Abstract
Purpose
Adolescents and young adults (AYAs) with cancer have not experienced improvements in survival to the same extent as children and older adults. We compared outcomes among children (<15 years), AYAs (15-40 years) and older adults (>40 years) receiving allogeneic hematopoietic cell transplant (HCT) for acute myeloid leukemia (AML).
Patients and Methods
Our cohort consisted of 900 children, 2708 AYA and 2728 older adult recipients of HLA-identical sibling or unrelated donor (URD) transplant using myeloablative or reduced-intensity/non-myeloablative conditioning. Outcomes were assessed over three time periods (1980-1988, 1989-1997, 1998-2005) for sibling and two time periods (1989-1997, 1998-2005) for URD HCT. Analyses were stratified by donor type.
Results
Overall survival for AYAs using either siblings or URD improved over time. Although children had better and older adults had worse survival compared to AYAs, improvements in survival for AYAs did not lag behind those for children and older adults. After sibling donor HCT, five-year adjusted survival for the three time periods was 40%, 48% and 53% for children, 35%, 41% and 42% for AYAs and 22%, 30% and 34% for older adults. Among URD HCT recipients, five-year adjusted survival for the two time periods was 38% and 37% for children, 24% and 28% for AYAs and 19% and 23% for older adults. Improvements in survival occurred due to a reduction in risk of treatment-related mortality. The risk of relapse did not change over time.
Conclusion
Improvements in survival among AYAs undergoing allogeneic HCT for AML have paralleled those among children and older adults.
Introduction
Cancer is the leading cause of non-accidental death among adolescents and young adults (AYA) in the United States (U.S.).1,2 Tremendous improvements in cancer treatments have taken place over the last several decades; however, survival for AYAs with cancer has not improved to the same extent as for children and older adults with cancer.1-4 The reasons for these outcome disparities among AYA cancer patients are not well understood.3,5 Contributing factors may include age-related differences in biology, socioeconomic status, access to care, adherence to medical plans, participation rates in clinical trials, treatment in centers experienced with the care of AYA patients, caregiver availability, and other psychosocial issues.6
Innovations in transplantation care since the 1980s have led to incremental improvements in survival and reduction of treatment-related mortality (TRM) among hematopoietic cell transplantation (HCT) recipients.7,8 Whether improvements in transplant outcomes have occurred to the same degree across all age groups, particularly AYAs, is not well known. Using data from the Center for International Blood and Marrow Transplant Research (CIBMTR), we compared outcomes of children, AYAs and older adult recipients of allogeneic HCT for acute myeloid leukemia (AML) to determine whether AYAs have experienced improvements in survival at rates that are similar to their younger and older counterparts.
Methods
Data source and patients
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR) and the National Marrow Donor Program (NMDP) established in 2004, which comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplants to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority, and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Board (IRB) of the National Marrow Donor Program and the Medical College of Wisconsin since 1985.
For this study, we included patients who had received their first allogeneic HCT for AML using either an HLA-identical sibling donor (matched sibling donor, MSD) or unrelated donor (URD), from 1980 to 2005 at a U.S. transplant center. Patients transplanted using myeloablative, reduced-intensity (RIC) or non-myeloablative (NMA) conditioning regimens were included in this analysis. Patients with acute promyelocytic leukemia, recipients of cord blood grafts and recipients of prior autologous HCT were excluded. Patients were divided into three groups based on age at transplantation: children (<15 years), AYA (15-40 years),2 and older adults (>40 years).
All surviving recipients included in this analysis were contacted retrospectively and provided informed consent for participation in the NMDP research program. Informed consent was waived by the NMDP IRB for all deceased recipients. Approximately 10% of surviving patients would not provide consent for use of research data. To adjust for the potential bias introduced by exclusion of non-consenting surviving patients, a corrective action modeling process randomly excluded the same percentage of deceased patients using a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of the data in survivors.9
Outcomes and study definitions
The primary objective of this study was to compare change over time in rates of overall survival (OS), leukemia-free survival (LFS), relapse and TRM among children, AYAs and older adults. For OS, death from any cause was considered an event. LFS was defined as survival in complete remission (CR) after HCT. Relapse was defined as leukemia recurrence. TRM was defined as death in CR. All outcomes were assessed from the date of transplantation.
Disease status was classified as early, intermediate or advanced.10 Early disease included AML in CR1. AML in ≥ CR2 was categorized as intermediate and patients in relapse/primary induction failure were classified as advanced disease. The NMDP classification of HLA-matching status was used for URD recipients (well-matched, partially-matched or mismatched).11 Conditioning regimens were classified as myeloablative or RIC/NMA based on CIBMTR definitions.12 Where information was available, cytogenetic risk was classified according to standard criteria as good, intermediate or poor.13,14
Statistical methods
Summaries of patient-, disease- and treatment-related characteristics were produced for the three age groups. Univariate probabilities of OS and LFS were calculated using the Kaplan-Meier estimator.15 Probabilities of relapse and TRM were estimated using a cumulative incidence function method.16
Separate analyses were performed for MSD and URD recipients. To evaluate changes in outcomes over time, we divided the MSD cohort into three time periods (1980-1988, 1989-1997 and 1998-2005) based on year of transplantation. Because the NMDP began facilitating URD HCT in the U.S. in 1986, the URD cohort was evaluated over two time periods (1989-1997 and 1998-2005).
Cox proportional hazards models were used to adjust for significant covariates while comparing the three age groups. All factors were examined for proportional hazards using a time-dependent covariate approach. The non-proportional hazards were addressed by introducing a time-dependent covariate to appropriately model early versus later events in OS (<3 months and ≥ 3 months), relapse (<3 months and ≥ 3 months), and TRM (<6 months and ≥ 6 months) in multivariable analyses for MSD recipients. A backward regression model selection technique was used to identify significant covariates to be included in the models. The main effects tested in all multivariate analysis models for the outcomes of interest were age and time period of transplantation. Interaction between age and time period was also examined. All p-values were two-sided, and risk factors of P<0.05 were included in the final model. In addition to age and time period of transplant, the covariates considered in the multivariable models included: gender, Karnofsky/Lansky performance status at HCT (<90 vs. ≥ 90), disease status at HCT (CR1 vs. CR2 vs. other), time from diagnosis to HCT (<6 vs. 6-12 vs. >12 months), graft source (peripheral blood vs. bone marrow), donor-recipient gender mismatch, and donor-recipient cytomegalovirus (CMV) status. HLA matching status was also considered for URD recipients. Transplant practices such as use conditioning regimen intensity and graft-versus-host disease prophylaxis regimens changed at different rates over time among the three age-groups; hence, we did not adjust for these variables in multivariate analyses.
All computations were performed using the SAS Version 9.2 statistical package (SAS Institute, Cary, NC).
Results
Patient characteristics
Table 1 describes patient, disease and transplant characteristics. Overall, 900 children, 2708 AYA and 2728 older adults met the study inclusion criteria. The numbers of patients receiving a transplant increased over time for all age groups. This increase was largely due to greater utilization of URD transplants. Among URD recipients, a greater proportion received HLA well-matched grafts in the most recent time period. Other notable trends over time included increasing utilization of peripheral blood as a graft source (particularly among AYA and older adult recipients) and larger numbers of older adults undergoing RIC/NMA HCT.
Table 1.
