Abstract
Airborne hexavalent chromate, Cr(VI), has been identified by the Environmental Protection Agency as a possible health threat in urban areas, due to the carcinogenic potential of some of its forms. Particulate chromates are produced in many different industrial settings, with high levels of aerosolized forms historically documented. Along with an increased risk of lung cancer, a high incidence of allergic asthma has been reported in workers exposed to certain inhaled particulate Cr(VI) compounds. However, a direct causal association between Cr(VI) and allergic asthma has not been established. We recently showed that inhaled particulate Cr(VI) induces an innate neutrophilic inflammatory response in BALB/c mice. In the current studies we investigated how the inflammation induced by inhaled particulate Cr(VI) might alter the pathology of an allergic asthmatic response. We used a well-established mouse model of allergic asthma. Groups of ovalbumin protein (OVA)-primed mice were challenged either with OVA alone, or with a combination of OVA and particulate zinc chromate, and various parameters associated with asthmatic responses were measured. Co-exposure to particulate Cr(VI) and OVA mediated a mixed form of asthma in which both eosinophils and neutrophils are present in airways, tissue pathology is markedly exacerbated, and airway hyperresponsiveness is significantly increased. Taken together these findings suggest that inhalation of particulate forms of Cr(VI) may augment the severity of ongoing allergic asthma, as well as alter its phenotype. Such findings may have implications for asthmatics in settings in which airborne particulate Cr(VI) compounds are present at high levels.
Keywords: hexavalent chromium, asthma, inflammation, lung
INTRODUCTION
Epidemiological studies conducted in the U.K., Europe, Japan, and the U.S. show that long-term occupational exposure to certain inhaled particulate hexavalent chromium [Cr(VI)] compounds is associated with an elevated risk of developing lung cancer (reviewed in U.S. Environmental Protection Agency, 1998). These workers were also found to have increased incidences of other lung conditions, including pulmonary congestion and edema, lung tissue fibrosis, bronchiolization of the alveoli, and hyperplasia of the bronchial epithelium. Exposure to certain chromium compounds has also been associated with elevated incidences of asthma (Adams et al., 2006).
Asthma is a complex phenotype arising from genetic, environmental and social factors (Cookson, 1999). The most common form of asthma is allergic asthma. This form is initiated and perpetuated by Th2 CD4+ T lymphocytes that secrete cytokines (notably IL-4, IL-5 and IL-13) that promote the generation of allergen-specific IgE (reviewed in Cohn et al., 2004). The disease manifests itself as recurrent episodes of coughing, wheezing and breathlessness, triggered by the release of pro-inflammatory factors from tissue mast cells activated by IgE-receptor cross-linking. Most of these symptoms are the result of airway obstruction, which can be attributed to a combination of factors including changes in vasculature, mucus hypersecretion, airway hyperresponsiveness, and airway remodeling within lung tissues and airways.
As early as the 1930s, reports have been published proposing a link between occupational exposure to chromium compounds and the development of asthma in industrial workers (Joules, 1932; Smith, 1931). Since then, numerous case studies (Bright et al., 1997; Fernandez-Nieto et al., 2006; Hannu et al., 2005; Leroyer et al., 1998; Moller et al., 1986; Novey et al., 1983; Olaguibel et al., 1989; Olaguibel and Basomba, 1989; Park et al., 1994), as well as larger studies (reviewed in Leikauf, 2002), suggest an association between occupational exposure to chromates and asthma. It should be noted that for most of these studies the type of asthma (allergic versus non-allergic) was not identified. Interestingly, in the few studies in which skin prick tests and/or IgE levels were measured, the results were either mixed or showed no correlation with bronchial hyperresponsiveness, the primary parameter used to diagnose asthma in most studies. Such findings suggest that chromate compounds may be poor sensitizers for allergic asthma. Alternative explanations for the reported associations between chromium exposure and asthma include a capacity to mediate non-allergic forms of asthma, or the capacity to exacerbate pre-existing asthmatic conditions (also known as irritant-induced asthma). In the latter, chromium would act as a secondary inflammatory agent or adjuvant rather than a primary sensitizing agent.
Using a mouse model of intranasal chromium exposure we recently showed that a single inhaled dose of particulate Cr(VI) mediates an extensive inflammatory response characterized by an acute influx of neutrophils followed by macrophages, elevated levels of IL-6 and GROα IL-8 homolog) in airways, and progressive interstitial pneumonitis (Beaver et al., 2009a). A similar profile of inflammation was induced by repetitive exposures to the same compound, although the severity of the inflammation and injury within tissues was markedly increased (Beaver et al., 2009b). These earlier reports established that IN delivery of zinc chromate suspension efficiently targets the pulmonary tract of BALB/c mice, resulting in widespread distribution within lung tissues, with a preferential accumulation around small central airways. Such “hot spots” of particle accumulation have been reported in the same anatomical locations in the lungs of chromate workers (Ishikawa et al., 1994). The results from these previous studies suggest that inhaled particulate Cr(VI) is an efficient inducer of innate inflammatory responses. In the current studies we examined how exposure to Cr(VI) might impact on an ongoing allergic asthmatic response. Specifically, we investigated how the marked innate inflammation induced by inhaled particulate Cr(VI) might alter the pathology of the allergic response. We postulated that co-exposure to particulate chromate would either result in an exacerbated form of the ongoing allergic response, or might change the immunological phenotype of the response.
