Abstract
The human POK family members are transcription factors with a POZ domain and zinc fingers that act primarily as transcriptional repressors. Several members of this family are involved in oncogenesis and this prompted us to assess whether expression levels of individual POK family members are associated with clinical outcomes in cancer. We have observed that ZBTB4 is downregulated in breast cancer patients, and that its expression is significantly correlated with relapse-free survival. Further integrative analysis of mRNA and microRNA (miR) expression data from the NCI-60 cell lines revealed an inverse correlation between ZBTB4 and oncogenic miRs derived from the miR-17-92 cluster and its paralogues. The experimental results using MDA-MB-231 and MCF-7 human breast cancer cells confirm that miRNAs derived from these clusters, containing miR-17-5p, miR-20a, miR-106a, miR-106b and miR-93, negatively regulate ZBTB4 expression. Overexpression of ZBTB4 or restoration of ZBTB4 by using an antagomir inhibit growth and invasion of breast cancer cells, and this effect is due, in part, to ZBTB4-dependent repression of the specificity protein 1 (Sp1), Sp3, and Sp4 genes, and subsequent downregulation of several Sp-dependent oncogenes, in part, through competition between ZBTB4 and Sp transcription factors for GC-rich promoter sequences. These results confirm that ZBTB4 functions as a novel tumor suppressor gene with prognostic significance for breast cancer survival, and the oncogenic miR-17-92/ZBTB4/Sp axis may be a potential therapeutic target.
Keywords: ZBTB4, miR-17-92 cluster, breast cancer, prognostic, Sp transcription factors
INTRODUCTION
Transcription factors of the POK family are characterized by an N-terminal BTB/POZ domain, and a variable number of Krüppel-like zinc fingers (Perez-Torrado et al., 2006; Costoya, 2007). The human genome encodes over 40 members of this family, and several have causative roles in cancer (Perez-Torrado et al., 2006). ZBTB7 (also known as Pokemon, LRF, or FBI-1) controls cellular transformation (Pendergrast et al., 2002; Maeda et al., 2005), and ZBTB29 (also known as HIC-1) is a p53 effector lost in many neuroblastomas (Chen et al., 2003; Chen et al., 2005). ZBTB27 (also known as BCL6) regulates lymphocyte survival and is crucially involved in B-cell lymphoma (Kojima et al., 2001; Niu, 2002; Cattoretti et al., 2005). The functions of many other ZBTB proteins are characterized poorly or not at all; this prompted us to examine whether the expression of ZBTB genes is correlated with clinical outcome of cancer patients to identify new prognostic and functional roles of ZBTB proteins in cancer. Here we report that ZBTB4 protein and mRNA levels are downregulated in breast cancer patients and that its expression has powerful predictive value for relapse-free survival from this disease.
MicroRNAs (miRs) exhibit sequence-specific interactions with 3'-UTR regions of mRNAs to regulate their stability and translation and are crucial regulators of the cellular identity. Not surprisingly, different miRs exhibit tumor-suppressive and oncogenic activities and these can be tumor context-dependent (Volinia et al., 2006; Liu et al., 2010). Interaction of miRs with 3'-UTR regions results in translational repression, and POK family genes often have long and well-conserved 3'-UTRs, suggesting their potential regulation by miRs, and our previous studies have demonstrated that ZBTB10 expression is suppressed by miR-27a in several cancer cell lines (Mertens-Talcott et al., 2007; Jutooru et al., 2010). This led us to hypothesize that ZBTB4 expression may also be regulated by miRs in cancer cell lines and this possibility has been addressed using publically available mRNA and miR data sets. Analyses of these data indicate that ZBTB4 can be a potential target of multiple miRs derived from the components of the miR-17-92 cluster and its paralogues.
POK proteins generally function as sequence-specific transcriptional repressors (Tillotson, 1999; Filion et al., 2006; Weber et al., 2008; Jeon et al., 2008; Koh et al., 2009; Jeon et al., 2009; Sasai et al., 2010), and ZBTB4 binds several types of genomic target sites, including certain GC-rich sequences that bind specificity protein 1 (Sp1) (Filion et al., 2006; Weber et al., 2008; Sasai et al., 2010). Sp1, Sp3 and Sp4 are transcriptional activators that regulate a number of pro-survival and pro-invasion genes in breast cancer cells (Abdelrahim et al., 2002; Abdelrahim et al., 2004; Abdelrahim et al., 2007; Chadalapaka et al., 2008; Jutooru et al., 2010; Chadalapaka et al., 2010). We posited that ZBTB4 might interfere with the function of Sp1, Sp3 and Sp4 in breast cells and our results show that ZBTB4 indeed negatively regulates the expression of Sp1, Sp3, Sp4, and some of their important target genes such as vascular endothelial growth factor (VEGF), VEGF receptor 1, and survivin (Abdelrahim et al., 2002; Abdelrahim et al., 2004; Abdelrahim et al., 2007; Chadalapaka et al., 2008; Jutooru et al., 2010; Chadalapaka et al., 2010). The mechanism of ZBTB4-dependent repression of Sp1, Sp3, Sp4 and Sp-regulated genes involves competition between ZBTB4 and Sp proteins for binding to GC-rich promoter sequences.
Our results identify ZBTB4 as a new and important prognostic factor in human breast cancer that is regulated by oncogenic miR-17-92 cluster and its paralogues. Restoration of ZBTB4 by inhibiting expression of oncogenic miRs derived from miR-17-92 cluster and its paralogues suppress expression of Sp1, Sp3, and Sp4 and Sp-regulated pro-oncogenic gene products, resulting in inhibition of cancer cell proliferation and invasion.
METHODS AND MATERIALS
Chemicals, Antibodies, Plasmids and Reagents
Antibodies against Sp1, Sp3, Sp4, VEGF and VEGFR1 (Santa Cruz) and survivin and poly(ADP-ribose) polymerase (PARP) (Cell Signaling Technology) were purchased. ZBTB4 antibody was generated in the Defossez laboratory (Filion et al., 2006; Weber et al., 2008; Sasai et al., 2010). Antisense miRs, mimic miRs and their counterpart controls were purchased from Dharmacon, and the ZBTB4 expression vector and empty vector (pCMV-SPORT6) were from Open Biosystem. MISSION® siRNA (Sigma Aldrich) and control siRNA (Quiagen) were purchased, and MCF-7 and MDA-MB-231 human breast cancer cell lines were obtained from the American Type Culture Collection (Mertens-Talcott et al., 2007).
Immunoblotting, Transfection and Luciferase Assays
Whole cell lysates were analyzed by western blots essentially as described (Jutooru et al., 2010). Various construct (0.25 µg), β-galactosidase, siRNAs and other reagents and their corresponding controls were transfected (Lipofectamine 2000) and activity relative to β-galactosidase was determined.
Real-time PCR
Real time PCR was determined using total RNA extracts essentially as described (Mertens-Talcott et al., 2007; Jutooru et al., 2010), and PCR primers were purchased from Qiagen. Each gene value was normalized to the TATA-binding protein.
Cloning of 3’-UTR ZBTB4 reporter construct
The entire 2559 bp 3’-UTR of human ZBTB4 (2559 bp) was first amplified from human genomic DNA with following PCR primer set: 5’-TAT CTC GAG ACC CTG GGG CTC AAT CCC-3’ (sense) and 5’-TAT GCG GCC GCT CAT TTG TCT TGT TGG TTT ATT GG-3’ (antisense) and cloned into XhoI and NotI sites of psiCheck™ 2 vector (Promega). The 3’-UTR ZBTB4 reporter luciferase containing either core seed sequence (1836–1886 bp WT) or mutated sequence (1836–1886bp MT) was generated using following primers: For 1836–1886 WT, 5’-TCG AGA ATT CCC ACA AAT CTT GTT TCT GGC ACT TTA GAA AAA CTG CAA AAA AAT ACG TGC-3’ (sense) and 5’-GCG GCC GCA CGT ATT TTT TTG CAG TTT TTC TAA AGT GC CAG AAA CAA GAT TTG TGG GAA TTC-3’ (antisense); and For 1836–1886 MT, 5’-TCG AGA ATT CCC ACA AAT CTT GTT TCT GGA AAG GTA GAA AAA CTG CAA AAA AAT ACG TGC-3’ (sense) and 5’-GGC CGC ACG TAT TTT TTT GCA GTT TTT CTA CCT TTC CAG AAA CAA GAT TTG TGG GAA TTC-3’(antisense). Wild-type and mutated core seed sequences are shown in bold type.
Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation assay was performed as previously described (Lee et al., 2010). Briefly, MCF-7 cells 2.5 X 106) were seeded and after 24 hr, transfected with as-CT and As-miR-20a. After 48 hr, cells were cross-linked by formaldehyde. The equal amount of fragmented chromatin was immunoprecipitated using Sp1, RNA polymerase II (Santa Cruz Biotech) and ZBTB4 (from Dr. Defossez) antibodies. Rabbit immunoglobulin was used as control. The primer sets for negative and positive (GAPDH) were also described previously (Chadalapaka et al., 2010). Real-time PCR quantification was performed (see Table S3 for the primer sequence for the ChIP assay) and the total input was used as a control. The PCR amplified product from primer set 6 was resolved on a 2% agarose gel in the presence of ethdium bromide and visualized.
Invasion Assay
Transwell plates (Costar, 8.0 µm pore size) coated with Matrigel were used to measure tumor invasion upon ZBTB4 or as-miR-20a transfection. Cells were first transfected with either ZBTB4 or empty expression plasmid, or as-miR-20a or as-CT using lipofectamine 2000 (Invitrogen). The assay was carried out using the standard assay protocol and invading cells were photographed and counted under a microscope and the relative number was calculated.
Cancer Patient Gene Expression Data
Normalized gene expression data from the NKI and UNC data were obtained from the public Stanford microarray site (http://microarraypubs.stanford.edu/wound_NKI) and from the UNC microarray database (http://genome.unc.edu). Normalized lung cancer cohort gene expression data and B cell lymphoma were obtained from Gene Expression Omnibus (accession number GSE11969 and GSE10866) (Takeuchi et al., 2006; Lenz et al., 2008).
Statistical Significance and Survival Analysis
Statistical significance was determined by Student's t test (two-sided). Gene clustering was performed using Cluster and Treeview programs (Eisen et al., 1998). Kaplan-Meier analysis and log-rank test were applied to estimate patient survival.
RESULTS
Prognostic significance and expression of ZBTB4 in breast cancer patients
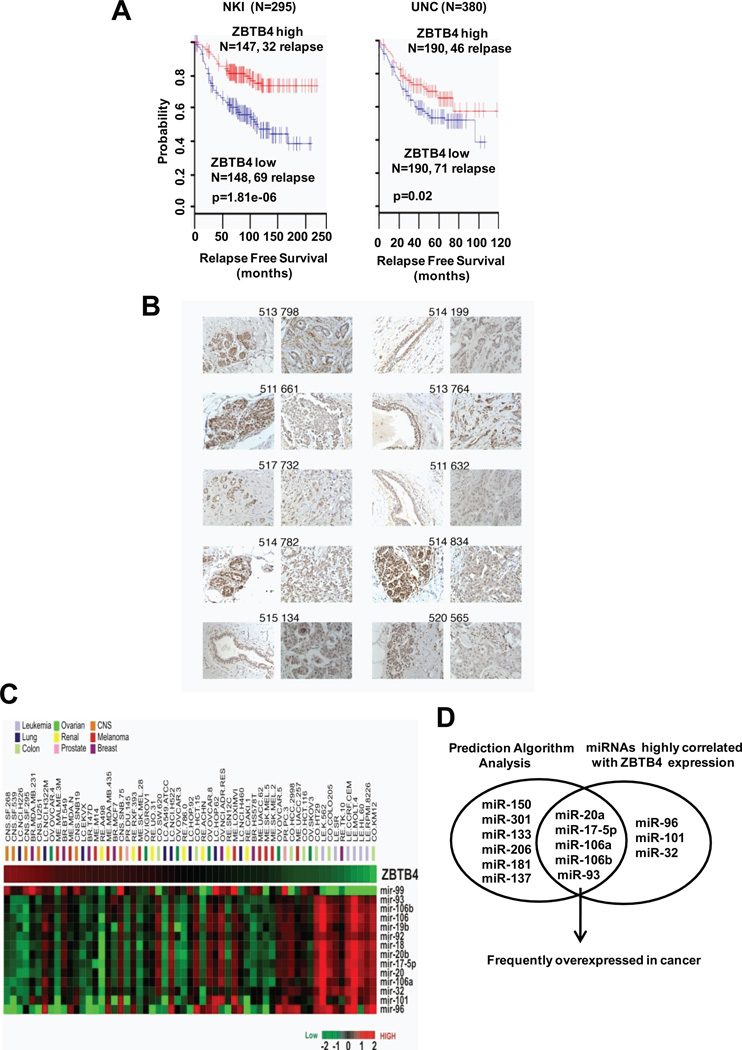
The potential prognostic and functional roles of ZBTB transcription factors in breast cancer was examined by Kaplan-Meier survival analysis of two publically available breast cancer patient datasets (van de Vijver et al., 2002; Oh et al., 2006) and, among this group of genes, only ZBTB4 exhibited consistent clinical correlations in these two independent data sets. High ZBTB4 expression correlated with longer relapse-free survival, while low ZBTB4 expression correlated with shorter relapse-free survival in two independent breast cancer patient cohorts [NKI cohort (n=295); UNC cohort (n=380)] (Fig. 1A). Survival differences were not observed for other ZBTB mRNAs (examples: ZBTB2, ZBTB5, ZBTB7, ZBTB10, ZBTB29, ZBTB33, and ZBTB38) expressed in these patients (Fig. S1). Expression of ZBTB4 protein was also determined in breast tumors and non-tumor tissues (Fig. 1B, Table S1, and Fig. S2) and there was consistently increased ZBTB4 protein staining in non-tumor vs. tumor tissue from the same patients.
Figure 1. Identification of ZBTB4 as miR-17-5p/miR-20a target.
(A) Breast cancer patients in the NKI (N=295) and UNC (N=380) cohort, and lung cancer patients (N=149, GSE11969) were dichotomized by ZBTB4 expression level. Patients were divided into two groups, high and low, by median ZBTB4 expression values. (B) Immunostaining of ZBTB4 protein expression in non-tumor (left) and tumor (right) tissue of breast cancer patients with invasive ductal carcinoma (also see Supplemental Table 1 and Supplemental Fig. 1) (C) ZBTB4 expression is inversely correlated with multiple miRs from miR-17-92 cluster and its paralogues. Each cell in the heat map represents expression level of each gene. The red and green color in cells stand for relatively high and low expression levels in log2 transformed scale. (D) Venn diagram displaying common set of miRs by integrating the predicted miRs targeting ZBTB4 by algorithm programs into miRs highly correlated with ZBTB4 expression by Pierson’s correlation analysis using NCI-60 miR and mRNA data sets.
Regulation of ZBTB4 by miR-17-92
To identify miRs that can potentially negatively regulate ZBTB4, the publically available NCI-60 cell line mRNA and miR expression profiling data sets were employed and the correlation between miR and mRNA expression was analyzed by Pierson’s R correlation test (Fig. 1C) (Shankavaram et al., 2007; Blower et al., 2007). The results were then further integrated into the prediction outcome from software algorithm programs of PicTar (Krek et al., 2005) and TargetScan (Lewis et al., 2003) and indicated that the ZBTB4 3’-UTR can be potentially targeted by miRs derived from miR-17-92 cluster such as miR-17-5p (R=−0.582, p<0.00001) and miR-20a (R=−0.642, p<0.00001) (Fig. 1D and Table S2). Since the miR-17-92 cluster and its paralogues contains previously described oncogenic miRs and is frequently overexpressed in breast cancer and metastatic breast cancer cells (Volinia et al., 2006; Blenkiron et al., 2007), we focused on the roles of these oncogenic miRs in regulating ZBTB4 expression.