Characteristics of patients who received HLA-identical sibling donor or unrelated donor allogeneic hematopoietic-cell transplant for acute myeloid leukemia in the United States from 1980-2005.
| Children | Adolescent and young adults | Older adults | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 1980-1988 | 1989-1997 | 1998-2005 | P- value |
1980- 1988 |
1989- 1997 |
1998- 2005 |
P- value |
1980- 1988 |
1989- 1997 |
1998- 2005 |
P- value |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||||
| Number of Patients | 110 | 373 | 417 | 462 | 1109 | 1137 | 57 | 683 | 1988 | |||
| Number of Centers | 19 | 60 | 67 | 36 | 105 | 111 | 17 | 87 | 102 | |||
| Male | 51 (46) | 209 (56) | 229 (55) | 230 (50) | 605 (55) | 610 (54) | 36 (63) | 360 (53) | 1068 (54) | |||
| Race/ethnicity | 0.01 | <0.01 | 0.09 | |||||||||
| White | 90 (82) | 299 (80) | 293 (70) | 414 (90) | 940 (85) | 936 (82) | 48 (84) | 617 (90) | 1809 (91) | |||
| Other | 19 (16) | 73 (20) | 123 (29) | 47 (10) | 168 (16) | 190 (19) | 9 ( 6) | 65 (10) | 163 ( 8) | |||
| Missing | 1 ( 1) | 1 (<1) | 1 (<1) | 1 (<1) | 1 (<1) | 11 ( 1) | 0 | 1 (<1) | 16 ( 1) | |||
| Performance status score at HCT | 0.90 | <0.01 | <0.01 | |||||||||
| < 90 | 20 (18) | 76 (20) | 75 (18) | 128 (28) | 389 (35) | 348 (31) | 19 (33) | 309 (45) | 681 (34) | |||
| ≥ 90 | 87 (79) | 294 (79) | 307 (74) | 323 (70) | 708 (64) | 691 (61) | 36 (63) | 370 (54) | 1093 (55) | |||
| Missing | 3 ( 3) | 3 ( 1) | 35 ( 8) | 11 ( 2) | 12 ( 1) | 98 ( 9) | 2 ( 4) | 4 ( 1) | 214 (11) | |||
| Disease status at HCT | <0.01 | <0.01 | <0.01 | |||||||||
| CR1 | 69 (63) | 169 (45) | 163 (39) | 263 (57) | 401 (36) | 397 (35) | 24 (42) | 203 (30) | 764 (38) | |||
| CR2 | 19 (17) | 67 (18) | 134 (32) | 52 (11) | 198 (18) | 275 (24) | 6 (11) | 94 (14) | 380 (19) | |||
| Primary induction failure | 5 ( 5) | 53 (14) | 42 (10) | 48 (10) | 151 (14) | 156 (14) | 9 (16) | 133 (19) | 331 (17) | |||
| Relapse | 16 (15) | 76 (20) | 69 (17) | 84 (18) | 318 (29) | 283 (25) | 17 (30) | 231 (34) | 443 (22) | |||
| Other/Missing | 1 (<1) | 8 ( 2) | 9 ( 2) | 15 ( 3) | 41 ( 4) | 26 ( 2) | 1 ( 2) | 22 ( 3) | 70 ( 6) | |||
| Cytogenetic risk at diagnosis | <0.01 | <0.01 | <0.01 | |||||||||
| Good | 7 ( 6) | 30 ( 8) | 34 ( 8) | 21 ( 5) | 83 ( 7) | 78 ( 7) | 4 ( 7) | 28 ( 4) | 87 ( 4) | |||
| Intermediate | 23 (21) | 185 (50) | 240 (58) | 111 (24) | 457 (41) | 703 (62) | 18 (32) | 353 (52) | 1217 (61) | |||
| Poor | 3 ( 3) | 60 (16) | 79 (19) | 26 ( 6) | 140 (13) | 193 (17) | 5 ( 9) | 96 (14) | 408 (21) | |||
| Unknown | 77 (70) | 98 (26) | 64 (15) | 304 (66) | 429 (39) | 163 (14) | 30 (53) | 206 (30) | 276 (14) | |||
| Time from diagnosis to HCT | <0.01 | <0.01 | 0.12 | |||||||||
| < 6 months | 73 (66) | 201 (54) | 200 (48) | 311 (67) | 480 (43) | 455 (40) | 33 (58) | 290 (42) | 831 (42) | |||
| 6-12 months | 18 (16) | 104 (28) | 83 (20) | 82 (18) | 340 (31) | 339 (30) | 10 (18) | 213 (31) | 603 (30) | |||
| > 12 months | 19 (17) | 66 (18) | 134 (32) | 69 (15) | 288 (26) | 339 (30) | 14 (25) | 178 (26) | 549 (28) | |||
| Missing | 0 | 2 ( 1) | 0 | 0 | 1 (<1) | 4 (<1) | 0 | 2 (<1) | 5 (<1) | |||
| Graft type | <0.01 | <0.01 | <0.01 | |||||||||
| Bone marrow | 110 (100) | 370 (99) | 340 (82) | 462 (100) | 1056 (95) | 609 (54) | 57 (100) | 627 (92) | 694 (35) | |||
| Peripheral blood | 0 | 3 ( 1) | 77 (18) | 0 | 53 ( 5) | 528 (46) | 0 | 56 ( 8) | 1294 (65) | |||
| HLA match* | <0.01 | <0.01 | <0.01 | |||||||||
| HLA-identical sibling | 110 | 171 (46) | 86 (21) | 462 | 604 (54) | 221 (19) | 57 | 421 (62) | 553 (28) | |||
| Unrelated, well-matched | 0 | 37 (10) | 134 (32) | 0 | 91 ( 8) | 446 (39) | 0 | 74 (11) | 897 (45) | |||
| Unrelated, partially matched | 0 | 72 (19) | 116 (28) | 0 | 206 (19) | 318 (28) | 0 | 111 (16) | 382 (19) | |||
| Unrelated, mismatched | 0 | 87 (23) | 75 (18) | 0 | 207 (19) | 140 (12) | 0 | 73 (11) | 135 ( 7) | |||
| Missing | 0 | 6 ( 2) | 6 ( 1) | 0 | 1 (<1) | 12 ( 1) | 0 | 4 ( 1) | 21 ( 1) | |||
| Conditioning regimen | 0.16 | <0.01 | <0.01 | |||||||||
| Myeloablative | 109 (99) | 354 (95) | 387 (93) | 453 (98) | 1051 (95) | 944 (83) | 56 (98) | 657 (96) | 1145 (58) | |||
| RIC/NMA | 1 ( 1) | 19 ( 5) | 19 ( 5) | 9 ( 2) | 58 ( 5) | 161 (14) | 1 ( 2) | 25 ( 4) | 776 (39) | |||
| Missing | 0 | 0 | 11 ( 3) | 0 | 0 | 32 ( 3) | 0 | 1 (<1) | 67 ( 3) | |||
| GVHD prophylaxis | <0.01 | <0.01 | <0.01 | |||||||||
| CSA + MTX ± other | 14 (13) | 193 (52) | 207 (50) | 58 (13) | 576 (52) | 445 (39) | 8 (14) | 350 (51) | 550 (28) | |||
| CSA ± other (no MTX) | 20 (18) | 44 (12) | 18 ( 4) | 162 (35) | 229 (21) | 74 ( 7) | 25 (44) | 139 (20) | 360 (18) | |||
| FK506 + MTX | 0 | 6 ( 2) | 66 (16) | 0 | 60 ( 5) | 370 (33) | 0 | 39 ( 6) | 669 (34) | |||
| MTX | 74 (67) | 36 (10) | 12 ( 3) | 178 (39) | 33 ( 3) | 9 ( 1) | 8 (14) | 12 ( 2) | 11 ( 1) | |||
| T-cell depletion | 2 ( 2) | 92 (25) | 98 (24) | 59 (13) | 183 (17) | 142 (12) | 16 (28) | 106 (16) | 125 ( 6) | |||
| Other | 0 | 2 ( 1) | 16 ( 4) | 5 ( 1) | 28 ( 3) | 97 ( 9) | 0 | 37 ( 5) | 273 (14) | |||
| Recipient/Donor gender match | 0.23 | <0.01 | <0.01 | |||||||||
| Recipient male/Donor male | 24 (22) | 126 (34) | 144 (35) | 136 (29) | 350 (32) | 391 (34) | 22 (39) | 206 (30) | 675 (34) | |||
| Recipient male/Donor female | 28 (25) | 90 (24) | 99 (24) | 113 (24) | 243 (22) | 297 (26) | 9 (16) | 156 (23) | 544 (27) | |||
