Abstract
Novel topoisomerase I (Top1) inhibitors are in clinical development to circumvent the drawbacks of camptothecins. Here we report molecular investigations into LMP-400, an indenoisoquinoline Top1 inhibitor in Phase 1 clinical trial, by itself and in combination with the cell cycle checkpoint inhibitor, AZD7762. We examined drug effects on DNA replication and killing of cancer cells and found that LMP-400 showed synergistic antiproliferative activity when combined with AZD7762 in human colon carcinoma cells. Inhibition of S-phase progression and bromodeoxyuridine incorporation were similarly induced by LMP-400 and camptothecin (CPT) and were abrogated by AZD7762. Replication studied by single DNA molecule analyses and immunofluorescence microscopy (molecular combing) showed rapid inhibition of fork progression in response to LMP-400 treatment with subsequent recapitulation after AZD7762 addition. AZD7762 inhibited both the activation/autophosphosphorylation of Chk1 and Chk2 at nanomolar concentrations in LMP-400-treated cells. This potent dual inhibition of Chk1 and Chk2 by AZD7762 was below the drug concentrations required to abrogate cell cycle inhibition and produce synergism with LMP-400. Also, the synergism was independent of Chk2 both in Chk2-complemented cells and Chk2 knockout cells, suggesting additional mechanisms for cell cycle abrogation by AZD7762. Together, our findings demonstrate a rationale for combining cell cycle checkpoint inhibitors with the novel non-camptothecin indenoisoquinoline Top1 inhibitors.
Keywords: Indenoisoquinoline, AZD7762, LMP-400, Chk1, Chk2
Introduction
Topoisomerases are enzymes essential for relieving torsional strain inflicted upon DNA by replication, transcription or other chromatin processes (1). Topoisomerase I (Top1) functions to unwind supercoiled DNA by the reversible cleavage and concomitant formation of a 3′ tyrosyl-DNA covalent catalytic intermediate, which is commonly referred to as the Top1 cleavage complex (Top1cc) (2, 3). Top1-targeted drugs act as Top1 poisons because they stabilize the Top1cc intermediates by slowing down their reversal and the religation of DNA, thereby producing deleterious DNA damage when replication and transcription collide with the drug-stabilized Top1cc (1).
Clinical camptothecin derivatives, though highly effective against a wide range of tumors, are limited by their chemical instability, solubility, ABCG2-mediated efflux and the rapid reversibility of Top1cc upon drug treatment (4–6). Indenoisoquinolines were first identified as Top1 inhibitors by the COMPARE analysis of the NCI drug screen database (7). Although structurally similar to camptothecin, idenoisoquinolines are chemically stable, able to overcome ABCG2 efflux while forming more persistent Top1-DNA complexes and maintaining potent antitumor activity (8, 9). LMP-400 (NSC-724998, NSC-743400) (Figure 1A) is one of the two indenoisoquinolines currently in clinical trial (8, 10, 11).
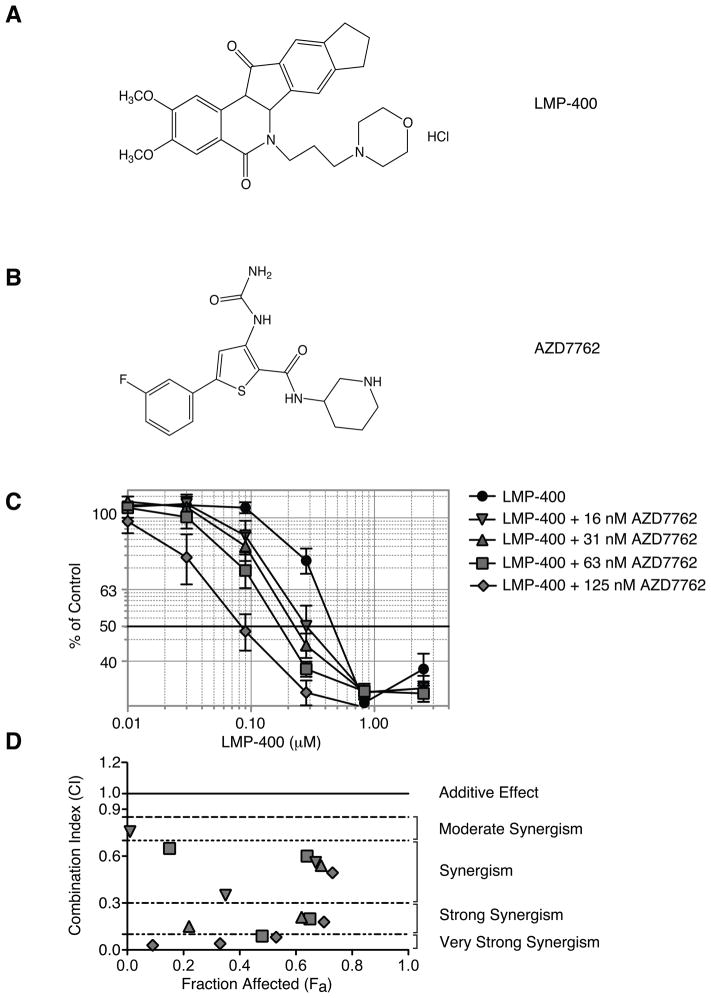
Figure 1. Synergistic inhibition of cellular proliferation in human colon carcinoma HT29 cells treated with LMP-400 and AZD7762.
A) LMP-400. B) AZD7762. C) Viability of HT29 cells treated with increasing concentrations of LMP-400 alone or in the presence of fixed non-cytotoxic concentrations of AZD7762 (16, 31, 62 and 125 nM) for 48 h. Data represent mean ± S.E. of four independent experiments. D) Graph of CI (combination Index) values vs Fa (Fraction affected) for data points shown in panel C on the combination (AZD7762 + LMP-400).
The potential for indenoisoquinoline derivatives as possible clinical agents raises the prospect of rationale combinations with other anticancer agents. Prior studies demonstrated a remarkable synergism between camptothecin and checkpoint kinase 1/2 (Chk1/2) inhibitors (12–15). 7-hydroxystaurosporine, UCN-01, was initially found to potentiate camptothecin-mediated cytotoxicity in p53-mutant cells by abrogating the cell cycle checkpoint (12). More recent studies extended this finding to more specific Chk1 inhibitors and showed that Chk1 inhibition abrogates the intra-S-phase checkpoint, which normally manifests by reduced replication fork speed and increased origin firing (15). Recently, novel Chk1 and Chk2 inhibitors have been introduced into therapeutic development (16–20).
