxp3 (150D), and LAG-3 (4-10-C9; ref. (31)). For intracellular cytokine staining, cells were activated with PMA (100ng/ml) plus ionomycin (500ng/ml) for 4 hours in the presence of GolgiPlug™ (32), processed with a Cytofix/Cytoperm™ kit (32), and stained as indicated. Measurement of IFN-γ, TNF-α and MCP-1 in serum was determined by IFN-γ- or TNF-α-specific ELISA kits (eBioscience, San Diego, CA) and a MCP-1 specific bead based kit (Millipore, Billerica, MA).
Tumor growth experiments and TIL preparation
B16 melanoma and MC38 colon adenocarcinoma models were performed as previously described with some modifications (33, 34). Briefly, on day 0 mice were injected with 1.25 – 5.0×105 B16 cells intradermally (i.d.) in the back or 2.0 – 5.0×106 MC38 cells subcutaneously (s.c) in the right flank. Lag3−/−Pdcd1−/− (and appropriate controls) were used at approximately 5 weeks of age. Tumor diameter was measured every 2-3 days with an electronic caliper and reported as volume using the formula m1 2 × m2 × π/6 (35). To isolate tumor infiltrating lymphocytes (TILs), solid tumors were excised after 12-14 days, single-cell suspensions prepared by mechanical dissociation, followed by density gradient centrifugation on an 80%/40% Percoll (GE Healthcare, Piscataway, NJ) gradient. For CD4+ and CD8+ T cell depletion experiments, anti-mouse CD4 (GK1.5) and anti-mouse CD8 (2.43) ascites were administered i.p. on day −1, 2, 5, 8 and 11 (pre-titered for maximal deletion).
Dual antibody blocking experiments
Sa1N fibrosarcoma cells or MC38 cells (2×106) were implanted s.c. into A/J mice (Harlan, Indianapolis, IN) or C57Bl/6 mice (Charles River, Wilmington, MA), respectively. Tumors volumes were measured using an electronic caliper (l × w × h/2) and randomized by size (10 mice/group). Mice with palpable tumors (Sa1N ~60 mm3/2; MC38 ~40 mm3/2) were injected i.p. at a dosage of 10 mg/kg for chimeric mouse anti-PD-1 (4H2, IgG1; ref. (36)) and/or rat anti-mouse LAG-3 (C9B7W, IgG1; ref. (37)). Control murine IgG1 (MOPC 21; BioXCell) was dosed at 20 mg/kg or added to individual anti-PD-1 or anti-LAG-3 antibody treatments at 10 mg/kg. Tumor Growth Inhibition (TGI) was calculated when all mice within a group were available for tumor measurement.
Adoptive transfer into Rag-1−/− mice
Splenocytes and lymph node cells from female mice (5-7 weeks old) were pooled, and 107 cells injected i.v. into age matched female Rag-1−/− (5-6 weeks old) mice. CD4+ or CD8+ cells were depleted from splenocytes and LNs cells using biotinylated anti-CD4 or anti-CD8 by MACS separation using streptavidin-coupled beads (Milteny Biotec, Auburn, CA) to achieve purity above 95%.
Histopathology
Full necropsies were completed independently at St Jude Children’s Research Hospital and Johns Hopkins. Tissues from knockout and control mice were fixed in 10% neutral buffered formalin, except eyes, which were fixed for 24 h in Davidson’s fixative, embedded in paraffin, sectioned at 4 μm and stained with hematoxylin and eosin for histopathologic examination. Collagen deposition was detected using a Masson’s Trichrome stain. Macrophages and T cells were detected using rat anti-mouse MAC2 (CL8942AP, Accurate Chemical, Westbury, NY) and goat anti-human CD3 (sc-1127, Santa Cruz Biotechnology, Santa Cruz, CA) antibodies, respectively. Heat-induced epitope retrieval was performed by heating slides in ER2 (AR9640, Leica, Bannockburn, IL) for 30 minutes and the Refine system (DS9800, Leica Microsystems, Bannockburn, IL) used for detection. Treg cells were detected using a rat anti-mouse FoxP3 antibody (14-5773-82, eBioscience, San Diego, CA) with heat induced retrieval, pH 6.0 Target Retrieval Buffer (S699, DAKO, Carpinteria, CA) followed by a horseradish peroxidase labeled streptavidin detection system (TS-125-HR, Thermo Shandon, Pittsburgh, PA). In all immunohistochemical assays, 3,3′-diaminobenzidine was used as the chromogenic substrate with a light hematoxylin counterstain.
Autoantibody analysis
Mouse sera were analyzed by indirect ELISA, alongside a positive control (serum from a MRL/lpr mouse, a SLE disease model), using 96-well Nunc MaxiSorp plates (Nalgene Nunc). Multiple antigen blot assay (MABA) was performed as described (38).
Clone 4 TCR transgenic T cell experiments
CL4 adoptive transfer and in vivo CTL studies were performed as previously described (25, 39).
Statistical analyses
Summary statistics are presented as mean ± standard error of the mean (SEM). Group means were compared with two-sample t-tests. Event-free survival (moribund) estimates were calculated using the Kaplan-Meier method; mouse groups were compared by logrank test. The proportions of tumor-free mice were evaluated with the binomial distribution; synergy hypotheses were tested based on the Maximum Likelihood method. Trends in weight over time and tumor growth over time among different mice groups were analyzed using mixed models. All p-values are two-sided and statistical significance was assessed at the 0.05 level. Analysis was conducted using SAS Version 9.2 (Cary, NC).
