Abstract
This review and meta-analysis aims at summarizing and integrating the human neuroimaging studies that report periaqueductal gray (PAG) involvement; 250 original manuscripts on human neuroimaging of the PAG were identified. A narrative review and meta-analysis using activation likelihood estimates is included. Behaviors covered include pain and pain modulation, anxiety, bladder and bowel function and autonomic regulation. Methods include structural and functional magnetic resonance imaging, functional connectivity measures, diffusion weighted imaging and positron emission tomography. Human neuroimaging studies in healthy and clinical populations largely confirm the animal literature indicating that the PAG is involved in homeostatic regulation of salient functions such as pain, anxiety and autonomic function. Methodological concerns in the current literature, including resolution constraints, imaging artifacts and imprecise neuroanatomical labeling are discussed, and future directions are proposed. A general conclusion is that PAG neuroimaging is a field with enormous potential to translate animal data onto human behaviors, but with some growing pains that can and need to be addressed in order to add to our understanding of the neurobiology of this key region.
1. Introduction
The periaqueductal gray (PAG) (a.k.a. central gray or substantia grisea centralis) is conserved across vertebrate species (cartilaginous and bony fishes, amphibians, reptiles, birds and mammals, and probably also in jawless fish (Fiebig, 1988; Kingsbury et al., 2011; Kittelberger et al., 2006; Pezalla, 1983; Stephenson-Jones et al., 2011; ten Donkelaar and de Boer-van Huizen, 1987)). It is well situated at the crossroads of ascending sensory information and inputs from higher centers that modulate these processes. The PAG is involved in neurobiological functions that include pain modulation, anxiety and reproductive behavior (Behbehani, 1995). Some of these functions, for example descending modulation of pain, have been more clearly defined than others, but the putative functions all seem to play a homeostatic defense of the individual’s response, integrating afferent information from the periphery and information from higher centers. These functions may be segregated within the PAG (e.g., anxiety and pain (Mendes-Gomes et al., 2011)) based on current understandings of its anatomical subdivisions (see below). Conceptually the structure may be involved in balancing or segueing information related to survival salience. Here, we review the current human neuroimaging literature indicating PAG structural alterations, functional activations, tractography and functional connectivity (Figure 1). For a comprehensive overview of non-neuroimaging studies of the PAG, Carrive and Morgans chapter on the PAG in Paxinos & Mai’s “The human nervous” system (2004) is recommended.
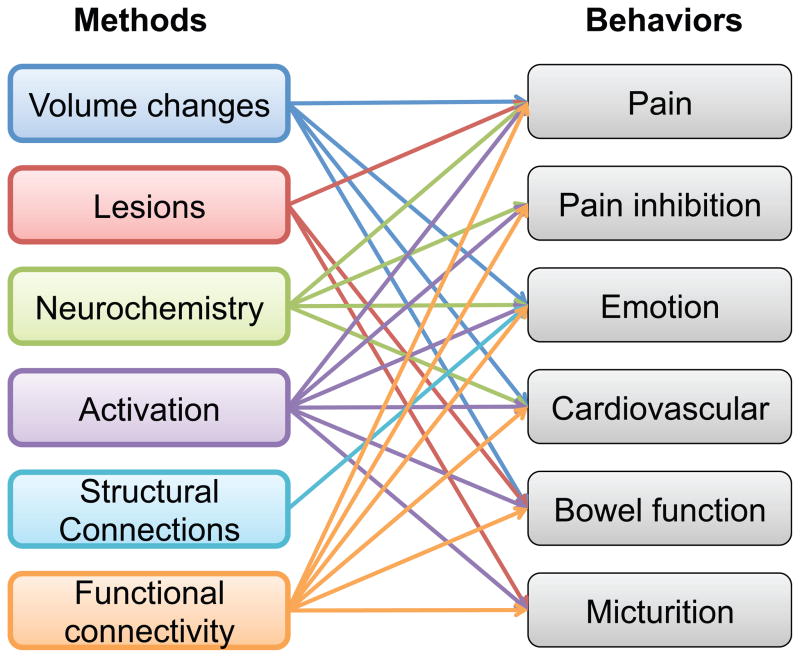
Figure 1. Methods and behaviors in review.
Arrows indicate that a method (for example volumetric studies) has demonstrated involvement in a behavior (i.e. pain, emotion, cardiovascular and bowel function).
The human PAG is about 14mm long and 4–5mm wide and consists of poorly differentiated gray matter that encircles the mesencephalic aqueduct. It extends from near the posterior commissure rostrally to the level of the locus coeruleus caudally. Contrary to its name, it does not completely encircle the aqueduct, but is more like a celery stalk with the regions in the midline ventral to the aqueduct arranged into separate well-differentiated nuclei (Paxinos and Mai, 2004). The neurons of the PAG are formed between days E13 and E17 in the rat embryo (Altman and Bayer, 1981). No data is available in human development.
Forebrain projections to the PAG arise mainly from the prefrontal cortex, the insular cortex and the amygdala (Mantyh, 1982). Further, the PAG receives highly organized projections from the central nucleus of the amygdala and, in turn, has reciprocal connections with the central nucleus (Rizvi et al., 1991). The PAG also projects to the thalamus, hypothalamus, brainstem and deep layers of the spinal cord (Mantyh, 1983) with some somatotopic organization (Wiberg et al., 1987), but projections to cortical regions have not been identified.
There are no clear cytoarchitectonical boundaries within the PAG, and the nomenclature and definitions of different subregions are evolving. The current model of the mammalian PAG proposes an organization into four longitudinal columns parallel with the aqueduct (Carrive, 1993) (Figure 2). The four columns are the dorsomedial (dmPAG), dorsolateral (dlPAG), lateral (lPAG) and ventrolateral (vlPAG). All but the dlPAG project directly to the lower brainstem. Afferents from the medial prefrontal cortex project primarily in the dlPAG, dorsomedial cortex and cingulate cortex areas project mainly to the lPAG and orbital cortex afferents project primarily in the vlPAG (An et al., 1998). Central amygdala nucleus projections terminate in the dmPAG, lPAG and vlPAG, but not in the dlPAG. However, basolateral amygdala projections terminate in the dlPAG (Figure 3).
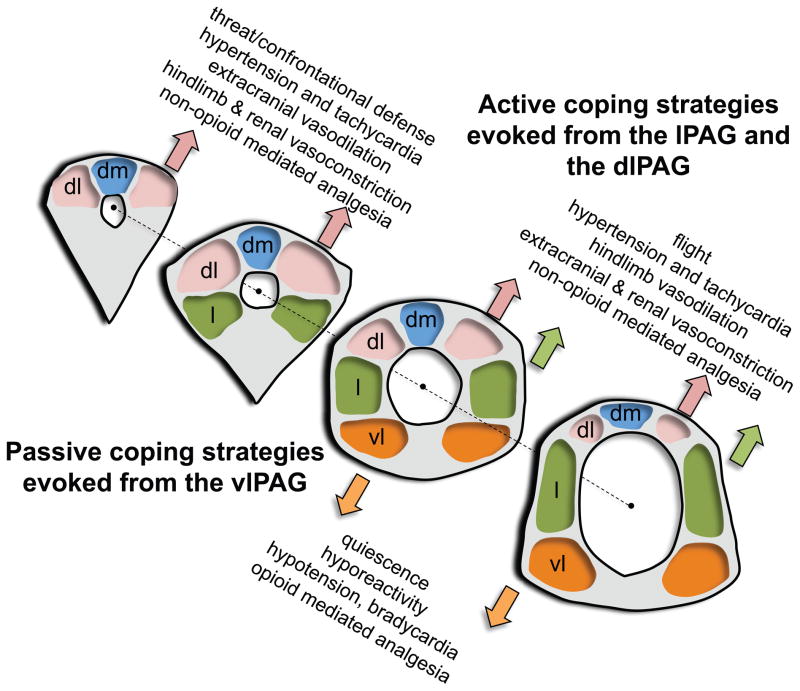
Figure 2.
Schematic illustration of the dorsomedial, dorsolateral, lateral and ventrolateral neuronal columns within (from left to right) the rostral periaqueductal gray (PAG), the intermediate PAG (two sections) and the caudal PAG. Injections of excitatory amino acids (EAA) within the dorsolateral (dlPAG)/lateral (lPAG; green) vs. ventrolateral (vlPAG; orange) columns evoke fundamentally opposite, active vs. passive emotional coping strategies. EAA injections made within the rostral portions of dlPAG and lPAG columns evoke a confrontational defensive reaction, tachycardia, and hypertension (associated with decreased blood flow to limbs and viscera and increased blood flow to extracranial vascular beds). EAA injections made within the caudal portions of the dlPAG and lPAG evoke flight, tachycardia and hypertension (associated with decreased blood flow to visceral and extracranial vascular beds and increased blood flow to limbs). In contrast, EAA injections made within the vlPAG evoke cessation of all spontaneous activity (quiescence), a decreased responsiveness to the environment (hyporeactivity), hypotension and bradycardia. A nonopioid-mediated vs. an opioid-mediated analgesia is evoked from the dlPAG/lPAG vs. vlPAG. Adapted from Bandler et al. (1994) Fig. 1 and Bandler et at. (2000), Fig. 1 with permission.
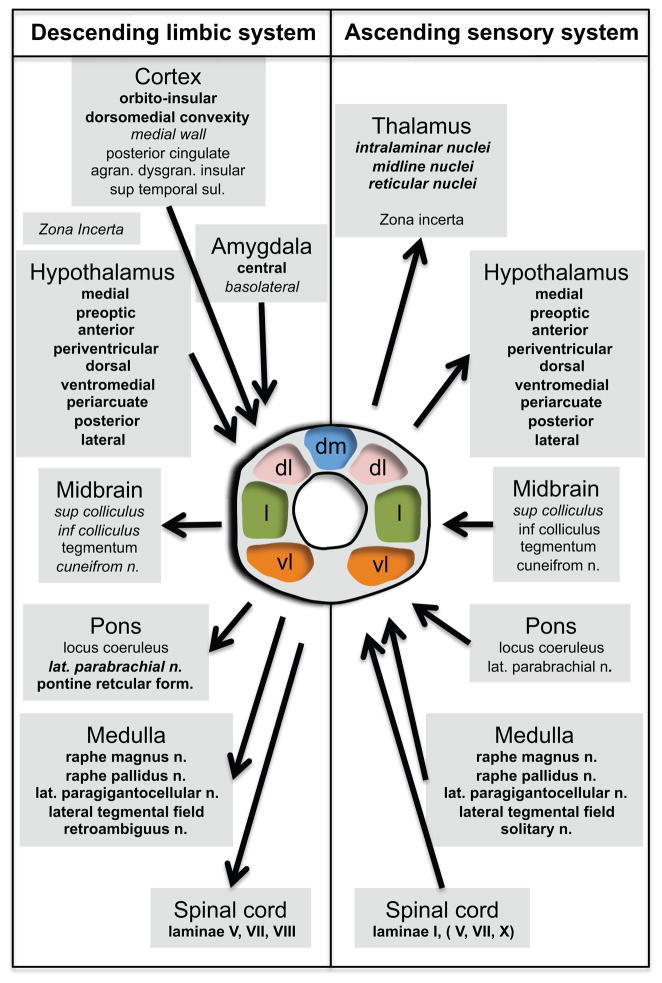
Figure 3. Anatomical Organization of the PAG.
Schematic overview of the organization of the PAG afferent and efferent connections. Represented on the left are the connections forming the descending limbic system and on the right are the connections forming the ascending sensory system. The two systems interact in the PAG. Structures indicated in bold are connected to either the dorsomedial, lateral or ventrolateral columns, or two of them or all of them. Structures indicated in italic are connected to the dorsolateral column. Structures indicated in bold and italic are connected to all four columns. The specific connections of the structures indicated in regular style have not been established. Adapted from (Paxinos and Mai, 2004), Figure 12.13 with permission.
A large body of evidence from animal studies indicates that the PAG is involved in control and expression of pain, analgesia, fear, anxiety, vocalization, lordosis and cardiovascular function (Behbehani, 1995; Paxinos and Mai, 2004). The lPAG appears to coordinate active defensive behaviors, non-opioid analgesia and has a hypertensive effect. The vlPAG appears to coordinate passive defensive behaviors, opioid analgesia and also has a hypotensive effect. In rats, lateral and dorsolateral PAG stimulation evokes active coping strategies such as fight/flight behaviors, hypertension, tachycardia and non-opioid mediated analgesia. Ventrolateral stimulation, on the other hand, evokes passive coping behaviors such as quiescence, hypotension, bradycardia and opioid mediated analgesia, see Bandler et al. (2000) and Behbehani (1995) for reviews, and An et al. (1998) for a detailed analysis of cortical projections to the PAG in the macaque.
The most convincing evidence for functional segregation within the human PAG comes from deep brain stimulation studies. Following Reynolds pivotal discovery that PAG stimulation could induce analgesia in rats (1969), DBS of the PAG area has been used in patients to ameliorate chronic pain since the late 1960’s (Hosobuchi et al., 1977; Nashold et al., 1969; Richardson and Akil, 1977a, b), see Bittar et al. (2005) for a review. Of note, dorsal PAG stimulation acutely elevates blood pressure, and ventral stimulation decreases blood pressure and increases high frequency heart rate variability (Green et al., 2006; Pereira et al., 2010), in line with the animal data.
Recent imaging advances have allowed for non-invasive measures of brain function. For large brain structures, particularly cortical regions, evaluation of functional-structural relationships is relatively straightforward. However, for subcortical and brainstem structures, there are challenges given the resolution of functional imaging in the millimeter range. It is therefore important to evaluate brain activations in smaller brain structures in a robust, reproducible and sensitive way. There are many reasons for this including: (i) interpreting data in the human condition; (ii) defining novel functions for a structure; (iii) understanding how the structure integrates with general brain function; (iv) and having the ability to infer or compare information across studies.
In a broader sense, the PAG can be seen as a model structure to evaluate the nature of the above four issues. We sought to highlight the multiple roles of the PAG, and we hypothesized that a similar pattern of functional segregation within the PAG previously observed in animals would emerge from reports of functional neuroimaging across behaviors in human studies. The review is presented in 3 sections; (1) Methods, defining the approach to evaluating data from the literature; criteria for including data; (2) Results, divided into subsections that include the number of reports that met our inclusion criteria and that were used in the evaluation of imaging methods on the PAG; and then summaries of data on structural, neurochemical, functional, and connectivity findings (Figure 1); and (3) Discussion of the state of the field, technical aspects of PAG neuroimaging and future directions.
2. Methods
2.1 Search Methods
Pubmed searches (http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed) using the criteria “fMRI and periaqueductal”, “Volume AND periaqueductal”, “MRI AND periaqueductal NOT fMRI”, “PET AND periaqueductal”, “structural AND periaqueductal”, “central gray AND MRI”, “central gray AND fMRI” “central gray AND PET”. Additional publications were identified through searches for “periaqueductal” and “PAG” on the Surface Management system database (http://sumsdb.wustl.edu/sums/index.jsp) and the neurosynth database (http://neurosynth.org); through references in the identified papers; Science Direct searches and searches on publications by well-known research groups.
