Abstract
Spurred by the recent isolation of a novel hantavirus, named Imjin virus (MJNV), from the Ussuri white-toothed shrew (Crocidura lasiura), targeted trapping was conducted for the phylogenetically related Asian lesser white-toothed shrew (Crocidura shantungensis). Pair-wise alignment and comparison of the S, M and L segments of a newfound hantavirus, designated Jeju virus (JJUV), indicated remarkably low nucleotide and amino acid sequence similarity with MJNV. Phylogenetic analyses, using maximum likelihood and Bayesian methods, showed divergent ancestral lineages for JJUV and MJNV, despite the close phylogenetic relationship of their reservoir soricid hosts. Also, no evidence of host switching was apparent in tanglegrams, generated by TreeMap 2.0β.
Keywords: Hantavirus, Crocidura, Shrews, Phylogeny, Jeju Island, Korea
Introduction
For more than four decades, the only known non-rodent-associated hantavirus was Thottapalayam virus (TPMV), isolated from an Asian house shrew (Suncus murinus), captured in 1964 near Vellore in India (Carey et al., 1971). Recently, the discovery of genetically distinct hantaviruses in crocidurine and soricine shrews (Order Soricomorpha, Family Soricidae), including Tanganya virus (TGNV) in the Therese's shrew (Crocidura theresae) (Klempa et al., 2007), Azagny virus (AZGV) in the West African pygmy shrew (Crocidura obscurior) (Kang et al., 2011b), Camp Ripley virus (RPLV) in the northern short-tailed shrew (Blarina brevicauda) (Arai et al., 2007), Cao Bang virus (CBNV) in the Chinese mole shrew (Anourosorex squamipes) (Song et al., 2007b), Seewis virus (SWSV) in the Eurasian common shrew (Sorex araneus) (Song et al., 2007a), Ash River virus (ARRV) in the masked shrew (Sorex cinereus) and Jemez Spring virus (JMSV) in the dusky shrew (Sorex monticolus) (Arai et al., 2008a), and Kenkeme virus (KKMV) in the flat-skulled shrew (Sorex roboratus) (Kang et al., 2010), has challenged the conventional view that rodents are the principal reservoir hosts. That genetically divergent hantaviruses are also harbored by moles (Family Talpidae), including Asama virus (ASAV) in the Japanese shrew mole (Urotrichus talpoides) (Arai et al., 2008b), Oxbow virus (OXBV) in the America shrew mole (Neurotrichus gibbsii) (Kang et al., 2009b), Nova virus (NVAV) in the European common mole (Talpa europaea) (Kang et al., 2009c) and Rockport virus (RKPV) in the eastern mole (Scalopusaquaticus) (Kang et al., 2011a), has spurred the search for other soricomorph-borne hantaviruses, in hopes of clarifying their evolutionary history.
Beginning with the seminal identification of Hantaan virus (HTNV) (Lee et al., 1978), as the prototype virus of hemorrhagic fever with renal syndrome in the striped field mouse (Apodemus agrarius), the Republic of Korea has been the epicenter of hantavirus discovery. In fact, Seoul virus (SEOV) in the brown rat (Rattus norvegicus) (Lee et al., 1982), Soochong virus (SOOV) in the Korean field mouse (Apodemus peninsulae) (Baek et al., 2006), and Muju virus (MUJV) in the royal vole (Myodes regulus) (K. J. Song et al., 2007) were first reported in Korea.
Recently, the isolation of Imjin virus (MJNV) from the Ussuri white-toothed shrew (Crocidura lasiura) (Song et al., 2009), captured near the Imjin River in the Republic of Korea, prompted us to search for a hantavirus in the Asian lesser white-toothed shrew (Crocidura shantungensis). Because of the close phylogenetic relationship between C. lasiura and C. shantungensis and the separate lineages of Asian and African Crocidura shrews, we conjectured that a hantavirus harbored by C. shantungensis would be closely related to MJNV. Furthermore, we posited that the more distant phylogenetic relationship between C. shantungensis in Asia and C. theresae and C. obscurior in Africa (Dubey et al., 2008) would be similarly reflected by the divergence in their hantaviruses. Genetic and phylogenetic analyses, however, indicated that a newfound hantavirus, designated Jeju virus (JJUV), in C. shantungensis captured on Jeju Island, off the southern coast of Korea, differed significantly from MJNV, and shared a common ancestry with TGNV and AZGV, hantaviruses hosted by crocidurine shrews in sub-Saharan Africa. That is, despite the close phylogenetic relationship between their reservoir soricid hosts, JJUV and MJNV exhibited divergent ancestral lineages. Intensified efforts to detect JJUV in C. shantungensis on mainland Korea and phylogeographic studies of JJUV throughout the vast geographic range of the Asian lesser white-toothed shrew should provide greater insights into the complex evolutionary history of crocidurine shrew-borne hantaviruses.
