Abstract
The foreskin is the main site of heterosexual HIV acquisition in uncircumcised men, but functional data regarding T cells subsets present at this site are lacking. Foreskin tissue and blood were obtained from Ugandan men undergoing elective adult circumcision. Tissue was treated by mechanical and enzymatic digestion followed by T cell subset identification and assessment of cytokine production using flow cytometery. Foreskin CD4+ T cells were predominantly an effector memory phenotype, and compared to blood they displayed a higher frequency of CCR5 expression (42.0% vs. 9.9%) and IL-17 production. There was no difference in T regulatory cell frequency, but IFNγ and TNFα production were increased in foreskin CD8+ T cells. These novel techniques demonstrate that the foreskin represents a pro-inflammatory milieu that is enriched for HIV-susceptible T cell subsets. Further characterization of foreskin T cell subsets may help to define the correlates of HIV susceptibility in the foreskin.
INTRODUCTION
As of 2009 there were 33.3 million people infected with HIV-1 (HIV), and only a third of those requiring treatment were receiving it.1 In addition there were an estimated 2.6 million new infections in that year, the majority transmitted through heterosexual sex, emphasizing the urgent need for better HIV prevention strategies. Clinical trials have demonstrated that circumcision reduces HIV acquisition by 50–60% in heterosexual men, proving that the foreskin is the site of most acquisition in uncircumcised men exposed to HIV during insertive vaginal sex.2–4 Although other penile sites such as the urethra may also play a role,5 the central role of the foreskin in HIV acquisition was further supported by the observation that an increased foreskin surface area correlated with increased risk of HIV acquisition.6 However, the immune events that surround acquisition and establishment of productive infection in the foreskin are poorly defined.7 Understanding the immunopathogenesis of HIV acquisition in the foreskin remains an important priority for the development of new prevention modalities, despite the efficacy of male circumcision, as evidenced by the fact that only a third of eligible men opted to avail themselves for free male circumcision during a recent HIV vaccine trial in South Africa.8
In the cervix, HIV and SIV infection is initiated by a small founder population of infected CD4+ T cells that expands through the local production of chemoattractant cytokines, followed by subsequent recruitment of activated memory CD4+ T cells.9,10 It is likely that the efficiency with which this founder virus population expands depends on the immune milieu in the genital mucosa at the time of exposure to HIV.11 While resting CD4+ T cells can be infected, viral replication within such cells is less efficient, and HIV propagation and dissemination from the site of initial infection is driven by the rapid recruitment of activated CD4+ T cells in which the virus can more readily replicate.9,12 Recruitment of these activated CD4+ T cells to the initial site of exposure may be assisted by HIV-induced changes in the local immune milieu, including the expression of chemokines such as MIP-3α and MIP-1β by epithelial and plasmacytoid dendritic cells.9,13
The presence or absence of certain T cell subsets at the mucosal site of HIV exposure may be an important determinant of HIV susceptibility. Genital herpes is associated with an increase in activated CD4+ T cells within the foreskin and female genital tract,14–16 perhaps contributing to the three-fold increase in HIV susceptibility associated with this infection.17 The pro-inflammatory Th17 cells that normally protect skin and mucosal sites against bacterial and fungal infection are present at high frequency in the female genital mucosa and display enhanced HIV-susceptibility.18,19 IL22 is an important effector molecule of Th17 cells, playing a role in epithelial integrity and repair. IL22 is also produced by pro-inflammatory Th22 cells, which may be preferentially infected by HIV.20 Conversely, CD25+/FoxP3+ (Forkhead box P3) T regulatory cells (Tregs) play an important role in controlling inflammation, and higher Treg frequencies in the blood have been linked to reduced HIV susceptibility.21 Furthermore, individuals who are HIV exposed but seronegative (HESN) show a quiescent immune phenotype with reduced basal T cell cytokine production and lower proportions of activated T cells.21–24
While immunohistochemistry is able to demonstrate the tissue position of specific cells in three dimensions, the ability of this technique to define cellular immune function is very limited. Therefore we have developed techniques to isolate a single cell suspension from fresh foreskin tissues, and to characterize the frequency and function of foreskin T cell subsets using multiparameter flow cytometry. Our results demonstrate that the foreskin constitutes a pro-inflammatory immune environment that is enriched for HIV-susceptible T cell subsets.
RESULTS
Study Population
Participants were recruited from a longstanding community cohort in Rakai, Uganda.25 Foreskin and whole blood were collected from 46 men between the ages 15–49 who had requested elective circumcision at the Rakai Health Sciences Program clinic in Kalisizo, Uganda, and who had provided written, informed consent. All men were free of symptomatic STIs at the time of surgery.