Results
Combinatorial anti-LAG-3/anti-PD-1 immunotherapy inhibits tumor growth
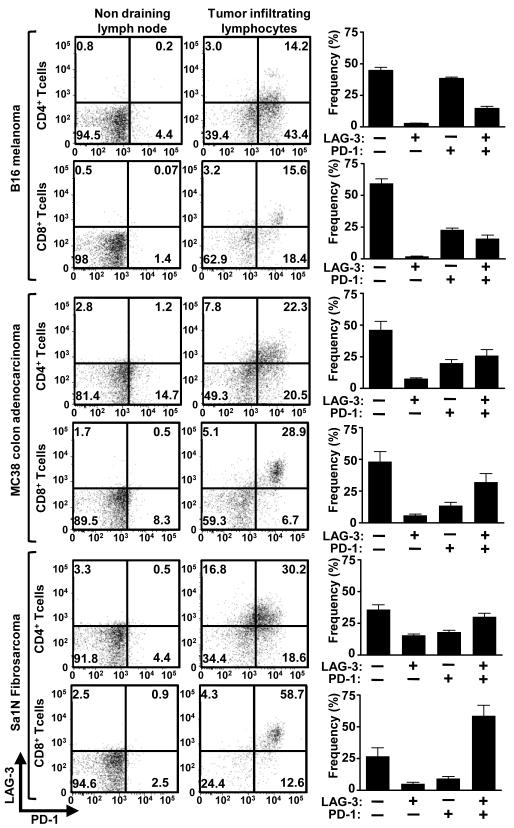
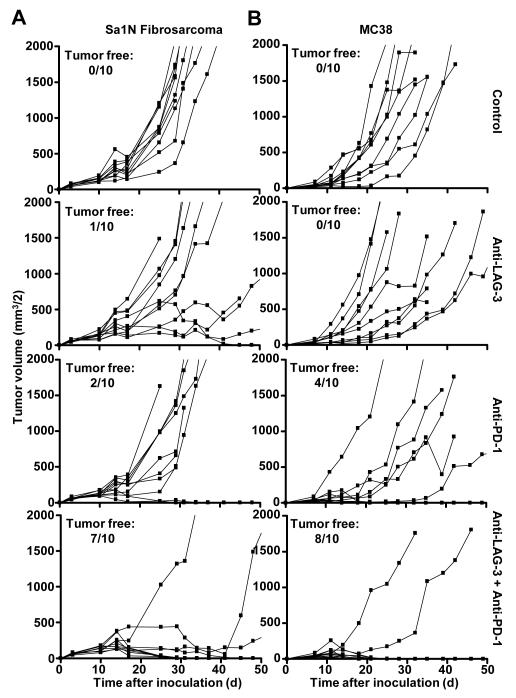
PD-1 monoclonal antibody treatment has shown clinical efficacy against multiple malignancies including melanoma, prostate, renal cell, and lung cancer (27). LAG-3 has been suggested to directly modulate the activity of PD-1+ cells (5); further, co-expression of LAG-3 and PD-1 has been demonstrated in malignant mouse and human tissue (5, 24). Given these data, we hypothesized that LAG-3 and PD-1 act synergistically to control immune homeostasis and mediate tumor-induced tolerance. Consistent with previous reports, a significant percentage of CD4+ and CD8+ TILs from transplanted B16 melanoma, MC38 colorectal adenocarcinoma, and Sa1N fibrosarcoma expressed high levels of LAG-3 and PD-1 (32, 34), whereas similar up-regulation was not observed on peripheral T cell populations (Fig. 1). Next, we asked if antibody-mediated dual blockade of these pathways would reduce tumor growth by assessing the potential efficacy of combined anti-LAG-3 and anti-PD-1 blockade in mice with established tumors. Reduced growth of Sa1N fibrosarcoma and MC38 colorectal adenocarcinoma (32, 40-42) was observed in some but not all mice treated with the anti-LAG-3 or anti-PD-1 monotherapy (Fig. 2); only a few mice were tumor-free after 50 days (0-40%). For anti-LAG-3, this is the first demonstration of tumor growth inhibition with anti-LAG-3 as a monotherapy. In striking contrast, 70% and 80% of the Sa1N- and MC38-inoculated mice, respectively, were tumor-free after 50 days following combinatorial anti-LAG-3/anti-PD-1 immunotherapy (Fig. 2). However, this regimen had no effect against established B16 tumors. Using the Maximum Likelihood method, there appeared to be a synergistic benefit of anti-LAG-3/anti-PD-1 combinatorial immunotherapy that is superior to either the additive effect of anti-LAG-3 and anti-PD-1 or monotherapy. Dual treatment with anti-LAG-3/anti-PD-1 did not result in immunopathological manifestations such as lymphocytic infiltration in the Sa1N fibrosarcoma model as determined by detailed histologic analysis of multiple tissues. Despite efficient tumor clearance, no evidence of systemic or organ-specific autoimmunity was observed.
Figure 1.
Tumor-infiltrating lymphocytes express LAG-3 and PD-1. TILs were isolated from B16, MC38, and Sa1N tumors resected from WT mice 13 days post-inoculation (average sizes: B16=350mm3, MC38=1000mm3, Sa1N=750mm3) and stained for flow cytometric analysis. Representative data (left; gated on live CD4+ or CD8+ lymphocytes as indicated) or pooled data (right; n=8-10) of LAG-3/PD-1 expression on CD4+ or CD8+ TILs are shown.