| Recipient female/Donor male | 27 (25) | 82 (22) | 84 (20) | 94 (20) | 253 (23) | 218 (19) | 14 (25) | 151 (22) | 392 (20) | |||
| Recipient female/Donor female | 31 (28) | 74 (20) | 88 (21) | 119 (26) | 256 (23) | 229 (20) | 12 (21) | 165 (24) | 373 (19) | |||
| Missing | 0 | 1 (<1) | 2 (<1) | 0 | 7 ( 1) | 2 (<1) | 0 | 5 ( 1) | 4 (<1) | |||
| Recipient/Donor CMV status | <0.01 | <0.01 | <0.01 | |||||||||
| Recipient and donor negative | 16 (15) | 164 (44) | 154 (37) | 75 (16) | 304 (27) | 357 (31) | 7 (12) | 142 (21) | 482 (24) | |||
| Recipient or donor positive | 22 (20) | 202 (54) | 249 (60) | 153 (33) | 779 (70) | 768 (68) | 36 (63) | 534 (78) | 1478 (74) | |||
| Missing | 72 (65) | 7 ( 2) | 14 ( 3) | 234 (51) | 26 ( 2) | 12 ( 1) | 14 (25) | 7 ( 1) | 28 ( 1) | |||
| Occurrence of acute GVHD | 0.02 | 0.04 | 0.78 | |||||||||
| Yes | 42 (38) | 167 (45) | 222 (53) | 225 (49) | 537 (48) | 627 (55) | 27 (47) | 342 (50) | 1002 (50) | |||
| No | 67 (61) | 204 (55) | 194 (47) | 235 (51) | 560 (50) | 502 (44) | 30 (53) | 340 (50) | 981 (49) | |||
| Missing | 1 ( 1) | 2 ( 1) | 1 (<1) | 2 (<1) | 12 ( 1) | 8 ( 1) | 0 | 1 (<1) | 5 (<1) | |||
| Occurrence of chronic GVHD | 0.95 | <0.01 | <0.01 | |||||||||
| Yes | 31 (28) | 98 (26) | 122 (29) | 154 (33) | 348 (31) | 464 (41) | 15 (26) | 192 (28) | 799 (40) | |||
| No | 79 (72) | 259 (69) | 294 (71) | 308 (67) | 691 (62) | 669 (59) | 42 (74) | 467 (68) | 1189 (60) | |||
| Missing | 0 | 16 ( 4) | 1 (<1) | 0 | 70 ( 6) | 4 (<1) | 0 | 24 ( 4) | 0 | |||
| Follow-up, months | ||||||||||||
| Median | 163 | 116 | 59 | 147 | 120 | 50 | 158 | 130 | 47 | |||
| Range | 37 - 315 | 4-214 | 3-132 | 6-328 | 3-239 | 3-133 | 49-302 | 4-235 | 2-121 | |||
KPS – Karnofsky performance status, HCT – hematopoietic cell transplantation, US – United States, CR – complete remission, RIC – reduced intensity conditioning, NMA – non-myeloablative conditioning, GVHD – graft-versus-host disease, CSA – cyclosporine, MTX – methotrexate, FK506 – tacrolimus, CMV – cytomegalovirus
Prior to 1989, 40 URD HCT recipients (all age groups) met the study eligibility criteria; these patients were not considered in this analysis.
Outcomes after HLA-identical sibling donor HCT
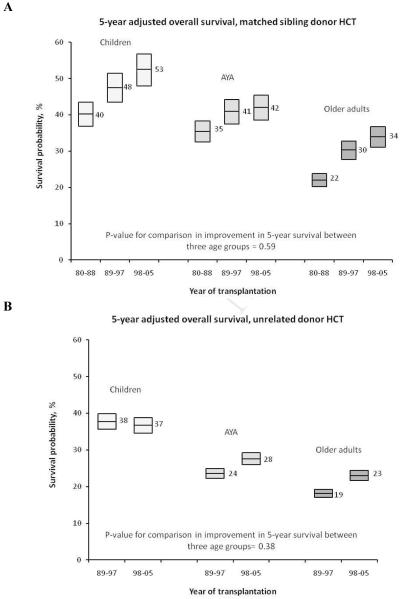
Univariate analysis showed that the probability of OS at five-years post transplant improved significantly for patients transplanted from 1998-2005, compared to 1980-1988, for all age groups (Table 2, Figure 1A). Also, survival was inversely correlated with age for all three time periods; five-year OS for children, AYAs and older adults receiving transplants for AML in 1998-2005 were 64%, 43% and 31%, respectively. Change in five-year OS for AYAs paralleled that for children and older adults for the time periods studied (Table 2). In multivariate analyses of the MSD cohort, older age at HCT was associated with increased risk of overall mortality. Transplantation in a more recent time period, in general, was associated with a lower risk of mortality (Table 3). We also examined survival over time within each age group using multivariate analyses (Table 4); the AYA and older adult age groups improved the most in OS over time, especially in the early post-transplant period for 1998-2005.
Table 2.
Unadjusted probability of overall survival and leukemia-free survival, and cumulative incidence of relapse and treatment-related mortality at five years after transplantation
| Outcome | 1980-1988* | 1989-1997* | 1998-2005* | P-value† |
|---|---|---|---|---|
| Matched sibling donor HCT | ||||
| Overall survival | 0.23 | |||
| Children | 45 (41-50)% | 53 (49-57)% | 64 (57-71)% | |
| Adolescent and young adults | 37 (35-39)% | 40 (38-42)% | 43 (40-46)% | |
| Older adults | 21 (19-23)% | 26 (25-27)% | 31 (30-33)% | |
| Leukemia-free survival | 0.76 | |||
| Children | 45 (36-55)% | 47 (39-55)% | 58 (47-69)% | |
| Adolescent and young adults | 35 (31-40)% | 36 (32-40)% | 42 (35-49)% | |
| Older adults | 20 (10-31)% | 26 (22-30)% | 29 (25-34)% | |
| Relapse | <0.01 | |||
| Children | 32 (24-42)% | 43 (35-50)% | 26 (17-37)% | |
| Adolescent and young adults | 26 (22-30)% | 34 (30-38)% | 38 (31-45)% | |
| Older adults | 13 (5-23)% | 31 (26-35)% | 40 (36-44)% | |
| Treatment related mortality | 0.01 | |||
| Children | 22 (15-31)% | 10 (6-15)% | 16 (8-25)% | |
| Adolescent and young adults | 39 (35-44)% | 30 (26-34)% | 20 (15-26)% | |
| Older adults | 67 (55-79)% | 44 (39-48)% | 31 (27-35)% | |
| Unrelated donor HCT | ||||
| Overall survival | 0.87 | |||
| Children | - | 28 (26-30)% | 38 (36-40)% | |
| Adolescent and young adults | - | 17 (16-17)% | 29 (28-30)% | |
| Older adults | - | 15 (14-16)% | 26 (25-27)% | |
| Leukemia-free survival | 0.77 | |||
| Children | - | 25 (19-31)% | 34 (29-40)% | |
| Adolescent and young adults | - | 16 (13-19)% | 27 (24-30)% | |
| Older adults | - | 15 (11-19)% | 24 (21-26)% | |
| Relapse | 0.03 | |||
| Children | - | 42 (36-49)% | 40 (35-46)% | |
| Adolescent and young adults | - | 29 (25-33)% | 34 (31-37)% | |
| Older adults | - | 25 (20-30)% | 39 (37-42)% | |
| Treatment related mortality | 0.05 | |||
| Children | - | 33 (27-39 )% | 26 (21-31)% | |
| Adolescent and young adults | - | 56 (51-60)% | 39 (36-43)% | |
| Older adults | - | 60 (54-66)% | 37 (34-40)% |
Probability and 95% confidence intervals
P-value for comparison in improvement in 5-year outcomes between the three age groups (2 degrees of freedom)
Figure 1.