2.2 Selection Criteria and Data Extraction
For the functional neuroimaging studies, for inclusion, manuscripts needed to make explicit mention of the PAG in the results section, or discuss mesencephalon findings in terms of the PAG. Unfortunately, this criterion resulted in excluding papers that describe activations more conservatively (i.e. calling the midbrain clusters just that). However, voxel based morphometry (VBM) studies that found midbrain changes in clusters encompassing the PAG were included. Single subject reports were excluded. Manuscripts on neuroimaging findings in Wernicke’s encephalopathy (Cerase et al., 2011), tumors (Rilliet et al., 1990; Steinbok and Boyd, 1987) and magnetic resonance spectroscopy studies of the PAG are beyond the scope of this review.
From each manuscript, we identified study population, gender distribution, methods, coordinates of regions reported as PAG and contrast that identified PAG alterations. Coordinates reported in Talairach space (Talairach and Tournoux, 1988) were converted to MNI space using the Brett tal2mni algorithm described at http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/tal2mni.m. Activation likelihood estimates were calculated using GingerALE 2.0 (Eickhoff et al., 2009; Turkeltaub et al., 2011)
3. Results
The results are divided into 5 sections (1) General considerations, (2) Structural Alterations in the PAG; (3) Neurochemical Alterations of the PAG; (4) Functional Activation of the PAG; and (5) Connectivity of the PAG.
3.1 General Considerations
From our initial searches, we identified a total of 194 manuscripts, whereof 89 reported PAG coordinates in MNI space, 43 in Talairach space, and six that allowed for localization through figures. An additional 56 manuscripts, which did not report standard coordinates of the PAG, were also included in the qualitative overview. Of the 194 manuscripts, 107 studies employed functional magnetic resonance imaging (fMRI), 39 studies measured regional cerebral blood flow (rCBF) with PET, 11 studies used receptor ligand PET, 20 studies used voxel-based morphomety (VBM), 12 studies used diffusion weighted imaging (DWI), and 5 studies measured MRI signal intensity.
Study Population
A total of 6617 subjects were included, of these 2377 were patient/clinical studies. The average study population (± standard deviation) was 34 (±81) subjects, with a median study population of 18 subjects. The smallest included study (Hsieh et al., 1996) had 4 subjects, the largest study (Tomasi and Volkow, 2011) had 979 subjects. Within the clinical studies, the median patient population was 15 subjects and the median control group was 12 subjects. The overall gender distribution was close to 50:50 (48% male subjects); thus there were no significant differences in the number (or ratio) of men and women studied in the healthy or the clinical populations.
PAG Coordinate Distribution
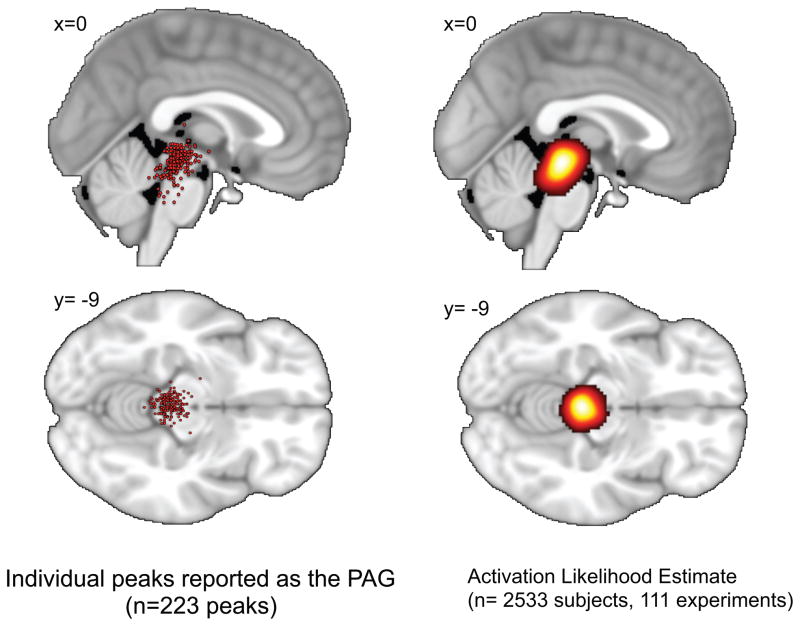
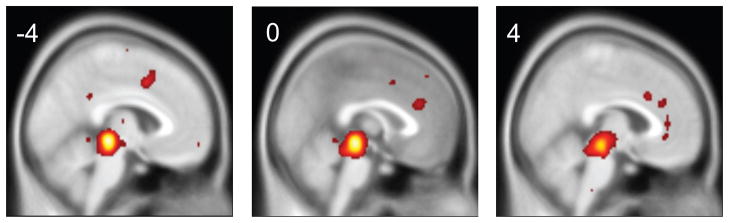
225 coordinates labeled as the PAG in the manuscripts were identified: 100 were on the left, 100 on the right and 25 at the midline. The average (±SD) MNI coordinates reported as the PAG were x=−4 (left) or 4 (right) (±3mm), y=−29 (±5), z=−12 (±7). The reported coordinates ranged 34 mm in the left-right (x) direction, 35 mm in the anterior-posterior (y) direction, and 46 mm in the dorsal-caudal (z) direction. The peak activation likelihood estimate fell at MNI x= 1, y=−29, z=−12. The distribution of coordinates is illustrated in Figure 4 in terms of cluster peaks and in terms of activation likelihood estimates. There were no significant differences in coordinates described in MNI space and coordinates described in Talairach space after transformation into MNI space through the Brett tal2mni transform algorithm.
Figure 4. Functional Activations in the PAG across Studies.
Regions reported as the PAG. Red dots represent individual peaks projected to the sagittal and axial plane. The activation likelihood estimate for all included studies is illustrated on the right.
3.2 Structural Alterations of the PAG
Volumetric studies
Voxel based morphometry (VBM) involves a statistical voxel-wise test of the local concentration of gray matter, usually identified through T1 contrast structural MRIs (Ashburner and Friston, 2000). The method relies on precise between subject brain volume normalization. As white matter bundles surround the PAG, it is identifiable on 1×1×1 mm structural MRIs despite its smallness. Higher PAG VBM signal, suggestive of higher gray matter volume, has been reported in a few clinical conditions: patients with primary dysmenorrhea relative to controls (Tu et al., 2010), migraine relative to controls and migraine without aura relative to migraine with aura (Rocca et al., 2006), in patients with Tourette’s syndrome compared to healthy controls (Garraux et al., 2006), and, at non-significant levels, in patients with panic disorder (Protopopescu et al., 2006). A positive correlation between PAG VBM signal and creativity has also been reported (Takeuchi et al., 2010).
Reduction in PAG VBM signal has been found in a wide range of clinical conditions: irritable bowel syndrome (independent of anxiety and depression) (Seminowicz et al., 2010), heart failure patients (Woo et al., 2003) and frontotemporal dementia (Boccardi et al., 2005). More general midbrain gray matter volume decreases are present in both Alzheimer and in dementia with Lewy bodies (Whitwell et al., 2007). In patients with Alzheimer’s disease, pathological changes and the presence of β-amyloid peptide and abnormally phosphorylated tau protein have been found in the PAG (Iseki et al., 1989; Parvizi et al., 2000), and gray matter alterations in the midbrain have been related to amyloid beta levels in healthy elderly (Glodzik et al., 2011). Midbrain volume decreases have also been reported for neurocardiogenic syncope (simple fainting) (Beacher et al., 2009), narcolepsy (Kim et al., 2009b), chronic fatigue syndrome in relation to blood pressure (Barnden et al., 2011), major depression (Lee et al., 2011), late onset depression with history of suicide attempts (Hwang et al., 2010), severe Huntington disease (Ruocco et al., 2008), pre-clinical subjects carrying the Huntington disease gene mutation (Thieben et al., 2002), postanoxic persistent vegetative state (Juengling et al., 2005), progressive supranuclear palsy (Price et al., 2004) and spinocerebellar ataxias (Schulz et al., 2010). Patients with multiple system atrophy have decreases in PAG volume (Kassubek et al., 2007) and R2 relaxation rate, indicative of increased water content and tissue atrophy (Minnerop et al., 2007). Patients with Kennedy disease (X-linked spinobulbar muscular atrophy) also have decreased white matter signal in the PAG region (Kassubek et al., 2007).
Lesions of the PAG
Lesions of the PAG have most systematically been studied in multiple sclerosis. In a group of 277 multiple sclerosis patients, lesions in the PAG were associated with a four-fold increase in migraine-like headaches, a 2.5-fold increase in tension-type headaches and a 2.7-fold increase in combination of migraine and tension-type headaches (Gee et al., 2005). In another large multiple sclerosis study with 452 patients (Charil et al., 2003), PAG lesions were associated with bowel and bladder dysfunction, but not with sensory function on the Kurtzke Functional Systems Scores.
Diffusion weighted imaging of the PAG
Diffusion weighted imaging (DWI) is a procedure that can measure the directional diffusivity of water molecules. Several DTI based indices are used, including apparent diffusion coefficient (ADC), fractional anisotropy (FA, describing the degree of anisotropy of diffusion), axial diffusivity (a measure of diffusivity parallel to axons) and radial diffusivity (a measure of diffusivity perpendicular to fibers) (Song et al., 2002). These measures have been used to indicate white matter abnormalities in patient populations.
Interictal migraine patients without aura have lower fractional anisotropy in the PAG (DaSilva et al., 2007). Patients with idiopathic rapid eye movement sleep behavior disorder have reduced fractional anisotropy and mean diffusivity in the PAG. Patients with traumatic brain injury and in the vegetative state have lower ADC, FA and radial diffusivity in the midbrain (Newcombe et al., 2010). When comparing traumatic brain injury patients that have recovered with patients with persistent symptoms, DTI demonstrated higher FA and lower ADC in symptomatics than in asymptomatics in the midbrain (Hartikainen et al., 2010). Compared to healthy controls, patients with complete cervical spinal cord injury have lower midbrain FA and higher midbrain mean diffusivity, indicative of retrograde Wallerian degeneration (Guleria et al., 2008).
Children with congenital central hypoventilation syndrome show increased axial diffusivity in the PAG (Kumar et al., 2008), possibly indicative of inadequate development or processes secondary to hypoxia, leading to lower axonal density or caliber. In children with diabetic ketoacidosis, ADC of the PAG was found to be elevated during treatment and reduced post recovery.
For white matter tractography studies implicating the PAG, see section Connectivity of the PAG-Diffusion tensor imaging below.
3.3 Neurochemical Alterations of the PAG
The PAG, like other periventricular structures, contains a number of neurotransmitter and neuromodulator systems (Paxinos and Mai, 2004). Of these, the opioidergic system and its role in analgesia have been most extensively studied in human neuroimaging. PET studies can demonstrate ligand receptor distribution and competition with endogenous factors, while pharmacological MRI (phMRI) studies may produce direct effects and/or secondary effects on PAG activation mediated through other circuits. A third approach has been to study long-term effects of drugs on function and structure.
The Opioid system
phMRI and PET studies of opioidergic effects would be expected to produce PAG responses given that the PAG contains high levels of opioid receptors and peptides, and that opioids when injected in small amounts into the PAG in animal studies produce profound analgesia (Yaksh and Rudy, 1978). This is largely the case across methods and patient populations, with several studies furthering our knowledge of the human condition by using placebo protocols and relating opioid related signal to subjective experiences.
PET studies
Zubieta et al. (2001) found PAG 11C-carfentanil binding change negatively correlated to pain sensations induced by hypertonic saline injections, suggesting endogenous opioid release. In a similar study (Zubieta et al., 2005), a significant reduction of 11C-carfentanil binding in response to pain was observed in the PAG, but with no effect of placebo administration. Placebo effects on PAG μ-opioid binding were however reported in a subsequent study using similar methods (Scott et al., 2008), and the change in receptor occupancy was positively correlated with subject’s analgesic expectations. A study employing heat pain and topical placebo demonstrated pain specific opioid activation of the PAG, which was also correlated with self-reports of placebo analgesia (Wager et al., 2007). Moreover, anticipatory opioid activation was also observed in the PAG in this study
Patients with central neuropathic pain have significantly lower 11C diprenorphine binding in PAG regions contralateral to their pain, an effect not seen in peripheral neuropathic pain (Maarrawi et al., 2007a). In patients with chronic intractable central neuropathic pain, two months of chronic motor cortex stimulation (Maarrawi et al., 2007b) led to significantly reduced 11C diprenorphine binding in the PAG, and this change was correlated to pain reduction. In contrast to these pain studies, Prossin et al. (Prossin et al., 2010) found that sustained sadness led to increased PAG 11C-carfentanil binding in patients with borderline personality disorder, but with no significant differences in the PAG when compared to healthy subjects.
phMRI studies
In a pharmacological MRI study, Becerra et al. (2006a) found that administration of a small dose of morphine (4mg/70kg) led to significant negative BOLD signal change in the PAG. A study on the effects of remifentanil on control of respiration (Pattinson et al., 2009) found that PAG activation by volitional breath holding was significantly reduced by the μ receptor agonist remifentanil. This study is important as it employed a midbrain/brainstem specific imaging sequence and controlled for confounding factors such as movement, end-tidal CO2 levels, and drug induced changes in cerebral blood flow.
A series of recent fMRI studies have used the opioid receptor competitive antagonist naloxone to investigate the effects of opioid blockage on BOLD signal. Borras et al. (2004) found that naloxone administration (compared to saline) lead to sub-threshold activation of the PAG, but found no effects of pain+naloxone in the BOLD signal of the PAG. Eippert et al. (2008a) investigated the effects of naloxone on fear conditioning, and found that blocking endogenous opioid neurotransmission with naloxone led to more sustained responses to the unconditioned stimulus across trials, less fear-induced PAG activation, and a lower functional connectivity between the PAG and the rostral ventromedial medulla. Interestingly, there were no effects of naloxone on PAG signal to the unconditioned, painful stimulus. A subsequent study (Schoell et al., 2010) also found PAG activation to painful stimulus, but no effect of naloxone.
The effects of naloxone administration on topical placebo analgesia have also been investigated with fMRI (Eippert et al., 2009). Placebo + pain led to significantly higher PAG activations as compared to pain alone, and these activations were significantly reduced by naloxone. Moreover, PAG activation magnitude was positively correlated to pain ratings, and this correlation was reduced by naloxone. The functional connectivity between the PAG and the rostral anterior cingulate was higher during placebo + pain than during pain alone, and this relationship was abolished by naloxone.
Interestingly, similar effects to those noted above have been reported in a study employing heterotopic noxious conditioning stimulation (Sprenger et al., 2011) to induce pain inhibition (i.e., “one pain inhibits another pain”). Subjects were exposed to phasic heat pain in combination with a cold pressor task (i.e. cooling of the leg with icebags). Heat pain without cold pressor pain activated the PAG, and while there were no significant effects of cold pressor on the PAG activation compared to control conditions without naloxone. Thus, administration of naloxone significantly reduced the difference in PAG activity during the two conditions, suggesting an opioidergic PAG involvement in endogenous analgesia during heterotopic noxious conditioning stimulation. Moreover, the cold pressor task led to a significant increase in functional connectivity between the PAG and the subgenual anterior cingulate, and this connectivity change was abolished by naloxone. Naloxone did not abolish the “pain inhibited by other pain” effect on pain ratings, thus suggesting that not only endogenous opioids, but also factors such as attention and distraction may be important in endogenous analgesia.
Another line of evidence comes from opioid dependent subjects, who have decreased resting functional connectivity between the centromedial amygdala and the PAG, reductions that were positively correlated with the duration of prescription opioid dependence (Upadhyay et al., 2010).