Results and Discussion
Detection of hantavirus RNA
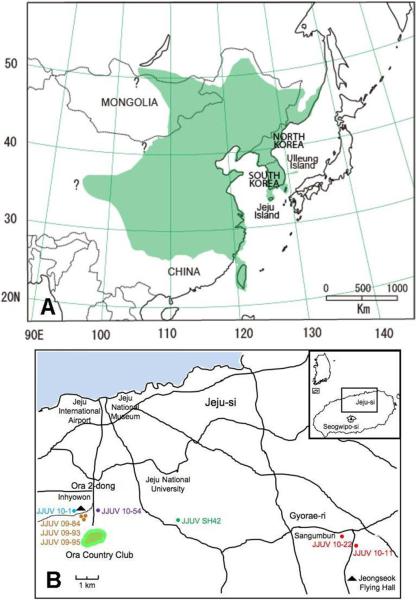
Hantavirus RNA, designated JJUV, was detected by reverse transcription polymerase chain reaction (RT-PCR) in eight of 51 Asian lesser white-toothed shrews, captured on Jeju Island (Fig. 1B), whereas none of 63 C. shantungensis captured on Ulleung Island were infected (Table 1). Also, JJUV RNA was not found in 28 Asian lesser white-toothed shrews from locales on mainland Korea, 200 or more km from Jeju Island. The failure to detect JJUV infection in Asian lesser white-toothed shrews on the mainland may be due to the small sample size. On the other hand, the inability to find JJUV on Ulleung Island is reminiscent of the absence of HTNV-infected striped field mice on Jeju Island, compared to mainland Korea (Lee et al., 1981).
Fig. 1.
(A) Geographic distribution, shaded in green (Bannikova et al., 2009; Ohdachi et al., 2004), of the Asian lesser white-toothed shrew, showing the locations of Jeju and Ulleung Islands. (B) Map of the Republic of Korea, showing locations of trap sites on Jeju Island, where hantavirus-infected shrews were captured.
Table 1.
Prevalence of hantavirus infection in Crocidura shantungensis captured on Jeju and Ulleung Islands.
| Island | Trap Site | Month Year | No. Tested | JJUV RNA |
|---|---|---|---|---|
| Jeju | Ora-dong | May 2009 | 1 | 0 |
| Sep 2009 | 7 | 1 | ||
| Oct 2009 | 7 | 2 | ||
| Mar 2010 | 1 | 1 | ||
| Apr 2010 | 3 | 0 | ||
| Dec 2010 | 2 | 1 | ||
| Gosan-ri, Hangyeong-myeon | Apr 2009 | 1 | 0 | |
| Sinheung-ri, Namwon-eup | May 2009 | 4 | 0 | |
| Ara-dong | Nov 2007 | 4 | 1 | |
| Oct 2009 | 5 | 0 | ||
| Gyorae-ri, Jocheon-eup | Apr 2010 | 8 | 1 | |
| Oct 2010 | 8 | 1 | ||
| Ulleung | Nari-ri, Buk-myeon | Sep 2009 | 48 | 0 |
| Sadong-ri, Ulleung-eup | Sep 2009 | 15 | 0 |
Recently, in a capture-mark-recapture study of Asian lesser white-toothed shrews in Taiwan, both females and males were found to mate with multiple partners, as evidenced by parentage analyses of 8 microsatellites in litters (Lin et al., 2009). As such, monogamous mating behaviors are unlikely to be responsible for the differential prevalence of JJUV carriage. Instead, founder groups of Asian lesser white-toothed shrews, naturally occurring on Jeju Island before the geological separation from the Korean peninsula approximately 12,000 to 16,000 years before present (Ohshima, 1990), might have already been infected. Alternatively, humans may have unintentionally transported JJUV-infected shrews to Jeju Island in the more recent past. Studies are underway to clarify the evolutionary history and phylogeography of JJUV, particularly in coastal regions on mainland Korea near the late Pleistocene land bridges.