Chk1 and Chk2 are serine/threonine kinases, members of the Ca2+/calmodulin-dependent protein kinase family in the kinome (21). In spite of their structural differences, both Chk1 and Chk2 can inactivate CDC25A/C by phosphorylation and can be targeted by similar drugs (19, 22, 23). Chk1 and Chk2 regulate cell cycle and DNA damage response downstream from ATM, ATR and DNA-PK, which are activated by genomic lesions (17, 24). Chk1 is essential for stabilizing replication forks and for the progression of cells through G1/S and G2/M (19, 24–26). Whereas Chk1 activation is primarily dependent on stalled replication forks, ultraviolet radiation and DNA crosslinks, Chk2 is mainly activated by DNA double-strand breaks (27).
Recently AZD7762 (Figure 1B), a Chk1/2 inhibitor, has been shown to synergize with DNA-damaging agents and radiation to induce apoptosis in a variety of cell types (28–32). Examination of a panel of neuroblastoma cell lines with variations in the p53/MDM2/p14ARF status showed a sensitization to DNA damaging agents, such as cisplatin and doxorubicin, when combined with AZD7762 (32). In combination with gemcitabine, AZD7762 was able to potentiate cell killing in colon and pancreatic cell lines via mitotic catastrophe (28, 29, 31). In the case of HCT116, this enhancement of apoptosis coincided with an increase in firing of suppressed replication origins as measured by DNA fiber combing (29). These data exemplify the breadth of DNA damaging agents and tumor types that can benefit from combinatorial treatment with checkpoint kinase inhibitors.
The present study was undertaken to determine whether novel checkpoint kinase inhibitors such as AZD7762 can potentiate the antiproliferative effects of the indenoisoquinolines.
Methods and Materials
Drugs, Cell Culture and Cell Viability Assay
Indenoisoquinoline LMP-400 and camptothecin (CPT) were obtained from the Drug Synthesis and Chemistry Branch of the Developmental Therapeutics Program, NCI (Rockville, MD). AZD7762 was provided by AstraZeneca (Wilmington, DE). AZD7762 and CPT were prepared as DMSO stocks at 10 mM and 5 mM, respectively. LMP-400 was prepared in sterile H2O as a 5 mM stock. HT29 and HCT116 cells were obtained from the Developmental Therapeutics Program (DTP, National Cancer Institute, Bethesda, MD, USA), grown in DMEM supplemented with 10% FBS and authenticated by STR DNA fingerprinting in 2009. HCT116 Chk2−/− cells (kindly provided by Dr. Bert Vogelstein, Johns Hopkins University, Baltimore, MD) were grown in DMEM supplemented with 10% FBS. HCT15 KD and HCT15 Chk2 cells (kindly provided by Dr. Carol Prives, Columbia University, New York, NY) were grown in RPMI supplemented with 10% FBS and 400 μg/ml G418 (Invitrogen, Carlsbad, CA). Cells were seeded at a density of 3,500 cells per well in 96-well plates and incubated at 37°C for 48 h before drug addition. At 48 h, serial dilutions of each drug starting at 0.02% DMSO for AZD7762, from stock solutions, were prepared in medium. After medium was aspirated, 100 μl of drug solution was added to appropriate wells with cells exposed to no more than 0.02% DMSO. The plates were incubated at 37°C for 48 h. Cell proliferation was assayed by adding 20 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3 carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium(MTS) (Promega, Madison, WI) to each well, incubating at 37°C for 25 min and then reading plates at 490 nm. Data were plotted using GraphPad Prism (GraphPad Software Inc., San Diego, CA) from raw absorbance values.
BrdU incorporation and Cell Cycle Analysis
HT29 cells were exposed to 0.3 μM LMP-400 for 1 h, media was removed and cells washed with phosphate buffered saline (1X PBS). Cells were then treated with 100 nM AZD7762 (0.01% DMSO) for indicated time-points with the addition of 50 μM BrdU 30 min prior to harvesting. Following fixation with 70% ethanol, DNA was denatured using 2 N HCl and 0.5% Triton X-100 and then neutralized with 0.1 M sodium borate (pH 8.5). Samples were washed twice with 0.5% Tween 20 and 0.5% bovine serum albumin (BSA) in PBS, anti-BrdU fluorescein isothiocyanate (Becton Dickinson, Franklin Lakes, NJ) was added for 1 h. Following two washes, samples were incubated with RNase-propidium iodide and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Western Blotting
After treatment at the indicated drug concentrations, cells were harvested, lysed (2 % SDS, 0.06 M Tris-HCl pH 6.8) and protein concentrations determined. Proteins (60 μg) were electrophoresed on 4–20 % polyacrylamide gels and the gels were transferred onto nitrocellulose membranes that were incubated with antibodies. All Chk1 and Chk2 antibodies were obtained from Cell Signaling Technologies (Beverly, MA) and the γH2AX antibody (clone JBW301 used at 1:1000 dilution from Upstate, Charlottesville, VA). After incubation with anti-mouse or rabbit secondary antibody (1:10,000 Sigma, St.Louis, MO), signals were detected by enhanced chemiluminescence (Pierce, Madison, WI).
DNA fiber Analysis
Approximately 5 ×105 cells were plated in each well of a six-well plate. Cells were pulse-labeled with 100 nM iododeoxyuridine (IdU) for 45 min, washed with prewarmed (37°C) PBS, and pulsed with 100 nM chlorodeoxyuridine (CldU) for 45 min (15, 29). The medium was prewarmed before pulses. To investigate the impact of LMP-400 on fork progression, 10 μM LMP-400 was added during the first 30 min of the CldU pulse. The checkpoint kinase inhibitor, AZD7762 (0.01% DMSO), was added during both pulses at a concentration of 100 nM. After drug treatments, cells were embedded in agarose at a density of 5 × 105 per 100 μl, the resulting plugs were treated with 1 mg/ml proteinase K and 1% N-Lauroyl sarcosine in 0.5 M EDTA (Ethylenediaminetetraacetic acid) (pH 8) overnight at 50°C. The plugs were then washed three times with 1x Tris-EDTA (TE, pH 8), melted in 0.1 M 2-(N-morpholino)ethane sulphonic acid (MES) for 20 min at 70°C and digested with β-agarase (NEB, Ipswich, MA) at 42°C overnight. The DNA was then stretched onto silane-treated slides (15), which were then baked for 1.5 h at 60°C and the DNA denatured with 0.5 M NaOH for 5 min. The slides were rinsed three times in PBS and incubated with the following antibodies: mouse anti-BrdU fluorescein isothiocyanate (Becton Dickinson) and rat anti-CldU (Accurate Chemical and Science Co., Westbury, NY) diluted in 1% BSA. After incubation in a humid chamber for 1 h at room temperature, slides were washed three times, each time for 3 min in PBS containing 0.1% Triton X-100. The slides were incubated with secondary fluorescent antibodies (Alexa anti-mouse 488 [Molecular Probes/Invitrogen (Carlsbad, CA)] and Alexa anti-rat 594 [Molecular Probes/Invitrogen (Carlsbad, CA)] diluted in 1% BSA) for 20 min at 37°C. Slides were washed three times for 5 min in PBS and mounted by using Vectashield (Vector Laboratories, Burlingame, CA). Pictures were acquired with the Pathway microscope and Attovision software (Becton Dickinson, San Jose, CA). Signals were measured by using ImageJ software (NCI/NIH), with some modifications made specifically to measure DNA fibers.