Other neurochemical alterations
Aside from opioid receptors and peptides present in the structure, prior studies in animals and in vitro studies in humans have provided insights into the chemical anatomy of the PAG, implicating the monoamines, neuropeptides and simple gases. Human neuroimaging studies have contributed and confirmed some of the animal literature, and some of the human findings await further detailing in animal models.
Higher iron levels in the PAG have been reported in patients with episodic migraine with and without aura and in patients with chronic daily headache as compared to healthy controls (Welch et al., 2001). PAG iron levels were further correlated to the duration of the disorder.
Linnman and colleagues found that neurokinin 1 receptor availability in the PAG, as measured by 11C-GR205171 PET, is reduced in chronic whiplash patients as compared to healthy controls (Linnman et al., 2010). This may reflect alterations in receptor density, altered endogenous levels of substance P, or both, as has been reported in the animal literature (Duric and McCarson, 2007).
PAG serotonin transporter binding levels, as measured by 11C-DASB, are elevated in major unipolar depression compared to both bipolar depression and healthy controls (Cannon et al., 2007). PAG activation in irritable bowel syndrome is reported to be reduced a 5-HT3 receptor antagonist, Alosetron, (Berman et al., 2002) and significantly increased PAG 5-HT1 A and 5-HT2 A receptor immunoreactivity has been demonstrated in sudden infant death syndrome (Ozawa and Okado, 2002).
Kumakura et al (2010) demonstrated elevated 18F-DOPA utilization in the PAG in early Parkinson patients as compared to age matched healthy controls.
In a study of the analgesic gabapentin, the drug led to reduced activation of the midbrain (not specific to PAG) during central sensitization following a capsaicin model of secondary hyperalgesia (Iannetti et al., 2005).
3.4 Functional Activation of the PAG
3.4.1. Pain-Induced Activation
The earliest studies on hemodynamic correlates of pain using 133Xe (Lassen et al., 1978), PET (Jones et al., 1991; Talbot et al., 1991) and fMRI (Davis et al., 1995) lacked the spatial resolution to identify the PAG unequivocally, yet pain induced regional cerebral blood flow increases in the dorsal midbrain were reported in several studies, with different pain modalities including noxious heat (Casey et al., 1994), angina pectoris (Rosen et al., 1994), intracutaneous ethanol injection (Hsieh et al., 1996) capsaicin injection (Iadarola et al., 1998), and cold pressor test (Petrovic et al., 2000). Since the early days, imaging methods and experimental refinement have improved enormously. We identified 54 studies that reported specific PAG activation to pain. The main choice of stimulation was heat pain (25 studies (Becerra et al., 2001; Becerra et al., 1999; Bingel et al., 2007; Bingel et al., 2011; Cahill and Stroman, 2011a; Casey et al., 1994; Derbyshire et al., 2002; Derbyshire et al., 1994; Derbyshire and Osborn, 2009; Eippert et al., 2008b; Eippert et al., 2009; Fairhurst et al., 2007; Helmchen et al., 2008; Kong et al., 2008b; Kong et al., 2009; Kong et al., 2010a; Salomons et al., 2004a; Salomons et al., 2007; Schoell et al., 2010; Strigo et al., 2008; Tracey et al., 2002b; Valet et al., 2004; Villemure and Bushnell, 2009; von Leupoldt et al., 2009a; von Leupoldt et al., 2009b; Yelle et al., 2009a)) followed by electrical (6 studies (Dunckley et al., 2005a; Freund et al., 2011; Gray et al., 2009; Niddam et al., 2007; Piche et al., 2009; Seminowicz and Davis, 2007)), brushing on allodynia regions (5 studies (Iadarola et al., 1998; Lebel et al., 2008; Mainero et al., 2007; Moisset et al., 2011; Petrovic et al., 1999)) rectal distention (4 studies (Mayer et al., 2005; Naliboff et al., 2003; Rosenberger et al., 2009b; Wilder-Smith et al., 2004)), von Frey stimulation (4 studies (Ghazni et al., 2010; Gwilym et al., 2009; Lee et al., 2008; Zambreanu et al., 2005)), various cold pain (Mochizuki et al., 2003; Mohr et al., 2008; Petrovic et al., 2000), chemical pain (Hsieh et al., 1996; Iadarola et al., 1998), laser stimulation (Helmchen et al., 2008; Mobascher et al., 2010), gastric pain (Ladabaum et al., 2001), pressure pain (Giesecke et al., 2006) painful sound (Lamm et al., 2007) and spontaneous pain in fibromyalgia (Napadow et al., 2010) and migraine (Cao et al., 2002).
Dysfunction of modulatory processing has been postulated in clinical conditions. Specifically, either altered inhibition or increased facilitation of modulatory circuits including the PAG are postulated to contribute to the chronic pain state (Apkarian et al., 2009). Accordingly, alterations in pain induced PAG reactivity has been reported for a number of clinical conditions.
Lower back pain
With heat pain and H215O PET, patients with non-specific chronic lower back pain and healthy controls both showed a positive correlation between PAG rCBF and subjective pain ratings, with no group differences in PAG activation (Derbyshire et al., 2002). Using fMRI and a thumb pressure probe, equally painful pressure stimulation resulted in a significantly lower increase of PAG activation in the LBP patients, suggesting dysfunctional PAG inhibitory systems as a possible pathogenic mechanism in chronic low back pain (Giesecke et al., 2006).
Neuropathic pain
Patients with mononeuropathy and dynamic mechanical allodynia in the lower extremity were studied with brush evoked rCBF, measured with [15O]butanol PET, during stimulation of the allodynic and contralateral homologous region. Brush stimuli to the allodynic region activated the PAG significantly more than the control region (Petrovic et al., 1999). Patients with classical (idiopathic) trigeminal neuralgia located within the maxillary and/or mandibular branch (V2 V3) of the trigeminal nerve were studied with fMRI. Brush stimulation evoked pain in 50% of the patients, and associated PAG activation, suggesting “compensatory mechanisms and reflect abnormal (i.e. ineffective) overactivation of inhibitory processes and/or correspond to pain facilitatory processes.” (Moisset et al., 2011). Similarly, both brush and cold stimulation evoked PAG responses in chronic neuropathic pain involving the maxillary region (V2) of the trigeminal nerve. Notably, for cold stimulation, activation in the PAG was increased in the more rostral portion and decreased in a more caudal location (Becerra et al., 2006b).
Complex regional pain syndrome
In children with complex regional pain syndrome (CRPS), brush stimuli of the affected limb led to PAG activation, but brushing the unaffected limb led to PAG deactivation (Lebel et al., 2008). Of note, this difference persisted even after resolution of the CRPS. In adults with CRPS, the early phase of pain stimulation led to higher PAG activation in controls compared to patients, regardless of stimulating the affected or unaffected region (Freund et al., 2011).
Other pain conditions
PAG activation has been reported in coronary artery disease patients after angina pectoris induction (Rosen et al., 1994), in patients with atypical facial pain (similar to controls) (Derbyshire et al., 1994), and in a subset of patients with visually triggered migraine (Cao et al., 2002). In myofacial pain patients, low-intensity low-frequency electrostimulation treatment of myofacial trigger points led to increased PAG activation to electrical pain in treatment responders (Niddam et al., 2007). In osteoarthritis patients and controls, Von Frey punctate stimulation to referred pain areas led to significantly higher PAG activation in patients (Gwilym et al., 2009). In fibromyalgia patients, greater spontaneous pain led to less functional connectivity between the PAG and the executive attention network (Napadow et al., 2010).
Irritable bowel syndrome
See section Bowel function and the PAG below.
Pain-induced PAG activation in other clinical groups
In contrast to healthy subjects, clinically depressed patients display an almost complete absence of PAG activation to pain (Strigo et al., 2008). Patients with asthma, compared to healthy controls, displayed higher PAG activation to both pain and dyspnea (von Leupoldt et al., 2009b). Alzheimer’s patients show a trend towards elevated PAG activity to pressure pain (Cole et al., 2006).
Pain modulation in the PAG
Descending control of pain involves a large number of structures and neurochemical systems, of which the PAG plays a pivotal role (Millan, 2002). In line with the putative role in pain modulation, several neuroimaging studies have identified roles for the PAG in pain modulation including through habituation, attention, placebo and acupuncture.
Habituation
Pain and pain-related brain activity do not remain constant when a subject is repeatedly exposed to a painful stimulus. Instead, processes like habituation and/or sensitization modify the painful experience. A study on rapid habituation to laser evoked (Mobascher et al., 2010) found that subjects with a faster habituation of electrodermal responses to pain showed larger PAG response. In a study on heat pain habituation over several days (Bingel et al., 2007), pain stimuli led to PAG activation, but while pain ratings decreased and pain thresholds increased over time, no changes over time were observed for the PAG response. These findings are of importance as neuroimaging protocols routinely group several repeated activations in order to increase signal to noise ratio, often without accounting for temporal effects.
A potent analgesia is evoked by slight incremental decreases in noxious stimulus temperatures (Grill and Coghill, 2002). This phenomenon, called offset analgesia, also engages the PAG (Derbyshire and Osborn, 2009; Yelle et al., 2009b).
Attention and Distraction
The effect of attention on pain processing was investigated by having subjects either receive pain passively, explicitly attend to the pain or attend to an auditory stimulus (Peyron et al., 1999). Midbrain activations were found in both attention and distraction conditions. Pain modulation during parallel cognitive processing was investigated by combining a cold pressor task with a distracting cognitive task. The PAG was activated by the cold pressor task, and there was an interaction between the cognitive task and pain, indicating less pain specific PAG increase during the cognitive task. Of note, the absolute PAG rCBF values were highest during the cognitive task in combination with non-painful cold stimulation (Petrovic et al., 2000).
Tracey et al. (2002a) used high field strength (4 Tesla) fMRI and a midbrain/brainstem specific sequence to investigate the effects of attention to/distraction from a painful stimulus. Subjects were instructed to either “pay full attention to the stimulus” or “to think of something else and not attend”. Not attending led to significantly lower intensity and averseness ratings, and a significantly higher BOLD signal in the PAG. Increased PAG signal was also related to decreased pain intensity, suggesting that distraction led to descending pain inhibition. These findings have been confirmed using heat pain and the Stroop task as the distractor, where distraction led to PAG activation and significantly reduced pain unpleasantness and intensity ratings (Valet et al., 2004). Functional connectivity analyses further revealed a higher connectivity between the genual anterior cingulate cortex and the PAG specific to the pain and distraction condition.
However, no effects on PAG activation to pain were observed using a multisource interference task (Seminowicz and Davis, 2007). A study using pleasant and aversive odors (to influence mood) in combination with instructions to attend to pain stimuli indicated that while neither mood nor attention had a main effect on PAG signal, the functional connectivity between the anterior cingulate and the PAG was modulated by mood (Villemure and Bushnell, 2009).
Higher PAG activation in healthy subjects than in patients with complex regional pain syndrome has been observed during tonic painful stimulation and instructions to “distract yourself from the feeling of pain by thinking of a nice holiday or by imagining that the finger is far away from you.” (Freund et al., 2011) Of note, individuals with CRPS were able to suppress the feeling of pain with similar efficiency as healthy individuals during constant maximal pain stimulation.
Placebo
Placebo and opioid analgesia both are associated with increased activity in the rostral anterior cingulate, and this activation covaries with midbrain/PAG activation (Petrovic et al., 2002). Placebo can also lead to greater PAG activity in the anticipation of pain, but not during the actual pain experience, and the PAG covaried with the dorsolateral prefrontal cortex in the placebo condition (Wager et al., 2004). Similarly, the PAG activates to heat pain, but not more so during placebo (Bingel et al., 2006). However, psychophysiological connectivity analyses revealed that placebo increased rostral ACC to PAG connectivity. This latter finding has been replicated, and is also abolished by naloxone, indicating an opioidergic component. Moreover, using midbrain/brainstem specific imaging, an increased PAG pain response has been demonstrated (Eippert et al., 2009).
Sham acupuncture and expectation/conditioning manipulation model has been used to investigate the neural substrates of nocebo hyperalgesia. While pain led to PAG activation, and nocebo led to higher pain ratings, no effects of nocebo were observed in the PAG (Kong et al., 2008a). Placebo modulation is also reviewed under section Neurochemical alterations of the PAG and section State specific connectivity.
Acupuncture
Despite the lack of a demonstrable and irrefutable analgesic effect, acupuncture may recruit PAG activation that may relate to endogenous modulatory processes. A handful of neuroimaging studies on acupuncture indicate PAG involvement. While the clinical efficacy is still under debate, the procedure (with or without sham needles (Moffet, 2009; White et al., 2001)) has been shown to involve the PAG across a handful of studies.
Acupuncture with deep needling led to higher PAG rCBF than did a rest condition, but with no statistical differences in the PAG when comparing deep to shallow needling, or needling in a non-acupuncture point (Hsieh et al., 2001). Acupuncture evokes PAG BOLD signal in the awake state, but not under propofol general anesthesia (Wang et al., 2007) suggesting that active cognitive processes including placebo/expectation may be at play. Using cardiac gated midbrain/brainstem specific fMRI, verum electroacupuncture induced deactivation in the caudal PAG and activation in the rostral ventrolateral PAG, which was greater for verum compared to sham electroacupuncture (Napadow et al., 2009). Other studies have found transient and sustained PAG activation to various acupuncture protocols (Bai et al., 2009; Bai et al., 2010; Liu et al., 2004; Zhang et al., 2007; Zhang et al., 2009). Also acupuncture-induced connectivity changes have been reported with increased PAG connectivity to various regions including the default mode network (Dhond et al., 2008), amygdala (Qin et al., 2008) and posterior cingulate (Zyloney et al., 2010).
Other mechanisms
In a PET study, hypnotic suggestion, be it to increase or decrease pain unpleasantness, as compared to hypnotic relaxation, led to higher rCBF in the red nucleus, adjacent to the PAG (Rainville et al., 1999). The perceived control over pain, while the stimulus itself was held constant, has also been studied (Salomons et al., 2004b). Uncontrollable pain led to higher PAG activations, and in a follow-up analysis, the difference between PAG activation to uncontrollable versus controllable pain was positively correlated to the difference in pain ratings between uncontrollable versus controllable pain (Salomons et al., 2007).
Pain and Emotional Activation of the PAG
In line with animal studies on the PAG as a part of the defensive behavior systems (Fanselow, 1994), and human stimulation studies (Nashold et al., 1969) evoking strong emotions, several human neuroimaging studies have delineated the role of the PAG in emotion processing. Damasio et al. (2000) found that self generated feelings of sadness, anger, happiness and fear all led to midbrain activation. Subsequent studies have largely focused on fear and stress, although there are some investigations on positive emotions as noted below.
Pain Anticipation
Anticipating pain can lead to PAG activation. Using fear-conditioning paradigms, several groups have reported midbrain activation (in the vicinity of the PAG) to conditioned cues (Fischer et al., 2000; Linnman et al., 2011; Yaguez et al., 2005) that are predictive of an aversive unconditioned stimulus. Late in the cue phase, deactivations of the PAG, which are blocked by naloxone, have also been reported (Eippert et al., 2008a). Patients with spinal cord injury display an elevated PAG response to conditioned cues, possibly due to diminished afferent spinal information flow or a consequence of psychological and emotional adjustment (Nicotra et al., 2006).