For the present studies, we made use of a well-established animal model of allergic asthma previously described by our laboratory (Balsley et al., 2010; Gwinn et al., 2006). Mice are initially primed with ovalbumin protein (OVA) in Alum adjuvant and then given repeated intranasal challenges of OVA to induce an inflammatory response dominated by an influx of eosinophils, elevated levels of Th2-associated cytokines (notably IL-13) in airways, goblet cell hyperplasia, mucus secretion, and bronchial hyperreactivity. In the current studies, groups of OVA-primed mice were challenged either with OVA alone, or with a combination of OVA and Cr(VI), and various parameters associated with asthmatic responses were measured.
MATERIALS AND METHODS
Chromium preparation
Endotoxin-free particulate basic zinc chromate [ZnCrO4 4Zn(OH)2] was obtained from Rockwood Pigments (Beltsville, MD). The particles (previously determined to be 4.7 μm in size, (Beaver et al., 2009b) were suspended in sterile 0.9% sodium chloride solution, pH 7.39, (saline) by sonication and then allowed to spin on a stir plate for 1 hour prior to use to ensure a homogeneous mixture.
Mouse model of allergic lung inflammation and intranasal delivery of particulate chromium
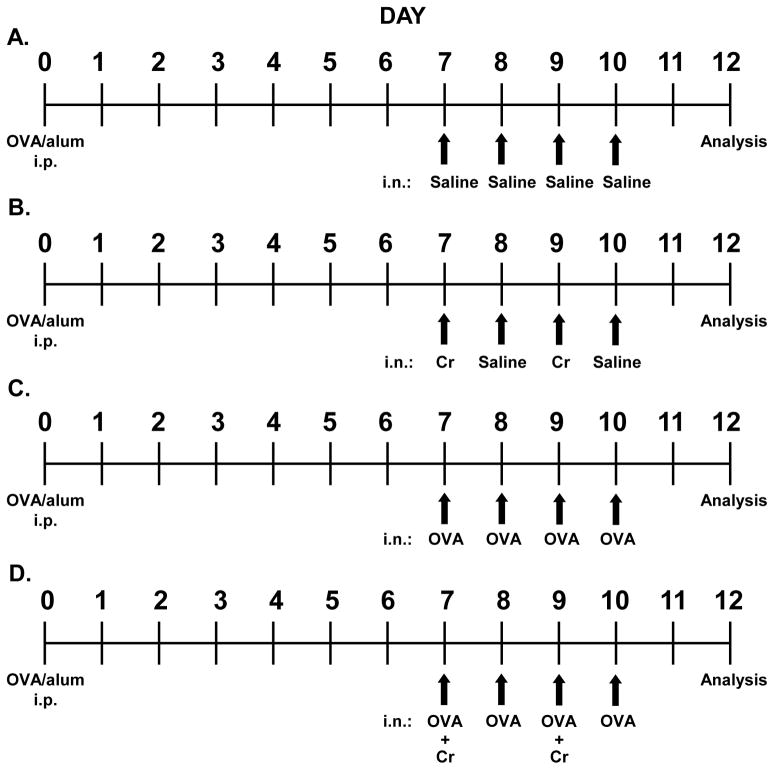
All experiments were conducted under the approval of The George Washington University Institutional Animal Care and Use Committee. A previously described (Balsley et al., 2010; Gwinn et al., 2006) mouse model of acute allergic lung inflammation was used. Briefly, female BALB/c mice aged 8–10 weeks (purchased from the Jackson Laboratory, Bar Harbor, Maine) were primed by intraperitoneal (i.p.) injection of 100 μg ovalbumin (OVA) protein in saline mixed 1:1 with Imject alum adjuvant (Pierce, Rockford, IL). Alum was included as an adjuvant to help promote priming of OVA-specific Th2 CD4+ T cells. Seven days later the mice were challenged under light anesthesia (Isoflurane) with 50 μg OVA in saline via intranasal (i.n.) delivery for four consecutive days (days 7–10). Most analyses were conducted two days later (day 12). In some experiments, mice were given i.n. challenges with saline alone, or were challenged with OVA plus 24 μg chromate solution (Cr) included on days 7 and 9, or were challenged with only Cr on days 7 and 9. Of importance is the fact that Cr was only included during the challenge phase of each regimen so that we could specifically observe its impact on effector inflammatory responses, rather than immune sensitization. Figure 1 shows a summary of the four different treatment groups.
Figure 1.