T cell proportions in the blood and foreskin
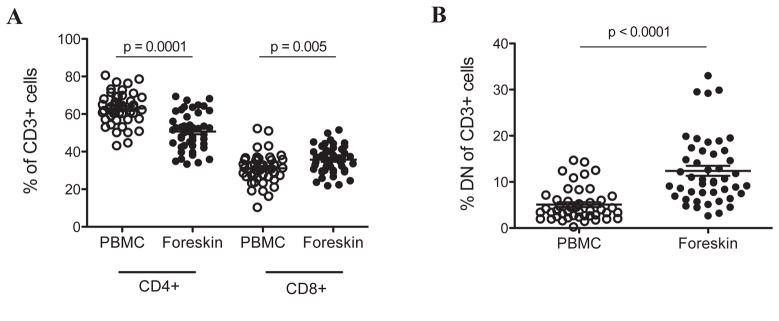
Foreskin T cells were identified based on the expression of CD3, and comprised between 0.1–0.6% of total recorded events from digested, filtered foreskin tissue (Supplementary material). Upon permeabilization, many contaminating events (non-CD3+) were removed from the cell solution, so that CD3+ events constituted 10–15% of total recorded events, allowing for easier identification of lymphocytes based on forward and side scatter alone. Due to differences in the size of foreskin samples and to variation in tissue physical properties leading to differential cell loss during the digestion procedure, the absolute number of CD3 cells per foreskin was not defined. Rather, we report proportions of cells, standardized to CD3+, CD3/4+ or CD3/8+. The majority of foreskin CD3+ cells were found to express either CD4 (mean, 51.4% of CD3+ cells) or CD8 (mean, 35.1%). Peripheral blood cells isolated from the same participants in parallel contained a higher proportion of CD4+ cells (63.4%, p=0.0001) and slightly lower proportion of CD8+ cells (31.8%, p=0.005, Figure 1A), resulting in a substantially reduced CD4/CD8 ratio in the foreskin compared to blood (1.53 vs. 2.27; p<0.0001). A small proportion of CD3+ cells in both the foreskin and peripheral blood were found to express both CD8and CD4 (0.41% and 0.84%, respectively, not significantly different). Of note, the foreskin contained more than twice as many CD4−/CD8− (double negative) CD3+ cells as the blood (12.4% vs. 5.1%, p<0.0001, Figure 1B).
Figure 1. CD4+ and CD8+ T cell subsets within the foreskin and peripheral blood.
PBMC and foreskin cells from 46 men were stained with CD3-FITC, CD4-PE, and CD8-PerCP. Graphs show percentages of CD3+ cells within PBMC or foreskin cells that co-express (A) either CD4 or CD8, or (B) expressed neither CD4 nor CD8 (double negative, DN, T cells).
CCR5 expression and CD4+ Th17 and T regulatory subsets in the foreskin
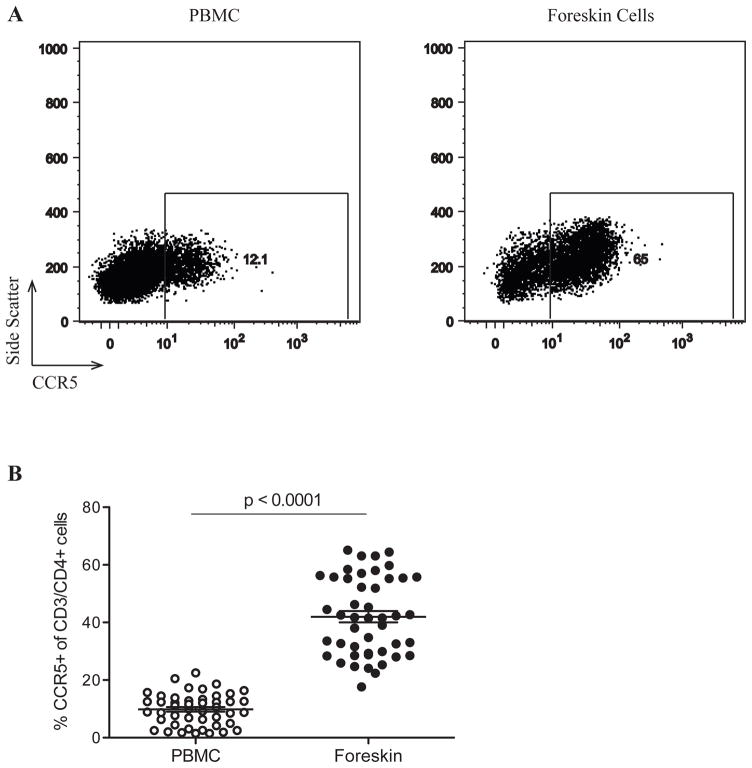
The great majority of sexually transmitted viruses use CCR5 as a co-receptor.26 Therefore, we compared the expression of CCR5 on CD3/CD4+ between T cells isolated from the foreskin and the blood of study participants (Figure 2A). The proportion of foreskin CD4+ T cells expressing CCR5 was over four-fold higher than that in blood (41.7% in the foreskin vs. 9.9% in PBMCs, p<0.0001, Figure 2B).
Figure 2. CCR5 expression on CD4+ T cells from the foreskin and peripheral blood.
PBMC and foreskin cells from 46 men were stained with CD3-APC, CD4-PE, and CCR5-FITC. Plots in (A) were created by gating on CD3+/CD4+ events. The gate defining CCR5+ events was created based on PBMC staining for this marker and applied to foreskin plots. (B) Proportions of CD3+/CD4+ cells in PBMC and foreskin cells co-expressing CCR5.