Both implicit (Hasler et al., 2007) and explicit (Hsieh et al., 1999) instructions that pain may be delivered or is about to be delivered (Drabant et al., 2011; Fairhurst et al., 2007) lead to PAG activation. Such anticipatory PAG activation influences the subsequent pain activation in the posterior insula (Fairhurst et al., 2007), and the connectivity between the PAG and the anterior insula prior to a stimulus delivery determines if the stimulus is perceived as painful or not (Ploner et al., 2010). Anticipatory PAG activation is also enhanced by placebo administration (Wager et al., 2004).
Observing Pain
Observing aversive images (physical assaults, poor children abandoned in the streets, war scenes, body lesions, dangerous animals, body products etc.) leads to PAG activation (Moll et al., 2002; Petrovic et al., 2005). In one of the few studies on gender differences in the PAG, reactivity to aversive images was comparable between men and women in the early follicular menstrual stage, but significantly lowered in the late follicular-midcycle menstrual phase (Goldstein et al., 2005).
Observing others in pain may be a subclass of emotional stimuli and leads to an empathetic response that engages emotional pain regions (Singer et al., 2004) including the PAG (Lamm and Decety, 2008). Empathetic PAG responses to observing pain in others have also been reported in healthy children (Decety et al., 2008), and in adolescents with and without aggressive conduct disorder (Decety et al., 2009). In a further dissection of the PAG empathy response, the effect of knowledge of the person you sympathize with (Decety et al., 2010), responsibility and social stigma (Lamm et al., 2010) have been evaluated. Theses studies are of particular interest as they demonstrate that empathetic responding, including differential PAG recruitment, is influenced by cognitive knowledge about the person you empathize with. Also when observing sad facial expression in others, the PAG is activated. Observing others when in a compassionate state of mind leads to higher PAG responses, both to neutral and to sad facial expressions (Kim et al., 2009a).
Fear
Electrical stimulation of the PAG in humans has been reported to induce extreme fear (Nashold et al., 1969), discomfort, distress, anxiety and weeping (Tasker, 1982). In line with this, the more proximal a tarantula spider is to a subject’s foot, the more active the PAG (Mobbs et al., 2010); this activation correlates to individual variations in the fear of spiders. Similarly, the more proximal a “virtual predator” is, the more active the PAG, and the dread of being captured is positively correlated to PAG signal (Mobbs et al., 2007). See also Pain anticipation.
Positive Affect
PAG activation has been reported by reading pleasant words (Maddock et al., 2003), in mothers who view their own infants (Noriuchi et al., 2008), and in feeling unconditional love towards individuals with intellectual disabilities (Beauregard et al., 2009). The PAG activates also by listening to music that elicits the highly pleasurable experience of “shivers-down-the-spine” (Blood and Zatorre, 2001). Also sexual affect involves the PAG. When experiencing orgasm, men display significantly higher PAG rCBF than women (Georgiadis et al., 2009).
Other Emotions
The disappointment of making a bad gamble activates the PAG (Canessa et al., 2009; Coricelli et al., 2005), and activity in the PAG tracks gambling outcomes associated with reducing risk seeking (Canessa et al., 2011). Social rejection (i.e. being excluded from participation in a boll tossing game) leads to PAG activation. Additionally, the magnitude of activation is correlated to feeling greater social distress during daily real world social interactions (Eisenberger et al., 2007). Also aversive sounds, such as nails scratching a blackboard, activate the PAG (Zald and Pardo, 2002).
Psychiatric populations
Midbrain activation is related to anxiety during symptom provocation in obsessive-compulsive disorder, simple phobia, and posttraumatic stress disorder (PTSD) (Pissiota et al., 2002; Rauch et al., 1997). Patients with pedophilia display a deactivation of the PAG in response to erotic pictures of adults (Walter et al., 2007).
3.4.2. Homeostatic and Physiological Processes
The PAG plays a pivotal role in the integration of emotional aspects of homeostatic regulation via the autonomic nervous system. A handful of human neuroimaging studies have directly addressed this.
Cardiovascular regulation
Angina pectoris, induced by the beta-adrenergic receptor agonist dobutamine, led to increased PAG blood flow (Rosen et al., 1994). Subsequent studies, where dobutamine was used only to induce an increase in mean arterial blood pressure, and not pain, found no effects in the PAG (Liu et al., 2006).
In an elegant study on cardiovascular and gustatory midbrain sites, Topolovec et al. (2004) demonstrated that maximal inspiration and the Valsalva maneuver (a moderately forceful attempted exhalation against a closed airway) both led to increased PAG BOLD signal along with increases in mean arterial pressure and heart rate. Isometric hand gripping also led to increases in mean arterial pressure and heart rate, but no PAG activation. In another sophisticated study using cardiac gating and a midbrain/brainstem specific scan sequence, Napadow et al. (2008) studied grip induced increases in heart rate, and associated variance in high frequency (HF) heart rate variability power. The change in HF power, reflective of parasympathetic modulation, was negatively correlated to PAG BOLD fluctuations. In a PET study, HF heart rate variability positively correlated with emotion specific PAG blood flow (Lane et al., 2009).
Stressful cognitive tasks increase PAG activation and mean arterial pressure (Gianaros et al., 2005), suppress baroreflex sensitivity (Gianaros et al., 2011), and alter connectivity to the anterior insula. The threat of social evaluation induces anxiety and increases heart rate and PAG activity (Wager et al., 2009). Moreover, the relationship between the ventromedial prefrontal cortex and heart rate increases is mediated by the PAG, but not the relationship between the cingulate and heart rate increases.
Respiratory Function
Animal studies indicate that the PAG exerts a strong influence on respiration, and it has been suggested that the PAG serves as the behavioral modulator of breathing (Subramanian et al., 2008). In humans, breathing carbon dioxide enriched air leads to increased rCBF in the midbrain including the PAG, and also in the amygdala and the basal ganglia, but reduced rCBF in the cingulate and frontal gyri (Brannan et al., 2001). While under the influence of remifentanil (a potent ultra short-acting synthetic opioid) the PAG response to volitional breath holding is significantly reduced (Pattinson et al., 2009). During severe dyspnea, induced by breathing through inspiratory flow resistive loads, the PAG appears deactivated in healthy controls and activated in asthma patients (von Leupoldt et al., 2009a).
Related to larynx function, voiced speech, as compared to whispered speech, lead to increased PAG rCBF and an increased correlation between PAG rCBF and rCBF in the ventromedial prefrontal cortex, the inferior operculum and the medial temporal gyrus (Schulz et al., 2005).
Micturition
A brainstem micturition circuit including the PAG has been well characterized in animals (Blok and Holstege, 1998). In human neuroimaging, midbrain and PAG involvement in normal voluntary micturition has been demonstrated (Blok et al., 1998; Blok et al., 1997; Fukuyama et al., 1996) and confirmed using bladder infusion and cystometry (Nour et al., 2000). At the first desire to void, slight increases in PAG rCBF have been reported (Takao et al., 2008), and the more the bladder is filled, the higher the PAG rCBF, an effect that was unrelated to the sensation of urgency (Athwal et al., 2001). Similarly, increased PAG rCBF when subjects had a full bladder, but not during intravesical ice water stimulation, suggests that the PAG activation is specific to bladder distention and not other bladder sensation (Matsuura et al., 2002). Voluntary enhancement of the urge to void (Kuhtz-Buschbeck et al., 2005), imitation voiding by releasing — and imitating interruption by contracting — pelvic floor muscles (Seseke et al., 2006, 2008) all lead PAG activation in women (Seseke et al., 2006) and in men (Seseke et al., 2008) with no gender differences in PAG activation magnitude.
Also clinical studies indicate a role of the PAG in abnormal micturition. In subjects with detrusor overactivity, bladder filling led to similar PAG activation as in healthy subjects (Griffiths et al., 2005). A subsequent study showed only minimal PAG responses in both controls and detrusor overactive patients (Griffiths et al., 2007), but follow up analyses on the same data indicated rostral insula and anterior cingulate connectivity to the PAG during bladder filling in the healthy group (Tadic et al., 2008) and a slight effect of age on PAG connectivity (Griffiths et al., 2009). In women with Fowler’s syndrome (an urethral sphincter abnormality) sacral neuromodulation led to increased (normalized) PAG reactivity to bladder filling (Kavia et al., 2010).
In Parkinson patients, detrusor over-activity is associated with PAG activation (Kitta et al., 2006), and PAG activity influences activity in the thalamus and insula only during subthalamic nucleus deep brain stimulation, suggesting improved sensory gating of bladder afferents (Herzog et al., 2008).
PAG activation to bladder filling has also been studied in spinal cord injury patients. During bladder filling and during bladder filling in combination with acute pudendal nerve stimulation, the PAG was activated. After 2 weeks of pudendal stimulation treatment, PAG activation to bladder filling was decreased. The authors suggest that the PAG may be overactive in spinal cord injured patients due to a decompensatory mechanism following the sudden loss of the spinal afferent input (Zempleni et al., 2010).
PAG lesions in multiple sclerosis are associated with bowel and bladder dysfunction (Charil et al., 2003).
Bowel Function and the PAG
The rectum is innervated by visceral afferents and the somatosensory pudendal nerve innervates the anal canal. This innervation has been capitalized on to test for differences in central domains of intestinal sensations. Non-painful anal stimulation resulted in greater cortical activation than did rectal stimulation, and only anal stimulation resulted in above threshold PAG activation, although there was no significant difference in the PAG for the two conditions (Lotze et al., 2001). Similarly, equally painful visceral and somatic stimuli both activated the PAG, which was slightly greater for visceral pain (Dunckley et al., 2005b). Anxiety was higher during visceral stimulation, and PAG activity was correlated with anxiety during visceral stimulation, suggesting greater nocifensive responses and greater emotive salience of visceral pain. In an investigation on the effects of acute stress on visceral pain in women, PAG BOLD signal to both non-painful and painful rectal distention correlated with chronic stress levels, but there were no effects of acute stress on PAG reactivity (Rosenberger et al., 2009a). Intense rectal distention led to PAG activation that was also associated with increased heart rate and with increased plasma adrenaline (Suzuki et al., 2009).
Patients with irritable bowel syndrome (IBS) show evidence of altered perceptual responses to visceral stimuli, consistent with altered processing of visceral afferent information by the brain. Involvement of the PAG in responses to rectal balloon distention has been reported in four studies, all indicating altered PAG signal in IBS (Mayer et al., 2005; Naliboff et al., 2003; Naliboff et al., 2001; Wilder-Smith et al., 2004) with some specificity, as ulcerative colitis patients have normal PAG function (Mayer et al., 2005). PAG activation in IBS is reduced by the 5-HT3 receptor antagonist Alosetron (Berman et al., 2002), and PAG gray matter density, as measured by VBM, is reduced in IBS (Seminowicz et al., 2010).
Other Modalities that evoke PAG Activation
Several other homeostatic functions have indicated PAG involvement. Thirst, elicited by rapid IV infusion of hypertonic saline, led to increased PAG blood flow (Denton et al., 1999). Hypoglycema, elicited by insulin infusion, led to increases in heart rate, and increased plasma levels of epinephrine, norepinephrine, and pancreatic polypeptide. While cerebral blood flow generally decreased at hypoglycema, PAG rCBF was elevated(Teves et al., 2004). Painful gastric distention, induced by balloons passed orally to the distal stomach, led to increased rCBF in the PAG (Ladabaum et al., 2001).
3.5 Connectivity of the PAG
Diffusion tensor tractography
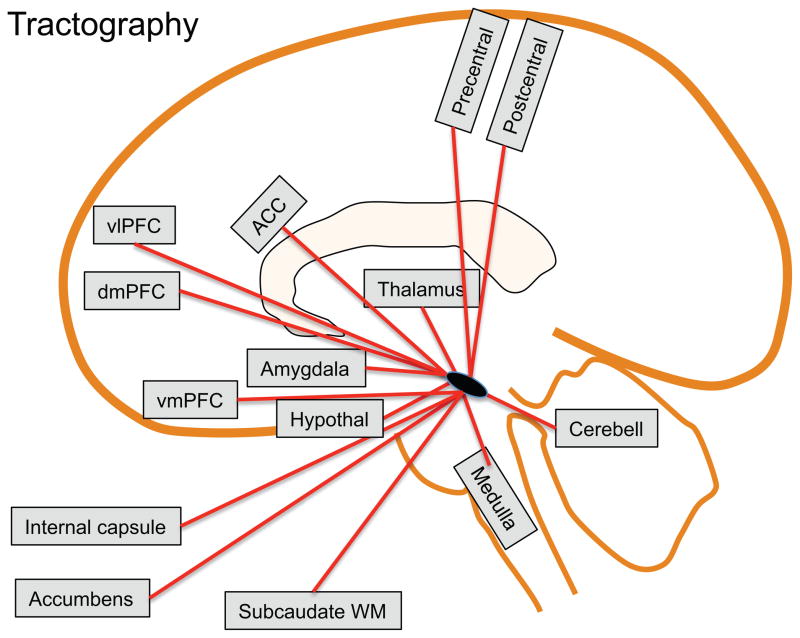
Diffusion weighted imaging allows for white matter tract identification through probabilistic Diffusion tensor imaging (DTI) tractography. This identification is done by following the principal diffusivity direction in a voxel to voxel manner. While the gold standard remains anterograde and retrograde tract tracing in animals, particularly in primates (Aggleton et al., 1980; An et al., 1998; Dujardin and Jurgens, 2005; Mantyh, 1982, 1983; Price and Amaral, 1981), DTI can confirm these pathways in humans. Current resolution constraints make tractography sensitive primarily to large fiber pathways, therefore smaller pathways, or those through regions of fiber crossing or complexity may not be detected. It should be noted that current DTI methods do not allow for inference on the directionality of information flow within tracts. An overview of the connection identified by DTI studies is presented in Figure 5.
Figure 5. PAG Connections based on DTI.
Schematic representation of regions connected to the PAG identified in diffusion-weighted tractography studies. ACC=anterior cingulate cortex, Cerebell=cerebellum, dmPFC=dorsomedial prefrontal cortex, vmPFC=ventromedial prefrontal cortex, vlPFC=ventrolateral prefrontal cortex, WM=white matter.
While not directly addressing the PAG connections, DTI has been used to identify the corticospinal tract, medial lemniscus, the frontopontine tract, the temporo-/parieto-/occipitopontine tract and the superior, medial, and inferior cerebellar peduncles (Stieltjes et al., 2001). Diffusion imaging tractography using the PAG as the starting point has been performed in five studies (Hadjipavlou et al., 2006; Owen et al., 2008; Owen et al., 2007; Pereira et al., 2010; Sillery et al., 2005). Tracts have been identified to the thalamus (medial dorsal nucleus), the middle frontal and frontopolar gyri, through the thalamus and hypothalamus to terminate in the amygdala, the rostral ventral medulla, and the dorsomedial, ventromedial and ventrolateral prefrontal cortex. Similar results were found in two studies on pre-operative probabilistic tractography in patients with PAG deep brain stimulation for chronic pain (Owen et al., 2008; Owen et al., 2007), and it was concluded that with further technical improvement, probabilistic tractography might have utility as a surgical planning tool. Further anatomical refinement indicated dominant ventral PAG connections include amygdala, nucleus accumbens, anterior cingulate cortex and ventromedial prefrontal cortex whereas prominent dorsal PAG connections included ventral posterior thalamus and primary somatosensory cortex (Pereira et al., 2010). Of note, in the above studies, tracts to the dorsal anterior cingulate were rare, possibly due to difficulties in following tracts perpendicular to, and passing through the corpus callosum bundle.