Statistical Analysis
Synergism was assessed by using CompuSyn Software (Paramus, NJ). All other statistical analyses were carried out on GraphPad Prism.
Results
AZD7762 sensitizes HT29 colon carcinoma cells to LMP-400
To evaluate the potential impact of AZD7762 on LMP-400-induced cell killing, human colon carcinoma HT29 cells were exposed to a combination of AZD7762 and LMP-400 or to each drug alone over a 48 h time-course of continuous treatment. This protocol was chosen based on the fact that the antiproliferative effect of Top1 inhibitors is optimum with long exposure and that the checkpoint response and cell cycle arrest induced by Top1 inhibitors are observed several hours after the initiation of treatment. LMP-400’s IC50 was 0.47 μM (Figure 1C), similar to that of CPT (IC50 =0.56 μM) (Supplemental S1). The concentration-dependent effect of AZD7762 on LMP-400-mediated cytotoxicity was examined at four fixed non-cytotoxic concentrations of AZD7762 (16, 31, 63 and 125 nM) (Figure 1). A concentration response for AZD7762 in HT29 as well as the other cell lines tested can be seen in supplemental Figure S2. A synergistic effect of AZD7762 was observed at all four doses tested, with the strongest effect at 125 nM of AZD7762, as assessed by CompuSyn analysis (Figure 1D), which showed CI (Combination Index) values below 0.1. These data show the synergistic effect of the combination of AZD7762 with LMP-400.
AZD7762 abrogates the S-phase arrest mediated by both LMP-400 and CPT
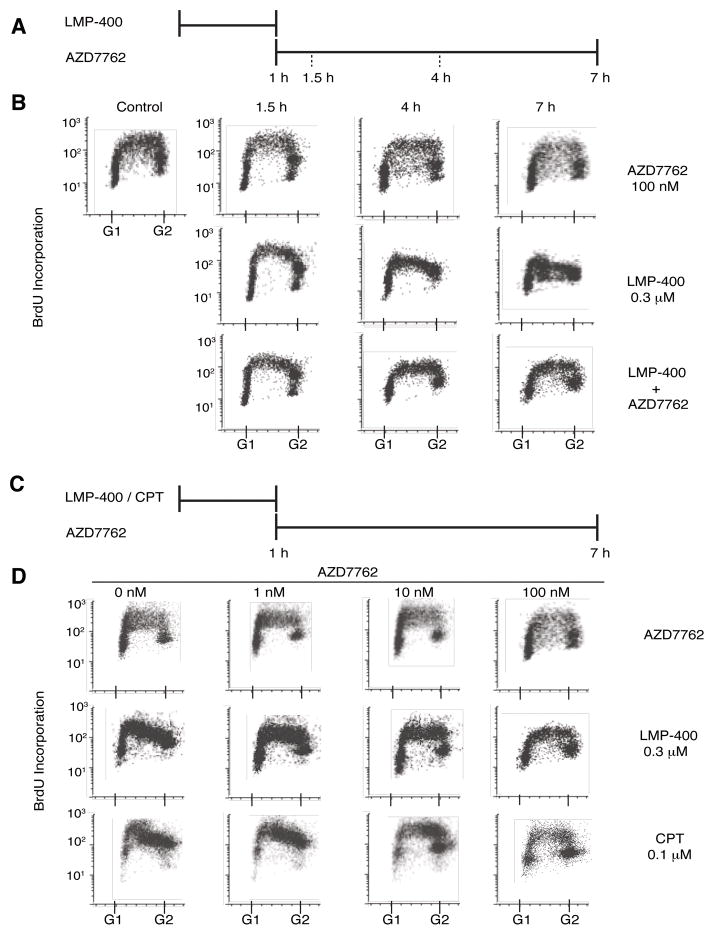
Top1 inhibition is known to result in intra-S phase arrest due to activation of Chk1/2 (8, 15, 33, 34). The ability of LMP-400 to induce such a pattern of replicative arrest was evaluated by a time-dependent exposure to 0.3 μM of LMP-400 and assessed via BrdU incorporation. The protocol for addition of both drugs is outlined in Figure 2A. A modest effect on the cell S-phase progression was observed after 1.5 h of LMP-400 treatment (Figure 2B). However, the effect of LMP-400 shows a definite time-dependence due to the marked decrease in the number of cells staining positive for BrdU in the late S-phase at the 4 and 7 h time-points. Consistent with the interpretation that this S-phase arrest is checkpoint-dependent, 100 nM AZD7762 was able to abrogate this arrest (Figure 2B).
Figure 2. AZD7762 abrogates the S-phase arrest induced by LMP-400 or CPT.
A) Treatment schedule for the time course experiments shown in panel B. HT29 cells were treated with LMP-400 for 1 h. AZD7762 was added immediately after removal of LMP-400 and kept for up to 6 h. B) Representative time-course experiments showing loss of S-phase progression in response to LMP-400 and recapitulation of cell cycle by AZD7762. DNA synthesis was monitored by adding 50 μM BrdU 30 min prior to cell harvesting. C) Treatment schedule for the AZD7762 concentration-response experiments shown in panel D. D) Representative concentration-response FACS experiments showing LMP-400-and CPT-mediated S-phase arrest and S-phase arrest abrogation by AZD7762. DNA synthesis was monitored by adding 50 μM BrdU 30 min prior to cell harvesting. Data are representative of at least three independent experiments.
The DMSO soluble analog of LMP-400, NSC 724998, has previously been shown to induce both S and G2-M phase arrests like CPT (8). Addition of AZD7762 or CPT was performed as per Figure 2C. To further establish the similarity between LMP-400 and camptothecins, the effects of LMP-400 and CPT on S-phase arrest were assessed in parallel (Figure 2D). As expected, both CPT and LMP-400 abrogated BrdU incorporation (Figure 2D, left). In conjunction with this, a dose-responsive treatment with AZD7762 was carried out. Under these conditions, 6 h of continuous treatment with AZD7762 attenuated the effect of both drugs at concentrations as low as 10 nM AZD7762. Thus, not only is LMP-400 as effective at inducing S-phase arrest as camptothecins but also AZD7762 is a potent inhibitor of this arrest.