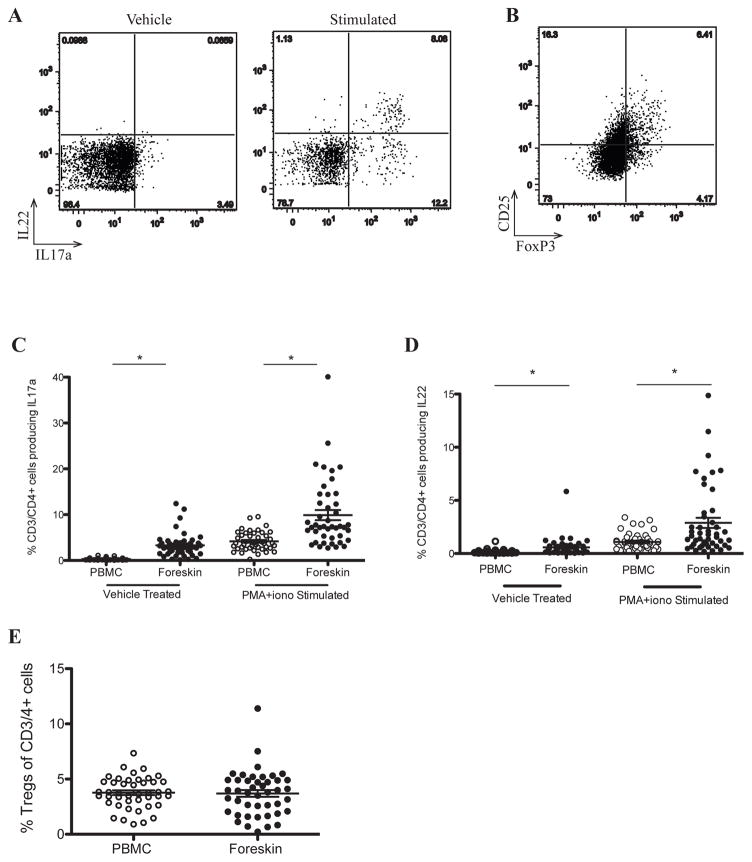
Th17 cells may be preferentially infected by HIV18 and the ratio of mucosal Th17/Treg cells is important in HIV immunopathogenesis.27 Regulatory T cells (Tregs) were defined as CD3+/CD4+ cells that co-expressed CD25 and the transcription factor FoxP3, and Th17 cells were defined as CD4+ T cells producing IL17a, either at rest or upon stimulation with PMA-ionomycin. Substantial differences in Th17 subsets were seen between foreskin and blood. Specifically, a much higher proportion of isolated foreskin CD4+ T cells produced IL17a, both unstimulated (3.3% of CD3/4+ cells vs. 0.30%, p<0.0001) and after stimulation (7.4% vs. 3.8%, p<0.0001; Figures 3A and C). In addition, a higher proportion of CD4+ T cells from the foreskin produced the Th17-associated cytokine IL22 than in the peripheral blood, both at rest (0.166% vs. 0.579%; p<0.0001), and after stimulation (1.09% vs. 2.88%; p<0.0001, Figures 3A and D). However, no difference was observed in the frequency of Tregs between the foreskin and blood (3.9% of foreskin CD3/4+ cells, vs. 3.7% in blood; Figures 3B and E). As a consequence, the Th17/Treg ratio was considerably higher in the foreskin that in the blood (4.1 vs. 1.3, respectively; p<0.0001).
Figure 3. Increased production of IL17a and IL22 by foreskin CD4+ T cells, with no increase in Treg frequency.
PBMC and foreskin cells from 46 men were either left unstimulated (vehicle/Treg) or treated with PMA-ionomycin (stimulated). Representative plots of foreskin cells are shown (A, B). The gates in (A) defining IL17a+ and IL22+ events were created based on unstimulated PBMC staining for each patient, and then applied to stimulated PBMC and foreskin plots. The gate defining CD25+ events in (B) was created based on CD25-FMOs (fluorescence minus one = CD3, CD4 and FoxP3). (C) Proportions of Th17 cells in PBMC and foreskin samples (CD3+/CD4+/IL17a+). (D) IL22 production in stimulated PBMC and foreskin CD4 T cells. (E) Proportions of PBMC and foreskin CD3+/CD4+ cells that are Tregs. *p<0.0001
Foreskin CD4+ T cells display a predominantly effector memory phenotype
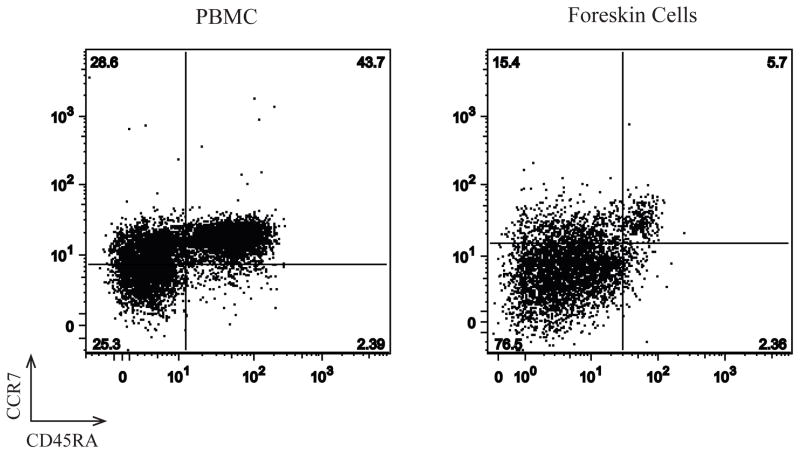
The memory phenotype of foreskin and blood CD4+ T cells was assessed in a subset of 3 individuals (representative plots; Figure 4) by staining with CD45RA to distinguish naïve (CD45RA+) from memory T cells (CD45RA−), and CCR7 to further delineate central (TCM; CD45RA−/CCR7+) and effector (TEM; CD45RA−/CCR7−) memory cells.28 While blood contained approximately equal proportions of naïve and memory T cells, the foreskin contained few naïve T cells (ranging from 1.2 to 5.8%). Of the memory CD4+ T cells in the foreskin, the majority was of the TEM phenotype (72.6–89.5%).