Figure 2.
Combinatorial anti-LAG-3/anti-PD-1 treatment inhibits tumor growth. Mice [(A) A/J; (B) C57BL/6] were randomized on (A) on day 6 when Sa1N fibrosarcoma tumor volumes were ~60 mm3/2, or (B) on day 7 when MC38 colon adenocarcinoma tumor volumes were ~40 mm3/2, and treated with isotype control, anti-PD-1, anti-LAG-3, or anti-PD-1/LAG-3 combination on days 8, 11 and 14 and tumor volume determined. Tumor growth inhibition (TGI) on day 18: (A) anti-LAG-3 - 18.9%; anti-PD-1 - 26.2%; anti-PD-1/LAG-3 - 77.3%. (B) anti-LAG-3 - 1%; anti-PD-1 - 55%; anti-PD-1/LAG-3 - 79%. Data represent 3 (Sa1N) or 4 (MC38) repeated experiments with 10 mice per group. Data were analyzed using the Maximum Likelihood method to determine synergy p values: (A) 0.0622 (for experiment shown; 0.0002 with all three experiments combined) and (B) 0.0455 (for experiment shown; 0.0366 with all four experiments combined) for the anti-PD-1/LAG-3 combinatorial treatment compared to anti-PD-1 and anti-LAG-3 treatments alone.
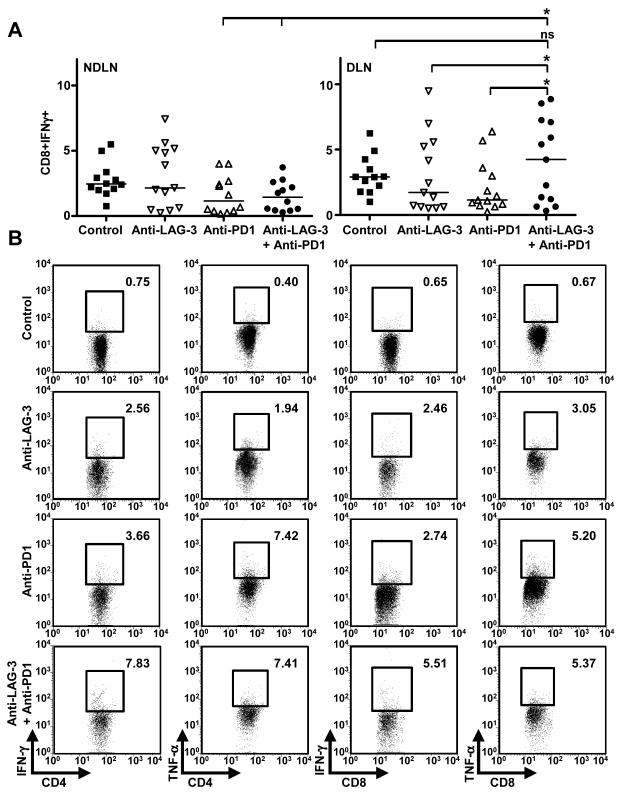
In order to investigate the mechanism underlying decreased tumor growth in antibody-treated mice, MC38 tumor-bearing mice were treated with the antibody combinations used above and draining lymph node (DLN) T cells, non-draining lymph node (NDLN) T cells, and TILs analyzed by flow cytometry for phenotype and effector function. As expected, average tumor size of anti-PD1-treated or dual-antibody-treated mice was significantly smaller than isotype control or anti-LAG3-treated mice (Supplementary Fig. S1). A significantly higher percentage of IFN-γ+CD8+ T cells were found in the tumor-associated DLNs of dual antibody-treated mice compared with the monotherapy groups, or cells analyzed from NDLNs (Figure 3A). Likewise, a higher percentage of IFN-γ+CD4+ and IFN-γ+CD8+ TILs, and to a lesser extent TNF-α+ CD4+ and CD8+ TILs, were observed in anti-LAG-3/anti-PD-1 treated mice than in control groups (Figure 3B). Taken together, these data suggest that anti-LAG-3/anti-PD-1 combinatorial immunotherapy may act synergistically to reduce tumor growth by increasing the proportion of effector T cells in the tumor and DLNs.
Figure 3.
Combinatorial anti-LAG-3/anti-PD-1 treatment results in enhanced adaptive immune responses. Mice were inoculated on day 0 with 2×106 MC38 cells s.c. in the right flank, euthanized at day 15, and tissues analyzed by flow cytometry. (A) Tumor draining inguinal (DLN) and non-draining brachial and axillary lymph nodes (NDLN) were isolated and activated with PMA + ionomycin for 4 h in the presence of brefeldin A, then analyzed by intracellular staining and flow cytometry (gated on lymphocytes). Nonparametric one-way ANOVA with Kruskal-Wallis test (p = 0.0074) was used for part A. (B) Tumor-infiltrating lymphocytes were analyzed by intracellular staining and flow cytometry; plots represent 5-8 animals per group. Numbers are percentage cytokine-positive infiltrating lymphocytes.