In an attempt to better understand the effects of 20th century surgical brain ablation procedures for the treatment of refractory depression, DTI tractography was done in healthy controls using reported lesion sites as the seeds. Seeds in the sites for anterior capsulotomy, subcaudate tractotomy and limbic leucotomy were all found to project to the PAG (Schoene-Bake et al., 2010).
Functional connectivity
Functional connectivity has been defined as the correlations between spatially remote neurophysiological events. In neuroimaging, this usually means a temporal correlation between regional fluctuations in cerebral blood flow or BOLD signal. There are numerous analytical approaches, either based on blind signal separation strategies such as independent component analysis, or model based. One common model based approach is analyzing functional connectivity of a seed region, either with the subject performing no explicit task (resting state) or while accounting for influences of other regions or behavioral tasks. Moreover, there is an ongoing discussion on how functional correlations, especially negative correlations, should be interpreted.
Below, we review the majority of PAG functional connectivity studies in the literature, and provide an activation likelihood estimate of findings. The resulting ALE map from this section summarizes both positive, negative and task-modulated functional connectivity results from various methods, indicating PAG midbrain autocorrelation, amygdala and anterior middle cingulate connectivity; see Figure 6 and Table 1.
Figure 6. Activation likelihood estimates of regions found to be functionally connected to the PAG.
Also, the amygdala and putamen displayed connectivity peaks.
Table 1.
Activation likelihood estimates for coordinates reported as the PAG
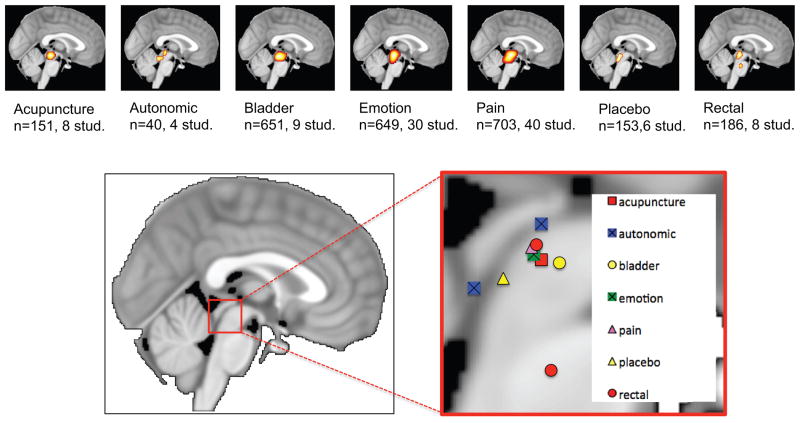
| Function | Subjects | Experiments | peaks | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| All | 2515 | 111 | 188 | 1 | −29 | −12 |
| Acupuncture | 151 | 8 | 13 | 0 | −28 | −12 |
| Autonomic | 40 | 4 | 4 | 1 | −38 | −16 |
| −3 | −38 | −6 | ||||
| Bladder | 651 | 9 | 25 | 1 | −25 | −12 |
| Emotion | 649 | 30 | 55 | 1 | −29 | −11 |
| Pain | 703 | 40 | 69 | 1 | −29 | −10 |
| Placebo | 153 | 6 | 13 | −1 | −33 | −15 |
| Rectal | 186 | 8 | 10 | 0 | −29 | −10 |
| 1 | −26 | −29 | ||||
Resting state connectivity of the PAG
Resting state functional connectivity MRI (fcMRI) is based on the observation that the brain regions show correlated slow fluctuations in cerebral blood flow (Friston et al., 1993) and in blood-oxygenation-level-dependent (BOLD) signal (Biswal et al., 1995). While the method does not provide direct measurement of anatomic connectivity or directionality, accumulating evidence suggests it is sufficiently constrained by anatomy to allow the architecture of distinct brain systems to be characterized (Van Dijk et al., 2010).
The brain’s intrinsic activity at rest is an expanding field of study, and resting state modeling has largely focused on cortical structures, but there are several publications on PAG resting functional connectivity. In a data driven functional hubs analysis of 979 healthy subjects (Tomasi and Volkow, 2011), the PAG was found in three subcortical networks, with the network hubs in the cerebellum, the thalamus and the amygdala. In an independent component analysis, the PAG was found to belong to a “salience network,” with the main nodes in dorsal anterior cingulate and orbital frontoinsular cortices (Seeley et al., 2007). Resting state connectivity of the PAG in 100 healthy controls using a seed based approach indicated significant functional connectivity to the PAG include the rostral and pregenual ACC, the cerebellum, the ventromedial medulla, the globus pallidus, the hippocampus and the anterior insula. Negative functional connectivity was seen to the post-central gyrus, the middle occipital gyrus, the posterior insula and the lateral orbital prefrontal cortex. Moreover, women had a higher functional connectivity to the mid-cingulate cortex, and men to the right insula, the left uncus, the left medial orbital prefrontal cortex and the right prefrontal cortex (Kong et al., 2010b).
We identified three clinical studies on PAG resting state connectivity: The dorsal putamen is functionally connected to the PAG in healthy subjects, but significantly less so in patients with obsessive-compulsive disorder (Harrison et al., 2009). The centromedial amygdala connectivity to the PAG is higher than the basolateral amygdala connectivity to the PAG, but with no significant differences between healthy controls and patients with generalized anxiety disorder (Etkin et al., 2009). The right executive attention network (dorsolateral prefrontal cortex and posterior parietal regions overlapping the superior parietal lobule and intraparietal sulcus) connectivity to the PAG is compromised in fibromyalgia patients with high levels of spontaneous pain (Napadow et al., 2010).
State specific connectivity
Pain specific connectivity
Stimulus induced alterations in functional connectivity during pain is a subject of intense investigation, particularly in studies investigating pain placebo effects, as discussed above, but also in pain anticipation, empathy to pain and in non-painful sensory stimulation.
Moulton et al. (2011) found that regions of the cerebellum that are involved in pain processing (but not processing of aversive images) display functional connectivity to the PAG.
The functional activation and connectivity of the midbrain, brainstem and spinal cord, spanning between the thalamus and the C7/T1 intervertebral disc has been investigated using a dedicated spinal receiver coil. Non-noxious temperatures (15–32°C) were applied to the right thenar eminence (base of the thumb). In the PAG, the signal changes were highest with stimulation at 15°C and increased monotonically from a low at 18°C to a high with stimulation at 29°C. Connectivity analyses showed correlations between right dorsal areas in C6 and the PAG (Stroman, 2009).
Pre-stimulus connectivity between the PAG and the anterior insula determines if a subsequent stimuli is perceived as painful (Ploner et al., 2010), and the connectivity between PAG and anterior insula is higher during self experienced pain as compared to observing pain in others (Lamm et al., 2010; Zaki et al., 2007).
Placebo
The rCBF of the PAG and the anterior cingulate covaries during both placebo and opioid analgesia (Petrovic et al., 2002). The functional connectivity between the PAG and the anterior cingulate is increased during placebo analgesia (Bingel et al., 2006; Eippert et al., 2009), and during heterotopic noxious conditioning stimulation (Sprenger et al., 2011), both effects that are abolished by naloxone administration. Similar effects have been reported in a study on pain and placebo modulation of 11C-carfentanil binding (Wager et al., 2007), where there was a placebo specific correlation between PAG and rostral anterior cingulate opioid binding potential, and between PAG and the orbitofrontal cortex. A negative relationship between PAG opioid activity during placebo and that of the nucleus accumbens and the amygdala, and a trend towards a negative correlation to the subgenual anterior cingulate, has also been reported (Scott et al., 2008). Of note, the functional connectivity between the dorsal anterior cingulate and the PAG is also influenced by mood, in that pain in combination with a bad odor led to increased connectivity (Villemure and Bushnell, 2009).
See also section on Acupuncture, The opioid system and Placebo
Emotion specific connectivity
In a study on fight and flight, the effect of proximity of a virtual predator on the activity and connectivity was investigated. As the predator grew closer, brain activity shifted from the ventromedial prefrontal cortex to the periaqueductal gray (Mobbs et al., 2007). With higher risk of being captured by the predator, midbrain (including the PAG) functional connectivity shifted, to higher functional connectivity with the dorsal ACC, ventral striatum, medial dorsal thalamus, anterior insula, and lateral midbrain, and lower connectivity with the right amygdala, hippocampus, insula, vmPFC, PCC, and subgenual ACC (Mobbs et al., 2009).
In an interesting meta-analytical approach (Kober et al., 2008), 162 emotion specific functional neuroimaging studies were identified and brain regions that were consistently activated in emotional tasks were identified. Moreover, regions that co-activated across studies were grouped into functional groups, thereby providing information on potential organization of brain regions into large-scale networks. The periaqueductal gray was only reported in 6 studies, but clustered together with the thalamus, the PAG was found to be activated in 36% of studies. These activations were associated with a core limbic group including the hypothalamus, the amygdala, the ventral striatum, the ventral globus pallidus and the thalamus. The PAG co-activated with the dorsomedial prefrontal cortex, and the rostral anterior cingulate. Mediation analyses of these co-activations suggested that the dorsomedial prefrontal cortex influences the hypothalamus through the PAG.
4. Discussion
The human neuroimaging studies of the PAG confirm many of the known processes previously observed in animal studies. As sub regions of the PAG exert contrasting influences on behavior, the wide range of behaviors identified is not surprising. One of the major issues in neuroimaging relate to improving methods of specificity and reproducibility. The case of the PAG illustrates that more rigorous approaches may be needed when imaging including appropriate methodological approaches, controls and how the data is evaluated and interpreted. Notably, the specificity of PAG findings may be rather low, as a wide range of behaviors engages the structure and PAG structure and function appears altered in a wide range of disease states.
In this meta-analysis, we hypothesized that different functions of the PAG would have different peak localizations based on the known organization of the PAG (Figure 2). The average peak locations in the included studies were grouped into seven categories: acupuncture, autonomic function, bladder control, emotion, pain, placebo and rectal function, see Table 2 and Figure 7 for activation likelihood peak locations. As can be seen in Figure 7, there is not clear separation of the peaks, most likely due to lack of spatial resolution and differences in imaging methods and data processing procedures.
Table 2.
Activation likelihood estimates for PAG connectivity, p<0.05 FDR
| Cluster size | Cluster peak | Peak label |
|---|---|---|
| 11256 mm3 | 0, −26, 9 | Midbrain |
| 1152 mm3 | −28, −1, −17 | Left amygdala |
| 928 mm3 | −5, 10, 44 | Left anterior middle cingulate |
| 616 mm3 | 1, 34, 24 | Right anterior middle cingulate |
| 456 mm3 | 7, 19, 31 | Right anterior middle cingulate |
| 368 mm3 | −35, 27, −15 | Left inferior frontal gyrus |
| 352 mm3 | 12, 7, 44 | Right middle cingulate |
| 288 mm3 | 29, −10, −22 | Right amygdala |
| 232 mm3 | 20, 7, −1 | Right putamen |
Figure 7. Activation likelihood estimates across behavioral domains.
The activation likelihood peaks are indicated in a sagittal slice of the MNI template.
Technical considerations in PAG imaging
Neuroimaging of the PAG is challenging due to a number of factors. While it is difficult to give exact estimates, most fMRI results are reliable in the intraclass correlation (ICC) = 0.33–0.66 range (Bennett and Miller, 2010), but ICCs of 0.76 and higher have also been reported (Aron et al., 2006). Older studies often employed slice thickness up to 7 mm, and current standard neuroimaging protocols usually have a resolution of around 2–5 mm, enough to cover the PAG with only a few voxels. The limited resolution makes identification of unique activation very difficult and it also introduces partial volume effects (PVE), a phenomenon that degrades the quantitative accuracy of images. PVEs have two causes: the finite spatial resolution of the scanner and the voxel size at which the image is sampled. The former causes a displacement of activity between neighboring regions, whereas the latter gives rise to the tissue-fraction effect where multiple tissue types, like gray matter and cerebrospinal fluid, can exist within a voxel. Not only does the blurring effect of the spatial resolution cause signal to spill-out of a region, signal from surrounding regions spills in (Thomas et al., 2011). Due to its dorsal location and the cerebral aqueduct, the PAG moves with heart and CSF pulsation and breathing. Although some movement artifacts can be accounted for (Van Dijk et al., 2011), the role of the PAG in autonomic regulation makes movement artifacts prone to be temporally correlated to the behavior being studied. Functional MRI also has difficulty imaging regions near tissue interfaces due to distortions from macroscopic susceptibility effects, which become more severe at higher magnetic field strengths. Moreover, standard normalization and spatial smoothing procedures are optimized for cortical structures, such that residual between subject structural variability may add further noise. Methods used to solve these problems include higher voxel resolution, cardiac gating (Becerra et al., 2006b; Guimaraes et al., 1998; Mainero et al., 2007; Napadow et al., 2008), midbrain optimized imaging sequences (Fairhurst et al., 2007; Napadow et al., 2008; Napadow et al., 2009; Pattinson et al., 2009; Topolovec et al., 2004; Tracey et al., 2002a), physiological noise modeling (Brooks et al., 2008), multiple channel (Wiggins et al., 2009) or spinal cord coils (Cahill and Stroman, 2011b; Ghazni et al., 2010; Stroman, 2009; Stroman et al., 2011), field mapping (Dhond et al., 2008; Etkin et al., 2009; Gray et al., 2009; Mobbs et al., 2010; Ploner et al., 2010; Salomons et al., 2007), and midbrain/brainstem dedicated normalization procedures (Beissner et al., 2011; Napadow et al., 2006), see (Oldfield et al., 2011) for further discussion and a recent example. Another potential concern are new findings that MRI magnetic fields may stimulate rotational sensors of the brain, leading to nystagmus (Roberts et al., 2011) inside the MRI bore. This may influence midbrain regions involved in eye movements (Tilikete and Pelisson, 2008).
Critical Assessment
As evident in Figure 4, several peaks fell outside of the PAG region, possibly due to large clusters encompassing several structures of the midbrain, non-optimal normalization procedures, typographical errors and mislabeling. While this review and analysis focused on the periaqueductal gray, a larger theme is the accuracy and specificity of neuroimaging methods. The complex and expensive experimental setups, the easily obtained high uncorrected p-values and correlation coefficients (Vul et al., 2009), and the beautiful brain images may lead scientists and the public to be over-confident in results (McCabe and Castel, 2008; Weisberg et al., 2008). Recent reports even suggest that the a large proportion, if not the majority, of published research findings are false (Ioannidis, 2005; Matullo et al., 2005; Wacholder et al., 2004) or make erroneous statistical interpretations (Nieuwenhuis et al., 2011). There is little reason to believe that the PAG literature is free from the over-interpretation (Logothetis, 2008), publication bias (Ioannidis, 2011; Jennings and Van Horn, 2011) double dipping (Kriegeskorte et al., 2009) and other methodological shortcomings observed in other neuroimaging fields (Smith, 2010). While part of the variability in functional localization may be attributed to methodological differences over the years and across statistical packages (Brett et al., 2002; Poline et al., 2006), careful and detailed neuroanatomical labeling is necessary for the ability to compare results across labs and functional domains (Devlin and Poldrack, 2007).