Figure 4. The foreskin contains primarily effector memory CD4 T cells (TEM).
Memory phenotype of PBMC and foreskin mononuclear cells was assessed on a sub-group of three men by staining with CD3-FITC, CD4-PerCP, CCR7-PE and CD45RA-APC. Representative plots were created by gating on CD3+/CD4+ events. The gates defining CD45RA+ and CCR7+ events were created based on FMO (Fluorescence minus one) staining for each cell type.
Capacity of foreskin T cells to produce pro-inflammatory cytokines
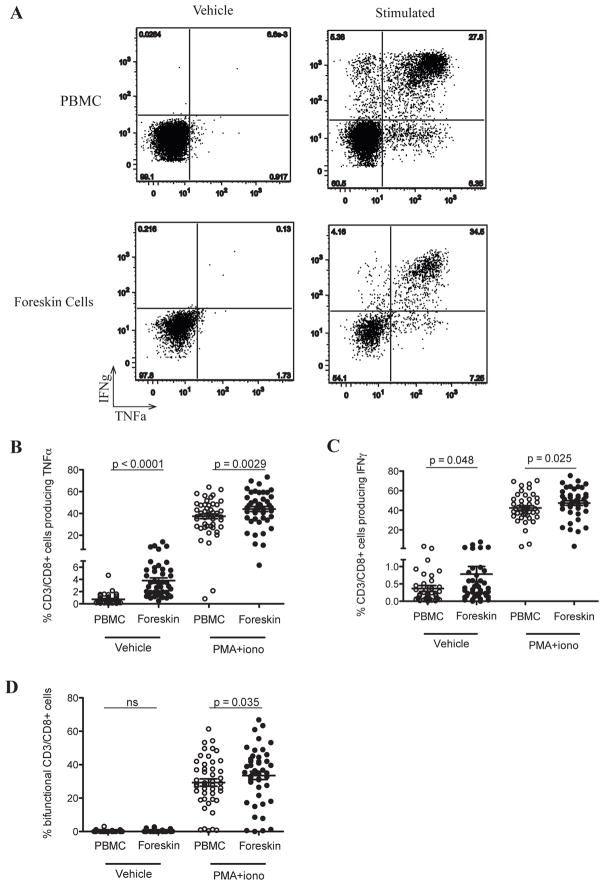
Since an inflammatory mucosal immune milieu may enhance HIV acquisition,11 we next assessed the production of the cytokines IFNγ and TNFα by CD8+ and CD8− T cell subsets, both at rest and after stimulation (Figure 5A). A relatively low frequency of foreskin T cells produced pro-inflammatory cytokines prior to stimulation, but this frequency was higher than blood T cells for both TNFα (2.8% vs. 0.47%; p<0.0001; Figure 5B) and IFNγ (0.33% vs. 0.19%; p=0.048; Figure 5C). Likewise, a higher frequency of foreskin CD8+ T cells produced pro-inflammatory cytokines after mitogen stimulation: this was the case for both TNFα (45.3% vs. 39.4%, p=0.0029; Figure 5B), IFNγ (48.2% vs. 41.3%, p=0.025; Figure 5C), and for bi-functional cells co-producing both cytokines (35.2% vs. 28.6%, p=0.035; Figure 5D).
Figure 5. Enhanced production of pro-inflammatory cytokines by foreskin CD8+ T cells.
PBMC and foreskin cells from 46 men were either left unstimulated (vehicle) or treated with PMA-ionomycin. Cells were then stained with CD3-FITC, CD8-PerCP, TNFα-PE and IFNγ-APC. Plots in (A) were created by gating on CD3+/CD8+ events. The gates defining TNFα + and IFNγ+ events were created based on unstimulated PBMC staining for each patient, and then applied to stimulated PBMC and foreskin plots. (B) Proportions of CD8 T cells producing TNFα, (C) IFNγ, and (D) of bi-functional CD8 T cells (producing both TNFα and IFNγ).
While both peripheral blood and foreskin contained a small proportion of “double negative” (CD4−/CD8−) T cells, the great majority of CD3+/CD8− cells were CD4+ T cells (Figure 1). Therefore, we also quantified TNFα and IFNγ production in these CD8− T cells as a proxy for CD4+ T cells. A greater frequency of foreskin CD8− T cells than blood produced pro-inflammatory cytokines prior to stimulation (0.98% of foreskin cells produced TNFα vs. 0.29% of those from blood, p<0.0001; 0.48% of foreskin cells produced IFNγ vs. 0.18% of blood, p<0.01). After mitogen stimulation the foreskin contained more CD8− T cells producing IFNγ (45.1% of foreskin T cells vs. 40.9% of blood T cells, p=0.0006) and more bi-functional cells (20.6% vs. 15.9%, p<0.0027), although no differences in the frequency of cells producing TNFα were apparent between compartments.