S-phase arrest induced by high concentrations LMP-400 is abrogated by AZD7762
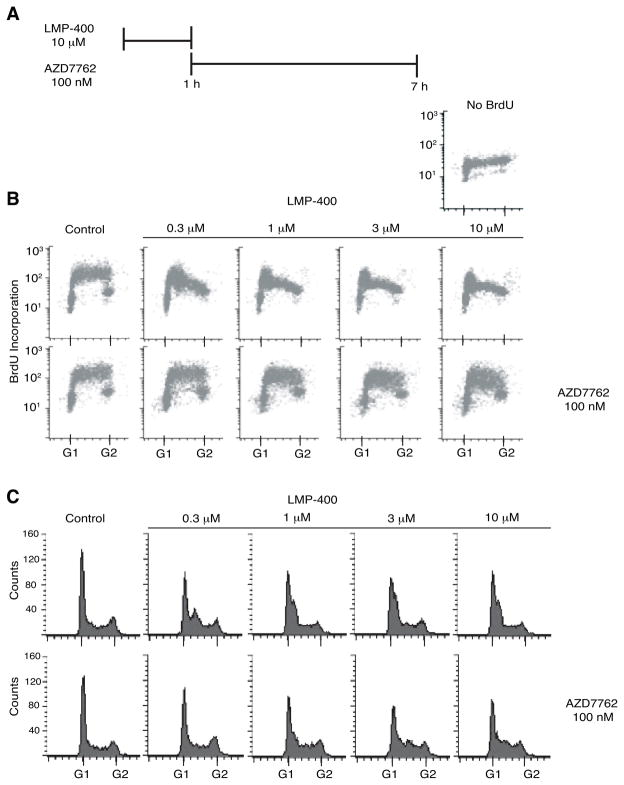
To test the efficacy of LMP-400 for inducing a substantial S-phase arrest and the extent to which this arrest could be abrogated by AZD7762, experiments were carried out over a broad range of LMP-400 concentrations. Prior to the addition of AZD7762 for 6 h, cells were treated with LMP-400 for 1 h (Figure 3A). DNA synthesis was monitored by BrdU incorporation at the end of the 6 h incubation with or without AZD7762, as described above. The DNA synthesis inhibition induced by LMP-400 was observed over a broad concentration range and was clearly observed at 0.3 μM LMP-400 (Figure 3B). At the same time, cells were arrested in early S-phase, and the arrest tended to occur earlier in the cell cycle as the LMP-400 concentration increased (Figure 3C). Regardless of the extent of S-phase arrest induced by LMP-400, 100 nM AZD7762 was able to reactivate DNA synthesis as measured by BrdU incorporation and to re-establish a normal cell cycle profile, albeit with a decreased G1 population and a residual population of cell arrested in S-phase at the highest LMP-400 concentrations (3 and 10 μM) (Figure 3B-C). These results demonstrate the selectivity of LMP-400 for S-phase cells and the effectiveness of AZD7762 in abrogating the cell cycle and DNA synthesis effects of LMP-400.
Figure 3. S-phase arrest induced by LMP-400 is abrogated by AZD7762.
A) Treatment schedule. B) DNA synthesis was monitored by adding 50 μM BrdU 30 min prior to harvesting HT29 cells treated as indicated. C) Cell cycle effects were determined simultaneously by propidium iodide staining. Data are representative of at least three independent experiments.
Reduction of replication fork velocity and inter-origin distances in cells treated with LMP-400 and AZD7762
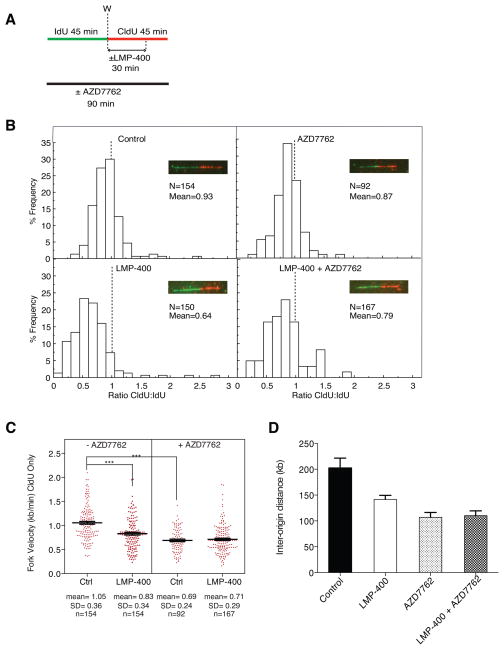
To elucidate the effect of LMP-400 and AZD7762 on DNA replication and checkpoint control, cells treated with each drug alone or with a combination of both were subjected to single molecule DNA analyses by molecular combing (15, 35, 36). Individual replicons were visualized by immunofluorescence microscopy with two specific antibodies after sequential DNA incorporation of the thymidine analogs IdU and CldU for 45 min each (Figure 4A). To assess the impact of LMP-400 and AZD7762 on DNA replication fork velocity, the percentage frequency of the ratio of red to green pulses were plotted (Figure 4B). As expected the ratios for both untreated (control) and AZD7762-treated HT29 cells were close to one (0.93 and 0.87, respectively), indicating an equal replication fork velocity over both pulses.
Figure 4. Effects of LMP-400 and AZD7762 on replication fork velocity and origin firing.
A) Treatment protocol. B) Distribution of Idu:CldU ratios (red:green signals) determined in single DNA molecules after molecular combing; N = number of signals measured. C) Fork velocity responses to LMP-400 and AZD7762 alone or in combination. Each dot represents a data point for an individual DNA fiber. Mean, standard deviation (SD) and number of signals measured (N) are indicated under each scatter plot. D) Inter-origin distances measured for each treatment. A minimum of 30 inter-origin distances were taken to measure their average distance. The bars represent SD. All data are representative from at least three independent experiments.
A reduction in the CldU:IdU ratio to 0.64 was observed for LMP-400-pulsed cells (Figure 4B), inferring that the 30 min pulse with LMP-400 during CldU incubation was sufficient to rapidly impact ongoing replication forks (15). Analysis of replication fork velocities showed a mean velocity of 1.05 kb/min for control cells and 0.83 kb/min for LMP-400 treated cells, a 20% decrease in the mean velocity for LMP-400 as compared to untreated cells (Figure 4C). The LMP-400-induced slowdown of replication forks was partially abrogated in the presence of AZD7762, as seen by the recapitulation of the CldU:IdU ratio to 0.79 (Figure 4B, compare the upper and lower panels).
Notably, AZD7762 by itself produced a consistent reduction in replication velocity (Figure 4C). In the AZD7762-treated cells there was no additional decrease in fork velocity upon addition of LMP-400 (Figure 4C, right panel), indicating that AZD7762 abrogates the LMP-400-induced intra-S-phase checkpoint component associated with reduction of fork velocity.
Distances between individual replication fork origins can also be measured by molecular combing (15, 36). Inter-origin distances in drug-treated cells are reported in Figure 4D. Consistent with the fact that one of the components of the intra-S-phase checkpoint consists in an increased origin firing (15, 36, 37), LMP-400 induced on average a 25% decrease in the inter-origin distances. In line with the inhibitory effect of AZD7762 on replication velocity, we found that AZD7762, by itself increased origin firing, as measured by a reduction of inter-origin distances. In the AZD7762-treated cells, LMP-400 also failed to significantly impact origin firing. Taken together, our results are consistent with a rapid activation of the intra-S-phase checkpoint by LMP-400 and with AZD7762 acting as a cell cycle checkpoint abrogator (28, 29, 34).