Trends over time for five-year adjusted overall survival after HLA-identical sibling donor (A) and unrelated donor (B) hematopoietic cell transplantation for AML. The edges of the box plots represent 95% confidence intervals.
Table 3.
Results of multivariate analyses for overall survival, leukemia-free survival, relapse and treatment-related mortality among recipients of HLA-identical sibling donor transplantation
| Variable | Hazard ratio* | 95% confidence intervals |
P-value |
|---|---|---|---|
| Overall survival† | |||
| Age at transplant, years | |||
| Children | 1.0 | - | <0.01‡ |
| Adolescent and young adults | 1.22 | 1.03-1.44 | 0.02 |
| Older adults | 1.68 | 1.41-2.00 | <0.01 |
| Year of transplant | |||
| 1980-1988 | 1.0 | - | <0.01§ |
| 1989-1997 | 0.83 | 0.73-0.95 | <0.01 |
| 1998-2005 (> 3 months after HCT) | 0.55 | 0.44-0.70 | <0.01 |
| 1998-2005 (≥ 3 months after HCT) | 0.88 | 0.74-1.05 | 0.15 |
| Leukemia-free survival∥ | |||
| Age at transplant, years | |||
| Children | 1.0 | - | <0.01‡ |
| Adolescent and young adults | 1.14 | 0.97-1.34 | 0.11 |
| Older adults | 1.47 | 1.24-1.75 | <0.01 |
| Year of transplant | |||
| 1980-1988 | 1.0 | - | 0.06‡ |
| 1989-1997 | 0.87 | 0.77-1.00 | 0.05 |
| 1998-2005 | 0.83 | 0.70-0.99 | 0.02 |
| Relapse¶ | |||
| Age at transplant, years | |||
| Children | 1.0 | - | 0.02‡ |
| Adolescent and young adults | 0.76 | 0.62-0.93 | <0.01 |
| Older adults | 0.77 | 0.62-0.96 | 0.02 |
| Year of transplant | |||
| 1980-1988 | 1.0 | - | <0.01§ |
| 1989-1997 | 1.10 | 0.90-1.34 | 0.35 |
| 1998-2005 (> 3 months after HCT) | 1.66 | 1.24-2.22 | <0.01 |
| 1998-2005 (≥ 3 months after HCT) | 1.18 | 0.92-1.52 | 0.20 |
| Treatment-related mortality** | |||
| Age at transplant, years | |||
| Children | 1.0 | - | <0.01‡ |
| Adolescent and young adults | 2.06 | 1.55-2.73 | <0.01 |
| Older adults | 3.40 | 2.53-4.56 | <0.01 |
| Year of transplant | |||
| 1980-1988 | 1.0 | - | <0.01§ |
| 1989-1997 | 0.65 | 0.55-0.77 | <0.01 |
| 1998-2005 (> 6 months after HCT) | 0.38 | 0.30-0.48 | <0.01 |
| 1998-2005 (≥ 6 months after HCT) | 0.64 | 0.47-0.85 | <0.01 |
Hazard ratios refer to hazards of death or relapse
Other variables significantly associated with overall survival included cytogenetic risk at diagnosis, disease status at transplant, time from diagnosis to transplant, performance status score at transplant and patient gender (also see Appendix Table 3)
Overall P-value, 2 degrees of freedom test
Overall P-value, 3 degrees of freedom test
Other variables significantly associated with leukemia-free survival included gender, cytogenetic risk at diagnosis, disease status at transplant, time from diagnosis to transplant and performance status score at transplant (also see Appendix Table 3)
Other variables significantly associated with relapse included cytogenetic risk at diagnosis, disease status at transplant, time from diagnosis to transplant and performance status score at transplant (also see Appendix Table 3)
Other variables significantly associated with treatment-related mortality included disease status at transplant, time from diagnosis to transplant and performance status score at transplant (also see Appendix Table 3)
Table 4.
Contrasts between time periods of transplant for each of the three age groups after matched sibling donor and unrelated donor hematopoietic cell transplantation for acute myeloid leukemia. The numbers in the table represent hazard ratios from multivariate analysis adjusting for important patient and disease related variables (hazard ratio of >1.00 indicates increased hazards of death or relapse).
| Children | Adolescent and young adults |
Older adults | ||||
|---|---|---|---|---|---|---|
| MATCHED SIBLING DONOR TRANSPLANTATION | ||||||
| vs. 1980-1988 | vs. 1989-1997 | vs. 1980-1988 | vs. 1989-1997 | vs. 1980-1988 | vs. 1989-1997 | |
| Overall survival | ||||||
| 1989-1997 | 0.80 | -- | 0.85* | -- | 0.77 | -- |
| 1998-2005 (< 3 mos) | 0.77 | 0.96 | 0.40* | 0.47* | 0.54* | 0.71* |
| 1998-2005 (≥ 3 mos) | 0.65 | 0.81 | 1.65 | 1.25 | 0.78 | 1.02 |
| Leukemia free survival | ||||||
| 1989-1997 | 0.88 | -- | 0.90 | -- | 0.85 | -- |
| 1998-2005 | 0.82 | 0.93 | 0.94 | 1.05 | 0.88 | 1.04 |
| Relapse | ||||||
| 1989-1997 | 1.06 | -- | 1.08 | -- | 1.89 | -- |
| 1998-2005 (< 3 mos) | 1.28 | 1.21 | 1.29 | 1.19 | 3.01* | 1.59* |
| 1998-2005 (≥ 3 mos) | 0.60 | 0.57 | 1.35 | 1.25 | 2.06 | 1.08 |
|
Treatment related mortality |
||||||
| 1989-1997 | 0.48* | -- | 0.80* | -- | 0.63* | -- |
| 1998-2005 (< 6 mos) | 0.72 | 1.49 | 0.45* | 0.57* | 0.43* | 0.67* |
| 1998-2005 (≥ 6 mos) | 0.95 | 1.96 | 0.94 | 1.12 | 0.68 | 1.08 |
| UNRELATED DONOR TRANSPLANTATION | ||||||
| vs. 1989-1997 | vs. 1989-1997 | vs. 1989-1997 | ||||
| Overall survival | ||||||
| 1998-2005 | 1.06 | 0.59* | 0.49* | |||
| Leukemia free survival | ||||||
| 1998-2005 | 1.06 | 0.63* | 0.57* | |||
| 1998-2005 | 1.15 | 1.10 | 1.70* | |||
|
Treatment related mortality |
||||||
| 1998-2005 | 0.93 | 0.40* | 0.27* | |||
P < 0.05
A similar trend was observed for LFS. Five-year LFS rates improved over time for all age groups; better LFS was seen for children than AYAs, who in turn had better LFS than older adults (Table 2). Again, change in LFS for AYA’s paralleled that of children and older adults. Multivariate analysis showed that older adults had significantly worse LFS rates, while AYAs had comparable LFS rates to those of children (Table 3). The time period of transplantation was not found to be significantly associated with LFS, which was confirmed in multivariate analysis by age group (Table 4).