DISCUSSION
While circumcision reduces the incidence of HIV by up to 60% in heterosexual African men,2–4 providing strong evidence that the foreskin is the main site of male HIV acquisition during vaginal sex,29 the immunobiology of HIV acquisition in the foreskin is poorly understood. Previous studies of genital immunology as it relates to the sexual acquisition of HIV have focused on the female genital tract and gut since samples are more easily obtained from these sites.30,31 While results of the recent circumcision trials have focused interest on the foreskin, immunology studies have often used cadaveric or fixed/cryopreserved tissues, precluding functional immune studies14,32–37. In collaboration with a clinical site providing safe and free male circumcision as an HIV prevention tool,4 we have developed field techniques utilizing expedited tissue processing and use of collagenase I for tissue digestion to isolate viable T cells from foreskin tissue with retention of the expression of T cell markers and the functional ability to produce multiple cytokines. This has allowed for the characterization of functional foreskin T cell subsets.
Several clear differences were evident between foreskin and blood T cell subsets, both in terms of proportions, expression of HIV co-receptor CCR5, memory phenotypes, and the production of pro-inflammatory cytokines. There was a relative enrichment of CD8+ T cells in the foreskin compared to the blood, contributing to a significantly reduced CD4/CD8 ratio in the foreskin. However, although the proportion of CD4+ T cells was reduced, the proportion of CD4 T cells in the foreskin that co-expressed CCR5 was over four times higher than in blood, potentially enhancing susceptibility to HIV infection. HIV strains that use CCR5 as an entry co-receptor (R5 strains) are almost always responsible for sexual HIV transmission in vivo,26 and an ex vivo model has demonstrated that the foreskin is susceptible to infection with R5-tropic but not X4-tropic viruses.38,39 These results suggest that the substantial enhancement of CCR5 expression on foreskin-derived T cells may have direct implications for HIV acquisition.
Interestingly, the proportion of double negative T cells (i.e. CD3+ but CD4−/CD8−) was twice as high in the foreskin than in blood. Various CD3+ T cell populations may be contained within this subset, including NKT cells40 and T cells bearing the variant T cell receptors (TCR) γδ41 or the regulatory TCR αβ+.42 Double negative CD3+ T cells have been associated with protection against SIV immunopathogenesis in some primate species.43 Elucidating the identity of these double negative cells using multiparameter flow cytometry and investigating their possible relevance for HIV transmission will be important areas for future study.
While phenotypic characterization of foreskin T cells has been possible using fixed or cryopreserved tissues, we were particularly interested to define their function directly ex vivo. Two CD4+ T cell subsets that may be particularly relevant to HIV acquisition and pathogenesis are Th17 and regulatory T cells (Tregs).27 Th17 cells are CD4+ T cells producing the cytokine IL17a, and play a prominent pro-inflammatory role in mucosal immune defense against invading bacterial and fungal pathogens through the IL17 mediated recruitment of neutrophils, induction of antimicrobial peptides, and maintenance of epithelial integrity.44 Th17 cells display enhanced susceptibility to HIV in vitro18,45 and are preferentially depleted from the blood and particularly the mucosa of HIV-infected individuals,18,30 suggesting their enrichment at mucosal surfaces might enhance HIV acquisition. Tregs have immunomodulatory effects that are thought to play an important role in counterbalancing Th17-induced inflammation, despite sharing a common precursor, chemokine receptors, and mucosal homing properties with Th17 cells.27 We found that Th17 proportions were substantially increased in the foreskin compared to blood in the absence of any corresponding enrichment in Treg cells. This increased Th17/Treg ratio in the foreskin suggests that this tissue is biased towards a predominantly pro-inflammatory immune environment, which could enhance HIV acquisition.11
Our data showing enhanced production of the cytokines TNFα and IFNγ by foreskin T cells, both at rest and after non-specific stimulation, supports the concept of the foreskin as a pro-inflammatory tissue. This enhanced cytokine production is likely to be related to the high proportion of effector memory T cells (TEM) found in the foreskin tissues, since this cell subset is primed to migrate to tissue sites and to carry out immediate effector functions.28
It is likely that both the function and proportions of T cell subsets in the foreskin would be impacted by common bacterial and viral genital co-infections,14,15 and any such differences might well have implications for HIV susceptibility. While men with symptomatic genital infections were excluded from male circumcision due to the potential increased risk of post-surgical infection and other complications, asymptomatic genital infections such as HSV-2 and HPV are common in these men.46,47 While the purpose of our initial analysis was to compare T cell subsets in the foreskin and blood, recruitment of a larger participant sample size is ongoing with the goal of characterizing the immune impact of these infections.