Conclusion and Future Directions
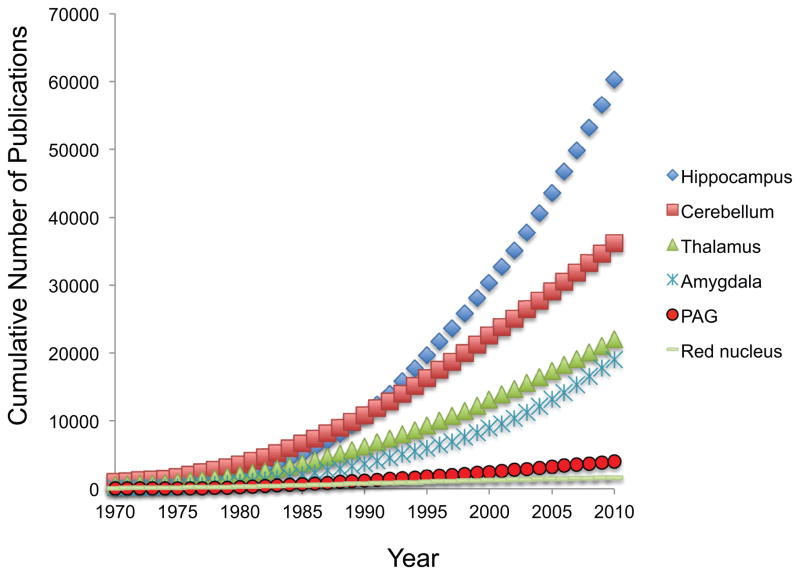
This meta-analysis points out the remarkable strength and potential the field holds. While the PAG literature has yet to experience the exceptional growth observable in other neuroscience fields (Figure 8), the detailed animal literature and the role of the PAG as an interface on salient stimuli between the forebrain and the lower brain stem makes it a highly interesting target for future studies and translational efforts. The effective spatial and temporal resolution of fMRI is increasing, with one example being the development of 7 Tesla fMRI with sub-millimeter resolution (Polimeni et al., 2010) (Figure 9). Such advances in technology permit the PAG anatomy to be readily visible in functional data. Simultaneous PET-fMRI (Judenhofer et al., 2008) and ultra high resolution diffusion weighted imaging (Miller et al., 2011) are other emerging technologies. While technical developments will allow us to ask new questions, improved experimental methods using currently available techniques may be a fast route to success. For example, contrasting PAG involvement across functional domains within a single subject may allow for a more detailed understanding (Fadiga, 2007). Using drugs with known pharmacology it is possible to examine the acute effects of the drug itself in the brain, alterations of the neurovascular coupling, and to investigate how neurotransmitter systems are involved in neural systems engaged by other processes (Anderson et al., 2008; Steward et al., 2005; Upadhyay et al., 2011). These studies come with their own set of unique challenges, as drugs can influence both the neuronal signaling and the neurovascular coupling (Borras et al., 2004; Choi et al., 2006; Shih et al., 2009). Recent studies indicate that genetics (Loggia et al, 2011) and gender (Linnman et al, 2011) influence PAG function and connectivity. Longitudinal studies across, for example, the menstrual cycle, aging, disease progression and treatments are another rich source awaiting exploitation.
Figure 8. PAG in the literature.
Cumulative number of PubMed citations mentioning various brain structures in the title/abstract. Notably, the PAG neuroimaging literature reviewed here compose less than 10% of the entire PAG literature.
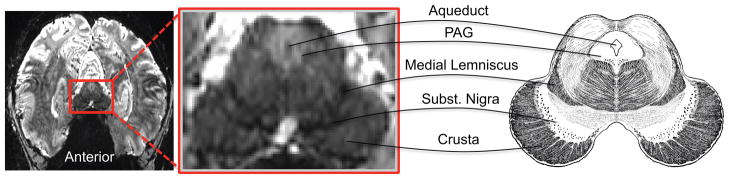
Figure 9. PAG at 7 Tesla.
A single functional EPI slice at 0.85 mm isotropic resolution from one subject obtained at 7 tesla with a 32-channel head coil. The midbrain anatomical illustration is adapted from (Gray and Lewis, 1918).
In conclusion, neuroimaging of the human PAG has made a substantial contribution to our understanding of behavior and disease, and translating animal studies to human conditions. We are now at a stage where the hardware, software and level of experimental sophistication are sufficient for direct hypothesis testing with highly specific probes. This will take human neuroimaging to a new level, as long as we continue to carefully scrutinize our results.
Highlights.
Neuroimaging of the human PAG is reviewed.
Pain and pain modulation, emotion, bladder and bowel function and autonomic regulation are covered.
Methods include function, structure, connectivity and neurochemistry imaging.
Human PAG neuroimaging replicates several animal findings, and methods can be improved upon.
Acknowledgments
a K24 Mentoring Grant (NINDS NS064050) to DB, a K01 (NIDA K01DA024289) grant to EM, an IASP early career grant to CL, and the Swedish Society for Medical Research (SSMF) supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. J Comp Neurol. 1981;198:677–716. doi: 10.1002/cne.901980409. [DOI] [PubMed] [Google Scholar]
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Anderson IM, McKie S, Elliott R, Williams SR, Deakin JF. Assessing human 5-HT function in vivo with pharmacoMRI. Neuropharmacology. 2008;55:1029–1037. doi: 10.1016/j.neuropharm.2008.06.029. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Gluck MA, Poldrack RA. Long-term test-retest reliability of functional MRI in a classification learning task. NeuroImage. 2006;29:1000–1006. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, Frackowiak RS, Fowler CJ. Brain responses to changes in bladder volume and urge to void in healthy men. Brain : a journal of neurology. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- Bai L, Qin W, Tian J, Liu P, Li L, Chen P, Dai J, Craggs JG, von Deneen KM, Liu Y. Time-varied characteristics of acupuncture effects in fMRI studies. Human Brain Mapping. 2009;30:3445–3460. doi: 10.1002/hbm.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Tian J, Zhong C, Xue T, You Y, Liu Z, Chen P, Gong Q, Ai L, Qin W, Dai J, Liu Y. Acupuncture modulates temporal neural responses in wide brain networks: evidence from fMRI study. Mol Pain. 2010;6:73. doi: 10.1186/1744-8069-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends in neurosciences. 1994;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain research bulletin. 2000;53(1):95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Barnden LR, Crouch B, Kwiatek R, Burnet R, Mernone A, Chryssidis S, Scroop G, Del Fante P. A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR in biomedicine. 2011 doi: 10.1002/nbm.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher FD, Gray MA, Mathias CJ, Critchley HD. Vulnerability to simple faints is predicted by regional differences in brain anatomy. NeuroImage. 2009;47:937–945. doi: 10.1016/j.neuroimage.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Courtemanche J, Paquette V, St-Pierre EL. The neural basis of unconditional love. Psychiatry Res. 2009;172:93–98. doi: 10.1016/j.pscychresns.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg. 2006a;103:208–216. doi: 10.1213/01.ane.0000221457.71536.e0. table of contents. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006b;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Beissner F, Deichmann R, Baudrexel S. fMRI of the brainstem using dual-echo EPI. NeuroImage. 2011;55:1593–1599. doi: 10.1016/j.neuroimage.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci. 2010;1191:133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, Fitzgerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD, Mayer EA. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist Alosetron. Gastroenterology. 2002;123:969–977. doi: 10.1053/gast.2002.35990. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Bingel U, Schoell E, Herken W, Buchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131:21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, Aziz TZ. Deep brain stimulation for pain relief: a meta-analysis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2005;12:515–519. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Blok BF, Holstege G. The central nervous system control of micturition in cats and humans. Behav Brain Res. 1998;92:119–125. doi: 10.1016/s0166-4328(97)00184-8. [DOI] [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain : a journal of neurology. 1998;121 ( Pt 11):2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain : a journal of neurology. 1997;120 ( Pt 1):111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Sabattoli F, Laakso MP, Testa C, Rossi R, Beltramello A, Soininen H, Frisoni GB. Frontotemporal dementia as a neural system disease. Neurobiol Aging. 2005;26:37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, Borsook D. fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol. 2004;91:2723–2733. doi: 10.1152/jn.00249.2003. [DOI] [PubMed] [Google Scholar]
- Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox PT. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nature reviews. Neuroscience. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Beckmann CF, Miller KL, Wise RG, Porro CA, Tracey I, Jenkinson M. Physiological noise modelling for spinal functional magnetic resonance imaging studies. NeuroImage. 2008;39:680–692. doi: 10.1016/j.neuroimage.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Stroman PW. Mapping of neural activity produced by thermal pain in the healthy human spinal cord and brain stem: a functional magnetic resonance imaging study. Magn Reson Imaging. 2011a;29:342–352. doi: 10.1016/j.mri.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Stroman PW. Mapping of neural activity produced by thermal pain in the healthy human spinal cord and brain stem: a functional magnetic resonance imaging study. Magn Reson Imaging. 2011b doi: 10.1016/j.mri.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Canessa N, Motterlini M, Alemanno F, Perani D, Cappa SF. Learning from other people’s experience: a neuroimaging study of decisional interactive-learning. NeuroImage. 2011;55:353–362. doi: 10.1016/j.neuroimage.2010.11.065. [DOI] [PubMed] [Google Scholar]
- Canessa N, Motterlini M, Di Dio C, Perani D, Scifo P, Cappa SF, Rizzolatti G. Understanding others’ regret: a FMRI study. PLoS One. 2009;4:e7402. doi: 10.1371/journal.pone.0007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biological Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Cao Y, Aurora SK, Nagesh V, Patel SC, Welch KM. Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology. 2002;59:72–78. doi: 10.1212/wnl.59.1.72. [DOI] [PubMed] [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- Cerase A, Rubenni E, Rufa A, Vallone I, Galluzzi P, Coratti G, Franchi F, Giannini F, Venturi C. CT and MRI of Wernicke’s encephalopathy. La Radiologia medica. 2011;116:319–333. doi: 10.1007/s11547-011-0618-x. [DOI] [PubMed] [Google Scholar]
- Charil A, Zijdenbos AP, Taylor J, Boelman C, Worsley KJ, Evans AC, Dagher A. Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: application to 452 patient data sets. NeuroImage. 2003;19:532–544. doi: 10.1016/s1053-8119(03)00117-4. [DOI] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. NeuroImage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, Gibson SJ. Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain : a journal of neurology. 2006;129:2957–2965. doi: 10.1093/brain/awl228. [DOI] [PubMed] [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O’Doherty JP, Sirigu A, Dolan RJ. Regret and its avoidance: a neuroimaging study of choice behavior. Nat Neurosci. 2005;8:1255–1262. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport. 2007;18:301–305. doi: 10.1097/WNR.0b013e32801776bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Wood ML, Crawley AP, Mikulis DJ. fMRI of human somatosensory and cingulate cortex during painful electrical nerve stimulation. Neuroreport. 1995;7:321–325. [PubMed] [Google Scholar]
- Decety J, Echols S, Correll J. The blame game: the effect of responsibility and social stigma on empathy for pain. J Cogn Neurosci. 2010;22:985–997. doi: 10.1162/jocn.2009.21266. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–2614. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc Natl Acad Sci U S A. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, Peterson AM, Firestone L. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. NeuroImage. 2002;16:158–168. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Devani P, Friston KJ, Feinmann C, Harris M, Pearce S, Watson JD, Frackowiak RS. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. Journal of neurology, neurosurgery, and psychiatry. 1994;57:1166–1172. doi: 10.1136/jnnp.57.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Osborn J. Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. NeuroImage. 2009;47:1002–1006. doi: 10.1016/j.neuroimage.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Poldrack RA. In praise of tedious anatomy. NeuroImage. 2007;37:1033–1041. doi: 10.1016/j.neuroimage.2006.09.055. discussion 1050–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136:407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, Cooper JR, Goldin PR, Hariri AR, Gross JJ. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. NeuroImage. 2011;55:401–410. doi: 10.1016/j.neuroimage.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin E, Jurgens U. Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Res. 2005;1034:114–131. doi: 10.1016/j.brainres.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005a;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005b;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Neurokinin-1 (NK-1) receptor and brain-derived neurotrophic factor (BDNF) gene expression is differentially modulated in the rat spinal dorsal horn and hippocampus during inflammatory pain. Mol Pain. 2007;3:32. doi: 10.1186/1744-8069-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell E, Yacubian J, Buchel C. Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. J Neurosci. 2008a;28:5465–5472. doi: 10.1523/JNEUROSCI.5336-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell E, Yacubian J, Buchel C. Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008b;28:5465–5472. doi: 10.1523/JNEUROSCI.5336-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–754. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Fadiga L. Functional magnetic resonance imaging: measuring versus estimating. NeuroImage. 2007;37:1042–1044. doi: 10.1016/j.neuroimage.2007.02.038. discussion 1066–1048. [DOI] [PubMed] [Google Scholar]
- Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128:101–110. doi: 10.1016/j.pain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fiebig E. Connections of the corpus cerebelli in the thornback guitarfish, Platyrhinoidis triseriata (Elasmobranchii): a study with WGA-HRP and extracellular granule cell recording. J Comp Neurol. 1988;268:567–583. doi: 10.1002/cne.902680407. [DOI] [PubMed] [Google Scholar]
- Fischer H, Andersson JL, Furmark T, Fredrikson M. Fear conditioning and brain activity: a positron emission tomography study in humans. Behav Neurosci. 2000;114:671–680. doi: 10.1037//0735-7044.114.4.671. [DOI] [PubMed] [Google Scholar]
- Freund W, Wunderlich AP, Stuber G, Mayer F, Steffen P, Mentzel M, Schmitz B, Weber F. The Role of Periaqueductal Gray and Cingulate Cortex During Suppression of Pain in Complex Regional Pain Syndrome. Clin J Pain. 2011 doi: 10.1097/AJP.0b013e31821d9063. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Matsuzaki S, Ouchi Y, Yamauchi H, Nagahama Y, Kimura J, Shibasaki H. Neural control of micturition in man examined with single photon emission computed tomography using 99mTc-HMPAO. Neuroreport. 1996;7:3009–3012. doi: 10.1097/00001756-199611250-00042. [DOI] [PubMed] [Google Scholar]
- Garraux G, Goldfine A, Bohlhalter S, Lerner A, Hanakawa T, Hallett M. Increased midbrain gray matter in Tourette’s syndrome. Ann Neurol. 2006;59:381–385. doi: 10.1002/ana.20765. [DOI] [PubMed] [Google Scholar]
- Gee JR, Chang J, Dublin AB, Vijayan N. The association of brainstem lesions with migraine-like headache: an imaging study of multiple sclerosis. Headache. 2005;45:670–677. doi: 10.1111/j.1526-4610.2005.05136.x. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Reinders AA, Paans AM, Renken R, Kortekaas R. Men versus women on sexual brain function: prominent differences during tactile genital stimulation, but not during orgasm. Human Brain Mapping. 2009;30:3089–3101. doi: 10.1002/hbm.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazni NF, Cahill CM, Stroman PW. Tactile sensory and pain networks in the human spinal cord and brain stem mapped by means of functional MR imaging. AJNR American journal of neuroradiology. 2010;31:661–667. doi: 10.3174/ajnr.A1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–635. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Human Brain Mapping. 2011 doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Clauw DJ, Nachemson A, Duck MH, Sabatowski R, Gerbershagen HJ, Williams DA, Petzke F. Central pain processing in chronic low back pain. Evidence for reduced pain inhibition. Schmerz. 2006;20:411–414. 416–417. doi: 10.1007/s00482-006-0473-8. [DOI] [PubMed] [Google Scholar]
- Glodzik L, Mosconi L, Tsui W, de Santi S, Zinkowski R, Pirraglia E, Rich KE, McHugh P, Li Y, Williams S, Ali F, Zetterberg H, Blennow K, Mehta P, de Leon MJ. Alzheimer’s disease markers, hypertension, and gray matter damage in normal elderly. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H, Lewis WH. Anatomy of the human body. 20. Lea & Febiger; Philadelphia and New York: 1918. [Google Scholar]