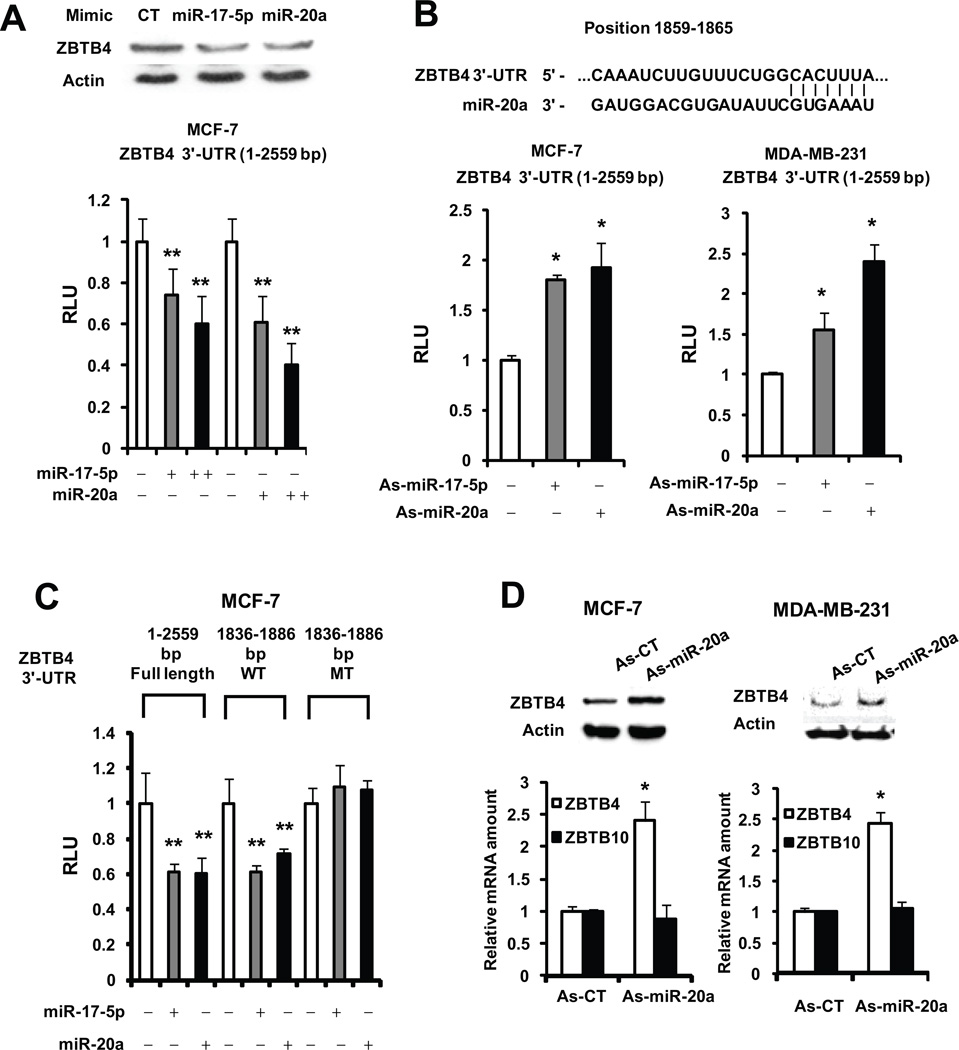
The 3’-UTR region of ZBTB4 has sequences that can interact with miR-17-5p and miR-20a, both of which have identical seed sequences (AAAGUG) (Figs. 2A and 2B). In cells transfected with 3’-UTR-ZBTB4-luc, luciferase activity was decreased after cotransfection with an increasing amount of miR-17-5p or miR-20a mimics (Fig. 2A) and this was also accompanied by decreased expression of ZBTB4. In contrast, transfection of as-miR-17-5p or as-miR-20a increased activity (Fig. 2B). Similar results were observed with antisense and mimics of miR-106a, miR-106b and miR-93 which are paralogues (identical seed sequences) of miR-20a/miR17-5p; however, miR-27a did not affect activity (Fig. S3). In cells transfected with a 3’-UTR-luc construct containing a wild-type or mutated core sequence (1836–1886 bp MT), the same miR mimics affected luciferase activity only in cells transfected with the wild-type construct (Fig. 2C). Since the miR-20a/miR-17-5p ratio was >8 in MCF-7 and MDA-MB-231 cells (data not shown), we used miR-20a as a model. Transfection of as-miR-20a into MCF-7 or MDA-MB-231 cells induced ZBTB4 but not ZBTB10 mRNA and this was accompanied by increased expression of the ZBTB4 protein (Fig. 2D). These results establish that oncogenic miR20a and miR17-5p negatively regulate ZBTB4 expression in breast cancer cells.
Figure 2. MiR-20a/miR-17-5p-dependent regulation of ZBTB4.
Effects of miR-20a and miR-17-5p and their antagomirs on interactions with wild-type ZBTB4-3’-UTR (A and B) and mutant (C) constructs. MCF-7 or MDA-MB-231 cells were transfected with miR-20a and miR-17-5p mimics or antagomirs (100 nM) and wild-type or mutant constructs containing ZBTB4 3’-UTR inserts linked to the luciferase gene, and after 48 hr, luciferase activity was determined as described in the Materials and Methods. (D) As-miR-20a induces ZBTB4 expression. MCF-7 or MDA-MB-231 cells were transfected with as-miR-20a (150 nM) and after 48 hr and expression of ZBTB4 protein and mRNA was determined as described in the Materials and Methods. Results are means ± SE (triplicate) and significant (p<0.05) induction (*) or inhibition (**) is indicated.
ZBTB4 represses Sp transcription factor gene expression
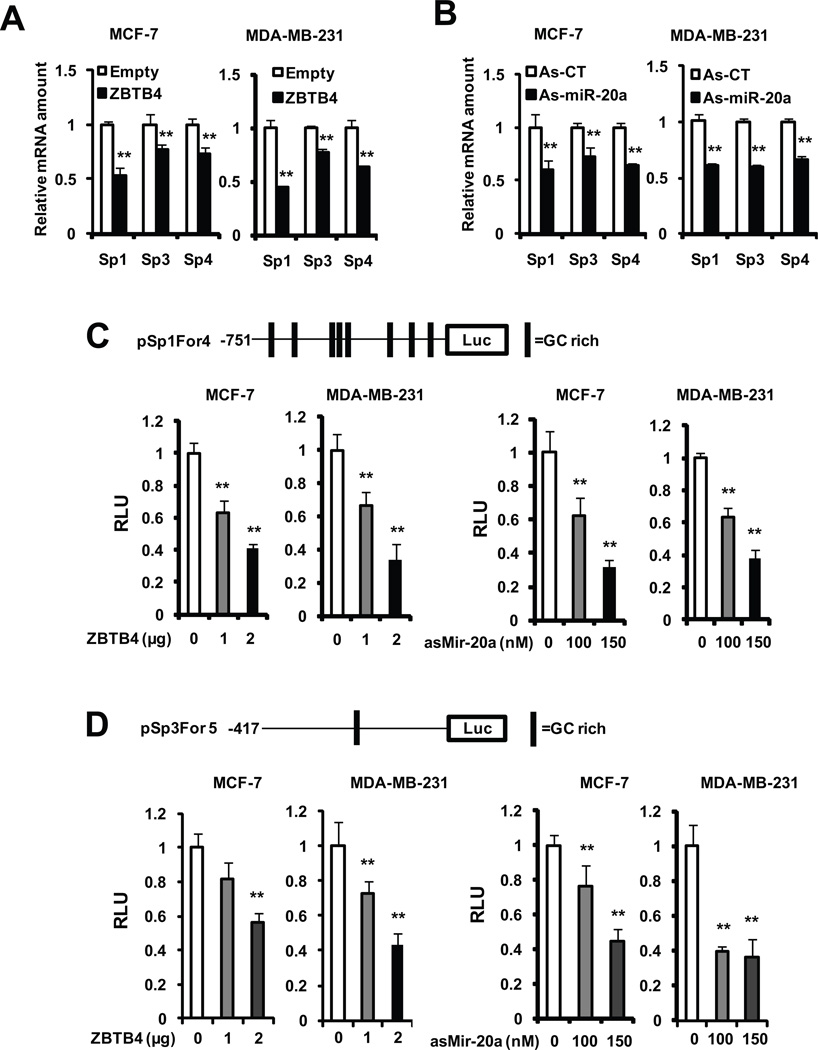
ZBTB4 represses p21 expression in cancer cells (Weber et al., 2008), and in this study, the potential role of this gene as a repressor of Sp1, Sp3, Sp4 and Sp-regulated genes was investigated in breast cancer cells. Figure 3A illustrates that overexpression of ZBTB4 in MCF-7 and MDA-MB-231 cells significantly decreased Sp1, Sp3 and Sp4 mRNA levels, and similar results were observed in these cells transfected with as-miR-20a (Fig. 3B). Both the Sp1 and Sp3 promoters contain GC-rich Sp binding sites (Nicolas et al., 2001; Tapias et al., 2008) and, in MCF-7 and MDA-MB-231 cells transfected with pSp1For4 (−751 to −20 Sp1 promoter insert) (Fig. 3C) or pSp3For5 (−417 to −38 Sp promoter) (Fig. 3D), cotransfection with ZBTB4 or as-miR-20a decreased luciferase activity. Similar results were observed in MDA-MB-231 and MCF-7 cells transfected with a GC-rich construct containing the −133 to +54 region of the VEGF promoter (Fig. S4).