Lag3−/−Pdcd1−/− mice develop lethal systemic autoimmunity
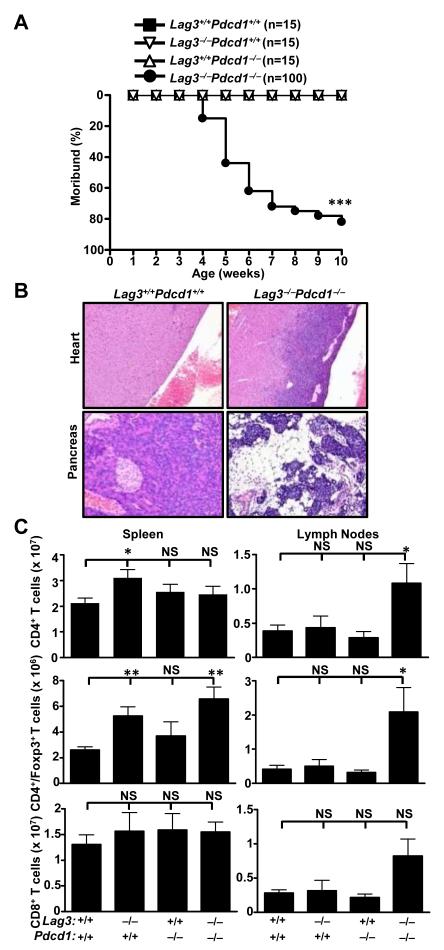
To further investigate the synergy between these two inhibitory molecules, we next assessed whether LAG-3 and PD-1 cooperate to control immune homeostasis and mediate tumor-induced tolerance using a genetic approach. Lag3−/−Pdcd1−/− C57BL/6 or B10.D2 mice were generated at two independent locations (see Methods), and disease manifestation and immune pathology analyzed over time. Lag3−/−Pdcd1−/− mice developed an early onset (~4 weeks of age), lethal autoimmune condition that resulted in ~80% of the mice moribund by ~10 weeks (Fig. 4A and Supplementary Fig. S2). The major histopathological manifestations included diffuse fibrosing lymphohistiocytic endocarditis, myocarditis and pancreatitis (Fig. 4B, Supplementary Table S1, Supplementary Fig. S3). Extensive infiltration by CD3+ T cells, Foxp3+ Treg cells and Mac2+-F4/80+ macrophages was observed, in conjunction with substantial collagen deposition but limited B cell and neutrophil infiltration; however, negligible autoantibody reactivity was seen in serum from Lag3−/−Pdcd1−/− mice but not single knockout or wild type mice (Supplementary Fig. S4). Lag3−/− and Pdcd1−/− single KO mice lacked any disease manifestations or histopathology over this period of observation. These results demonstrate that the PD-1 and LAG-3 pathways synergistically regulate self-reactivity.
Figure 4.
Lag3−/−Pdcd1−/− mice develop lethal systemic autoimmunity. (A) Disease incidence for Lag3−/−Pdcd1−/− mice, plus single knockout and WT littermate controls. Moribund curves were analyzed for statistical significance by logrank test- *** p<0.001. (B) Representative histopathology of the heart and pancreas of WT and Lag3−/−Pdcd1−/− mice. (C) Number of various T cell populations in the spleen and LNs (inguinal and brachial) is shown. Data represent three to four independent experiments with 4-7 mice total per group (5-7 weeks old). Error bars represent SEM - * p<0.05, ** p<0.01 (unpaired t-test).
Consistent with the histopathology observed, substantially increased numbers of CD4+ and CD8+ T cells were observed in the regional LNs, but not the spleens, of Lag3−/−Pdcd1−/− mice (Fig 4C, Supplementary Fig. S5). These cells possessed a predominantly activated/memory phenotype as indicated by CD69/CD44 staining. Nevertheless, there appeared to be minimal difference in the extent of division in vivo based on ex vivo Ki67 staining, even though Lag3−/− Pdcd1−/− T cells proliferate more in vitro following anti-CD3 stimulation (data not shown). The number of CD4+Foxp3+ Treg cells, B cells, and CD11c+ DC were also increased in Lag3−/−Pdcd1−/− mice (Fig 4C, Supplementary Fig. S5). Given that Lag3−/− Treg cells exhibit reduced suppressive activity (13, 14) and PD-L1 (PD-1 ligand) contributes to iTreg development (15), it is possible that the combined loss of LAG-3 and PD-1 alters Treg cell homeostasis.
To further probe the cellular defects in Lag3−/−Pdcd1−/− mice, we adoptively transferred splenocytes into lymphopenic Rag-1−/− mice. In contrast to healthy wild-type and single knockout controls, Lag3−/−Pdcd1−/− splenocyte recipients started to lose body weight ~6 days post-transfer with 100% morbidity by day 20 (Supplementary Fig. S6, A and B). Adoptive transfer experiments T cell-depleted Lag3−/−Pdcd1−/− splenocytes clearly showed that both CD4+ or CD8+ T cell populations contributed to the disease observed, with a dominant role for the former (Supplementary Fig. S6, C and D). Consistent with these survival and weight loss data, histological analysis of CD4+ T cell-depleted Lag3−/−Pdcd1−/− splenocyte recipients revealed relatively normal bone marrow cellularity and density, while Lag3−/−Pdcd1−/− splenocyte recipients exhibited a near total absence of hematopoietic cell precursors in bone marrow and severe lymphoid depletion in the spleen, LNs, and Peyer‘s patches (Supplementary Table S2, Supplementary Fig. S6E, and Supplementary Fig. S7). These data indicate that CD4+ T cells are primarily responsible for the pathology observed. Cytokine analysis revealed high levels of IFN-γ, TNF-α, and MCP-1 in the serum of Lag3−/−Pdcd1−/− recipients but not single knockout or wild type control recipients (Supplementary Fig. 6, F-H). Taken together, these data suggest that Lag3−/−Pdcd1−/− splenocyte recipients, in contrast with their single knockout and wild type controls, develop an autoimmune GvHD-like syndrome with evidence of aplastic anemia and bone marrow failure as a cause of death.