While our study examined pooled T lymphocytes derived from both the inner and outer foreskin, there is in vitro evidence to suggest that HIV acquisition may be more efficient across the inner surface of the foreskin,48 defined as the portion of the foreskin that sits against the glans on the non-erect penis but is exposed on the erect penis during intercourse. It was initially assumed that this increased susceptibility was due to a thinner keratin layer on the inner foreskin,36,48 but studies using freshly processed foreskin samples have shown no difference in this layer between the inner and outer foreskin.34,37 While reports of differences in the density of HIV target cells between these two sites have been contradictory,35,36,38,39,49 it does seem that cells of the inner foreskin may be functionally different to those of the outer foreskin, both in their responsiveness to cytokines such as TNFα and MIP1α49 and in their production of chemokines after HIV exposure.48–50 Better elucidation of the functional differences between T cells derived from the inner and outer foreskin will constitute an important area for future research.
In summary, we have developed novel techniques to purify a single-cell suspension from fresh foreskin tissues, and to characterize the functional characteristics of foreskin T cell populations. Compared to blood, the foreskin manifested a pro-inflammatory immune environment that was enriched for highly HIV-susceptible CD4+ T cell subsets such as Th17 cells and those expressing the HIV co-receptor CCR5. These observations have important implications for HIV susceptibility in the foreskin, and will permit larger immuno-epidemiology field studies aiming to define the immune correlates of HIV susceptibility in the foreskin.
METHODS
Participants
Participants were recruited from men in an established community cohort in Rakai, Uganda25 who had elected to undergo adult circumcision at the Rakai Health Sciences Program in Kalisizo, Uganda. Foreskins and whole blood samples were obtained from 46 HIV-negative men between the ages of 15–49. All participants provided written informed consent, and ethical approval was obtained through the research ethics boards of collaborating institutions (the University of Toronto, Uganda Virus Research Institute, and Western IRB). Surgery was deferred in the context of urethral discharge or clinically apparent genital ulceration. Participants were confirmed to be antibody-negative for HIV-1 and HIV-2 using two ELISAs (Murex HIV-1.2.O, Abbott, Abbott Park, Illinois, USA; and Vironistika HIV Uni-Form II plus O Mircoelisa System, bioMerieux; Marcy l’Etoile, France). Discordant results were confirmed by western blot (GS HIV-1 Western Blot, BioRad; Hercules, CA, USA). All participants were also screened for acute HIV infection by real time PCR. RNA was extracted from plasma samples using the Sample Preparation System (Abbott), and amplification was performed using the Real Time HIV-1 Amplification Reagent Kit (Abbott) run on the M2000rt (Abbott).
T cell isolation from the foreskin and blood
Foreskins were collected into RPMI 1640 media supplemented with: 10% heat-inactivated FBS, 10U/ml penicillin, 10μg/ml streptomycin, 250ng/ml amphotericin B, and 2mM L-Glutamine (all from Gibco, Invitrogen; Carlsbad, CA, USA; henceforth referred to as R10 medium). Foreskin samples were always processed within 15 minutes of surgery, since additional time caused the dermal morphology to change substantially, with gross macroscopic tissue edema (data not shown). Tissue was first sectioned into longitudinal strips including both inner and outer foreskin and containing both epidermal and dermal tissue. These strips were then further sectioned to create pieces of approximately 0.25cm2. Each piece was placed in a 1.5mL conical tube containing 1.0mL of 500U/mL Collagenase Type I (Gibco) and 42.5U/mL of DNAse (Invitrogen) in RPMI 1640 media supplemented with 10U/ml penicillin, 10μg/ml streptomycin, 250ng/ml amphotericin B, and 2mM L-Glutamine (henceforth referred to as RPMI, all from Gibco). Initial immune studies have used dispase for foreskin tissue digestion, but we found that treatment with as little as 1.0U/mL of dispase (Gibco) for 30 minutes at 37°C lead led to the loss of CD4 expression and decreased CD8 expression in both peripheral blood and foreskin-derived T cells (Supplementary Figure 1). Scissors were used to mechanically disrupt each piece of tissue, and tubes were then placed on a shaker (Eppendorf Thermomixer; Hamburg, Germany) for 30 minutes of enzymatic digestion at 37°C with shaking at 900rpm. The cellular suspension obtained from each tube was pooled and collagenase activity was inhibited by the addition of FBS to a final concentration of 10%. This cell suspension was then filtered through a 100μm cell strainer (BD Biosciences; Franklin Lakes, NJ USA) to remove any remaining undigested tissue. Filtered cells were washed once, resuspended in R10, and allowed to rest under normal growth conditions (37°C, 5% CO2, humidified atmosphere) for 3–7 hours. This combination of collagenase 1 and gentle mechanical digestion allowed for the retention of CD4 expression and gave a single cell suspension containing CD3+ T cells that showed a similar CD4 and CD8 expression profile to PBMCs from the same individual (Supplementary Figure 2).
PBMCs were isolated by density gradient centrifugation (Ficoll-Paque Plus; Amersham Biosciences; Uppsala, Sweden).