AZD7762 is a potent inhibitor of both Chk1 and Chk2 and abrogates their autophosphorylation-activation
Because the replication checkpoint is under the control of Chk1 and Top1-targets are well-established activators of both Chk1 and Chk2 (15, 33, 34), we determined the activation of Chk1 and Chk2 by LMP-400 and the impact of AZD7762 of these activations.
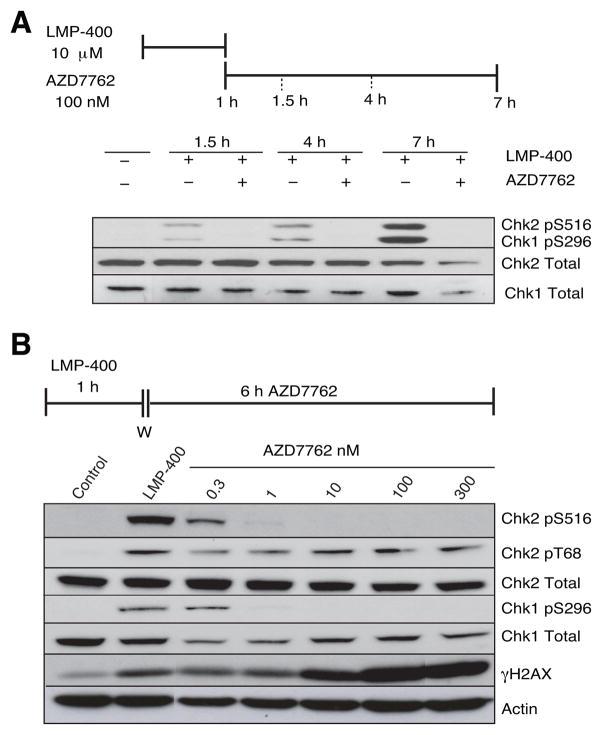
Both Chk1 and Chk2 are activated by autophosphorylation and Western blotting was used to measure the effects of LMP-400 and AZD7762 on Chk1 and Chk2 activations. The kinetics of checkpoint kinase activations by LMP-400 in the absence and presence of AZD7762 were first examined in time-course experiments. Reactions were followed over the course of 7 h and assessed by Western blotting for both Chk1 and Chk2 autophosphorylations (Chk1 pS296 and Chk2 pS516) (Figure 5A). LMP-400 induced detectable activation of both kinases at the 1.5 h time-point with further activation throughout the time-course. These data indicate that the full activation of both Chk1 and Chk2 checkpoint kinases by LMP-400 increases well after the removal of the drug. Figure 5A also shows that AZD7762 blocks LMP-400-induced autophosphorylations of both Chk1 and Chk2.
Figure 5. AZD7762 abrogates both the Chk1 and Chk2 activations induced by LMP-400 and enhances the histone H2AX response.
A) Time-dependent phosphorylations/activations of Chk1 and Chk2 by LMP-400 and their inhibition by AZD7762. The treatment schedule is shown at the top. HT-29 cells were harvested post LMP-400 treatment as indicated (upper panel). Autophosphorylation of Chk1 at Ser296 and Chk2 at Ser516 were analyzed by Western blotting. Chk1 and Chk2 total proteins were both used as loading controls. B) AZD7762-dependent concentration-response for abrogation of Chk1 or Chk2 autophosphorylations. Chk2 activation/phosphorylation (Chk2 T68) and histone H2AX phosphorylation (γH2AX) by PI3 kinases were also determined. Actin, Chk1 total and Chk2 total were used as loading controls.
Concentration-response experiments were then carried out to determine the potency of AZD7762 and its relative activity on Chk1 vs. Chk2 (Figure 5B). AZD7762 was able to inhibit both Chk1 and Chk2 autophosphorylations at sub-nanomolar concentrations (Figure 5B). Moreover, AZD7762 had no impact on the upstream kinases as demonstrated by its lack of activity against pT68-Chk2 (Figure 5B). These experiments demonstrate the potency of AZD7762 against Chk1 and Chk2 activation in cellular systems.
The impact of AZD7762 on the DNA damage response was evaluated using phosphorylation of histone H2AX as a sensitive biomarker (11). A significant induction of γH2AX was observed after treatment for 1 h with LMP-400 (Figure 5B, lane 2), which is consistent with prior experiments (8, 11). ThisγH2AX response was detectably enhanced by concentrations of AZD7762 at 10 nM and above (Figure 5B). The enhancement of γH2AX activation by AZD7762 is possibly due to stalled replication forks being converted to double-strand breaks (DSBs) (see Discussion).
Effect of LMP-400 and AZD7762 combination on a panel of colon carcinoma cell lines with Chk2 alterations
In order to demarcate the importance of Chk2 inhibition in the AZD7762-mediated synergistic effect observed with LMP-400, colon carcinoma cell lines with genetic Chk2 alterations were tested (Figure 6).
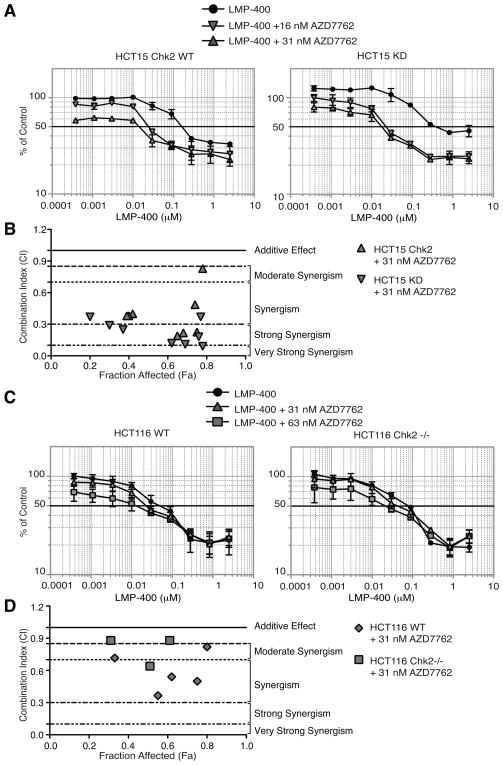
Figure 6. The synergistic effects of AZD7762 and LMP-400 in a panel of colon carcinoma cell lines is independent of Chk2.
A) HCT15 cells complemented with wild-type Chk2 (HCT15 Chk2 WT), kinase-dead Chk2 (HCT15 KD) were treated with LMP-400 alone or in combination with various fixed concentrations of AZD7762 for 48 h. B) Graph of CI (combination Index) values vs Fa (Fraction affected) for data points shown in panel A on the combination (AZD7762 + LMP-400) for HCT15 cells. C) wild-type HCT116 (HCT116 WT) and Chk2 knockout HCT116 cells (HCT116 Chk2 −/−) were treated with LMP-400 alone or in combination with various fixed concentrations of AZD7762 for 48 h. D) Graph of CI (combination Index) values vs Fa (Fraction affected) for data points shown in panel C on the combination (AZD7762 + LMP-400) for HCT116 cells. Cell survival was assessed by MTS assays. Data represent mean ± S.E. of three independent experiments.