Regimen of acute allergic lung inflammation and intranasal delivery of particulate chromium. On day 0, female BALB/c mice were primed i.p. with 50 μg OVA mixed 1:1 with Imject alum adjuvant. On days 7–10, mice were challenged intranasally (i.n.) with 50 μl of the indicated agent: (A) Saline-treated mice (control) received saline challenges. (B) Cr-treated mice were challenged with 24 μg Cr on days 7 & 9 and saline on days 8 & 10. (C) OVA-treated mice were administered 50 μg OVA for all challenges. (D) OVA+Cr-treated mice were challenged with 50 μg OVA + 24 μg Cr on days 7 & 9 and 50 μg OVA on days 8 & 10. All mice were sacrificed on day 12 and analyses were conducted.
Bronchoalveolar lavage and analysis of collected cells and fluid
Immediately following sacrifice (by exposure to carbon dioxide), bronchoalveolar lavage (BAL) was performed to isolate airway cells and fluid. For this, a cannula was inserted into the trachea and three 1-ml washes of cold PBS infused in and out of the airways. The fluid and cells were then separated by centrifugation and used for different analyses. The separated cells were treated with ammonium chloride lysis buffer and then quantified by hemocytometry. Eosinophils and neutrophils were identified using forward light scatter/side light scatter distribution as previously described (Balsley et al., 2010; Gwinn et al., 2006) and verified using cytospin analysis. To measure levels of airway cytokines, BAL fluids were pooled within treatment groups and then concentrated 4-fold using 4-kDa cut-off Centricon columns (Millipore, Billerica, MA). Levels of IL-4, IL-5, IL-6, IL-13, IL-17 and IFN-γ cytokines were measured using FlowCytomix™ bead-based simplex kits (eBioscience, San Diego, CA).
Lung tissue isolation and histology
Following BAL the lungs were perfused with 20-ml cold PBS and then inflated via the trachea with 1 ml paraformaldehyde-lysine-periodate (PLP) fixative. Suture thread was used to tie off the trachea and the lungs were then dissected out and placed in 5 ml of PLP fixative for 24 hours followed by storage in 5 ml 100% ethanol. Fixed lungs were embedded in paraffin and 6-μm sections were cut and stained with hematoxylin and eosin (H&E) stain to establish the severity of tissue pathology. Images were acquired at 10x magnification using a Nikon microscope equipped with a RT-Slider SPOT Camera.
Fixed lungs were also stained with periodic acid-Schiff (PAS) reagent to determine the presence and abundance of airway epithelium mucus (Histoserv, Inc., Germantown, MD). Images of airways containing PAS positive cells were acquired at 20x magnification. The percentage of PAS positive cells was determined by two independent investigators and the areas of the PAS positive cells were measured using Image J, an NIH Image system.
Measuring airway hyperresponsiveness
On day 12 of the regimen, mice were anesthetized by 300 μl i.p. injection of ketamine (10 mg/ml) and xylazine (1 mg/ml). A tracheostomy tube was inserted and connected to the FinePointe RC system (Buxco Research Systems, Wilmington, NC) for measurements of airway resistance and compliance. Mice were sequentially challenged with aerosolized PBS (baseline) followed by increasing doses of methycholine (acetyl-β-methacholine chloride; Sigma-Aldrich, St. Louis, MO) ranging from 6.25 to 50 mg/ml. Maximum resistance (RL, cm H2O/m/s) was recorded during a 3-minute period following each challenge.
Statistical analysis
Statistically significant differences among treatment groups were determined using 1-Way ANOVA for BAL leukocyte numbers and PAS-positive areas, a Student’s t-test for cytokine determinations, and a two-way analysis of variance (2-Way ANOVA) for airway resistance measurements. Statistical significance was reported at the 0.05 level.
RESULTS
Inhaled particulate Cr(VI) alters allergic airway cell profiles
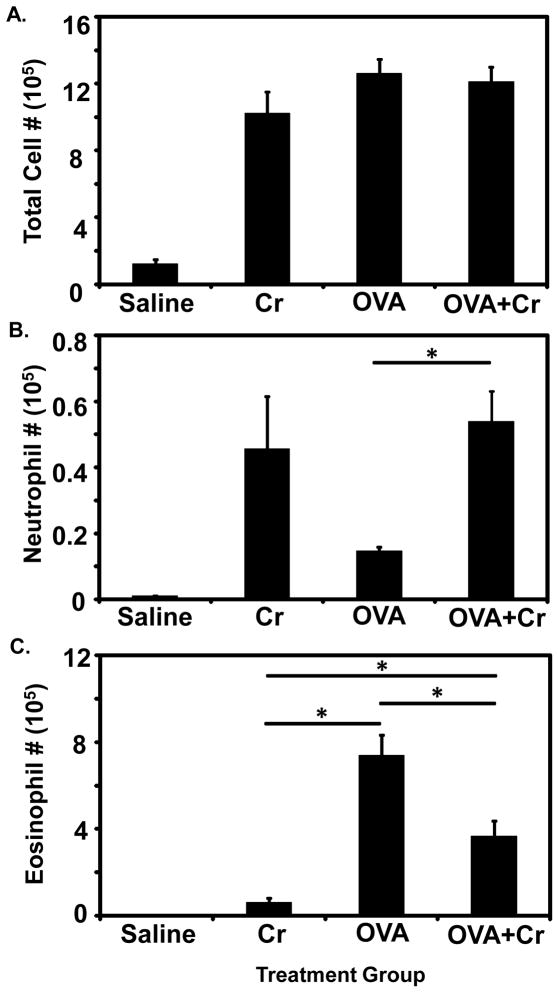