The data thus far suggested that while a reasonable level of tolerance is maintained in single knockout Lag3−/− or Pdcd1−/− mice, dual loss of LAG-3 and PD-1 expression results in a loss of peripheral self-tolerance of CD4+ and CD8+ T cells. To test this in an antigen-specific system, we asked if HA-specific tolerance induced in transgenic mice expressing HA as a self-antigen in multiple epithelial tissues (C3-HAlo mice) (13, 43), could also be broken if adoptively transferred HA-specific T cells (from Clone 4 [CL4] TCR transgenics) lacked both inhibitory molecules. Compared with wild type CL4 T cells, significant expansion of Lag3−/−Pdcd1−/− clonotypic CD8+ T cells was observed 5 days post-transfer (Supplementary Fig. S6I). Although this was not substantially greater than seen with Pdcd1−/− T cells, the Lag3−/−Pdcd1−/− T cells exhibited a significantly enhanced effector phenotype, as determined by intracellular expression of IFN-γ and IL-17, compared with their single knockout and wild type controls (Supplementary Fig. S6, J and K). Similarly, when tolerance was broken in C3-HAlo transgenic mice (39), adoptive transfer of antigen-specific Lag3−/−Pdcd1−/− CD8+ T cells expanded significantly more than their single knockout and wild type controls, although enhanced in vivo CTL activity was comparable in the Pdcd1−/− and Lag3−/−Pdcd1−/− CD8+ T cell recipients (Supplementary Fig. S8). Collectively, these data suggest that the loss of LAG-3 and PD-1 also results in loss of tolerance to a model self-antigen.
Reduced tumor growth and enhanced survival in Lag3−/−Pdcd1−/− mice
To continue our analysis of Lag-3/PD-1 synergy in the regulation of anti-tumor immunity, we assessed tumor growth in Lag3−/−Pdcd1−/− mice and controls over time. Of the three transplantable tumor models examined in this study, B16 is regarded as the least immunogenic and thus the hardest to eliminate by immunological intervention (32, 34). A low dose of B16 cells (1.25×105) progressively grew in wild-type and Lag3−/− mice inoculated intradermally at day 0, whereas limited growth was observed in Pdcd1−/− and Lag3−/−Pdcd1−/− mice (Supplementary Fig. S9A). Although previous studies suggested that PD-1 deletion did not affect subcutaneously-injected tumor growth (44), our experiments revealed reduced tumor growth in Pdcd1−/− mice compared with wild type mice. Paradoxically, Lag3−/− mice developed slightly larger tumors. Whether this is due to reported defects in natural killer cell cytolysis (23), or an unexpected role of pDCs, which highly express LAG-3 (45), remains to be determined. Statistical analysis using the Maximum Likelihood method for synergy found that the lack of tumor growth in the Lag3−/− Pdcd1−/− mice was greater than the additive effects of tumor growth in Lag3−/− mice and Pdcd1−/− mice at day 11 (p<0.05) and day 13 (p<0.0005) suggesting that LAG-3 and PD-1 synergize to mediate tumor-induced tolerance. Depletion of CD4+ and CD8+ T cells restored normal B16 tumor growth in compound-deficient mice, indicating the necessity of adaptive immunity to the anti-tumor response (Supplementary Fig. S9B).