HCT-15 colon carcinoma cells are naturally deficient for Chk2 due to biallelic CHEK2 mutations: R145W on one allele and A247D on the other, which both destabilize Chk2 polypeptides (38). HCT15 cells have also been used to study the functional roles of Chk2 by complementation with wild-type (WT) or kinase-dead (KD) Chk2 mutants (39). Figure 6A shows that the HCT15 cells complemented with a HA-tagged Chk2-expressing plasmid (left) are slightly more sensitive to LMP-400 than their kinase-dead counterpart (right) (IC50’s of 160 nM vs. 390 nM, respectively; Table 1). However, the synergistic effect of AZD7762 with LMP-400 was more profound on the HCT15 Chk2 KD line than in its wild-type counterpart due to the higher number of data points in the strong to very strong synergistic range (Figure 6B). Indeed, AZD7762 (16 nM) reduced the IC50 of LMP-400 by approximately 20-fold in the HCT15 KD cells compared to 10-fold for the HCT15 Chk2 WT cells (Figure 6A and Table 1).
Table 1.
IC50 of LMP-400, AZD7762 or a combination of both in different cell lines
| Cell lines | IC50 LMP-400 (nM) | IC50 AZD7762 (nM) | IC50 LMP-400 + AZD7762 (16 nM) |
|---|---|---|---|
| HCT116 WT | 50 ± 8 | 280 ± 16.2 | 40 ± 7.7 |
| HCT116 Chk2 −/− | 80 ± 2 | 290 ± 26.4 | 30 ± 5.6 |
| HCT15 Chk2 WT | 160 ± 2 | 50 ± 5.2 | 20 ± 1.9 |
| HCT15 Chk2 KD | 390 ± 3 | 80 ± 3.7 | 20 ± 2.9 |
HCT116 cells with homozygotous inactivation of Chk2 (40) were also studied. No synergistic effect was observed with either HCT116 cell line tested (Figure 6C). Both HCT116 cell lines showed a high sensitivity to LMP-400 and few synergistic data points when treated with AZD7762 (Figure 6D). Together, these results (Table 1) demonstrate that the synergistic effect of AZD7762 on LMP-400 induced cell killing is not directly related to the inhibitory activity of AZD7762 on Chk2.
Discussion
Clinically available Top1-targeted drugs, though highly successful, have warranted the development of novel non-camptothecin derivatives due to aforementioned chemical, biochemical and cellular limitations of camptothecin derivatives (41–44). As a class, the indenoisoquinolines induce Top1ccs that persist longer than camptothecins, have a markedly different DNA cleavage pattern, and avoid drug efflux (8). Although indenoisoquinolines have been shown to induce both S- and G2/M-phase arrest, the extent to which LMP-400 modulates cell cycle arrest and DNA replication has not been investigated previously. Critical for the progression of cells through S and G2/M phase, Chk1 and Chk2 are also essential for the DNA damage response. Checkpoint inhibition has been shown to potentiate the activity of chemotherapeutic agents such as IR, gemcitabine and camptothecin (16–20, 29).
Checkpoint kinase inhibitors, such as UCN-01 and CHIR-124 restore DNA replication and cell cycle progression in camptothecin treated cells (13, 22, 28, 34). Furthermore, indenoisoquolines have been shown at low concentrations to induced a G2/M arrest and S-phase at slightly higher doses after 6 h of continuous exposure (8). Our data shows that LMP-400-treated HT29 cells induce a marked inhibition on S-phase progression at submicromolar concentration treatments, consistent with Top1 inhibition. Activated by ATR in response to replication block, Chk1 is the primary kinase associated with S-phase arrest (45). Chk1 acts to maintain fork progression via its role in regulating Cdk2 and CDC25. Although Chk1 is the predominant kinase that affects S-phase progression, Chk2 has been shown to participate in the S-phase response after UV treatment (46). Consistent with the ability of checkpoint kinases to inhibit S-phase progression, AZD7762 treatment was able to reestablish a normal cell cycle profile after exposure to LMP-400.
Persistent Top1ccs lead to increased DNA DSBs detectable by histone H2AX activation as seen in Figure 5. LMP-400-induced slowdown of the replication fork velocity is consistent with DNA damage induced activation of checkpoint proteins inhibiting S-phase progression in order to maintain genomic stability. This is a significant finding as it illustrates that indenoisoquinolines are effective activators of the checkpoint kinases. Chk1 inhibition is implicated in the recapitulation of S-phase progression of cells after treatment with agents, such as camptothecin and IR, as it is activated by stalled DNA replication forks (13, 22, 34, 45). Moreover, inhibition of the ATR-Chk1 pathway has been shown to slowdown fork breakage and repair after inter-strand cross-linking (47). AZD7762 increases the origin firing in HCT116 cells, by itself and also when treated with gemcitabine (29). In addition to this, our data shows that the inter-origin distances of replication forks are decreased in HT29 cells along with the fork velocity. Taken together, these data infer a role for Chk1 in the overall slowdown of replication fork progression, independent of DNA damage. This study emphasizes the effectiveness of LMP-400 as a potent Top1 poison and potential for therapeutic enhancement that is afforded when combined with checkpoint inhibition.
Checkpoint inhibitors can be highly promiscuous at concentrations above those needed to inhibit the kinase activity in vitro. UCN-01 for example can inhibit PKC, PKA, PDK1 and v-SRC tyrosine kinase at nM concentrations (48, 49). AZD7762 is an equitoxic inhibitor of both checkpoint kinases, as demonstrated by the inhibition of both the autophosphorylation of Chk1 and Chk2 in AZD7762-treated cells is not surprising (28). Our study is the first demonstration by Western blot analysis of the downregulation of Chk2 autophosphorylation by AZD7762 in cells.
Our results indicate no further enhancement of the synergistic effect of LMP-400 by either up regulating or inhibiting Chk2 expression (31), which infers that the AZD7762-mediated synergism observed in these cell lines may be dependent on other cellular targets. The synergism between AZD7762 and Top1 inhibitors occurs at pharmacological concentrations where AZD7762 abrogrates the S- and G2-checkpoints induced by the Top1 inhibitors, but that these concentrations are above the threshold of AZD7762 concentrations required to inhibit Chk1 and Chk2. Thus, our findings that Chk1 (and Chk2) inhibition is observed at much lower concentration than those required to affect cell cycle arrest and produce synergy suggest that additional mechanisms are involved in the synergism and mechanisms of action of AZD7762. These observations point out to the need to test both Chk2 and Chk1 and correlate their inhibition with FACS analyses when developing cell cycle checkpoint inhibitors. Interestingly, In vivo data shows that Chk1 and Chk2 are cooperatively haplo-insufficient in their ability to regulate apoptosis (50). It is altogether possible that other kinases are important in AZD7762-mediated cell death, as AZD7762 was shown to inhibit members of the CAM kinase and SRC-like family (28).