Figure 3. ZBTB4 and as-miR-20a suppress Sp1, Sp3 and Sp4 transcription.
Decreased mRNA levels in cells transfected with ZBTB4 (A) or as-miR-20a (B). MCF-7 cells were transfected with ZBTB4 (2 µg) or as-miR-20a (100 nM) (and as-CT), and after 48 hr, Sp1, Sp3 and Sp4 mRNA levels were determined as described in the Materials and Methods. As-miR-20a and ZBTB4 decreased luciferase activity in cells transfected with pSp1For4 (C) and pSp3For5 (D). MCF-7 and MDA-MB-231 cells were transfected with the different constructs (as indicated), ZBTB4 expression plasmid (0, 1 or 2 µg), and as-miR-27a (0, 100 or 150 nM) and, after 48 hr, luciferase activity determined as described in the Materials and Methods. Results (A-D) are means ± SE (triplicate) and significant (p<0.05) inhibition (**) is indicated.
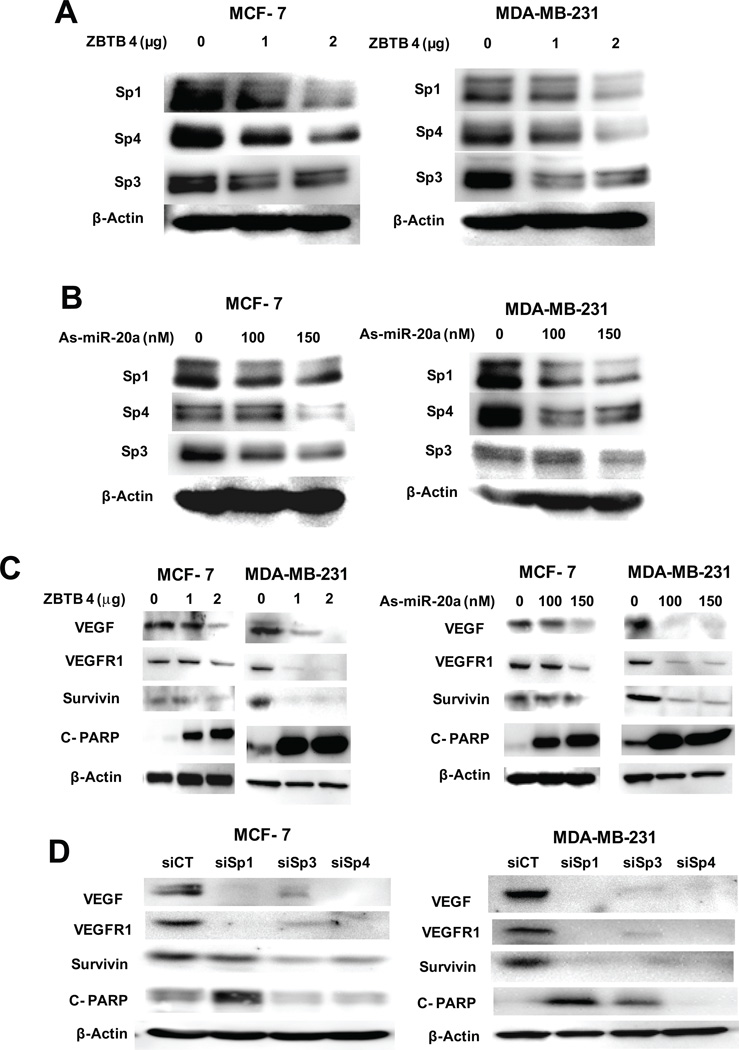
Confirmation that ZBTB4 repressed Sp mRNA levels in MCF-7 cells was determined by transfecting small interfering RNAs (siRNAs) against ZBTB4 (siZBTB4 I and siZBTB4 II). Both siRNAs increased expression of Sp1, Sp3 and Sp4 mRNA and proteins in MCF-7 cells (Fig. S5A and S5B), but not in MDA-MB-231 cells which express very low endogenous levels of ZBTB4 (data not shown). The ZBTB4 siRNAs increased luciferase activity in MCF-7 and MDA-MB-231 cells transfected with pSp1For4 (Fig. S5C) and pSp3For5 (Fig. S5D) confirming that ZBTB4 suppresses Sp1, Sp3 and Sp4 mRNA expression in breast cancer cells.
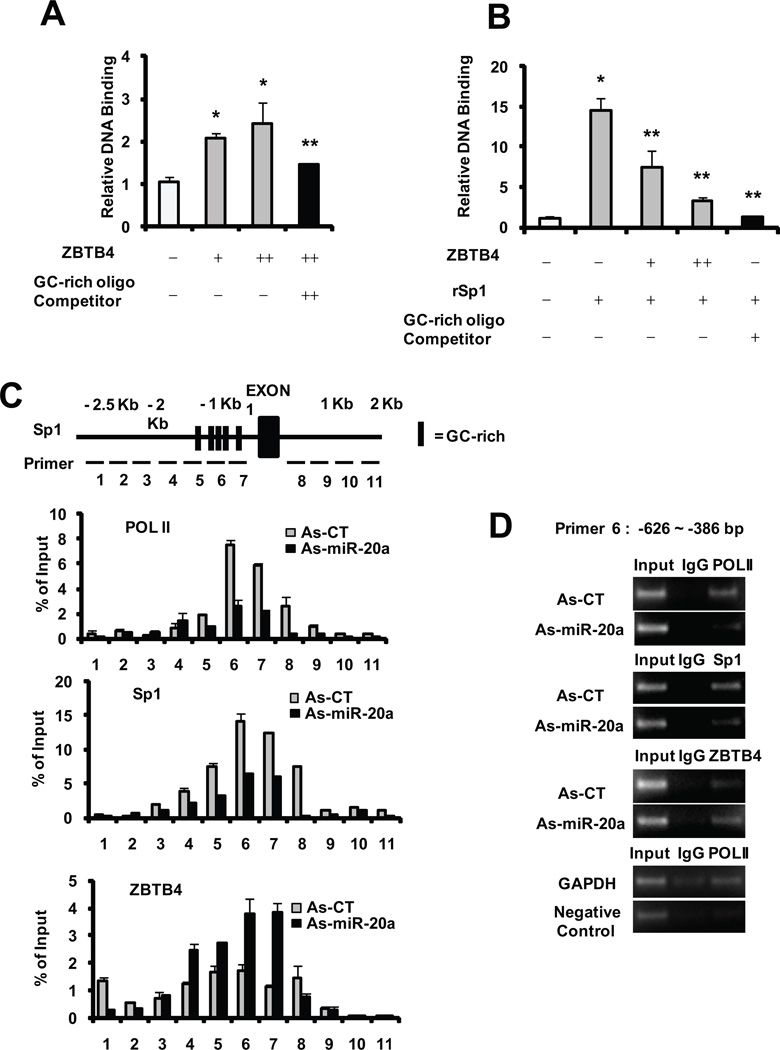
The mechanism of ZBTB4-dependent repression of Sp transcription factors was investigated using in vitro translated ZBTB4 and rSp1 in EMSA assays. Incubation of a concensus GC-rich oligonucleotide shows that ZBTB4 binds directly to the sequence and binding is repressed after co-incubation with excess GC-rich oligonucleotide (Fig. 4A). Moreover, using the same EMSA approach, the intensity of the rSp1-DNA complex is decreased after coincubation with ZBTB4 or excess GC-rich oligonucleotide (Fig. 4B). Results of a ChIP scanning analysis (−2.5 kB to +2.0 kB) over the GC-rich region of the Sp1 gene promoter showed an enhanced peak at −626 to −386 (GC-rich) (Fig. 4C). After transfection of MCF-7 cells with as-miR-20a/as-Ctl, we observed binding of Sp1 and pol II in cells transfected with as-Ctl, whereas as-miR-20a decreased Sp1 and pol II binding but increased ZBTB4 binding to this region (Figs. 4C and 4D). The results demonstrate that ZBTB4 competitively binds at GC-rich sites to displace Sp1 and thereby decreased transactivation.