Our initial studies were designed to establish the impact of co-exposure to particulate Cr(VI) on airway cells associated with OVA-induced allergic asthma. Four different intranasal challenge regimens were compared (Figure 1): saline alone, Cr alone, OVA alone and OVA+Cr. Interestingly, we found that the total number of leukocytes infiltrating the airways (BAL cells) were comparable between the Cr, OVA, and OVA+Cr groups while all of these groups had significantly greatly numbers of leukocytes than saline alone (Figure 2A). As expected, mice challenged with saline alone showed only minimal cellular infiltration. When leukocyte subsets associated with Cr(VI) inhalation (neutrophils) versus allergic asthma (eosinophils) were examined, the responses mediated by Cr alone and OVA alone segregated accordingly into predominantly neutrophilic or eosinophilic infiltrations, respectively, while responses in the OVA+Cr group were mixed, with both neutrophils and eosinophils present (Figures 2B and 2C). The numbers of neutrophils for both Cr and OVA+Cr were significantly greater compared to saline, and OVA+Cr also had more neutrophils than OVA alone. Furthermore eosinophil numbers for both OVA and OVA+Cr were significantly greater compared to saline. While the number of neutrophils in the OVA+Cr group was comparable to that in the Cr alone group, eosinophil numbers were reduced by approximately 50% in the OVA+Cr group relative to the OVA alone group. For all groups, cell numbers not accounted for by eosinophils or neutrophils consisted predominantly of monocytes and a small percentage of lymphoid cells.
Figure 2.
Cellular infiltrate in BAL fluid. Mice were primed and challenged as indicated in Figure 1 and sacrificed on day 12. BAL was performed on each mouse, and airway cells were isolated, counted, and leukocyte subtypes were identified by flow cytometric analysis using forward light scatter/side light scatter distribution. Bar graphs show: (A) The total number of living cells in the airways, (B) Neutrophil cell numbers in the airways, and (C) Eosinophil cell numbers in the airways. Data are the mean ± SE from two independent experiments, with a total of 8–12 animals per group. Statistically significant differences among treatment groups were determined using a 1-Way ANOVA, * p < 0.05.
Inhaled chromium alters pathology of asthmatic lung tissue
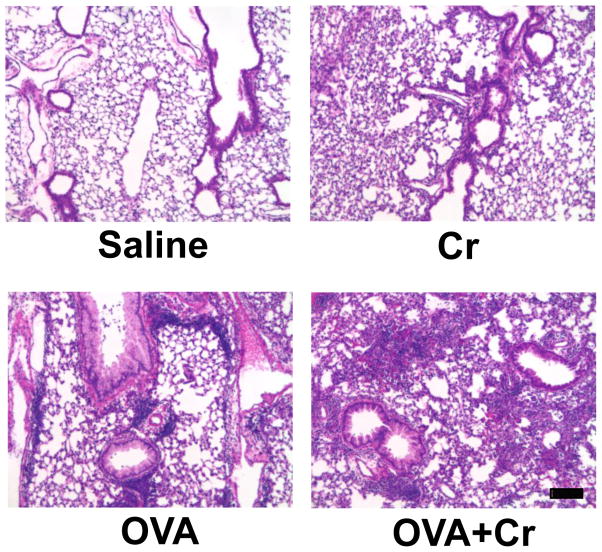
We next examined the effect of each challenge regimen on lung tissue pathology, using histological analysis. Striking differences in both the severity and phenotype of the pathology were observed between the four groups (Figure 3). As expected, saline treatment did not induce any additional obvious leukocyte infiltration or tissue damage (by H&E staining). Lungs exposed to Cr alone showed diffuse inflammation or pneumonitis with alveolar hemorrhage. In contrast, the inflammation induced in the OVA alone group was concentrated into foci of leukocytes accumulating adjacent to small airways and blood vessels. These findings fit well with our previous findings that exposure to Cr alone mediates a pneumonitic type of inflammatory response (Beaver et al., 2009a; Beaver et al., 2009b) whereas the OVA regimen induces responses resembling allergic asthma (Balsley et al., 2010; Gwinn et al., 2006). Co-exposure to Cr and OVA resulted in a mixed response that included diffuse inflammation throughout the tissue, and localized inflammatory foci around airways and blood vessels. Based on the size and frequency of the leukocytic foci, the asthmatic inflammatory response also appeared to be more severe relative to that in the OVA alone group.
Figure 3.
Histology of lung tissue. Mice were primed and challenged as indicated in Figure 1 and sacrificed on day 12. Whole lungs were isolated, embedded in parafin, cut into 6-μm sections, and stained. H&E staining was performed on lung sections representative of 4–6 animals of each treatment group from 2 independent experiments. All images, captured at 10X magnification, show tissue areas surrounding bronchioles. Scale bar: 10 μm.