In summary, we have shown that LMP-400 is a potent Top1 inhibitor whose effect can be enhanced by checkpoint inhibition. These data provide a rationale for combining cell cycle checkpoints inhibitors with LMP-400 and Top1-targeted drugs. Also, they suggest that cancer cells with intrinsic checkpoint defects might be selectively sensitive to LMP-400. Finally, although AZD7762 is a highly active Chk1/2 inhibitor, it is possible that contributions from additional kinase inhibitions exist. In any case, the unambiguous improvement in antiproliferative activity when combined with Top1 inhibition re-enforces the rationale for pursuit of combination of Top1-targeted drugs with checkpoint kinase inhibitors.
Acknowledgments
Financial Support: This work was supported by the Center for Cancer Research and the Intramural Program of the National Cancer Institute.
This work was supported by the Center for Cancer Research, the Intramural Program of the National Cancer Institute and by a CRADA with Astra Zeneca. We would like to thank Dr. Lyuba Varticovski, CCR-NCI for her generous help with the CompuSyn program.
References
- 1.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–33. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev. 2009;109:2894–902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champoux JJ. DNA topoisomerases: Structure, Function, and Mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 4.Teicher BA. Next generation topoisomerase I inhibitors: Rationale and biomarker strategies. Biochem Pharmacol. 2008;75:1262–71. doi: 10.1016/j.bcp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Pommier Y, Cushman M. The indenoisoquinoline noncamptothecin topoisomerase I inhibitors: update and perspectives. Mol Cancer Ther. 2009;8:1008–14. doi: 10.1158/1535-7163.MCT-08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pommier Y, Barcelo JM, Rao VA, Sordet O, Jobson AG, Thibaut L, et al. Repair of topoisomerase I-mediated DNA damage. Prog Nucleic Acid Res Mol Biol. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohlhagen G, Paull K, Cushman M, Nagafufuji P, Pommier Y. Protein-linked DNA strand breaks induced by NSC 314622, a non-camptothecin topoisomerase I poison. Mol Pharmacol. 1998;54:50–8. doi: 10.1124/mol.54.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Antony S, Agama KK, Miao ZH, Takagi K, Wright MH, Robles AI, et al. Novel indenoisoquinolines NSC 725776 and NSC 724998 produce persistent topoisomerase I cleavage complexes and overcome multidrug resistance. Cancer Res. 2007;67:10397–405. doi: 10.1158/0008-5472.CAN-07-0938. [DOI] [PubMed] [Google Scholar]
- 9.Ioanoviciu A, Antony S, Pommier Y, Staker BL, Stewart L, Cushman M. Synthesis and Mechanism of Action Studies of a Series of Norindenoisoquinoline Topoisomerase I Poisons Reveal an Inhibitor with a Flipped Orientation in the Ternary DNA-Enzyme-Inhibitor Complex As Determined by X-ray Crystallographic Analysis. J Med Chem. 2005;48:4803–14. doi: 10.1021/jm050076b. [DOI] [PubMed] [Google Scholar]
- 10.Han B, Stockwin LH, Hancock C, Yu SX, Hollingshead MG, Newton DL. Proteomic analysis of nuclei isolated from cancer cell lines treated with indenoisoquinoline NSC 724998, a novel topoisomerase I inhibitor. J Proteome Res. 2010;9:4016–27. doi: 10.1021/pr100194d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinders R, Hollingshead MG, Lawrence S, Ji J, Tabb B, Bonner WM, et al. Development of a Validated Immunofluorescence Assay for {gamma}H2AX as a Pharmacodynamic Marker of Topoisomerase I Inhibitor Activity. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-09-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao R-G, Cao C-X, Shimizu T, O’Connor P, Kohn KW, Pommier Y. Abrogation of an S-phase checkpoint and potentiation of camptothecin cytotoxicity by 7-hydroxystaurosporine (UCN-01) in human cancer cell lines, possibly influenced by p53. Cancer Res. 1997;57:4029–35. [PubMed] [Google Scholar]
- 13.Shao RG, Cao CX, Pommier Y. Abrogation of Chk1-mediated S/G2 checkpoint by UCN-01 enhances ara-C-induced cytotoxicity in human colon cancer cells. Acta Pharmacol Sin. 2004;25:756–62. [PubMed] [Google Scholar]
- 14.Fracasso PM, Williams KJ, Chen RC, Picus J, Ma CX, Ellis MJ, et al. A Phase 1 study of UCN-01 in combination with irinotecan in patients with resistant solid tumor malignancies. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-010-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiler JA, Conti C, Syed A, Aladjem MI, Pommier Y. The Intra-S-Phase Checkpoint Affects both DNA Replication Initiation and Elongation: Single-Cell and -DNA Fiber Analyses. Mol Cell Biol. 2007;27:5806–18. doi: 10.1128/MCB.02278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pommier Y, Sordet O, Rao A, Zhang H, Kohn KW. Targeting Chk2 kinase: molecular interaction maps and therapeutic rationale. Curr Pharm Des. 2005;11:2855–72. doi: 10.2174/1381612054546716. [DOI] [PubMed] [Google Scholar]
- 17.Pommier Y, Weinstein JN, Aladjem MI, Kohn KW. Chk2 molecular interaction map and rationale for Chk2 inhibitors. Clin Cancer Res. 2006;12:2657–61. doi: 10.1158/1078-0432.CCR-06-0743. [DOI] [PubMed] [Google Scholar]
- 18.Antoni L, Sodha N, Collins I, Garrett MD. CHK2 kinase: cancer susceptibility and cancer therapy - two sides of the same coin? Nat Rev Cancer. 2007;7:925–36. doi: 10.1038/nrc2251. [DOI] [PubMed] [Google Scholar]
- 19.Garrett MD, Collins I. Anticancer therapy with checkpoint inhibitors: what, where and when? Trends Pharmacol Sci. 2011;32:308–16. doi: 10.1016/j.tips.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Dent P, Tang Y, Yacoub A, Dai Y, Fisher PB, Grant S. CHK1 Inhibitors in Combination Chemotherapy: Thinking Beyond the Cell Cycle. Mol Interv. 2011;11:133–40. doi: 10.1124/mi.11.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 22.Yu Q, La Rose J, Zhang H, Takemura H, Kohn KW, Pommier Y. UCN-01 inhibits p53 up-regulation and abrogates gamma-radiation-induced G(2)-M checkpoint independently of p53 by targeting both of the checkpoint kinases, Chk2 and Chk1. Cancer Res. 2002;62:5743–8. [PubMed] [Google Scholar]