- Gray MA, Rylander K, Harrison NA, Wallin BG, Critchley HD. Following one’s heart: cardiac rhythms gate central initiation of sympathetic reflexes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1817–1825. doi: 10.1523/JNEUROSCI.3363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Wang S, Owen SL, Xie K, Bittar RG, Stein JF, Paterson DJ, Aziz TZ. Stimulating the human midbrain to reveal the link between pain and blood pressure. Pain. 2006;124:349–359. doi: 10.1016/j.pain.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. The Journal of urology. 2005;174:1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths DJ, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the lower urinary tract: how age-related changes might predispose to urge incontinence. NeuroImage. 2009;47:981–986. doi: 10.1016/j.neuroimage.2009.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol. 2002;87:2205–2208. doi: 10.1152/jn.00730.2001. [DOI] [PubMed] [Google Scholar]
- Guimaraes AR, Melcher JR, Talavage TM, Baker JR, Ledden P, Rosen BR, Kiang NY, Fullerton BC, Weisskoff RM. Imaging subcortical auditory activity in humans. Human Brain Mapping. 1998;6:33–41. doi: 10.1002/(SICI)1097-0193(1998)6:1<33::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria S, Gupta RK, Saksena S, Chandra A, Srivastava RN, Husain M, Rathore R, Narayana PA. Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. Journal of neuroscience research. 2008;86:2271–2280. doi: 10.1002/jnr.21664. [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain. 2006;123:169–178. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, Menchon JM, Cardoner N. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hartikainen KM, Waljas M, Isoviita T, Dastidar P, Liimatainen S, Solbakk AK, Ogawa KH, Soimakallio S, Ylinen A, Ohman J. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. Journal of clinical and experimental neuropsychology. 2010;32:767–774. doi: 10.1080/13803390903521000. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen C, Mohr C, Roehl M, Bingel U, Lorenz J, Buchel C. Common neural systems for contact heat and laser pain stimulation reveal higher-level pain processing. Human Brain Mapping. 2008;29:1080–1091. doi: 10.1002/hbm.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog J, Weiss PH, Assmus A, Wefer B, Seif C, Braun PM, Pinsker MO, Herzog H, Volkmann J, Deuschl G, Fink GR. Improved sensory gating of urinary bladder afferents in Parkinson’s disease following subthalamic stimulation. Brain : a journal of neurology. 2008;131:132–145. doi: 10.1093/brain/awm254. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996;64:303–314. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Stone-Elander S, Ingvar M. Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neurosci Lett. 1999;262:61–64. doi: 10.1016/s0304-3940(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Tu CH, Chen FP, Chen MC, Yeh TC, Cheng HC, Wu YT, Liu RS, Ho LT. Activation of the hypothalamus characterizes the acupuncture stimulation at the analgesic point in human: a positron emission tomography study. Neurosci Lett. 2001;307:105–108. doi: 10.1016/s0304-3940(01)01952-8. [DOI] [PubMed] [Google Scholar]
- Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, Tsai CF. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. Journal of geriatric psychiatry and neurology. 2010;23:171–184. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, Bennett GJ. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121 ( Pt 5):931–947. doi: 10.1093/brain/121.5.931. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, Vennart W, Tracey I. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci U S A. 2005;102:18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP. Why most published research findings are false. PLoS medicine. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP. Excess significance bias in the literature on brain volume abnormalities. Arch Gen Psychiatry. 2011;68:773–780. doi: 10.1001/archgenpsychiatry.2011.28. [DOI] [PubMed] [Google Scholar]
- Iseki E, Matsushita M, Kosaka K, Kondo H, Ishii T, Amano N. Distribution and morphology of brain stem plaques in Alzheimer’s disease. Acta neuropathologica. 1989;78:131–136. doi: 10.1007/BF00688200. [DOI] [PubMed] [Google Scholar]
- Jennings RG, Van Horn JD. Publication Bias in Neuroimaging Research: Implications for Meta-Analyses. Neuroinformatics. 2011 doi: 10.1007/s12021-011-9125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proceedings Biological sciences/The Royal Society. 1991;244:39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Rocken M, Nutt RE, Machulla HJ, Uludag K, Cherry SR, Claussen CD, Pichler BJ. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Kassubek J, Huppertz HJ, Krause T, Els T. Separating functional and structural damage in persistent vegetative state using combined voxel-based analysis of 3-D MRI and FDG-PET. Journal of the neurological sciences. 2005;228:179–184. doi: 10.1016/j.jns.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Sperfeld AD. Widespread white matter changes in Kennedy disease: a voxel based morphometry study. Journal of neurology, neurosurgery, and psychiatry. 2007;78:1209–1212. doi: 10.1136/jnnp.2006.112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavia R, Dasgupta R, Critchley H, Fowler C, Griffiths D. A functional magnetic resonance imaging study of the effect of sacral neuromodulation on brain responses in women with Fowler’s syndrome. BJU international. 2010;105:366–372. doi: 10.1111/j.1464-410X.2009.08819.x. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kim SE, Kim JJ, Jeong B, Park CH, Son AR, Song JE, Ki SW. Compassionate attitude towards others’ suffering activates the mesolimbic neural system. Neuropsychologia. 2009a;47:2073–2081. doi: 10.1016/j.neuropsychologia.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Lee YS, Lee JY, Yoon SJ, Kim JE, Kim JH, Hong SJ, Jeong DU. Gray matter deficits in young adults with narcolepsy. Acta Neurologica Scandinavica. 2009b;119:61–67. doi: 10.1111/j.1600-0404.2008.01063.x. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitta T, Kakizaki H, Furuno T, Moriya K, Tanaka H, Shiga T, Tamaki N, Yabe I, Sasaki H, Nonomura K. Brain activation during detrusor overactivity in patients with Parkinson’s disease: a positron emission tomography study. The Journal of urology. 2006;175:994–998. doi: 10.1016/S0022-5347(05)00324-1. [DOI] [PubMed] [Google Scholar]
- Kittelberger JM, Land BR, Bass AH. Midbrain periaqueductal gray and vocal patterning in a teleost fish. J Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008a;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008b;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, Rosen B, Gollub R. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. NeuroImage. 2009;45:940–949. doi: 10.1016/j.neuroimage.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010a;148:257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010b;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP. Cortical representation of the urge to void: a functional magnetic resonance imaging study. The Journal of urology. 2005;174:1477–1481. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Danielsen EH, Gjedde A, Vernaleken I, Buchholz HG, Heinz A, Grunder G, Bartenstein P, Cumming P. Elevated [(18)F]FDOPA utilization in the periaqueductal gray and medial nucleus accumbens of patients with early Parkinson’s disease. NeuroImage. 2010;49:2933–2939. doi: 10.1016/j.neuroimage.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatric research. 2008;64:275–280. doi: 10.1203/PDR.0b013e31817da10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology. 2001;120:369–376. doi: 10.1053/gast.2001.21201. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J. Is the extrastriate body area (EBA) sensitive to the perception of pain in others? Cerebral cortex. 2008;18:2369–2373. doi: 10.1093/cercor/bhn006. [DOI] [PubMed] [Google Scholar]
- Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. J Cogn Neurosci. 2010;22:362–376. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. NeuroImage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Ingvar DH, Skinhoj E. Brain function and blood flow. Sci Am. 1978;239:62–71. doi: 10.1038/scientificamerican1078-62. [DOI] [PubMed] [Google Scholar]
- Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain : a journal of neurology. 2008;131:1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- Lee HY, Tae WS, Yoon HK, Lee BT, Paik JW, Son KR, Oh YW, Lee MS, Ham BJ. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: An optimized voxel-based morphometry study. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Lee MC, Zambreanu L, Menon DK, Tracey I. Identifying brain activity specifically related to the maintenance and perceptual consequence of central sensitization in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11642–11649. doi: 10.1523/JNEUROSCI.2638-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Appel L, Furmark T, Soderlund A, Gordh T, Langstrom B, Fredrikson M. Ventromedial prefrontal neurokinin 1 receptor availability is reduced in chronic pain. Pain. 2010;149:64–70. doi: 10.1016/j.pain.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bucking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2011a doi: 10.1016/j.pain.2011.11.006. doi:10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Rainey C, Lauer KK, Piacentine L, Bloom A, Risinger R, Ward BD, Stein E, Li SJ. Peripheral blood pressure changes induced by dobutamine do not alter BOLD signals in the human brain. NeuroImage. 2006;30:745–752. doi: 10.1016/j.neuroimage.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Liu WC, Feldman SC, Cook DB, Hung DL, Xu T, Kalnin AJ, Komisaruk BR. fMRI study of acupuncture-induced periaqueductal gray activity in humans. Neuroreport. 2004;15:1937–1940. doi: 10.1097/00001756-200408260-00021. [DOI] [PubMed] [Google Scholar]
- Loggia ML, Jensen K, Gollub RL, Wasan AD, Edwards RR, Kong J. The Catechol-O-Methyltransferase (COMT) valmet Polymorphism Affects Brain Responses to Repeated Painful Stimuli. PLoS One. 2011;6:e27764. doi: 10.1371/journal.pone.0027764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lotze M, Wietek B, Birbaumer N, Ehrhardt J, Grodd W, Enck P. Cerebral activation during anal and rectal stimulation. NeuroImage. 2001;14:1027–1034. doi: 10.1006/nimg.2001.0901. [DOI] [PubMed] [Google Scholar]
- Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain. 2007a;127:183–194. doi: 10.1016/j.pain.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 2007b;69:827–834. doi: 10.1212/01.wnl.0000269783.86997.37. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainero C, Zhang WT, Kumar A, Rosen BR, Sorensen AG. Mapping the spinal and supraspinal pathways of dynamic mechanical allodynia in the human trigeminal system using cardiac-gated fMRI. NeuroImage. 2007;35:1201–1210. doi: 10.1016/j.neuroimage.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW. Forebrain projections to the periaqueductal gray in the monkey, with observations in the cat and rat. J Comp Neurol. 1982;206:146–158. doi: 10.1002/cne.902060205. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Connections of midbrain periaqueductal gray in the monkey. I. Ascending efferent projections. J Neurophysiol. 1983;49:567–581. doi: 10.1152/jn.1983.49.3.567. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. The Journal of urology. 2002;168:2035–2039. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- Matullo G, Berwick M, Vineis P. Gene-environment interactions: how many false positives? Journal of the National Cancer Institute. 2005;97:550–551. doi: 10.1093/jnci/dji122. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Castel AD. Seeing is believing: the effect of brain images on judgments of scientific reasoning. Cognition. 2008;107:343–352. doi: 10.1016/j.cognition.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Mendes-Gomes J, Amaral VC, Nunes-de-Souza RL. Ventrolateral periaqueductal gray lesion attenuates nociception but does not change anxiety-like indices or fear-induced antinociception in mice. Behav Brain Res. 2011;219:248–253. doi: 10.1016/j.bbr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller KL, Stagg CJ, Douaud G, Jbabdi S, Smith SM, Behrens TE, Jenkinson M, Chance SA, Esiri MM, Voets NL, Jenkinson N, Aziz TZ, Turner MR, Johansen-Berg H, McNab JA. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. NeuroImage. 2011;57:167–181. doi: 10.1016/j.neuroimage.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnerop M, Specht K, Ruhlmann J, Schimke N, Abele M, Weyer A, Wullner U, Klockgether T. Voxel-based morphometry and voxel-based relaxometry in multiple system atrophy-a comparison between clinical subtypes and correlations with clinical parameters. NeuroImage. 2007;36:1086–1095. doi: 10.1016/j.neuroimage.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Mobascher A, Brinkmeyer J, Warbrick T, Musso F, Schlemper V, Wittsack HJ, Saleh A, Schnitzler A, Winterer G. Brain activation patterns underlying fast habituation to painful laser stimuli. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2010;75:16–24. doi: 10.1016/j.ijpsycho.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci U S A. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105:339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Moffet HH. Sham acupuncture may be as efficacious as true acupuncture: a systematic review of clinical trials. Journal of alternative and complementary medicine. 2009;15:213–216. doi: 10.1089/acm.2008.0356. [DOI] [PubMed] [Google Scholar]
- Mohr C, Leyendecker S, Mangels I, Machner B, Sander T, Helmchen C. Central representation of cold-evoked pain relief in capsaicin induced pain: an event-related fMRI study. Pain. 2008;139:416–430. doi: 10.1016/j.pain.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Moisset X, Villain N, Ducreux D, Serrie A, Cunin G, Valade D, Calvino B, Bouhassira D. Functional brain imaging of trigeminal neuralgia. European journal of pain. 2011;15:124–131. doi: 10.1016/j.ejpain.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourao-Miranda J, Andreiuolo PA, Pessoa L. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern M, Chang L, Mayer EA. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. NeuroImage. 2008;42:169–177. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Kennedy D, Hui KK, Makris N. Automated brainstem co-registration (ABC) for MRI. NeuroImage. 2006;32:1113–1119. doi: 10.1016/j.neuroimage.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Park K, Kim J, Makris N, Kwong KK, Harris RE, Purdon PL, Kettner N, Hui KK. Time-variant fMRI activity in the brainstem and higher structures in response to acupuncture. NeuroImage. 2009;47:289–301. doi: 10.1016/j.neuroimage.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold BS, Jr, Wilson WP, Slaughter DG. Sensations evoked by stimulation in the midbrain of man. J Neurosurg. 1969;30:14–24. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- Newcombe VF, Williams GB, Scoffings D, Cross J, Carpenter TA, Pickard JD, Menon DK. Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. Journal of neurology, neurosurgery, and psychiatry. 2010;81:552–561. doi: 10.1136/jnnp.2009.196246. [DOI] [PubMed] [Google Scholar]
- Nicotra A, Critchley HD, Mathias CJ, Dolan RJ. Emotional and autonomic consequences of spinal cord injury explored using functional brain imaging. Brain : a journal of neurology. 2006;129:718–728. doi: 10.1093/brain/awh699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niddam DM, Chan RC, Lee SH, Yeh TC, Hsieh JC. Central modulation of pain evoked from myofascial trigger point. Clin J Pain. 2007;23:440–448. doi: 10.1097/AJP.0b013e318058accb. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biological Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Nour S, Svarer C, Kristensen JK, Paulson OB, Law I. Cerebral activation during micturition in normal men. Brain : a journal of neurology. 2000;123 ( Pt 4):781–789. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- Oldfield EH, Brooks JC, Wise RJ, Padormo F, Hajnal JV, Beckmann CF, Ungless MA. Identification and characterisation of midbrain nuclei using optimised functional magnetic resonance imaging. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SL, Heath J, Kringelbach M, Green AL, Pereira EA, Jenkinson N, Jegan T, Stein JF, Aziz TZ. Pre-operative DTI and probabilisitic tractography in four patients with deep brain stimulation for chronic pain. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2008;15:801–805. doi: 10.1016/j.jocn.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Owen SL, Heath J, Kringelbach ML, Stein JF, Aziz TZ. Preoperative DTI and probabilistic tractography in an amputee with deep brain stimulation for lower limb stump pain. British journal of neurosurgery. 2007;21:485–490. doi: 10.1080/02688690701558358. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Damasio A. Selective pathological changes of the periaqueductal gray matter in Alzheimer’s disease. Ann Neurol. 2000;48:344–353. [PubMed] [Google Scholar]