Cumulative incidence of TRM decreased over time, especially among AYAs and older adults, and was again inversely related to patient age at time of transplantation (Table 2). Multivariate analysis showed there was a strong age correlation with relative risk of TRM, with AYAs having twice the risk and older adults having more than three times the risk of dying from transplant-related complications than children (Table 3). Patients had a lower relative risk of TRM in the 1989-1997 and 1998-2005 time periods than in the 1980-1988 timeframe. This was further confirmed in analyses for each age group (Table 4); TRM improved from 1980-1988 to 1989-1997 for all three age groups. Early TRM (<6 months post-transplant) improved significantly from 1989-1997 to 1998-2005 for AYAs and older adults.
Multivariate analysis of all MSD showed differences in relapse risks by age and time period (Table 3). In analyses within each age group, while there were no statistically significant changes in relapse rates for children and AYA patients over the three time periods considered, early relapse rates (<3 months after transplantation) increased among older patients (Table 4). The relative risk of early relapse among older patients transplanted from 1998-2005 was 1.6 times higher than those transplanted from 1989-1997 (hazard ratio [HR] 1.59, 95% CI 1.17-2.16, P=0.003) and 3 times higher than those transplanted from 1980-1988 (HR 3.01, 95% CI 1.37-6.60, P=0.006).
We found no significant interactions between age group and time period for any of the endpoints studied. We observed no notable trends in outcome disparities between the 15-25 and 26-40 year age groups (Appendix Table 1). Although limited by small numbers, unadjusted analyses for a subset of patients who had received myeloablative HCT for AML in CR1 did not show a negative trend in survival for AYAs (Appendix Table 2).
Appendix table 3 shows hazard ratios for factors other than time period of transplantation and age-group that were significantly associated with outcomes following MSD HCT.
Outcomes after unrelated donor HCT
Similar to recipients of MSD transplants, survival for URD HCT recipients also improved significantly over time (1989-1997 vs. 1998-2005), and was inversely related to age at transplant (Table 2, Figure 1B). Similar to sibling donor HCT, changes in five-year OS for AYAs paralleled those of children and older adults (Table 2). In multivariate analyses, older age at time of HCT was associated with increasing risk of overall mortality (Table 5). For the URD cohort, we also observed a significant association between time period of transplant and OS after URD HCT, with mortality risk improving for patients transplanted in the most recent time period. Furthermore, analysis by age group showed significant improvement in OS for AYAs and older adults, but not for children, from 1998-2005 when compared to 1989-1997 (Table 4).
Table 5.
Results of multivariate analyses for overall survival, leukemia-free survival, relapse and treatment-related mortality among recipients of unrelated donor transplantation
| Variable | Hazard ratio* | 95% confidence intervals |
P-value |
|---|---|---|---|
| Overall survival† | |||
| Age at transplant, years | |||
| Children | 1.0 | - | <0.01‡ |
| Adolescent and young adults | 1.33 | 1.18-1.50 | <0.01 |
| Older adults | 1.43 | 1.26-1.62 | <0.01 |
| Year of transplant | |||
| 1989-1997 | 1.0 | - | <0.01 |
| 1998-2005 | 0.74 | 0.67-0.81 | |
| Leukemia-free survival§ | |||
| Age at transplant, years | |||
| Children | 1.0 | - | <0.01‡ |
| Adolescent and young adults | 1.22 | 1.08-1.38 | <0.01 |
| Older adults | 1.28 | 1.14-1.45 | <0.01 |
| Year of transplant | |||
| 1989-1997 | 1.0 | - | <0.01 |
| 1998-2005 | 0.80 | 0.73-0.87 | |
| Relapse∥ | |||
| Age at transplant, years | |||
| Children | 1.0 | - | 0.03‡ |
| Adolescent and young adults | 0.80 | 0.68-0.95 | 0.01 |
| Older adults | 0.90 | 0.76-1.05 | 0.18 |
| Year of transplant | |||
| 1989-1997 | 1.0 | - | 0.10 |
| 1998-2005 | 1.13 | 0.98-1.30 | |
| Treatment-related mortality¶ | |||
| Age at transplant, years | |||
| Children | 1.0 | - | <0.01‡ |
| Adolescent and young adults | 1.83 | 1.53-2.18 | <0.01 |
| Older adults | 1.90 | 1.58-2.29 | <0.01 |
| Year of transplant | |||
| 1989-1997 | 1.0 | - | <0.01 |
| 1998-2005 | 0.57 | 0.51-0.64 |
Hazard ratios refer to hazards of death or relapse
Other variables significantly associated with overall survival included race/ethnicity, cytogenetic risk at diagnosis, disease status at transplant, time from diagnosis to transplant and performance status score at transplant (also see Appendix Table 4)
Overall P-value, 2 degrees of freedom test
Other variables significantly associated with leukemia-free survival included cytogenetic risk at diagnosis, disease status at transplant, time from diagnosis to transplant and performance status score at transplant (also see Appendix Table 4)
Other variables significantly associated with relapse included cytogenetic risk at diagnosis, disease status at transplant, time from diagnosis to transplant and performance status score at transplant (also see Appendix Table 4)
Other variables significantly associated with treatment-related mortality included race/ethnicity, disease status at transplant and performance status score at transplant (also see Appendix Table 4)
Results similar to those for OS were observed for LFS, with improved LFS for patients transplanted from 1998-2005 compared to 1989-1997 (Table 2). In multivariate analyses that included all URD HCT patients, children and those with more recent transplants (1998-2005) experienced better LFS (Table 5). Analyses restricted by age group showed that LFS had significantly improved in the most recent time period for AYAs and older adults but not for children (Table 4).
Cumulative incidence of TRM significantly decreased over time for all age groups (Table 2). Multivariate analyses showed that older age and transplant in an earlier time period were associated with higher risks of TRM (Table 5). Improvements in TRM over time were seen in AYAs and older adults but not in children (Table 4).
Cumulative incidence of relapse did not change over the two time periods for children and AYAs, but increased for older adults (Table 2). This was also observed on multivariate analyses by age group, where older adults had a significantly higher risk of relapse if transplanted in 1998-2005 compared to 1989-1997 (HR 1.70, 95% CI 1.01-2.87, P=0.05) (Table 4).
As with the analysis of MSD HCT recipients, we found no significant interactions between age group and time period for any of the endpoints studied. Exploratory analyses showed that the 15-25 year age group had superior OS compared to the 26-40 year age group in both time periods studied (Appendix Table 1). Again, unadjusted analyses in a small subset of patients who had received myeloablative HCT for AML in CR1 did not show a negative trend in survival for AYAs (Appendix Table 2).
Appendix table 4 shows hazard ratios for factors other than time period of transplantation and age-group that were significantly associated with outcomes following URD HCT.
Discussion
Our study shows that survival for AYAs after allogeneic HCT for AML using either MSD or URD has improved over time, and unlike survival rates of cancer in general, improvements in survival for AYAs after transplant have not necessarily lagged behind those for children and older adults. Children have the best survival, followed by AYAs, and older adults have the worst survival rates after allogeneic HCT for AML.