Figure 4. Competitive interactions of ZBTB4 and Sp1.
EMSA analysis of ZBTB4 (A) and competitive ZBTB4/Sp1 binding (B). In vitro expressed ZBTB4, rSp1 alone, or in combination were incubated with a GC-rich oligonucleotide (± excess competitor) and analyzed by EMSA as described in the Materials and Methods. (C) ChIP scanning of the Sp1 promoter. Different primers were used for analysis of Sp1 binding on the Sp1 promoter (−2.5 to +2.0 kB) as described in the Materials and Methods. (D) As-miR-20 recruitment of ZBTB4 to the Sp1 promoter. Cells were transfected with as-miR-20a or as-Ctl, and binding of polII, Sp1 and ZBTB4 to the GC-rich −620 to −386 region of the Sp1 promoter was determined as outlined in the Materials and Methods.
ZBTB4 and as-miR-27a repress Sp1, Sp3, Sp4 and Sp-regulated gene products
The effect of as-miR-20a and ZBTB4 on Sp1, Sp3 and Sp4 protein expression was investigated in MDA-MB-231 and MCF-7 cells. Transfection of a ZBTB4 expression plasmid (Fig. 5A) or as-miR-20 (Fig. 5B) in MCF-7 and MDA-MB-231 cells downregulated expression of Sp1, Sp3 and Sp4 proteins, and this is accompanied by downregulation of several Sp-dependent genes including VEGF, VEGFR1 and survivin and enhanced PARP cleavage (Fig. 5C). Moreover, RNA interference studies in cells transfected with siSp1, siSp3 and siSp4 also shows that knockdown of Sp transcription factors decreased VEGF, VEGFR1 and survivin protein levels and induced PARP cleavage (Fig. 5D). The role of ZBTB4 in mediating the effects of as-miR-20a was investigated in MDA-MB-231 cells transfected with as-miR-20a alone or in combination with siZBTB4. As-miR-20a-induced downregulation of Sp1, Sp3 and Sp4 proteins (Fig. S6A), Sp1 mRNA (Fig. S6B), and luciferase activity in cells transfected with pSp1For4 construct (Fig. S6C) were significantly inhibited after cotransfection with siZBTB4. Since induction of ZBTB4 was associated with downregulation of Sp1, Sp3 and Sp4, we reexamined the data sets for correlations between patient survival and expression of Sp1, Sp3, Sp4, VEGFR1, survivin, cyclin D1 and VEGF. Among these mRNAs, only VEGF levels were prognostic factors for patient survival (Fig. S6D).
Figure 5. Decreased Sp1, Sp3, Sp4 and Sp-regulated protein expression by ZBTB4 overexpression or endogenous miR-20a knockdown and EMSA results.
Effects of ZBTB4 expression (A) and as-miR-20a (B) on Sp1, Sp3 and Sp4 protein expression. MCF-7 or MDA-MB-231 cells were transfected with ZBTB4 (0, 1 or 2 µg) or as-miR-20a (0, 100 or 150 nM), and whole cell lysates were analyzed for Sp proteins by western blots as described in the Materials and Methods. Effects of ZBTB4 (C) and as-miR-20a (D) on Sp-regulated genes and PARP cleavage. Cells were transfected with either ZBTB4 or as-miR-20a, and whole cell lysates were analyzed by western blots as described in the Materials and Methods.
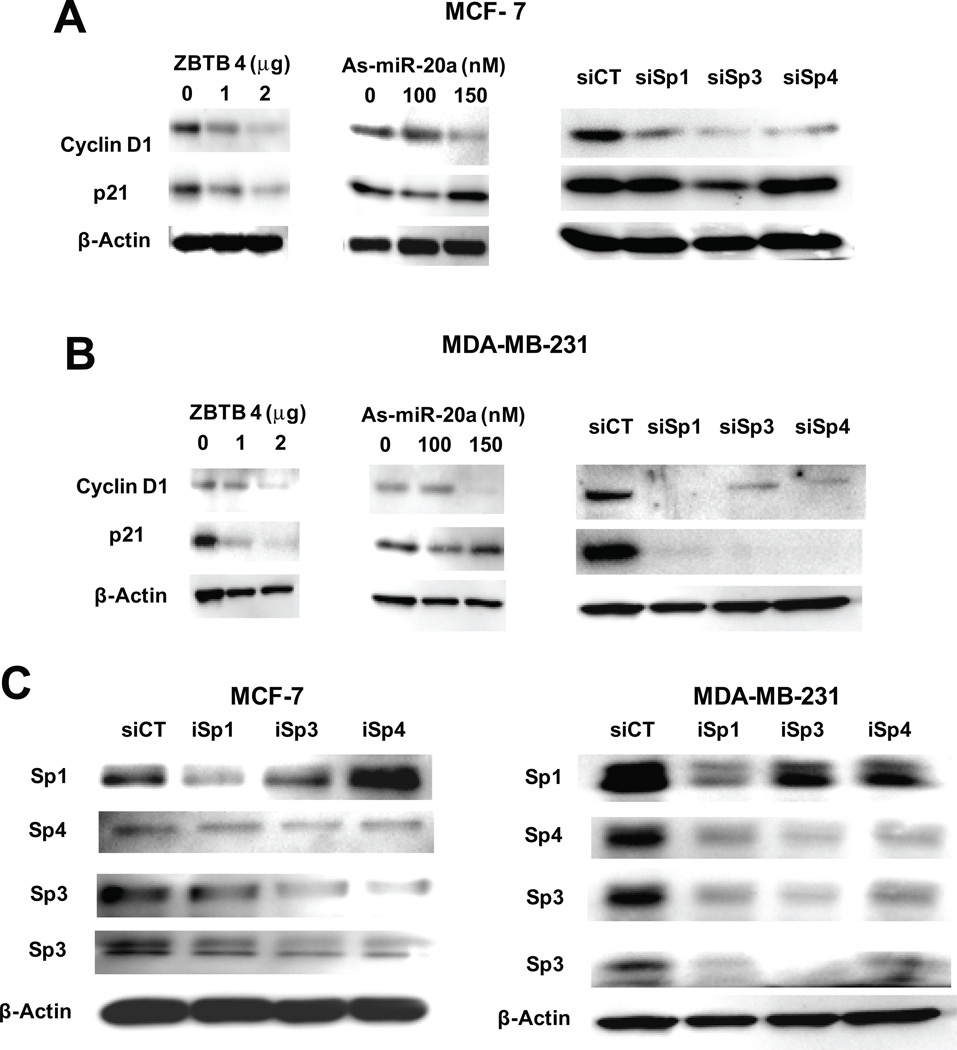
MiR-20a and miR-17-5p also regulate cyclin D1 and p21 expression in some cancer cell lines through 3'-UTR interactions (Yu et al., 2008; Inomata et al., 2009). Since both of these genes can also be regulated by Sp transcription factors and would therefore be impacted by ZBTB4, we further investigated expression of cyclin D1 and p21 in MCF-7 and MDA-MB-231 cells. Cyclin D1 expression was decreased by knockdown of Sp1, Sp3 and Sp4 in both cell lines and similar results were observed in cells transfected with ZBTB4 or as-miR-20a (Figs. 6A and 6B). These results indicate that cyclin D1 is regulated by ZBTB4-Sp and not by direct interactions with miR-20a. In MDA-MB-231 cells, both ZBTB4 overexpression and Sp1, Sp3 or Sp4 knockdown decreased p21 expression, whereas minimal effects were observed in cells transfected with as-miR-20a. This suggests the possibility of opposing pathways in which as-miR-20a directly enhances p21, whereas as-miR-20a activation of ZBTB4 and repression of Sp transcription factors decreases p21. As-miR-20a and knockdown of Sp1, Sp3 and Sp4 by RNA interference had minimal effects on p21 expression in MCF-7 cells, whereas ZBTB4 inhibited p21 expression and this is consistent with previous studies showing that ZBTB4 directly suppresses p21 (Filion et al., 2006). The effects of individual knockdown of Sp proteins was gene- and cell line-specific (Fig. 6C). The oligonucleotides used in this study were highly specific for their respective targets in bladder cancer cells (Chadalapaka et al., 2008); however, this specificity was not observed in breast cancer cells (Fig. 6C), suggesting that in these cells Sp1, Sp3 and Sp4 are autoregulatory and this is consistent with their GC-rich promoter regions (Nicolas et al., 2001; Tapias et al., 2008).