- Pattinson KT, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG. Opioids depress cortical centers responsible for the volitional control of respiration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8177–8186. doi: 10.1523/JNEUROSCI.1375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Mai JK. The human nervous system. 2. Elsevier Academic Press; Amsterdam ; Boston: 2004. [Google Scholar]
- Pereira EA, Lu G, Wang S, Schweder PM, Hyam JA, Stein JF, Paterson DJ, Aziz TZ, Green AL. Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Exp Neurol. 2010;223:574–581. doi: 10.1016/j.expneurol.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia--imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85:19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122 ( Pt 9):1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Pezalla PD. Morphine-induced analgesia and explosive motor behavior in an amphibian. Brain Res. 1983;273:297–305. doi: 10.1016/0006-8993(83)90854-5. [DOI] [PubMed] [Google Scholar]
- Piche M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14236–14246. doi: 10.1523/JNEUROSCI.2341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrikson M. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A. 2010;107:355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni JR, Fischl B, Greve DN, Wald LL. Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1. NeuroImage. 2010;52:1334–1346. doi: 10.1016/j.neuroimage.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline JB, Strother SC, Dehaene-Lambertz G, Egan GF, Lancaster JL. Motivation and synthesis of the FIAC experiment: Reproducibility of fMRI results across expert analyses. Human Brain Mapping. 2006;27:351–359. doi: 10.1002/hbm.20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S, Paviour D, Scahill R, Stevens J, Rossor M, Lees A, Fox N. Voxel-based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson’s disease. NeuroImage. 2004;23:663–669. doi: 10.1016/j.neuroimage.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am J Psychiatry. 2010;167:925–933. doi: 10.1176/appi.ajp.2010.09091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien A, Yang Y, Gorman J, LeDoux J, Stern E, Silbersweig D. Increased brainstem volume in panic disorder: a voxel-based morphometric study. Neuroreport. 2006;17:361–363. doi: 10.1097/01.wnr.0000203354.80438.1. [DOI] [PubMed] [Google Scholar]
- Qin W, Tian J, Bai L, Pan X, Yang L, Chen P, Dai J, Ai L, Zhao B, Gong Q, Wang W, von Deneen KM, Liu Y. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol Pain. 2008;4:55. doi: 10.1186/1744-8069-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. J Cogn Neurosci. 1999;11:110–125. doi: 10.1162/089892999563175. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA. The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biological Psychiatry. 1997;42:446–452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Richardson DE, Akil H. Long term results of periventricular gray self-stimulation. Neurosurgery. 1977a;1:199–202. doi: 10.1097/00006123-197709000-00018. [DOI] [PubMed] [Google Scholar]
- Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 1: Acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977b;47:178–183. doi: 10.3171/jns.1977.47.2.0178. [DOI] [PubMed] [Google Scholar]
- Rilliet B, Reverdin A, Haenggeli CA, Pizzolato GP, Berney J. Tumors of aqueduct of Sylvius. Presentation of 5 cases and review of the literature. Neuro-Chirurgie. 1990;36:336–346. [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Marcelli V, Gillen JS, Carey JP, Della Santina CC, Zee DS. MRI Magnetic Field Stimulates Rotational Sensors of the Brain. Current biology : CB. 2011 doi: 10.1016/j.cub.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke; a journal of cerebral circulation. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- Rosen SD, Paulesu E, Frith CD, Frackowiak RS, Davies GJ, Jones T, Camici PG. Central nervous pathways mediating angina pectoris. Lancet. 1994;344:147–150. doi: 10.1016/s0140-6736(94)92755-3. [DOI] [PubMed] [Google Scholar]
- Rosenberger C, Elsenbruch S, Scholle A, de Greiff A, Schedlowski M, Forsting M, Gizewski ER. Effects of psychological stress on the cerebral processing of visceral stimuli in healthy women. Neurogastroenterol Motil. 2009a;21:740–e745. doi: 10.1111/j.1365-2982.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- Rosenberger C, Elsenbruch S, Scholle A, de Greiff A, Schedlowski M, Forsting M, Gizewski ER. Effects of psychological stress on the cerebral processing of visceral stimuli in healthy women. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009b;21:740–e745. doi: 10.1111/j.1365-2982.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- Ruocco HH, Bonilha L, Li LM, Lopes-Cendes I, Cendes F. Longitudinal analysis of regional grey matter loss in Huntington disease: effects of the length of the expanded CAG repeat. Journal of neurology, neurosurgery, and psychiatry. 2008;79:130–135. doi: 10.1136/jnnp.2007.116244. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004a;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci. 2004b;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Shackman AJ, Davidson RJ. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J Cogn Neurosci. 2007;19:993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- Schoell ED, Bingel U, Eippert F, Yacubian J, Christiansen K, Andresen H, May A, Buechel C. The effect of opioid receptor blockade on the neural processing of thermal stimuli. PLoS One. 2010;5:e12344. doi: 10.1371/journal.pone.0012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene-Bake JC, Parpaley Y, Weber B, Panksepp J, Hurwitz TA, Coenen VA. Tractographic analysis of historical lesion surgery for depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2553–2563. doi: 10.1038/npp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: an H215O PET study. Cerebral cortex. 2005;15:1835–1847. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Borkert J, Wolf S, Schmitz-Hubsch T, Rakowicz M, Mariotti C, Schols L, Timmann D, van de Warrenburg B, Durr A, Pandolfo M, Kang JS, Mandly AG, Nagele T, Grisoli M, Boguslawska R, Bauer P, Klockgether T, Hauser TK. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. NeuroImage. 2010;49:158–168. doi: 10.1016/j.neuroimage.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: the brain stays on task. Cerebral cortex. 2007;17:1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. e42. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P. Voluntary pelvic floor muscle control--an fMRI study. NeuroImage. 2006;31:1399–1407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P. Gender differences in voluntary micturition control: an fMRI study. NeuroImage. 2008;43:183–191. doi: 10.1016/j.neuroimage.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chen YY, Chang C. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillery E, Bittar RG, Robson MD, Behrens TE, Stein J, Aziz TZ, Johansen-Berg H. Connectivity of the human periventricular-periaqueductal gray region. J Neurosurg. 2005;103:1030–1034. doi: 10.3171/jns.2005.103.6.1030. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Smith DF. Cognitive brain mapping for better or worse. Perspectives in biology and medicine. 2010;53:321–329. doi: 10.1353/pbm.0.0165. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sprenger C, Bingel U, Buchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain. 2011;152:428–439. doi: 10.1016/j.pain.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Steinbok P, Boyd MC. Periaqueductal tumor as a cause of late-onset aqueductal stenosis. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1987;3:170–174. doi: 10.1007/BF00717895. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Current biology : CB. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Steward CA, Marsden CA, Prior MJ, Morris PG, Shah YB. Methodological considerations in rat brain BOLD contrast pharmacological MRI. Psychopharmacology (Berl) 2005;180:687–704. doi: 10.1007/s00213-005-2213-7. [DOI] [PubMed] [Google Scholar]
- Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S. Diffusion tensor imaging and axonal tracking in the human brainstem. NeuroImage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroman PW. Spinal fMRI investigation of human spinal cord function over a range of innocuous thermal sensory stimuli and study-related emotional influences. Magn Reson Imaging. 2009;27:1333–1346. doi: 10.1016/j.mri.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Coe BC, Munoz DP. Influence of attention focus on neural activity in the human spinal cord during thermal sensory stimulation. Magn Reson Imaging. 2011;29:9–18. doi: 10.1016/j.mri.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Watanabe S, Hamaguchi T, Mine H, Terui T, Kanazawa M, Oohisa N, Maruyama M, Yambe T, Itoh M, Fukudo S. Brain activation associated with changes in heart rate, heart rate variability, and plasma catecholamines during rectal distention. Psychosomatic medicine. 2009;71:619–626. doi: 10.1097/PSY.0b013e31819b69ca. [DOI] [PubMed] [Google Scholar]
- Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. NeuroImage. 2008;39:1647–1653. doi: 10.1016/j.neuroimage.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao T, Tsujimura A, Miyagawa Y, Kiuchi H, Ueda T, Hirai T, Komori K, Takada S, Nonomura N, Osaki Y, Enomoto K, Hatazawa J, Okuyama A. Brain responses during the first desire to void: a positron emission tomography study. International journal of urology : official journal of the Japanese Urological Association. 2008;15:724–728. doi: 10.1111/j.1442-2042.2008.02076.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. Regional gray matter volume of dopaminergic system associate with creativity: evidence from voxel-based morphometry. NeuroImage. 2010;51:578–585. doi: 10.1016/j.neuroimage.2010.02.078. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Georg Thieme Verlag; 1988. [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Tasker RR. Identification of pain processing systems by electrical stimulation of the brain. Human neurobiology. 1982;1:261–272. [PubMed] [Google Scholar]
- ten Donkelaar HJ, de Boer-van Huizen R. A possible pain control system in a non-mammalian vertebrate (a lizard, Gekko gecko) Neurosci Lett. 1987;83:65–70. doi: 10.1016/0304-3940(87)90217-5. [DOI] [PubMed] [Google Scholar]
- Teves D, Videen TO, Cryer PE, Powers WJ. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci U S A. 2004;101:6217–6221. doi: 10.1073/pnas.0307048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, McCusker E, Frackowiak RS. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain : a journal of neurology. 2002;125:1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- Thomas BA, Erlandsson K, Modat M, Thurfjell L, Vandenberghe R, Ourselin S, Hutton BF. The importance of appropriate partial volume correction for PET quantification in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2011;38:1104–1119. doi: 10.1007/s00259-011-1745-9. [DOI] [PubMed] [Google Scholar]
- Tilikete C, Pelisson D. Ocular motor syndromes of the brainstem and cerebellum. Curr Opin Neurol. 2008;21:22–28. doi: 10.1097/WCO.0b013e3282f4097d. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Association between Functional Connectivity Hubs and Brain Networks. Cerebral cortex. 2011 doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol. 2004;471:446–461. doi: 10.1002/cne.20033. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002a;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002b;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu YT, Yeh TC, Lirng JF, Hsieh JC. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150:462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping. 2011 doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Anderson J, Schwarz AJ, Coimbra A, Baumgartner R, Pendse G, George E, Nutile L, Wallin D, Bishop J, Neni S, Maier G, Iyengar S, Evelhoch JL, Bleakman D, Hargreaves R, Becerra L, Borsook D. Imaging Drugs with and without Clinical Analgesic Efficacy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain : a journal of neurology. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Buchel C. Dyspnea and pain share emotion-related brain network. NeuroImage. 2009a;48:200–206. doi: 10.1016/j.neuroimage.2009.06.015. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Eippert F, Baumann HJ, Klose H, Dahme B, Buchel C. Down-regulation of insular cortex responses to dyspnea and pain in asthma. American journal of respiratory and critical care medicine. 2009b;180:232–238. doi: 10.1164/rccm.200902-0300OC. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognit–ion. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. Journal of the National Cancer Institute. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. NeuroImage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Witzel J, Wiebking C, Gubka U, Rotte M, Schiltz K, Bermpohl F, Tempelmann C, Bogerts B, Heinze HJ, Northoff G. Pedophilia is linked to reduced activation in hypothalamus and lateral prefrontal cortex during visual erotic stimulation. Biological Psychiatry. 2007;62:698–701. doi: 10.1016/j.biopsych.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Wang SM, Constable RT, Tokoglu FS, Weiss DA, Freyle D, Kain ZN. Acupuncture-induced blood oxygenation level-dependent signals in awake and anesthetized volunteers: a pilot study. Anesth Analg. 2007;105:499–506. doi: 10.1213/01.ane.0000270216.71234.f5. [DOI] [PubMed] [Google Scholar]
- Weisberg DS, Keil FC, Goodstein J, Rawson E, Gray JR. The seductive allure of neuroscience explanations. J Cogn Neurosci. 2008;20:470–477. doi: 10.1162/jocn.2008.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41:629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- White AR, Filshie J, Cummings TM. Clinical trials of acupuncture: consensus recommendations for optimal treatment, sham controls and blinding. Complementary therapies in medicine. 2001;9:237–245. doi: 10.1054/ctim.2001.0489. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, Knopman DS, Petersen RC, Benarroch EE, Josephs KA, Jack CR., Jr Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain : a journal of neurology. 2007;130:708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg M, Westman J, Blomqvist A. Somatosensory projection to the mesencephalon: an anatomical study in the monkey. J Comp Neurol. 1987;264:92–117. doi: 10.1002/cne.902640108. [DOI] [PubMed] [Google Scholar]
- Wiggins GC, Polimeni JR, Potthast A, Schmitt M, Alagappan V, Wald LL. 96-Channel receive-only head coil for 3 Tesla: design optimization and evaluation. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2009;62:754–762. doi: 10.1002/mrm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. Journal of applied physiology. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- Yaguez L, Coen S, Gregory LJ, Amaro E, Jr, Altman C, Brammer MJ, Bullmore ET, Williams SC, Aziz Q. Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology. 2005;128:1819–1829. doi: 10.1053/j.gastro.2005.02.068. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978;4:299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009a;29:10264–10271. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci. 2009b;29:10264–10271. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Social neuroscience. 2007;2:276–291. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The neural correlates of aversive auditory stimulation. NeuroImage. 2002;16:746–753. doi: 10.1006/nimg.2002.1115. [DOI] [PubMed] [Google Scholar]
- Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Zempleni MZ, Michels L, Mehnert U, Schurch B, Kollias S. Cortical substrate of bladder control in SCI and the effect of peripheral pudendal stimulation. NeuroImage. 2010;49:2983–2994. doi: 10.1016/j.neuroimage.2009.10.064. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Cao XD, Lie J, Tang WJ, Liu HQ, Fenga XY. Neuronal specificity of needling acupoints at same meridian: a control functional magnetic resonance imaging study with electroacupuncture. Acupuncture & electro-therapeutics research. 2007;32:179–193. [PubMed] [Google Scholar]
- Zhang JH, Li J, Cao XD, Feng XY. Can electroacupuncture affect the sympathetic activity, estimated by skin temperature measurement? A functional MRI study on the effect of needling at GB 34 and GB 39 on patients with pain in the lower extremity. Acupuncture & electro-therapeutics research. 2009;34:151–164. doi: 10.3727/036012909803861013. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- Zyloney CE, Jensen K, Polich G, Loiotile RE, Cheetham A, LaViolette PS, Tu P, Kaptchuk TJ, Gollub RL, Kong J. Imaging the functional connectivity of the Periaqueductal Gray during genuine and sham electroacupuncture treatment. Mol Pain. 2010;6:80. doi: 10.1186/1744-8069-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]