Several factors have been implicated in the lack of progress against cancer in AYAs.1-4 These include under-recognition of cancer risk and adverse cancer outcomes in this population, and restricted or delayed access to care given that AYAs have the highest uninsured rate of any age group in the country. AYAs have an exceedingly low participation rates in cancer clinical trials, which has resulted in a poor understanding of the patient and tumor biology that distinguishes cancers in this age group.1-3 Although AYAs with cancer, in general, have not seen the same improvement in survival compared to their younger and older counterparts,1-4 our study did not find age-related disparities in outcomes improvement in recent years. Children, who already had better outcomes than AYAs and older patients in the earliest years of the study, maintained but did not improve their outcomes over time, whereas both AYAs and older adults are doing better in recent years but still not achieving the success seen in children.
Surveillance Epidemiology and End Results (SEER) data show similar survival trends among patients with AML.17 Five-year survival rates for AML decline with advancing age. In analyses that evaluated five-year survival rates for AML by age according to era (four equal six-year intervals from 1975 to 1998), survival remained inversely correlated with age and improvements in survival in all age categories occurred over time. However, during the late 1980s and 1990s, improvement in AML survival in those less than 30 years of age was negligible and considerably less than in older patients. We observed a similar plateau in outcomes in children undergoing HCT for AML.
Similar to another CIBMTR analysis,7 improvement in survival for all age groups has largely been driven by decreasing risks of TRM after both MSD and URD HCT. Rates of relapse did not improve over time and in fact worsened among older adults. This likely reflects changes in transplantation practices over time. Improvements in understanding of AML prognostic factors and HCT techniques and supportive care have allowed earlier transplantation in high-risk patients more recently. Although data on cytogenetic risk was missing for a large proportion of patients transplanted in the earlier time periods, more patients received transplantation in 1998-2005 for higher risk disease. Also, older patients are inherently at higher risk for AML relapse and the number of older patients transplanted in the most recent time period increased substantially because of the availability of RIC/NMA regimens. Relapse continues to be a major reason for treatment failure after allogeneic HCT for AML, and more research and advancement in this area is still needed to optimize survival after allogeneic HCT for all age groups.18
Our study has the usual limitations of a retrospective cohort study using registry-level data. Our findings are specific to allogeneic HCT and AML and may not be generalizable to other malignancies prevalent among AYAs that are treated with HCT. Also, there may be a selection bias at the level of centers in offering transplantation as therapy to patients who were more likely to have favorable outcomes. For instance, patients with inadequate caregiver support and financial resources and those who were non-compliant with therapy may not have been referred for or offered allogeneic HCT as a treatment option for their leukemia. AYAs are more likely to encounter access barriers than other age groups.1,2 Our study did not address issues related to access to allogeneic HCT for AYAs.
Although we found no disparities in survival improvement over time, other issues remain in determining what causes the disparity in outcomes between AYAs and the other age groups. These include the availability of AYA-appropriate patient educational materials and resources, appropriate methods of patient communication, and AYA-specific post-transplant quality of life and transition to survivorship. AYAs who develop chronic GVHD face a chronic health condition that can be associated with significant long-term morbidity and quality of life impairment, and they may need age-specific treatments and supportive care.
In conclusion, our study shows that improvements in survival among AYAs undergoing allogeneic HCT for AML parallel those of other age groups.
Financial Disclosure Statement.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Appendix Tables
Appendix Table 1.
Unadjusted probability of overall survival and leukemia-free survival, and cumulative incidences of relapse and treatment-related mortality at five years after transplantation for patients aged 15-25 years and 26-40 years
| Age 15-25 years | Age 26-40 years | ||||
|---|---|---|---|---|---|
| Outcome | N | Probability (95% CI) |
N | Probability (95% CI) |
P-value |
| Matched sibling donor HCT | |||||
| Overall survival | |||||
| 1980-1988 | 176 | 42 (35-50)% | 286 | 34 (29-40)% | 0.08 |
| 1989-1997 | 194 | 46 (38-53)% | 410 | 37 (32-42)% | 0.06 |
| 1998-2005 | 77 | 47 (34-61)% | 144 | 41 (32-49)% | 0.41 |
| Leukemia-free survival | |||||
| 1980-1988 | 173 | 40 (33-48)% | 281 | 32 (27-38)% | 0.09 |
| 1989-1997 | 193 | 43 (35-50)% | 402 | 33 (29-38)% | 0.04 |
| 1998-2005 | 76 | 46 (33-60)% | 140 | 39 (31-48)% | 0.39 |
| Relapse | |||||
| 1980-1988 | 173 | 33 (26-40)% | 281 | 43 (37-49)% | 0.03 |
| 1989-1997 | 193 | 34 (27-41)% | 402 | 34 (29-39)% | 0.94 |
| 1998-2005 | 76 | 40 (27-53)% | 140 | 37 (29-46)% | 0.75 |
| Treatment related mortality | |||||
| 1980-1988 | 173 | 27 (21-34)% | 281 | 25 (20-30)% | 0.61 |
| 1989-1997 | 193 | 24 (18-30)% | 402 | 33 (28-38)% | 0.02 |
| 1998-2005 | 76 | 14 (6-23)% | 140 | 23 (16-31)% | 0.09 |
| Unrelated donor HCT | |||||
| Overall survival | |||||
| 1989-1997 | 189 | 22 (16-28)% | 316 | 13 (10-17)% | 0.02 |
| 1998-2005 | 381 | 33 (28-38)% | 535 | 26 (22-30)% | 0.04 |
| Leukemia-free survival | |||||
| 1989-1997 | 186 | 21 (15-27)% | 314 | 13 (9-17)% | 0.02 |
| 1998-2005 | 377 | 30 (25-35)% | 522 | 25 (21-29)% | 0.13 |
| Relapse | |||||
| 1989-1997 | 186 | 29 (23-36)% | 314 | 28 (24-33)% | 0.88 |
| 1998-2005 | 377 | 34 (29-39)% | 522 | 34 (30-38)% | 0.92 |
| Treatment related mortality | |||||
| 1989-1997 | 186 | 50 (43-57)% | 314 | 59 (54-64)% | 0.05 |
| 1998-2005 | 377 | 36 (31-41)% | 522 | 41 (37-46)% | 0.12 |
Appendix Table 2.