Characterization of CD4+ T cell subsets
Both PBMC and foreskin cell numbers were determined by trypan blue exclusion. 1×106 PBMCs and 10–20×106 foreskin cells (depending on yield) were plated in 500μl R10 and stimulated with either 1ng/ml phorbol-12-myristate-13-acetate (PMA) and 1μg/ml ionomycin (both from Sigma; St. Lousi, MO, USA) or vehicle (0.1% DMSO) with 5μg/ml Brefeldin A (GolgiPlug, BD Biosciences) for 9 hours at 37°C. Samples were then washed with cold 2% FBS in PBS and stained with fluorochrome-labeled monoclonal antibodies specific for CD3 (UCHT1), CD4 (RPA-T4), CD8 (SK1), CCR5 (2D7/CCR5), and CD25 (M-A251; all BD Biosciences). Excess surface antibody was removed by washing with 2% FBS in PBS. Samples for intracellular staining were permeabilized using either the eBioscience fixation/permeabilization solution for Treg identification (eBiosciences; San Diego, CA, USA) or the BD Cytofix/Cytoperm solution (BD Biosciences) for all others. Cells were washed in permeabilization wash buffer and stained with fluorochrome-labeled monoclonal antibodies specific for combinations of the following intracellular cytokines/transcription factors: TNFα (MAb11; BD Biosciences), IFNγ (B27; BD Biosciences), IL17a (eBio64DEC17; eBioscience), IL22 (22URTI; eBioscience), and FoxP3 (PCH101; eBioscience). Samples were acquired using a FACSCalibur flow cytometer (BD Systems) and data analysis performed using FlowJo analytical software version v.9.3 (Treestar; Ashland, OR, USA).
Unpermeabilized foreskin cells were gated based on forward and sideward scatter (Supplementary Figure 2). This gate was created based on the location of CD3+ T cells in the PBMC sample from the same patient. Back gating was used to confirm that this gate corresponded with the location of CD3+ cells in the foreskin sample (CD3+ cells representing ~0.1–0.6% of total events in the foreskin sample). A clearly visible population of CD3+ cells in the foreskin samples could then be identified. For unpermeabilized foreskin cells, 106 events were recorded, while only 105 events were recorded for PBMC and permeabilized foreskin cells, due to the large amount of other cell types present in unpermeabilized foreskin samples. After permeabilization the T cell population was enriched (3–5% of total events) and could be directly identified on the forward by side scatter plot (Supplementary Figure 2).
Statistical analysis
T cell populations were compared between blood and foreskin by paired Wilcoxon rank sum test. Statistical tests were run on SPSS v.17.0 for Mac (IBM; New York, NY, USA). Flow cytometry data was analyzed in FlowJo v.9.3 and Excel (Microsoft; Redmond, WA, USA) prior to statistical testing.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the support of the Rakai Health Sciences staff and thank the men who participated in this study.
Sources of support: Ontario HIV Treatment Network (JP, studentship); the Canada Research Chair Programme (RK, salary support); the Bill and Melinda Gates Foundation (RG, #22006.02); the Canadian Institutes of Health Research (CIHR # HBF-115704).
Footnotes
Disclosures
The authors have nothing to disclose and no conflicts of interest.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. 2010. pp. 1–364. [Google Scholar]
- 2.Auvert B, et al. Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk: The ANRS 1265 Trial. PLoS Medicine. 2005;2 doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey R, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. The Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 4.Gray RH, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. The Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 5.Anderson D, Politch JA, Pudney J. HIV infection and immune defense of the penis. Am J Reprod Immunol. 2011;65:220–229. doi: 10.1111/j.1600-0897.2010.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kigozi G, et al. Foreskin surface area and HIV acquisition in Rakai, Uganda (size matters) Aids. 2009;23:2209–2213. doi: 10.1097/QAD.0b013e328330eda8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganor Y, Bomsel M. HIV-1 transmission in the male genital tract. Am J Reprod Immunol. 2011;65:284–291. doi: 10.1111/j.1600-0897.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- 8.de Bruyn G, et al. Uptake of male circumcision in an HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2009;51:108–110. doi: 10.1097/QAI.0b013e3181a03622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z. Sexual Transmission and Propagation of SIV and HIV in Resting and Activated CD4+ T Cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 11.Kaul R, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. Journal of ReproductiveImmunology. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZQ. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proceedings of the National Academy of Sciences. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase AT. Early Events in Sexual Transmission of HIV and SIV and Opportunities for Interventions. Annual Review of Medicine. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 14.Johnson KE, et al. Effects of HIV-1 and Herpes Simplex Virus Type 2 Infection on Lymphocyte and Dendritic Cell Density in Adult Foreskins from Rakai, Uganda. Journal of Infectious Diseases. 2011;203:602–609. doi: 10.1093/infdis/jiq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebbapragada A, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. Aids. 2007;21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman EE, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 18.El Hed A, et al. Susceptibility of Human Th17 Cells to Human Immunodeficiency Virus and Their Perturbation during Infection. The Journal of Infectious Diseases. 2010;201:843–854. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinnon LR, et al. Characterization of a Human Cervical CD4+ T Cell Subset Coexpressing Multiple Markers of HIV Susceptibility. The Journal of Immunology. 2011;187:000–000. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 20.Kim CJ, Kaul R. Th22 cells constitute a highly HIV susceptible T cell subset that is associated with epithelial integrity in the sigmoid mucosa. 20th Annual Canadian Conference on HIV/AIDS Research (CAHR); Toronto, Ontario. 2011. [Google Scholar]