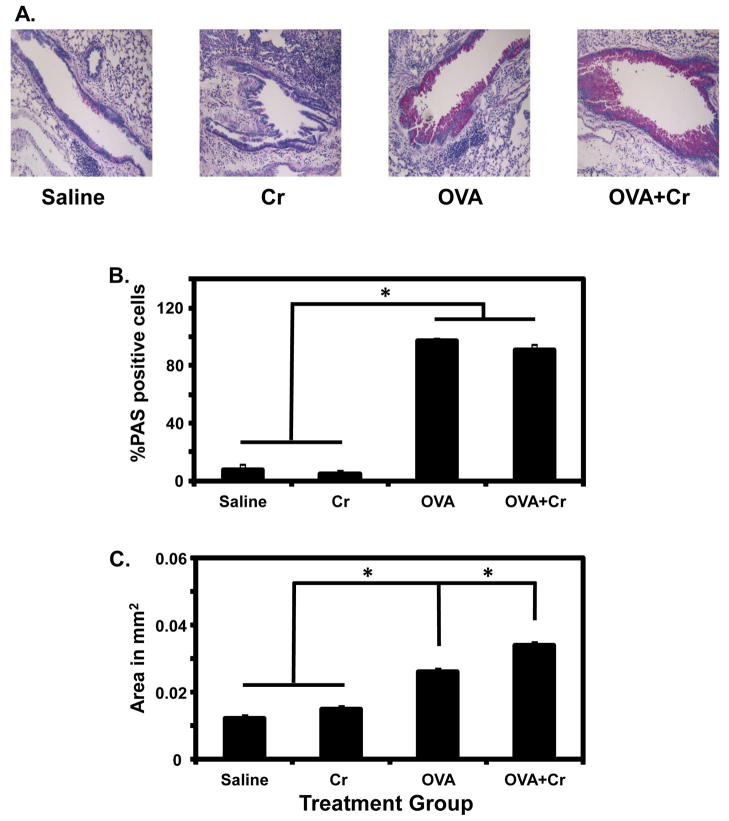
As shown in Figure 4A, PAS staining revealed the presence of an increased number of goblet cells (hyperplasia) as well as mucus secretion within the airways of the OVA group, but not in the Cr group. Interestingly, while the percentage of PAS-positive cells was similarly increased to over 90% in lungs of OVA and OVA+Cr groups (Figure 4B), the area of PAS-positive staining was significantly increased by the presence of Cr (Figure 4C) suggesting an increased abundance of mucus (Fahy, 2002). One of the major hallmarks of allergic asthma is the presence of cytokines associated with Th2 effector CD4+ T cells. Of these, IL-13 is known to be the most important cytokine for regulating mucus hypersecretion in asthmatic airways (Wills-Karp, 2004). In the current studies levels of several different cytokines were measured in pooled BAL fluid from the four groups of mice. Interestingly, IL-13 was the only cytokine that showed a consistent significant increase, ranging from 1.70 to 2.59-fold, in OVA+Cr versus OVA mice (for example, 247.9 ± 65.5 pg/ml versus 116.6 ± 15.8 pg/ml; P<0.05). This specific finding fits well with the increased abundance in airway mucus observed by PAS staining in this group (Figure 4C). Of the several other cytokines measured in BAL fluid, IL-4 was the only one also found above baseline levels in the OVA alone and OVA+Cr groups, although these levels were not significantly different between the two groups (average of 18.0 pg/ml versus 22.8 pg/ml, respectively, with a range of 1.5–4.4 pg/ml in the saline and Cr alone groups).
Figure 4.
PAS staining of lung tissue. (A) PAS staining was performed on lung sections representative of 4–6 animals of each treatment group from 1 independent experiment. Bar graphs show: (B) The percentage of PAS-positive cells in the airways, (C) Area of PAS-positive staining in the airways. Data are the mean ± SE from 4–6 animals per group. Statistically significant differences among treatment groups were determined using a 1-Way ANOVA, * p < 0.05.