Figure 6. Changes in cyclin D1 and p21 protein expression by Sp knockdown, ZBTB4 or as-miR-20a.
Effects of as-miR-20a, Sp knockdown or ZBTB4 overexpression on p21 and cyclin D1 in MCF-7 (A) and MDA-MB-231 (B) cells. After transfection of the indicated reagents (100 nM siSps) for 48 hr, whole cell lysates were analyzed by western blots as described in the Materials and Methods. (C) Effect of Sp protein knockdown of Sp-regulated genes on Sp protein expression. Cells were transfected with siCT (control), iSp1, iSp3 or iSp4 (100 nM) and, after 48 hr, whole cell lysated were analyzed by western blots as outlined in the Materials and Methods.
MiR-17-92: oncogenic or tumor suppressor-like activity?
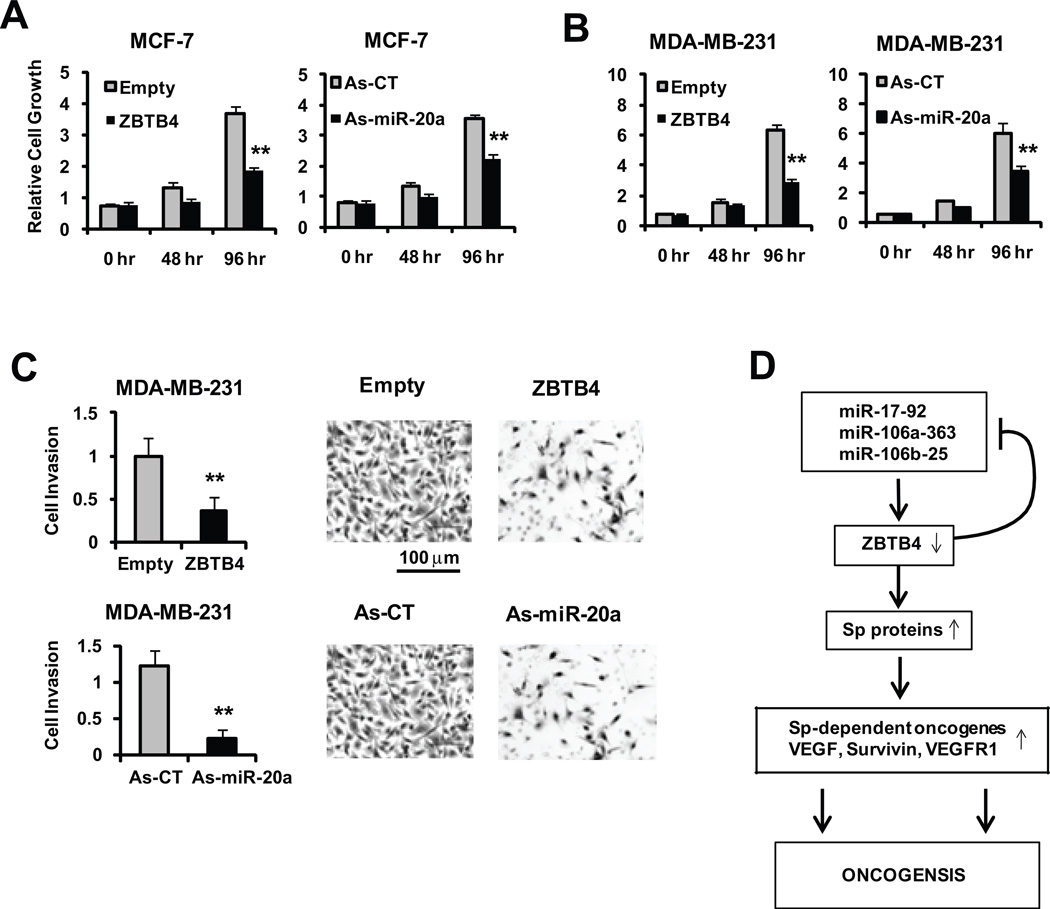
The functional role of as-miR-20a and ZBTB4 on cell proliferation was also investigated in MCF-7 and MDA-MB-231 cells. Cells were transfected with ZBTB4 or empty vector and the effects on cell proliferation were determined 48 and 96 hr after ZBTB4 overexpression (Fig. 7A). A similar approach was used for as-miR-20a (Fig. 7B) and the results showed that ZBTB4 and the antagomir decreased cell growth after 96 hr. These results are consistent with a previous report showing that Sp1 knockdown inhibited G0/G1 to S phase progression of MCF-7 cells (Abdelrahim et al., 2002). MDA-MB-231 (but not MCF-7) cells are highly invasive and metastatic, and the effects of ZBTB4 and as-miR-20a on MDA-MB-231 cell invasion were examined using the Boyden Chamber invasion assay. The results show that ZBTB4 and as-miR-20a decreased invasion in MDA-MB-231 cells (Fig. 7C), indicating that miR-20a-mediated suppression of ZBTB4 plays a role in the invasive phenotype. Overexpression of miR-20 mimic in MCF-7 or MDA-MB-231 cells did not significantly affect cell proliferation (Fig. S7A); however, in MCF-7 cells which express higher levels of ZBTB4, miR-17-5p and miR-20a mimics significantly enhanced invasion in a Boyden chamber assay (Fig. S7B).
Figure 7. ZBTB4 and as-miR-20 inhibit breast cancer cell growth and invasion.
Inhibition of MCF-7 (A) and MDA-MB-231 (B) cell growth. Cells were transfected with ZBTB4 (2 µg) or as-miR-20a (150 nM), and their effects on cell growth after 48 or 96 hr and cell invasion in a Boyden Chamber assay were determined as described in the Materials and Methods. (C) Boyden chamber assay. This assay was carried out as described for cell proliferation (32 hr) and cancer cell invasion was determined using a Boyden chamber as described in the Materials and Methods. Schematic Model to explain how miR-17-92/ZBTB4/Sp axis plays important role in oncogenesis (D). Results are expressed as means ± SE (triplicate) and significant (p<0.05) inhibition by ZBTB4 or as-miR-20a is indicated (*).