- 21.Card Catherine M, et al. Decreased Immune Activation in Resistance to HIV-1 Infection Is Associated with an Elevated Frequency of CD4+CD25+FOXP3+Regulatory T Cells. The Journal of Infectious Diseases. 2009;199:1318–1322. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- 22.Jennes W, et al. Suppressed cellular alloimmune responses in HIV-exposed seronegative female sex workers. Clinical and Experimental Immunology. 2006;143:435–444. doi: 10.1111/j.1365-2249.2006.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaren PaulJ, et al. HIV-Exposed Seronegative Commercial Sex Workers Show a Quiescent Phenotype in the CD4+T Cell Compartment and Reduced Expression of HIV-Dependent Host Factors. The Journal of Infectious Diseases. 2010;202:S339–S344. doi: 10.1086/655968. [DOI] [PubMed] [Google Scholar]
- 24.Bégaud E, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RH, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. AIDS. 2000;14:2371–2381. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 26.Grivel JC, Shattock RJ, Margolis LB. Selective transmission of R5 HIV-1 variants: where is the gatekeeper? Journal of Translational Medicine. 2010;9:S6. doi: 10.1186/1479-5876-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanwar B, Favre D, McCune JM. Th17 and regulatory T cells: implications for AIDS pathogenesis. Current Opinion in HIV and AIDS. 2010;5:151–157. doi: 10.1097/COH.0b013e328335c0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 29.Dinh MH, Fahrbach KM, Hope TJ. The Role of the Foreskin in Male Circumcision: An Evidence-Based Review. American Journal of Reproductive Immunology. 2011;65:279–283. doi: 10.1111/j.1600-0897.2010.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chege D, et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. Aids. 2011;25:741–749. doi: 10.1097/QAD.0b013e328344cefb. [DOI] [PubMed] [Google Scholar]
- 31.Kaul R, et al. HIV-1-Specific Mucosal CD8+ Lymphocyte Responses in the Cervix of HIV-1-Resistant Prostitutes in Nairobi. Journal of Immunology. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 32.Bailey RC, et al. HIV-1 Target Cells in Foreskins of African Men With Varying Histories of Sexually Transmitted Infections. American Journal of Clinical Pathology. 2006;125:386–391. [PubMed] [Google Scholar]
- 33.Hirbod T, et al. Abundant Expression of HIV Target Cells and C-Type Lectin Receptors in the Foreskin Tissue of Young Kenyan Men. The American Journal of Pathology. 2010;176:2798–2805. doi: 10.2353/ajpath.2010.090926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinh MH, McRaven MD, Kelley ZL, Penugonda S, Hope TJ. Keratinization of the adult male foreskin and implications for male circumcision. Aids. 2010;24:899–906. doi: 10.1097/QAD.0b013e3283367779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussain LA, Lehner T. Comparative investigation of Langerhans’ cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology. 1995;85:475–484. [PMC free article] [PubMed] [Google Scholar]
- 36.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 37.Qin Q, et al. Langerhans’ cell density and degree of keratinization in foreskins of Chinese preschool boys and adults. International Urology and Nephrology. 2009;41:747–753. doi: 10.1007/s11255-008-9521-x. [DOI] [PubMed] [Google Scholar]
- 38.Fischetti L, Barry SM, Hope TJ, Shattock RJ. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. Aids. 2009;23:319–328. doi: 10.1097/QAD.0b013e328321b778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson BK, et al. Susceptibility to Human Immunodeficiency Virus-1 Infection of Human Foreskin and Cervical Tissue Grown in Explant Culture. American Journal of Pathology. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annual Review of Immunology. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 41.Gollob K, Antonelli L, Faria D, Keesen T, Dutra W. Immunoregulatory mechanisms and CD4–CD8–(double negative) T cell subpopulations in human cutaneous leishmaniasis: A balancing act between protection and pathology. International Immunopharmacology. 2008;8:1338–1343. doi: 10.1016/j.intimp.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D, et al. New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood. 2007;109:4071–4079. doi: 10.1182/blood-2006-10-050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milush JM, et al. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J Clin Invest. 2011;121:1102–1110. doi: 10.1172/JCI44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor W, Zenewicz LA, Flavell RA. The dual nature of TH17 cells: shifting the focus to function. Nature Immunology. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 45.Gosselin A, et al. Peripheral Blood CCR4+CCR6+ and CXCR3+CCR6+ CD4+ T Cells Are Highly Permissive to HIV-1 Infection. The Journal of Immunology. 2009;184:1604–1616. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray RH, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. Journal of Infectious Diseases. 2010;201:1455–1462. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tobian Aaron AR, et al. Factors Associated with the Prevalence and Incidence of Herpes Simplex Virus Type 2 Infection among Men in Rakai, Uganda. The Journal of Infectious Diseases. 2009;199:945–949. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganor Y, et al. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans–T cell conjugates. Mucosal Immunology. 2010;3:506–522. doi: 10.1038/mi.2010.32. [DOI] [PubMed] [Google Scholar]
- 49.Fahrbach KM, Barry SM, Anderson MR, Hope TJ. Enhanced cellular responses and environmental sampling within inner foreskin explants: implications for the foreskin’s role in HIV transmission. Mucosal Immunology. 2010;3:410–418. doi: 10.1038/mi.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Z, et al. HIV-1 efficient entry in inner foreskin is mediated by elevated CCL5/RANTES that recruits T cells and fuels conjugate formation with Langerhans cells. PLoS Pathog. 2011;7:e1002100. doi: 10.1371/journal.ppat.1002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.