Exposure to inhaled Cr(V) exacerbates asthmatic airway hyperresponsiveness
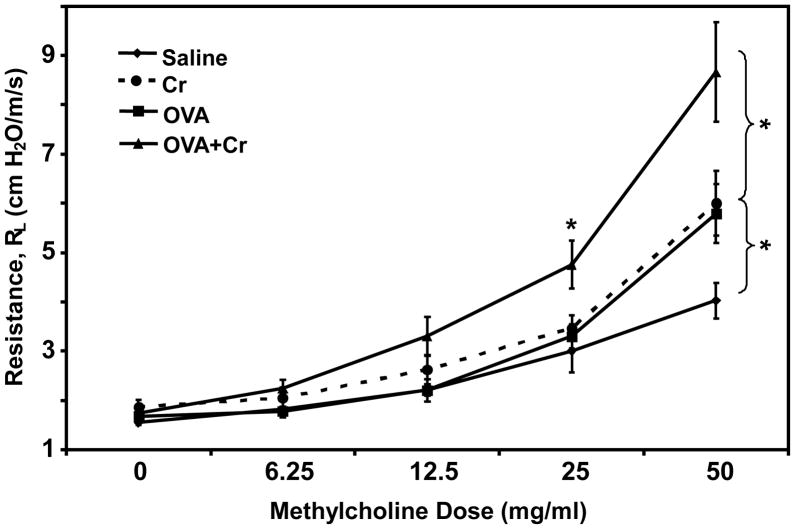
Our final question was whether the increase in asthmatic tissue pathology mediated by co-exposure to Cr(VI) would translate into changes in physiological lung function. Patients with allergic asthma demonstrate airway hyperresponsiveness, characterized by elevated bronchial constriction (resistance), upon challenge with known allergens or chemical bronchoconstrictors. In the current studies individual mice from our four exposure regimens were anesthetized and their airways challenged with increasing doses of methylcholine drug to induce airway constriction. As shown in Figure 5, the OVA alone (asthmatic) group demonstrated significantly greater airway resistance, relative to the saline (control) group, when given a high dose of methylcholine. Surprisingly, the Cr alone group showed the same increased airway resistance as the OVA alone group, suggesting the tissue pathology resulting from inhaled particulate Cr(VI) can also mediate bronchial dysfunction. Most striking was the dramatic increase in airway resistance observed in mice co-exposed to OVA+Cr. This significant increase was seen not only relative to control mice, but also to OVA alone and Cr alone mice. In addition, the airway resistance could also be detected using a lower dose of methylcholine.
Figure 5.
Airway hyperresponsiveness to methylcholine. Mice were primed and challenged as indicated in Figure 1. On day 12, individual mice were anesthetized i.p. with ketamine/xylazine, a tracheostomy tube was inserted and then attached to a respirator. The animals were challenged with aerosolized PBS (baseline) followed by increasing doses of methylcholine ranging from 0–50 mg/ml. Maximum resistance (RL, cm H2O/m/s) was recorded during a 3-minute period following each challenge. Data are the mean ± SE from two independent experiments, with a total of 8–12 animals per group. Statistically significant differences among treatment groups was determined using a 1-Way ANOVA, * p < 0.05.
DISCUSSION
The overarching focus of our studies was to establish the impact of particulate Cr(VI) inhalation on the phenotype and severity of allergic asthma. Several significant observations were generated in the current co-exposure studies. First was the demonstration of a mixed granulocytic leukocyte infiltration (both neutrophils and eosinophils) in the airways of OVA+Cr mice, compared to the asthmatic OVA alone group where only eosinophils were present. Interestingly, the total number of BAL leukocytes was comparable among all the groups, suggesting that the inclusion of Cr(VI) might be impacting the phenotype, but not the severity, of the airway inflammatory response. Histological analysis of H&E stained lung tissue also demonstrated a mixed response in the OVA+Cr group, with both diffuse (associated with Cr exposure) and focal (associated with OVA-induced allergic asthma) areas of inflammation present. Unlike that observed in airway spaces, the severity of the inflammation in lung tissues was greater in OVA+Cr mice, relative to the OVA alone and Cr alone groups. Activated neutrophils and eosinophils are both potent mediators of tissue injury due to the release of multiple damaging products, including reactive oxygen species, highly charged cationic proteins, matrix metalloproteases, and other tissue-degrading enzymes. In addition, although the extracellular reduction of Cr(VI) produces the essential element Cr(III), the process of reduction may result in direct oxidation of other tissue-associated macromolecules. Moreover, reduction of intracellular Cr(VI) produces genotoxic reactive intermediates with the capacity to cause cellular injury independent of immunological events. The presence of three different sources of tissue-damaging agents provides a potential explanation for the observed increase in pathology observed following co-exposure to OVA and particulate Cr(VI).
Of particular relevance to the pathology of allergic asthma was the finding of an increased abundance of airway mucus (based on the area of PAS staining) in the OVA+Cr group. Interestingly, when levels of cytokines were measured in BAL fluid from each group, the only significant finding was an increase in IL-13 levels in OVA+Cr mice, relative to the level determined in mice given OVA alone. This increase may explain the observed augmentation in airway mucus in the OVA+Cr group, since IL-13 is known to be the principal regulator of goblet cell hyperplasia and mucus secretion in the airways of allergic asthmatics (Wills-Karp, 2004). The mechanism for how Cr(VI) might be stimulating an increased production of IL-13 is not obvious. The main source of IL-13 during allergic asthmatic responses is activated antigen-specific Th2 cells, which in the current experimental model would be OVA-specific T cells. However, several non-Th2 cell sources of IL-13 have also been identified, including mast cells, basophils and NKT cells (Kasaian et al., 2008). While all three cell types can be activated to secrete IL-13 via antigen receptor stimulation (Gibbs et al., 1996; Kobayashi et al., 1998; Stock et al., 2008), IL-13 secretion can also be induced by cytokines associated with innate immunity (Kobayashi et al., 1998; Lauwerys et al., 2000; Pecaric-Petkovic et al., 2009). In the case of Cr(VI), we would postulate the more likely mechanism to be stimulation via cytokines. Indeed, inhaled particulate Cr(VI) is known to induce the production of several innate cytokines within airways (Beaver et al., 2009a; Beaver et al., 2009b). However, the presence of chromium-specific IgE reported in certain human occupational studies (Fernandez-Nieto et al., 2006; Novey et al., 1983) suggests that Cr(VI) may have the capacity to sensitize cells via antigen receptors, although it should be noted that the chromium exposure in these studies was over multiple years. At this point, neither mechanism can be ruled out without further studies.