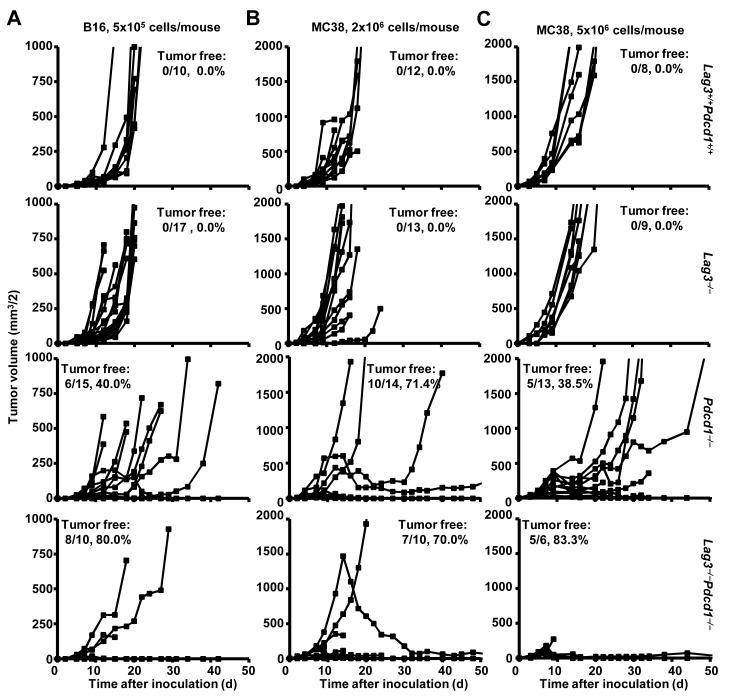
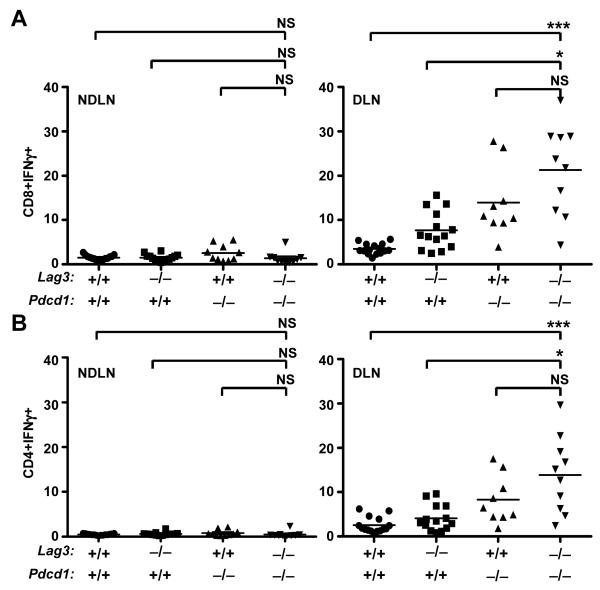
As the difference in resistance to B16 growth between Pdcd1−/− and Lag3−/−Pdcd1−/− mice seemed small, we evaluated tumor B16 and MC38 growth at different doses. At the higher B16 dose (5×105 cells/mouse), wild-type and Lag3−/− mice demonstrate uncontrolled tumor growth and lethality with an average survival time of less than 20 days (Fig. 5A and Supplemental Fig. S10). 80% of Lag3−/−Pdcd1−/− mice eliminated tumors compared with only 40% of Pdcd1−/− mice; however, B16 survivors did not display autoimmune vitiligo as is often seen with this model (46). We also investigated growth of subcutaneously-implanted MC38 adenocarcinoma cells at two different doses. Whereas MC38 growth and survival were comparable in Lag3−/− Pdcd1−/− and Pdcd1−/− mice when a low dose (2×105 cells/mouse) was used (70% vs. 71%, respectively; Figure 5B and Supplemental Fig. S10), Lag3−/−Pdcd1−/− mice were clearly more effective at preventing high-dose (5×105 cells/mouse) MC38 tumor growth and ensuring survival (83% vs. 38%, respectively; Fig. 5C and Supplemental Fig. S10). Phenotypic analysis revealed enhanced IFN-γ expression by Lag3−/−Pdcd1−/− CD4+ and CD8+ T cells in tumor-DLNs compared with wild-type and Lag3−/− mice, and NDLNs from all groups (Fig. 6). These data suggest that combined loss of LAG-3 and PD-1 limits tumor-mediated tolerization, enhances tumor-specific immunity and resistance to tumor growth.
Figure 5.
Reduced tumor growth in tumor-bearing Lag3−/−Pdcd1−/− mice. WT, Lag3−/−, Pdcd1−/−, and Lag3−/−Pdcd1−/− mice were inoculated on day 0 with 5×105 B16 cells i.d. (A), 2×106 MC38 cells s.c. (B), or 5×106 MC38 cells s.c. (C). Tumors were measured with an electronic caliper and reported as volume (see Methods). Data are combined from 2-3 repeated experiments, 3-5 animals per animals per group. Data were analyzed using the Maximum Likelihood method to determine synergy p values: (A) 0.0253, (B) 0.94, and (C) 0.0273 for the Lag3−/−Pdcd1−/− mice compared to the additive effect of the two single knockouts. Animals were euthanized when tumors became large, ulcerated, and/or necrotic.
Figure 6.
Tumor-draining (right panel) and non-draining (left panel) LN T cells were isolated on day 14 post-B16 inoculation and were activated with PMA + ionomycin for 4 h in the presence of brefeldin A. After intracellular cytokine staining, CD8+IFN-γ+ (A) and CD4+IFN-γ+ (B) cells were analyzed by flow cytometry. Data are representative of 10-15 animals per group. Nonparametric one-way ANOVA with Kruskal-Wallis test (p < 0.0001) was used; symbols represent * p<0.05, ** p<0.01, and *** p<0.001 vs Lag3−/−Pdcd1−/−.
To further investigate the killing efficacy of Lag3−/−Pdcd1−/− T cells in vivo in the presence of an established tumor, clonotypic CL4 CD8+ T cells were transferred into ProTRAMP male mice, which develop prostate cancer driven by the probasin promoter and express HA. After vaccination with HA-expressing Vaccinia virus, Lag3−/−Pdcd1−/− recipients showed significantly increased killing ability in comparison to the wild type, and slightly increased killing efficiency in comparison to Pdcd1−/− single knockouts (Supplementary Fig. S11). These data support the conclusion that Lag3−/−Pdcd1−/− CD8+ T cells are less susceptible to tumor-induced tolerance than wild type cells. Taken together, these data clearly show that Lag3−/−Pdcd1−/− mice are more capable of resisting high-dose tumor growth than Pdcd1−/− and wild-type mice.