Unadjusted outcomes at five years after transplantation in the subset of children, AYAs and older adults with AML in CR1 who received a myeloablative HCT
| Outcome | 1980-1988* | 1989-1997* | 1998-2005* | P-value | |||
|---|---|---|---|---|---|---|---|
| N | N | N | |||||
| Matched sibling donor HCT | |||||||
| Overall survival | |||||||
| Children | 68 | 60 (48-72)% | 120 | 58 (49-67)% | 56 | 69 (55-81)% | 0.41 |
| Adolescent and young adults | 262 | 49 (43-55)% | 299 | 57 (52-63)% | 96 | 52 (42-63)% | 0.16 |
| Older adults | 23 | 35 (17-55)% | 152 | 35 (28-43)% | 146 | 47 (38-56)% | 0.13 |
| Leukemia-free survival | |||||||
| Children | 67 | 61 (49-72)% | 119 | 54 (44-63)% | 55 | 65 (51-78)% | 0.33 |
| Adolescent and young adults | 256 | 46 (40-52)% | 297 | 53 (47-58)% | 95 | 51 (41-61)% | 0.32 |
| Older adults | 22 | 32 (14-52)% | 150 | 35 (28-43)% | 144 | 46 (37-55)% | 0.16 |
| Relapse | |||||||
| Children | 67 | 15 (7-24)% | 119 | 38 (30-47)% | 55 | 21 (11-33)% | <0.01 |
| Adolescent and young adults | 256 | 17 (13-22)% | 297 | 20 (16-25)% | 95 | 28 (20-38)% | 0.09 |
| Older adults | 22 | 14 (3-31)% | 150 | 22 (16-29)% | 144 | 25 (18-32)% | 0.40 |
| Treatment related mortality | |||||||
| Children | 67 | 24 (15-35)% | 119 | 8 (4-14)% | 55 | 14 (6-26)% | 0.02 |
| Adolescent and young adults | 256 | 37 (31-43)% | 297 | 27 (22-32)% | 95 | 20 (13-29)% | <0.01 |
| Older adults | 22 | 55 (34-74)% | 150 | 43 (35-51)% | 144 | 29 (22-37)% | 0.02 |
| Unrelated donor HCT | |||||||
| Overall survival | |||||||
| Children | - | 44 | 36 (23-51)% | 91 | 34 (24-45)% | 0.81 | |
| Adolescent and young adults | - | 93 | 22 (15-31)% | 235 | 41 (34-48)% | <0.01 | |
| Older adults | - | 45 | 22 (11-35)% | 261 | 34 (28-41)% | 0.09 | |
| Leukemia-free survival | |||||||
| Children | - | 44 | 32 (19-46)% | 91 | 35 (25-45)% | 0.71 | |
| Adolescent and young adults | - | 92 | 22 (14-31)% | 231 | 40 (33-46)% | <0.01 | |
| Older adults | - | 45 | 22 (11-35)% | 257 | 34 (28-41)% | 0.09 | |
| Relapse | |||||||
| Children | - | 44 | 30 (17-44)% | 91 | 35 (25-45)% | 0.53 | |
| Adolescent and young adults | - | 92 | 12 (6-19)% | 231 | 24 (19-30)% | <0.01 | |
| Older adults | - | 45 | 16 (7-27)% | 257 | 27 (21-33)% | 0.07 | |
| Treatment related mortality | |||||||
| Children | - | 44 | 39 (25-53)% | 91 | 30 (21-40)% | 0.33 | |
| Adolescent and young adults | - | 92 | 66 (56-76)% | 231 | 36 (30-43)% | <0.01 | |
| Older adults | - | 45 | 62 (48-76)% | 257 | 39 (33-45)% | <0.01 | |
Probability and 95% confidence intervals
Appendix Table 3.
Factors other than year of transplant and age group that were significantly associated with overall survival, leukemia-free survival, relapse and treatment-related mortality among recipients of HLA-identical sibling donor transplantation
| Variable | Hazard ratio* | 95% confidence intervals |
P-value |
|---|---|---|---|
| Overall survival | |||
| Cytogenetic risk | |||
| Good | 1.0 | . | <0.01 |
| Intermediate | 1.28 | 1.02-1.60 | 0.04 |
| Poor | 1.79 | 1.39-2.31 | <0.01 |
| Unknown | 1.48 | 1.17-1.87 | <0.01 |
| Disease status at transplant | |||
| First complete remission | 1.0 | . | <0.01 |
| Second complete remission | 1.49 | 1.24-1.78 | <0.01 |
| Other | 2.14 | 1.90-2.40 | <0.01 |
| Unknown | 4.56 | 2.26-9.23 | <0.01 |
| Time from diagnosis to transplant | |||
| < 6 months | 1.00 | . | <0.01 |
| 6-12 months | 1.24 | 1.09-1.40 | <0.01 |
| > 12 months | 0.95 | 0.81-1.10 | 0.46 |
| Unknown | 0.52 | 0.07-3.75 | 0.52 |
| KPS score at transplant | |||
| < 90 | 1.0 | . | <0.01 |
| ≥ 90 | 0.74 | 0.66-0.82 | <0.01 |
| Unknown | 0.74 | 0.55-0.99 | 0.05 |
| Gender | |||
| Male | 1.0 | . | <0.01 |
| Female | 0.87 | 0.79-0.96 | |
| Leukemia-free survival | |||
| Cytogenetic risk | |||
| Good | 1.0 | . | <0.01 |
| Intermediate | 1.31 | 1.05-1.64 | 0.02 |
| Poor | 1.89 | 1.47-2.43 | <0.01 |
| Unknown | 1.46 | 1.15-1.84 | <0.01 |
| Disease status at transplant | |||
| First complete remission | 1.0 | . | <0.01 |
| Second complete remission | 1.58 | 1.32-1.88 | <0.01 |
| Other | 2.20 | 1.96-2.47 | <0.01 |
| Unknown | 4.03 | 1.90-8.53 | <0.01 |
| Time from diagnosis to transplant | |||
| < 6 months | 1.0 | . | .<0.01 |
| 6-12 months | 1.23 | 1.08-1.39 | <0.01 |
| > 12 months | 0.90 | 0.78-1.05 | 0.18 |
| Unknown | 0.40 | 0.06-2.90 | 0.37 |
| KPS score at transplant | |||
| < 90 | 1.0 | . | <0.01 |
| ≥ 90 | 0.76 | 0.69-0.84 | <0.01 |
| Unknown | 0.82 | 0.61-1.10 | 0.18 |
| Gender | |||
| Male | 1.0 | . | <0.01 |
| Female | 0.86 | 0.78-0.95 | |
| Relapse | |||
| Cytogenetic risk | |||
| Good | 1.0 | . | <0.01 |
| Intermediate | 1.78 | 1.25-2.53 | <0.01 |
| Poor | 2.86 | 1.96-4.16 | <0.01 |
| Unknown | 1.70 | 1.17-2.45 | <0.01 |
| Disease status at transplant | |||
| First complete remission | 1.00 | . | <0.01 |
| Second complete remission | 2.23 | 1.72-2.86 | <0.01 |
| Other | 3.40 | 2.89-3.99 | <0.01 |
| Unknown | 4.28 | 1.36-13.45 | 0.01 |
| Time from diagnosis to transplant | |||
| < 6 months | 1.00 | . | <0.01 |
| 6-12 months | 1.17 | 0.99-1.39 | 0.07 |
| > 12 months | 0.58 | 0.47-0.72 | <0.01 |
| KPS score at transplant | |||
| < 90 | 1.00 | . | <0.01 |
| ≥ 90 | 0.73 | 0.63-0.84 | <0.01 |
| Unknown | 0.90 | 0.61-1.33 | 0.59 |
| Treatment-related mortality | |||
| Disease status at transplant | |||
| First complete remission | 1.0 | . | <0.01 |
| Second complete remission | 1.14 | 0.89-1.45 | 0.31 |
| Other | 1.41 | 1.19-1.67 | <0.01 |
| Unknown | 4.01 | 1.48-10.84 | <0.01 |
| Time from diagnosis to transplant | |||
| < 6 months | 1.0 | . | <0.01 |
| 6-12 months | 1.29 | 1.08-1.54 | <0.01 |
| > 12 months | 1.35 | 1.09-1.66 | <0.01 |
| Unknown | 2.75 | 0.38-19.98 | 0.32 |
| KPS score at transplant | |||
| < 90 | 1.0 | . | <0.01 |
| ≥ 90 | 0.79 | 0.68-0.92 | <0.01 |
| Unknown | 0.77 | 0.49-1.20 | 0.25 |
Hazard ratios refer to hazards of death or relapse
Appendix Table 4.