One of our most striking findings in the current studies was that co-exposure to OVA and Cr(VI) caused a significant increase in airway dysfunction, as measured by increased airway resistance (hyperresponsiveness) upon methylcholine challenge. Several factors have been suggested to contribute to asthma-induced airway hyperresponsiveness (AHR). In the case of allergic asthma, mucus hypersecretion is thought to be one of the most significant contributing factors (Wills-Karp, 2004). Thus, the augmentation in AHR observed in the OVA+Cr mice could be explained by the presence of increased airway mucus (Figure 4C). Intriguingly, exposure to Cr(VI) alone was sufficient to induce AHR, and the magnitude of the response was similar to that induced in OVA-mediated allergic asthma (OVA alone group). Since airway mucus was not detected in the Cr alone group, other factors must be responsible for this AHR. Of relevance to the current findings is the fact that non-allergic types of asthma, notably neutrophil-associated asthma, are associated with AHR yet airway mucus is not present (Borish et al., 2008). In addition, neutrophilic forms of asthma are commonly associated with environmental as well as occupational exposure to particulate air pollution (Douwes et al., 2002). Based on the current findings, it is tempting to speculate that particulate Cr(VI) could be an inducer of neutrophil-associated asthma. One argument against this possibility is that a hallmark of neutrophilic asthma in humans is high levels of the chemokine IL-8 (Gibson et al., 2001), yet we did not detect elevated levels of the mouse homolog of human IL-8, KC, in BAL fluid from either Cr alone or OVA+Cr exposed mice in the current studies (data not shown). Until additional hallmarks are excluded, an association between exposure to particulate chromium and neutrophilic asthma remains an open question.
In conclusion, our findings demonstrate that exposure to inhaled particulate zinc Cr(VI) augments the severity of allergic asthma in a mouse model. This exacerbation includes an increase in tissue pathology, as well as an altered inflammatory phenotype. The downstream impact is an augmentation in airway dysfunction, as measured by increased AHR. Thus, while the inflammation induced by Cr(VI) is distinct from that of an ongoing allergic response, the net effect is a mixed and more damaging inflammatory response resulting in increased tissue pathology and disease. It is important to note that since Cr was included with OVA only during the challenge phase of the asthma regimen, its impact was on the effector phase, and not the sensitizing phase, of the asthmatic response. Thus, our current findings lead us to propose that particulate Cr(VI) may be functioning primarily as an adjuvant that exacerbates ongoing allergic asthma. This conclusion fits well with the fact that persons with asthma are known to be at increased risk from the adverse effects of air pollution (reviewed in Leikauf, 2002). In addition, studies looking at workers with occupational reactive airways dysfunction syndrome concluded that individuals with established atopy or asthma were significantly more predisposed to develop the syndrome in response to other respiratory irritants (Brooks et al., 1998). The current studies provide evidence that the same types of exacerbated responses can be reproduced in animal models of co-exposure, opening up the potential to better understand lung diseases mediated by mixtures of allergens and pollutants, as well as testing future intervention regimens.
Highlights.
allergic asthma correlated with exposure to certain inhaled particulate chromates
direct causal association between Cr(VI) and allergic asthma not established
Cr exacerbated pathology and airway hyperresponsiveness in an OVA-challenged mouse
particulate Cr(VI) may augment severity and alter phenotype of ongoing allergic asthma
Acknowledgments
We thank Lindsay Lazarus for her skillful technical help. This work was supported by National Institutes of Health grants R21-ES017334 to SC and R21-ES017307 to SLC and SRP.