Discussion
The data presented here illustrate clear synergy between the inhibitory receptors LAG-3 and PD-1 in controlling immune homeostasis, preventing autoimmunity, and enforcing tumor-induced tolerance. First, we demonstrate co-expression of LAG-3 and PD-1 on tumor-infiltrating lymphocytes. Second, we show that dual blockade of these receptors leads to decreased tumor growth and enhanced anti-tumor immunity. Importantly, dual-antibody-treated mice demonstrate more robust immune responses than either single-treated group. Third, analysis of mutant mice revealed a cooperative requirement for LAG-3 and PD-1 in maintaining immune homeostasis. Consistent with our observations following antibody-mediated blockade of LAG-3 and PD-1, Lag3−/−Pdcd1−/− mice prevented growth of high-dose B16 and MC38 tumors and ensured survival while single knockout controls and wild-type mice succumbed to disease. Taken together, these data reveal an unappreciated synergistic cooperation between LAG-3 and PD-1 in limiting tumor growth.
Although anti-LAG-3/anti-PD-1 combinatorial immunotherapy effectively cleared established Sa1N and MC38 tumors, this therapy was not effective against established B16 tumors. In contrast, B16 tumors were more difficult to establish in Lag3−/−Pdcd1−/− mice. B16 is a more difficult tumor to eradicate than MC38 and Sa1N and thus there could be several possible explanations for this apparent discrepancy. First, expression of LAG-3/PD-1 on TILs from B16 is lower than for MC38 and Sa1N, and this may be below a required threshold for efficacy upon antibody blockade in tumor-bearing mice. Second, as the knockout mice would lack LAG-3/PD-1 at the initiation of tumor inoculation, the immune system would not have this impediment at the commencement of an anti-tumor response. In contrast, the initial stages of LAG-3/PD-1 upregulation may already have occurred in tumor-bearing mice at the time of antibody treatment thereby establishing sufficient tolerance to prevent effective anti-tumor immunity. Third, there may be additional regulatory mechanisms that contribute a greater role with B16 that may be sufficient to prevent anti-tumor immunity when LAG-3/PD-1 blockade is initiated in mice that already have tumors. Lastly, we can’t rule out the possibility that the genetic deletion of LAG-3/PD-1 results in Tregs that have a cell-intrinsic defect in their regulatory capacity which impacts early tumor establishment. Of course, a combination of these issues could also contribute to the difference in B16 clearance seen in LAG-3/PD-1 deficiency versus late stage antibody-mediated blockade. It is possible that alternate dosing regimens and therapeutic combinations could result in effective clearance of established B16 with anti-LAG-3/anti-PD-1 combinatorial immunotherapy.
It is noteworthy that the percentage of IFN-γ+ TILs is higher in Lag3−/−Pdcd1−/− mice versus anti-LAG-3/anti-PD-1-treated mice (Fig. 6 versus Fig. 3). These differences could be reflective of the differences in B16 elimination in LAG-3/PD-1-deficient versus mAb-treated mice. Alternatively, as discussed above, these observations could be due to the different tumors analyzed or temporal differences between these experiments, as IFN-γ expression was determined 1 week after mAb treatment compared with 2 weeks after tumor inoculation into Lag3−/−Pdcd1−/− mice. Finally, we cannot rule out the possibility that there is a phenotypic difference in the immune cells in the Lag3−/−Pdcd1−/− mice. However, there does not seem to be an active systemic defect at the time of the experiment as high IFN-γ expression is not observed in the NDLN.
Although Lag3−/−Pdcd1−/− mice develop a lethal autoimmune condition, the disease is slower (~10 weeks versus 3-4 weeks) and less penetrant (80% vs 100%) than the phenotype observed in Ctla4−/− mice (20). Recently, analogous observations were reported by Honjo and colleagues in which BALB/c mice harboring a loss-of-function mutation in Lag3 combined with genetic deletion of Pdcd1 develop lethal myocarditis (47). Heart-infiltrating T cells from these compound-deficient mice were shown to produce high amounts of IFNγ compared with distal lymphoid organs such as the spleen. Our results are consistent with their with their data, as we also observed enhanced production of pro-inflammatory cytokines by T cells infiltrating sites of inflammation, such as the heart and pancreas in Lag3−/−Pdcd1−/− mice and in tumors and DLNs in knockout and antibody-treated mice. Furthermore, the Honjo group also observed accelerated autoimmune diabetes in NOD mice expressing a loss-of-function LAG-3 mutant, consistent with our recent observations in Lag3−/− NOD mice (48). However, the mice used by Honjo and colleagues that lacked functional LAG-3/PD-1 expression were on a BALB/c background, whereas our data were derived from mice on a C57BL/6 or B10.D2 background. Strain-specific differences between these mice have been well-documented (49, 50), and may have contributed to subtle differences in phenotypic and mechanistic observations reported. For instance, loss of LAG-3/PD-1 on a B10.D2 background can lead to an increase in IL-17+ cells which was not seen in mice on a Balb/c background.
While CTLA-4, PD-1 and LAG-3 are all negative regulators expressed during T cell activation, high level, dual LAG-3/PD-1 expression is largely restricted to infiltrating TILs. Thus, LAG-3/PD-1 combinatorial immunotherapy may promote tumor-specific responses relative to non-specific or self-antigen specific immune responses and thus may be less toxic than CTLA-4 blockade. Given the recent Phase 3 results with anti-CTLA-4 treatment of patients with metastatic melanoma, demonstrating a clear survival benefit (albeit with notable immune toxicity) (28), our results suggest that combined blockade of PD-1 and LAG-3 is a highly promising combinatorial strategy for the immune-based therapy of cancer.