Factors other than year of transplant and age group that were significantly associated with overall survival, leukemia-free survival, relapse and treatment-related mortality among recipients of unrelated donor transplantation
| Variable | Hazard ratio* | 95% confidence intervals |
P-value |
|---|---|---|---|
| Overall survival | |||
| Cytogenetic risk | |||
| Good | 1.0 | . | <0.01 |
| Intermediate | 1.17 | 0.97-1.41 | 0.10 |
| Poor | 1.48 | 1.21-1.80 | <0.01 |
| Unknown | 1.35 | 1.11-1.63 | <0.01 |
| Disease status at transplant | |||
| First complete remission | 1.0 | . | <0.01 |
| Second complete remission | 1.18 | 1.04-1.35 | 0.01 |
| Other | 1.82 | 1.65-2.01 | <0.01 |
| Unknown | 1.65 | 0.86-3.17 | 0.13 |
| Time from diagnosis to transplant | |||
| < 6 months | 1.0 | . | <0.01 |
| 6-12 months | 1.12 | 1.02-1.24 | 0.02 |
| > 12 months | 0.87 | 0.77-0.97 | 0.01 |
| Unknown | 0.63 | 0.27-1.51 | 0.30 |
| KPS score at transplant | |||
| < 90 | 1.0 | . | <0.01 |
| ≥ 90 | 0.74 | 0.68-0.80 | <0.01 |
| Unknown | 0.69 | 0.59-0.81 | <0.01 |
| Ethnicity/Race | |||
| White | 1.0 | . | <0.01 |
| Black | 1.50 | 1.25-1.80 | <0.01 |
| Asian/Pacific-Islander | 1.06 | 0.78-1.43 | 0.73 |
| Hispanic | 1.04 | 0.87-1.25 | 0.66 |
| Other | 1.01 | 0.63-1.63 | 0.97 |
| Leukemia-free survival | |||
| Cytogenetic risk | |||
| Good | 1.0 | . | <0.01 |
| Intermediate | 1.19 | 0.99-1.42 | 0.07 |
| Poor | 1.50 | 1.23-1.82 | <0.01 |
| Unknown | 1.34 | 1.11-1.62 | <0.01 |
| Disease status at transplant | |||
| First complete remission | 1.0 | . | <0.01 |
| Second complete remission | 1.22 | 1.07-1.39 | <0.01 |
| Other | 1.99 | 1.80-2.19 | <0.01 |
| Unknown | 1.45 | 0.74-2.89 | 0.28 |
| Time from diagnosis to transplant | |||
| < 6 months | 1.0 | . | <0.01 |
| 6-12 months | 1.11 | 1.01-1.22 | 0.03 |
| > 12 months | 0.83 | 0.74-0.92 | <0.01 |
| Unknown | 0.66 | 0.27-1.62 | 0.36 |
| KPS score at transplant | |||
| < 90 | 1.0 | . | <0.01 |
| ≥ 90 | 0.74 | 0.68-0.80 | <0.01 |
| Unknown | 0.74 | 0.63-0.86 | <0.01 |
| Relapse | |||
| Cytogenetic risk | |||
| Good | 1.0 | . | <0.01 |
| Intermediate | 1.58 | 1.15-2.16 | <0.01 |
| Poor | 2.04 | 1.47-2.84 | <0.01 |
| Unknown | 1.73 | 1.25-2.39 | <0.01 |
| Disease status at transplant | |||
| First complete remission | 1.0 | . | <0.01 |
| Second complete remission | 1.39 | 1.13-1.70 | <0.01 |
| Other | 3.17 | 2.74-3.66 | <0.01 |
| Unknown | 1.55 | 0.59-4.08 | 0.40 |
| Time from diagnosis to transplant | |||
| < 6 months | 1.0 | . | <0.01 |
| 6-12 months | 1.08 | 0.95-1.23 | 0.26 |
| > 12 months | 0.59 | 0.50-0.69 | <0.01 |
| Unknown | 1.01 | 0.32-3.18 | 0.98 |
| KPS score at transplant | |||
| < 90 | 1.0 | . | <0.01 |
| ≥ 90 | 0.68 | 0.60-0.76 | <0.01 |
| Unknown | 0.86 | 0.71-1.05 | 0.13 |
| Treatment-related mortality | |||
| Disease status at transplant | |||
| First complete remission | 1.0 | . | <0.01 |
| Second complete remission | 1.07 | 0.93-1.24 | 0.34 |
| Other | 1.32 | 1.16-1.50 | <0.01 |
| Unknown | 0.91 | 0.43-1.93 | 0.80 |
| KPS score at transplant | |||
| < 90 | 1.0 | . | <0.01 |
| ≥ 90 | 0.79 | 0.71-0.89 | <0.01 |
| Unknown | 0.58 | 0.45-0.74 | <0.01 |
| Ethnicity/Race | |||
| White | 1.0 | . | <0.01 |
| Black | 1.68 | 1.33-2.13 | <0.01 |
| Asian/Pacific-Islander | 1.22 | 0.82-1,81 | 0.33 |
| Hispanic | 1.13 | 0.89-1.43 | 0.31 |
| Other | 0.99 | 0.45-2.00 | 0.99 |
Hazard ratios refer to hazards of death or relapse
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bleyer A. The adolescent and young adult gap in cancer care and outcome. Curr Probl Pediatr Adolesc Health Care. 2005;35:182–217. doi: 10.1016/j.cppeds.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Adolescent and Young Adult Oncology Progress Review Group . Closing the gap: Research and care imperatives for adolescents and young adults with cancer. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, and the LiveStrong Young Adult Alliance; Bethesda, MD: 2006. NIH Publication No. 06-6067. http://planning.cancer.gov/library/AYAO_PRG_Report_2006_FINAL.pdf. [Google Scholar]
- 3.Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol. 2002;38:1–10. doi: 10.1002/mpo.1257. [DOI] [PubMed] [Google Scholar]
- 4.Zebrack B, Mathews-Bradshaw B, Siegel S. Quality cancer care for adolescents and young adults: a position statement. J Clin Oncol. 2010;28:4862–4867. doi: 10.1200/JCO.2010.30.5417. [DOI] [PubMed] [Google Scholar]
- 5.Albritton K, Bleyer WA. The management of cancer in the older adolescent. Eur J Cancer. 2003;39:2584–2599. doi: 10.1016/j.ejca.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Wood WA, Lee SJ. Malignant hematologic diseases in adolescents and young adults. Blood. 2011;117:5803–5815. doi: 10.1182/blood-2010-12-283093. [DOI] [PubMed] [Google Scholar]
- 7.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. [accessed 05/01/2011]. Available at: http://www.cibmtr.org. [Google Scholar]
- 11.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 14.Tallman MS, Dewald GW, Gandham S, et al. Impact of cytogenetics on outcome of matched unrelated donor hematopoietic stem cell transplantation for acute myeloid leukemia in first or second complete remission. Blood. 2007;110:409–417. doi: 10.1182/blood-2006-10-043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Mattano L, Nachman J, Ross J, Stock W. Leukemias. In: Bleyer A, O’Leary M, Barr R, Ries LAG, editors. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. National Cancer Institute; Bethesda, MD: 2006. NIH Pub. No. 06-5767. http://seer.cancer.gov/publications/aya/aya_mono_complete.pdf. [Google Scholar]
- 18.Bishop MR, Alyea EP, 3rd, Cairo MS, et al. National Cancer Institute’s First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant. 2011;17:443–454. doi: 10.1016/j.bbmt.2010.12.713. [DOI] [PMC free article] [PubMed] [Google Scholar]