DISCUSSION
ZBTB4 : a novel tumor suppressor, targeted by OncomiRs derived from MiR-17-92 cluster and its paralogues
Since several POK proteins play a role in cancer, we performed an unbiased analysis of gene expression data from breast cancer patients to determine which of these proteins are clinically associated with disease progression or with patient survival. We found that the expression of one gene, ZBTB4, exhibited significant prognostic power; its expression was decreased in tumors, and low expression correlated with shorter relapse-free patient survival (Figs. 1A and 1B). Results of survival analysis from two independent breast cancer patient data sets correlated with ZBTB4 expression with patient outcomes (Fig. 1A) and similar results were observed for lymphoma and lung cancer patients (data not shown), confirming the prognostic significance of ZBTB4. There are over 40 POK proteins, and some of them, like ZBTB10, have previously been linked to breast cancer as inhibitors of several pro-oncogenic gene products (Mertens-Talcott et al., 2007); however, among several ZBTB mRNAs (Fig. S1), only ZBTB4 consistently exhibited a significant predictive value in our datasets, and therefore, we hypothesized that ZBTB4 may play a unique role in cancer. ZBTB4 has two close paralogs, namely, Kaiso (ZBTB33) and ZBTB38 (Perez-Torrado et al., 2006; Costoya, 2007), and they are also expressed in mammary tissue but display no association with this disease (Fig. S1), suggesting that ZBTB4 represses a unique gene set that are not regulated by Kaiso or ZBTB38. However, the specificity among these genes is probably not dictated by their intrinsic DNA-binding capacities since they have similar targets, at least in vitro (Sasai et al., 2010), suggesting that other mechanisms may explain why ZBTB4 exhibits unique activity.
Regulation of ZBTB4 by members of the miR-17-92 cluster and its paralogues
The NCI-60 mRNA and miR expression profiles were used to correlate ZBTB4 and miR expression (Fig. 1C). These correlations coupled with analysis of miRs that are overexpressed in breast cancer and target the 3'-UTR of ZBTB4 identified a relatively small number of candidate miRs that regulate ZBTB4 (Fig. 1D). MiR-20a and miR-17-5p are members of the miR-17-92 cluster, consisting of 6 miRNAs (miR-17-5p, miR-18a, miR-19a, miR-20a, and miR-19b and miR-92), and have two closely related paralogue clusters in different chromosomal locations (miR-106b-25: chromosome 7, miR-106a-363:chromosome X). Several tumor suppressor genes regulated by these oncogenic clusters are involved with cell survival, cell cycle progression, and apoptosis (He et al., 2005; Dews et al., 2006; Fontana et al., 2008; Inomata et al., 2009; Liu et al., 2009; Li et al., 2010). MiR-20a and miR-17-5p share the same seed sequences with miR-106a, miR-106b and miR-93, and mimics of these paralogs decrease ZBTB4 expression in breast cancer cells and also decrease luciferase activity in cells transfected with a ZBTB4-3'-UTR-luc construct (Figs. 2A, 2C and S3). Expression patterns of the miR paralogs in MCF-7 and MDA-MB-231 cells demonstrated that miR-20a was one of the most highly expressed miR in both cell lines and was used to further investigate the regulation and consequences associated with ZBTB4-miR-20a interactions.
Competition between ZBTB4 and Sp proteins for GC-rich sequences
ZBTB4 and some other members of the POK family interact with methylated DNA, other cis-elements and GC-rich sites that bind Sp transcription factors and thereby inhibit transcription (Filion et al., 2006). Results in Figure 3 demonstrate that ZBTB4 and as-miR-20a inhibit Sp1, Sp3 and Sp4 mRNA expression and this is accompanied by decreased expression of Sp1, Sp3 and Sp4 proteins and several Sp-regulated gene products including survivin, VEGF and VEGFR1 (Fig. 5). Using the GC-rich Sp1 promoter as a model, we show that results of EMSA and ChIP assays indicate that ZBTB4 competes with Sp1 for binding to GC-rich promoter elements (Fig. 4). Other members of the POK family such as ZBTB10 also bind GC-rich Sp binding sites (Tillotson, 1999); however, these genes do not show any clinical association with breast cancer in our study. ZBTB4 represses p21 expression in neuroblastoma cells (Pendergrast et al., 2002), and this was also observed in breast cancer cells (Figs. 6A and 6B). A previous report showed that ZBTB4 regulated p21 by interacting with the DNA-bound protein MIZ-1 (Weber et al., 2008). However, Sp1-binding sites are present in the p21 core promoter (Koutsodontis et al., 2002), and, in light of our results, it is now apparent that the regulation of p21 also involves the recognition of these sites and displacement of Sp transcription factors by ZBTB4.
ZBTB4 was initially identified as a transcriptional repressor that binds methylated CGs (Perez-Torrado et al., 2006; Costoya, 2007) and subsequent studies demonstrate the versatility of ZBTB4 which can also bind non-methylated GC-rich sequences containing consensus Sp1 binding sequences (GGGCGG) (Sasai et al., 2010). However, our results indicated that the methylated DNA binding activity of ZBTB4 is not necessarily required since the reporter plasmids are not methylated (Fig. 3), but were repressed after overexpression of ZBTB4. It is possible that ZBTB4 binds its target sites with more avidity when they are methylated, and DNA methylation can inhibit Sp1 binding to it cognate sites. This suggests that the observed ZBTB4/Sp protein competition for binding sites may also be influenced by the methylation status of the DNA.
MiR-17-92 as an oncogenic miR in breast cancer cells
The complementary effects of ZBTB4 and miR-20a antagomirs on downregulation of Sp transcription factors and Sp-regulated genes (Figs. 5 and 6) coupled with their inhibition of MCF-7 and MDA-MB-231 cell growth and invasion (MDA-MB-231 cells) (Figs. 7 and S7) are consistent with several reports on the oncogenic activity of the miR-17-92 cluster and other paralogues (He et al., 2005). In breast cancer cells, some studies report that miR-17-92 exhibits tumor suppressor-like activity (Yu et al., 2008; Yu et al., 2010); however, it was also reported that miR-17-92 was associated both with breast cancer cell growth, migration and invasion (Liu et al., 2009; Li et al., 2010). Differences in the conclusions of these studies likely originate in the use of different cellular systems and methodologies. However, using the most appropriate assay, namely loss of function by antagomirs, we clearly observe that miR-20a contributes to the transformed characteristics of two different breast cancer cell lines (Fig. 7) and this is due to perturbation of the ZBTB4/Sp protein axis (Fig. 7D) which is characterized by decreased expression of Sp-regulated genes that play critical roles in cancer cell growth, survival and angiogenesis/metastasis (Abdelrahim et al., 2004; Abdelrahim et al., 2007; Jutooru et al., 2010).
In summary, results of this study demonstrate that the mechanism of action of ZBTB4 as a transcriptional repressor is also due, in part, to its activity as a repressor of Sp1, Sp3, Sp4 and Sp-regulated genes through competitive binding at GC-rich sites. MiR-20a which is a member of the miR-17-92 cluster regulates ZBTB4 expression and is part of a miR-17-92-ZBTB4-Sp transcription factor network that determines the inversely correlated expression of ZBTB4 and Sp1, both of which are positive and negative prognostic factors, respectively, for survival/relapse-free survival of cancer patients (Fig. 1). MiR-20a-dependent suppression of ZBTB4 allows for enhanced expression of Sp transcription factors and Sp-regulated genes with pro-oncogenic activity suggesting that drugs targeting miR-20a and the oncogenic miR-17-92 cluster may have important clinical applications.
Supplementary Material
Acknowledgments
FUNDING
This research was supported by National Institutes of Health (CA136571) and is gratefully acknowledged. PAD is supported by Association pour la Recherche sur le Cancer, Ligue contre le Cancer, and Institut National du Cancer. DY was supported by a postdoctoral fellowship from Association pour la Recherche sur le Cancer.
Footnotes
DECLARATION OF INTEREST
The authors have nothing to disclose.