Supplementary Material
Acknowledgements
We wish to thank Many Jo Turk and Jim Allison for cell lines. At St. Jude, the authors wish to thank Karen Forbes, Amy Krause, and Ashley Castellaw for maintenance and breeding of mouse colonies, Cliff Guy for help with cytokine analysis, Paul Thomas for anti-CD4 and anti-CD8 depleting Abs, Richard Cross, Greig Lennon and Stephanie Morgan for FACS, Song Wu and Hui Zhang for help with statistical analysis, the staff of the Shared Animal Resource Center at St Jude for the animal husbandry, the Veterinary Pathology Core Laboratory at St. Jude for histology and immunohistochemistry support, and the Hartwell Center for Biotechnology and Bioinformatics at St Jude for real-time PCR primer/probe synthesis and MOG synthesis and purification. At Johns Hopkins, the authors wish to thank Dih-Dih Huang for the maintenance and breeding of the mouse colonies, and technical support. At Bristol-Myers-Squibb, the authors wish to thank David Klitzing and the staff of the Milpitas animal facility for performing the tumor experiments and Rangan Vanganipuram, Brian Lee and Shilpa Mankikar for provision of antibodies.
Financial Support: This work was supported by the National Institutes of Health (R01 AI39480 to D.A.A.V., and R01 CA127153 and P50 CA58236-15 to C.G.D.), a Hartwell Postdoctoral Fellowship (to M.L.B.), NCI Comprehensive Cancer Center Support CORE grant (CA21765, to D.A.A.V.), the American Lebanese Syrian Associated Charities (ALSAC, to D.A.A.V.), the Patrick C. Walsh Fund (to C.G.D.), the Koch Fund (to C.G.D.), NHLBI contract - HHSN-268201999934C and CIHR - 20R92141 to P.J.U., and NIAID F32 AI080086 to C.C.L. C.G.D. is a Damon Runyon-Lilly Clinical Investigator.
Footnotes
Disclosure of Potential Conflict of Interest: The authors declare competing financial interests. D.P., D.A.A.V., C.G.D. and C.J.W. have submitted patents that are pending and are entitled to a share in net income generated from licensing of these patent rights for commercial development. A.J.K., M.S. and J.F.G. are employees of Bristol-Myers Squibb.
References
- 1.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229:126–44. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–92. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2010;344:269–78. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169:5392–5. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–95. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 15.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, et al. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11:129–35. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885–95. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 18.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 19.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–26. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 20.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki T, Dierich A, Benoist C, Mathis D. Independent modes of natural killing distinguished in mice lacking Lag3. Science. 1996;272:405–8. doi: 10.1126/science.272.5260.405. [DOI] [PubMed] [Google Scholar]
- 24.Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182:6659–69. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. European journal of immunology. 2003;33:970–9. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. International immunology. 1998;10:1563–72. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 31.Woo SR, Li N, Bruno TC, Forbes K, Brown S, Workman C, et al. Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4. European journal of immunology. 2010;40:1768–77. doi: 10.1002/eji.200939874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocak E, Lute K, Chang X, May KF, Jr., Exten KR, Zhang H, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–84. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 33.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–82. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park D, Lapteva N, Seethammagari M, Slawin KM, Spencer DM. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat Biotechnol. 2006;24:1581–90. doi: 10.1038/nbt1262. [DOI] [PubMed] [Google Scholar]
- 36.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–34. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 37.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) European journal of immunology. 2002;32:2255–63. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 38.Noya O, Alarcon de Noya B. The multiple antigen blot assay (MABA): a simple immunoenzymatic technique for simultaneous screening of multiple antigens. Immunol Lett. 1998;63:53–6. doi: 10.1016/s0165-2478(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–92. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paradis TJ, Floyd E, Burkwit J, Cole SH, Brunson B, Elliott E, et al. The anti-tumor activity of anti-CTLA-4 is mediated through its induction of IFN gamma. Cancer Immunol Immunother. 2001;50:125–33. doi: 10.1007/s002620100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–21. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 43.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–64. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. International immunology. 2005;17:133–44. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 45.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–91. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Cote AL, de Vries VC, Usherwood EJ, Turk MJ. Induction of postsurgical tumor immunity and T-cell memory by a poorly immunogenic tumor. Cancer Res. 2007;67:6468–76. doi: 10.1158/0008-5472.CAN-07-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X, et al. Cutting Edge: Accelerated Autoimmune Diabetes in the Absence of LAG-3. J Immunol. 187:3493–8. doi: 10.4049/jimmunol.1100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenner GJ, Cohen N, Moynihan JA. Similar immune response to nonlethal infection with herpes simplex virus-1 in sensitive (BALB/c) and resistant (C57BL/6) strains of mice. Cell Immunol. 1994;157:510–24. doi: 10.1006/cimm.1994.1246. [DOI] [PubMed] [Google Scholar]
- 50.Niewiesk S, Brinckmann U, Bankamp B, Sirak S, Liebert UG, ter Meulen V. Susceptibility to measles virus-induced encephalitis in mice correlates with impaired antigen presentation to cytotoxic T lymphocytes. J Virol. 1993;67:75–81. doi: 10.1128/jvi.67.1.75-81.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.