Abbreviations
- AHR
airway hyperresponsiveness
- Cr
chromium
- Cr(VI)
hexavalent chromium
- Cr(III)
trivalent chromium
- H&E
hematoxylin and eosin
- IgE
immunoglobulin E
- IL
interleukin
- i.p
intraperitoneal
- i.n
intranasal
- NKT
natural killer T-cells
- OVA
ovalbumin protein
- PAS
periodic acid-Schiff
- Th2
helper T-cell type 2
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams RJ, Wilson DH, Taylor AW, Daly A, Tursan dE, Dal Grande E, Ruffin RE. Coexistent chronic conditions and asthma quality of life: a population-based study. Chest. 2006;129:285–291. doi: 10.1378/chest.129.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Balsley MA, Malesevic M, Stemmy EJ, Gigley J, Jurjus RA, Herzog D, Bukrinsky MI, Fischer G, Constant SL. A cell-impermeable cyclosporine A derivative reduces pathology in a mouse model of allergic lung inflammation. J Immunol. 2010;185:7663–7670. doi: 10.4049/jimmunol.1001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaver LM, Stemmy EJ, Constant SL, Schwartz A, Little LG, Gigley JP, Chun G, Sugden KD, Ceryak SM, Patierno SR. Lung injury, inflammation and Akt signaling following inhalation of particulate hexavalent chromium. Toxicol Appl Pharmacol. 2009a;235:47–56. doi: 10.1016/j.taap.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaver LM, Stemmy EJ, Schwartz AM, Damsker JM, Constant SL, Ceryak SM, Patierno SR. Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environ Health Perspect. 2009b;117:1896–1902. doi: 10.1289/ehp.0900715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borish L, Culp JA. Asthma: a syndrome composed of heterogeneous diseases. Ann Allergy Asthma Immunol. 2008;101:1–8. doi: 10.1016/S1081-1206(10)60826-5. [DOI] [PubMed] [Google Scholar]
- 6.Bright P, Burge PS, O’Hickey SP, Gannon PF, Robertson AS, Boran A. Occupational asthma due to chrome and nickel electroplating. Thorax. 1997;52:28–32. doi: 10.1136/thx.52.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks SM, Hammad Y, Richards I, Giovinco-Barbas J, Jenkins K. The spectrum of irritant-induced asthma: sudden and not-so-sudden onset and the role of allergy. Chest. 1998;113:42–49. doi: 10.1378/chest.113.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. 789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 9.Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999;402:B5–11. doi: 10.1038/35037002. [DOI] [PubMed] [Google Scholar]
- 10.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122:320S–326S. doi: 10.1378/chest.122.6_suppl.320s. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Nieto M, Quirce S, Carnes J, Sastre J. Occupational asthma due to chromium and nickel salts. Int Arch Occup Environ Health. 2006;79:483–486. doi: 10.1007/s00420-005-0078-z. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs BF, Haas H, Falcone FH, Albrecht C, Vollrath IB, Noll T, Wolff HH, Amon U. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol. 1996;26:2493–2498. doi: 10.1002/eji.1830261033. [DOI] [PubMed] [Google Scholar]
- 14.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 15.Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, Vanpouille C, Lee JJ, Dent LA, Leitenberg D, Bukrinsky MI, Constant SL. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol. 2006;177:4870–4879. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannu T, Piipari R, Kasurinen H, Keskinen H, Tuppurainen M, Tuomi T. Occupational asthma due to manual metal-arc welding of special stainless steels. Eur Respir J. 2005;26:736–739. doi: 10.1183/09031936.05.00130504. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. “Hot spots” of chromium accumulation at bifurcations of chromate workers’ bronchi. Cancer Research. 1994;54:2342–2346. [PubMed] [Google Scholar]
- 18.Joules H. Asthma from sensitisation to chromium. The Lancet. 1932;220:182–183. [Google Scholar]
- 19.Kasaian MT, Miller DK. IL-13 as a therapeutic target for respiratory disease. Biochem Pharmacol. 2008;76:147–155. doi: 10.1016/j.bcp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi H, Okayama Y, Ishizuka T, Pawankar R, Ra C, Mori M. Production of IL-13 by human lung mast cells in response to Fcepsilon receptor cross-linkage. Clin Exp Allergy. 1998;28:1219–1227. doi: 10.1046/j.1365-2222.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- 21.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165:1847–1853. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- 22.Leikauf GD. Hazardous air pollutants and asthma. Environ Health Perspect. 2002;110(Suppl 4):505–526. doi: 10.1289/ehp.02110s4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leroyer C, Dewitte JD, Bassanets A, Boutoux M, Daniel C, Clavier J. Occupational asthma due to chromium. Respiration. 1998;65:403–405. doi: 10.1159/000029303. [DOI] [PubMed] [Google Scholar]
- 24.Moller DR, Brooks SM, Bernstein DI, Cassedy K, Enrione M, Bernstein IL. Delayed anaphylactoid reaction in a worker exposed to chromium. J Allergy Clin Immunol. 1986;77:451–456. doi: 10.1016/0091-6749(86)90179-x. [DOI] [PubMed] [Google Scholar]
- 25.Novey HS, Habib M, Wells ID. Asthma and IgE antibodies induced by chromium and nickel salts. J Allergy Clin Immunol. 1983;72:407–412. doi: 10.1016/0091-6749(83)90507-9. [DOI] [PubMed] [Google Scholar]
- 26.Olaguibel JM, Basomba A. Occupational asthma induced by chromium salts. Allergol Immunopathol(Madr) 1989;17:133–136. [PubMed] [Google Scholar]
- 27.Park HS, Yu HJ, Jung KS. Occupational asthma caused by chromium. Clin Exp Allergy. 1994;24:676–681. doi: 10.1111/j.1365-2222.1994.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 28.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AR. Chrome poisoning with manifestations of sensitization. Journal of the American Medical Association. 1931;97:95–98. [Google Scholar]
- 30.Stock P, Akbari O. Recent advances in the role of NKT cells in allergic diseases and asthma. Curr Allergy Asthma Rep. 2008;8:165–170. doi: 10.1007/s11882-008-0027-5. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Environmental Protection Agency. Toxicological Review of Hexavalent Chromium. 1998 http://www.epa.gov/ncea/iris/toxreviews/0144tr.pdf.
- 32.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]