REFERENCES
- Abdelrahim M, Baker CH, Abbruzzese JL, Sheikh-Hamad D, Liu S, Cho SD, et al. Regulation of vascular endothelial growth factor receptor-1 (VEGFR1) expression by specificity proteins 1,3 and 4 in pancreatic cancer cells. Cancer Res. 2007;67:3286–3294. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- Abdelrahim M, Samudio I, Smith R, Burghardt R, Safe S. Small inhibitory RNA duplexes for Sp1 mRNA block basal and estrogen-induced gene expression and cell cycle progression in MCF-7 breast cancer cells. J. Biol. Chem. 2002;277:28815–28822. doi: 10.1074/jbc.M203828200. [DOI] [PubMed] [Google Scholar]
- Abdelrahim M, Smith R, III, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 2004;64:6740–6749. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower PE, Verducci JS, Lin S, Zhou J, Chung JH, Dai Z, et al. MicroRNA expression profiles for the NCI-60 cancer cell panel. Mol. Cancer Ther. 2007;6:1483–1491. doi: 10.1158/1535-7163.MCT-07-0009. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, Shen Q, et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 2005;7:445–455. doi: 10.1016/j.ccr.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Chadalapaka G, Jutooru I, Burghardt R, Safe S. Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol. Cancer Res. 2010;8:739–750. doi: 10.1158/1541-7786.MCR-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, III, Li X, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–5354. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chen WY, Zeng X, Carter MG, Morrell CN, Chiu Yen RW, Esteller M, et al. Heterozygous disruption of Hic1 predisposes mice to a gender-dependent spectrum of malignant tumors. Nat. Genet. 2003;33:197–202. doi: 10.1038/ng1077. [DOI] [PubMed] [Google Scholar]
- Costoya JA. Functional analysis of the role of POK transcriptional repressors. Brief. Funct. Genomic. Proteomic. 2007;6:8–18. doi: 10.1093/bfgp/elm002. [DOI] [PubMed] [Google Scholar]
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat. Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion GJ, Zhenilo S, Salozhin S, Yamada D, Prokhortchouk E, Defossez PA. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol. Cell Biol. 2006;26:169–181. doi: 10.1128/MCB.26.1.169-181.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS. One. 2008;3:e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- Jeon BN, Choi WI, Yu MY, Yoon AR, Kim MH, Yun CO, et al. ZBTB2, a novel master regulator of the p53 pathway. J. Biol. Chem. 2009;284:17935–17946. doi: 10.1074/jbc.M809559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BN, Yoo JY, Choi WI, Lee CE, Yoon HG, Hur MW. Proto-oncogene FBI-1 (Pokemon/ZBTB7A) represses transcription of the tumor suppressor Rb gene via binding competition with Sp1 and recruitment of co-repressors. J. Biol. Chem. 2008;283:33199–33210. doi: 10.1074/jbc.M802935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutooru I, Chadalapaka G, Abdelrahim M, Basha MR, Samudio I, Konopleva M, et al. Methyl 2-Cyano-3,12-dioxooleana-1,9-dien-28-oate (CDDO-Me) decreases specificity protein (Sp) transcription factors and inhibits pancreatic tumor growth: role of microRNA-27a. Mol. Pharmacol. 2010;78:226–236. doi: 10.1124/mol.110.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DI, Choi WI, Jeon BN, Lee CE, Yun CO, Hur MW. A novel POK family transcription factor, ZBTB5, represses transcription of p21CIP1 gene. J. Biol. Chem. 2009;284:19856–19866. doi: 10.1074/jbc.M109.025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Hatano M, Okada S, Fukuda T, Toyama Y, Yuasa S, et al. Testicular germ cell apoptosis in Bcl6-deficient mice. Development. 2001;128:57–65. doi: 10.1242/dev.128.1.57. [DOI] [PubMed] [Google Scholar]
- Koutsodontis G, Moustakas A, Kardassis D. The role of Sp1 family members, the proximal GC-rich motifs, and the upstream enhancer region in the regulation of the human cell cycle inhibitor p21WAF-1/Cip1 gene promoter. Biochemistry. 2002;41:12771–12784. doi: 10.1021/bi026141q. [DOI] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nat. Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, et al. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010;70:6824–6836. doi: 10.1158/0008-5472.CAN-10-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Li H, Bian C, Liao L, Li J, Zhao RC. miR-17-5p promotes human breast cancer cell migration and invasion through suppression of HBP1. Breast Cancer Res. Treat. 2010 doi: 10.1007/s10549-010-0954-4. in press. [DOI] [PubMed] [Google Scholar]
- Liu H, D'Andrade P, Fulmer-Smentek S, Lorenzi P, Kohn KW, Weinstein JN, et al. mRNA and microRNA expression profiles of the NCI-60 integrated with drug activities. Mol. Cancer Ther. 2010;9:1080–1091. doi: 10.1158/1535-7163.MCT-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69:8742–8751. doi: 10.1158/0008-5472.CAN-09-1541. [DOI] [PubMed] [Google Scholar]
- Maeda T, Hobbs RM, Merghoub T, Guernah I, Zelent A, Cordon-Cardo C, et al. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature. 2005;433:278–285. doi: 10.1038/nature03203. [DOI] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein (Sp) transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- Nicolas M, Noe V, Jensen KB, Ciudad CJ. Cloning and characterization of the 5'-flanking region of the human transcription factor Sp1 gene. J. Biol. Chem. 2001;276:22126–22132. doi: 10.1074/jbc.M010740200. [DOI] [PubMed] [Google Scholar]
- Niu H. The proto-oncogene BCL-6 in normal and malignant B cell development. Hematol. Oncol. 2002;20:155–166. doi: 10.1002/hon.689. [DOI] [PubMed] [Google Scholar]
- Oh DS, Troester MA, Usary J, Hu Z, He X, Fan C, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J. Clin. Oncol. 2006;24:1656–1664. doi: 10.1200/JCO.2005.03.2755. [DOI] [PubMed] [Google Scholar]
- Pendergrast PS, Wang C, Hernandez N, Huang S. FBI-1 can stimulate HIV-1 Tat activity and is targeted to a novel subnuclear domain that includes the Tat-P-TEFb-containing nuclear speckles. Mol. Biol. Cell. 2002;13:915–929. doi: 10.1091/mbc.01-08-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Torrado R, Yamada D, Defossez PA. Born to bind: the BTB protein-protein interaction domain. BioEssays. 2006;28:1194–1202. doi: 10.1002/bies.20500. [DOI] [PubMed] [Google Scholar]
- Sasai N, Nakao M, Defossez PA. Sequence-specific recognition of methylated DNA by human zinc-finger proteins. Nucleic Acids Res. 2010;38:5015–5022. doi: 10.1093/nar/gkq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankavaram UT, Reinhold WC, Nishizuka S, Major S, Morita D, Chary KK, et al. Transcript and protein expression profiles of the NCI-60 cancer cell panel: an integromic microarray study. Mol. Cancer Ther. 2007;6:820–832. doi: 10.1158/1535-7163.MCT-06-0650. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tomida S, Yatabe Y, Kosaka T, Osada H, Yanagisawa K, et al. Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J. Clin. Oncol. 2006;24:1679–1688. doi: 10.1200/JCO.2005.03.8224. [DOI] [PubMed] [Google Scholar]
- Tapias A, Ciudad CJ, Noe V. Transcriptional regulation of the 5'-flanking region of the human transcription factor Sp3 gene by NF 1, c-Myb, B-Myb, AP 1 and E2F. Biochim. Biophys. Acta. 2008;1779:318–329. doi: 10.1016/j.bbagrm.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Tillotson LG. RIN ZF, a novel zinc finger gene, encodes proteins that bind to the CACC element of the gastrin promoter. J. Biol. Chem. 1999;274:8123–8128. doi: 10.1074/jbc.274.12.8123. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Marquardt J, Elzi D, Forster N, Starke S, Glaum A, et al. Zbtb4 represses transcription of P21CIP1 and controls the cellular response to p53 activation. EMBO J. 2008;27:1563–1574. doi: 10.1038/emboj.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, et al. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J. Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Willmarth NE, Zhou J, Katiyar S, Wang M, Liu Y, et al. microRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8231–8236. doi: 10.1073/pnas.1002080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.