- 23.Jobson AG, Cardellina JH, 2nd, Scudiero D, Kondapaka S, Zhang H, Kim H, et al. Identification of a Bis-guanylhydrazone [4,4′-Diacetyldiphenylurea-bis(guanylhydrazone); NSC 109555] as a Novel Chemotype for Inhibition of Chk2 Kinase. Mol Pharmacol. 2007;72:876–84. doi: 10.1124/mol.107.035832. [DOI] [PubMed] [Google Scholar]
- 24.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 26.Peddibhotla S, Lam MH, Gonzalez-Rimbau M, Rosen JM. The DNA-damage effector checkpoint kinase 1 is essential for chromosome segregation and cytokinesis. Proc Natl Acad Sci U S A. 2009;106:5159–64. doi: 10.1073/pnas.0806671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zabludoff SD, Deng C, Grondine MR, Sheehy AM, Ashwell S, Caleb BL, et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol Cancer Ther. 2008;7:2955–66. doi: 10.1158/1535-7163.MCT-08-0492. [DOI] [PubMed] [Google Scholar]
- 29.McNeely S, Conti C, Sheikh T, Patel H, Zabludoff S, Pommier Y, et al. Chk1 inhibition after replicative stress activates a double strand break response mediated by ATM and DNA-dependent protein kinase. Cell Cycle. 2010;9:995–1004. doi: 10.4161/cc.9.5.10935. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell JB, Choudhuri R, Fabre K, Sowers AL, Citrin D, Zabludoff SD, et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin Cancer Res. 2010;16:2076–84. doi: 10.1158/1078-0432.CCR-09-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan MA, Parsels LA, Zhao L, Parsels JD, Davis MA, Hassan MC, et al. Mechanism of Radiosensitization by the Chk1/2 Inhibitor AZD7762 Involves Abrogation of the G2 Checkpoint and Inhibition of Homologous Recombinational DNA Repair. Cancer Res. 2010;70:4972–81. doi: 10.1158/0008-5472.CAN-09-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Cheung IY, Wei XX, Tran H, Gao X, Cheung NK. Checkpoint kinase inhibitor synergizes with DNA-damaging agents in G(1) checkpoint-defective neuroblastoma. Int J Cancer. 2010 doi: 10.1002/ijc.25842. [DOI] [PubMed] [Google Scholar]
- 33.Takemura H, Rao VA, Sordet O, Furuta T, Miao ZH, Meng L, et al. Defective Mre11-dependent Activation of Chk2 by Ataxia Telangiectasia Mutated in Colorectal Carcinoma Cells in Response to Replication-dependent DNA Double Strand Breaks. J Biol Chem. 2006;281:30814–23. doi: 10.1074/jbc.M603747200. [DOI] [PubMed] [Google Scholar]
- 34.Tse AN, Rendahl KG, Sheikh T, Cheema H, Aardalen K, Embry M, et al. CHIR-124, a novel potent inhibitor of Chk1, potentiates the cytotoxicity of topoisomerase I poisons in vitro and in vivo. Clin Cancer Res. 2007;13:591–602. doi: 10.1158/1078-0432.CCR-06-1424. [DOI] [PubMed] [Google Scholar]
- 35.Conti C, Caburet S, Bensimon A. Targeting the molecular mechanism of DNA replication. Drug Discov Today. 2001;6:786–92. doi: 10.1016/s1359-6446(01)01854-2. [DOI] [PubMed] [Google Scholar]
- 36.Conti C, Seiler J, Pommier Y. The Mammalian DNA Replication Elongation Checkpoint: Implication of Chk1 and Relationship with Origin Firing as Determined by Single DNA Molecule and Single Cell Analyses. Cell Cycle. 2007;6:2760–7. doi: 10.4161/cc.6.22.4932. [DOI] [PubMed] [Google Scholar]
- 37.Courbet S, Gay S, Arnoult N, Wronka G, Anglana M, Brison O, et al. Replication fork movement sets chromatin loop size and origin choice in mammalian cells. Nature. 2008;455:557–60. doi: 10.1038/nature07233. [DOI] [PubMed] [Google Scholar]
- 38.Lee SB, Kim SH, Bell DW, Wahrer DC, Schiripo TA, Jorczak MM, et al. Destabilization of CHK2 by a Missense Mutation Associated with Li- Fraumeni Syndrome. Cancer Res. 2001;61:8062–7. [PubMed] [Google Scholar]
- 39.Urist M, Tanaka T, Poyurovsky MV, Prives C. p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev. 2004;18:3041–54. doi: 10.1101/gad.1221004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jallepalli PV, Lengauer C, Vogelstein B, Bunz F. The Chk2 tumor suppressor is not required for p53 responses in human cancer cells. J Biol Chem. 2003;278:20475–9. doi: 10.1074/jbc.M213159200. [DOI] [PubMed] [Google Scholar]
- 41.Covey JM, Jaxel C, Kohn KW, Pommier Y. Protein-linked DNA strand breaks induced in mammalian cells by camptothecin, an inhibitor of topoisomerase I. Cancer Res. 1989;49:5016–22. [PubMed] [Google Scholar]
- 42.Burke TG, Mi Z. The structural basis of camptothecin interactions with human serum albumin: impact on drug stability. J Med Chem. 1994;37:40–6. doi: 10.1021/jm00027a005. [DOI] [PubMed] [Google Scholar]
- 43.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002;99:15387–92. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 45.Feijoo C, Hall-Jackson C, Wu R, Jenkins D, Leitch J, Gilbert DM, et al. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J Cell Biol. 2001;154:913–23. doi: 10.1083/jcb.200104099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falck J, Petrini JH, Williams BR, Lukas J, Bartek J. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat Genet. 2002;30:290–4. doi: 10.1038/ng845. [DOI] [PubMed] [Google Scholar]
- 47.Le Breton C, Hennion M, Arimondo PB, Hyrien O. Replication-fork stalling and processing at a single psoralen interstrand crosslink in Xenopus egg extracts. PLoS One. 2011;6:e18554. doi: 10.1371/journal.pone.0018554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi I, Kobayashi E, Asano K, Yoshida M, Nakano H. UCN-01, a selective inhibitor of protein kinase C from Streptomyces. J Antibiot (Tokyo) 1987;40:1782–4. doi: 10.7164/antibiotics.40.1782. [DOI] [PubMed] [Google Scholar]
- 49.Sato S, Fujita N, Tsuruo T. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine) Oncogene. 2002;21:1727–38. doi: 10.1038/sj.onc.1205225. [DOI] [PubMed] [Google Scholar]
- 50.Niida H, Murata K, Shimada M, Ogawa K, Ohta K, Suzuki K, et al. Cooperative functions of Chk1 and Chk2 reduce tumour susceptibility in vivo. EMBO J. 2010;29:3558–70. doi: 10.1038/emboj.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]