Genetic analysis
Genome amplification of the newfound JJUV was accomplished from tissues of Asian white-toothed shrews captured in Ara-dong, Ora-dong and Gyorae-ri (Table 2), using oligonucleotide primers based on conserved regions. Pair-wise alignment and comparison with TPMV and MJNV, two crocidurine shrew-borne hantaviruses from Asia, showed low nucleotide similarity in the S, M and L segments, ranging from 50.8–63.1%. By contrast, much higher nucleotide sequence similarities of 62.2–79.1% were found between JJUV and hantaviruses harbored by soricine shrews from Africa. The deduced-amino acid sequences of the JJUV S and M segment products diverged by more than 15% from that of all known rodent- and soricomorph-borne hantaviruses, indicating that JJUV was a distinct hantavirus species (Fauquet et al., 2005; Maes et al., 2009).
Table 2.
Summary of JJUV strains detected in tissues of Crocidura shantungensis captured on Jeju Island, Korea.
| Nucleotides and GenBank Accession Numbers |
|||||
|---|---|---|---|---|---|
| JJUV strain | Capture site | Capture date | S segment | M segment | L segment |
| SH42 | Ara-dong | 15-Nov-2007 | 1,736 nt (1~1,736) HQ663933 |
2,312 nt (1,259~3,570) HQ663934 |
6,588 nt (1~6,588) HQ663935 |
| 09–84 | Ora-dong | 16-Sep-2009 | 2,311 nt (1,260~3,570) HQ834699 |
1,390 nt (1~1,390) HQ834705 |
|
| 09–93 | Ora-dong | 15-Oct-2009 | 2,311 nt (1,260~3,570) HQ834700 |
1,390 nt (1~1,390) HQ834706 |
|
| 09–95 | Ora-dong | 15-Oct-2009 | 2,158 nt (1,413~3,570) HQ834701 |
1,390 nt (1~1,390) HQ834707 |
|
| 10–1 | Ora-dong | 31-Mar-2010 | 2,311 nt(1,260~3,570) HQ824702 |
1,390 nt(1~1,390) HQ834708 |
|
| 10–11 | Gyorae-ri | 29-Apr-2010 | 1,754 nt (1~1,754) HQ834695 |
3,570 nt (1~3,570) HQ834696 |
6,588 nt (1~6,588) HQ834697 |
| 10–22 | Gyorae-ri | 14-Oct-2010 | 812 nt(1,419~2,230) HQ834703 |
||
| 10–54 | Ora-dong | 10-Dec-2010 | 597 nt (1,410~2,006) HQ834704 |
||
Amplification of the S-genomic segment was achieved for two of the eight JJUV strains. The full-length 1,754-nucleotide S-genomic segment of JJUV strains 10–11 and SH42 contained a single open reading frame, encoding a predicted N protein of 428 amino acids (nucleotide positions 37 to 1320), and 36- and 431-nucleotide 5'- and 3'- noncoding region (NCR), respectively. The sequence variation in the entire S-genomic segment between the JJUV strains was 2.5% and 0.3% at the nucleotide and amino acid levels, respectively.
The predicted secondary structure of the JJUV N protein, as determined by various software available in the @NPS structure server, was composed of 45.79% α-helices, 8.18% extend strands and 46.03% random coils (data not shown), and comprised two major α-helical domains packed against a central β-pleated sheet, resembling that of other rodent- and soricomorph-borne hantaviruses.
Partial to full-length sequences of the M-genomic segment, exhibiting up to 11.1% nucleotide sequence variation, were available for all eight JJUV strains. The 3,570-nucleotide full-length M segment of JJUV strain 10–11 encoded an entire glycoprotein of 1,143 amino acids, with 38- and 100-nucleotide 5'- and 3'-NCR, respectively. Four potential N-linked glycosylation sites in Gn and Gc at positions 142, 355, 407, 936 and 1058 were found in JJUV, as determined by the NetNGlyc 1.0 server. In addition, the highly conserved WAASA amino-acid motif was present at amino acid positions 652–656.
The full-length 6,588-nucleotide L-genomic segment of JJUV strains 10–11 and SH42 contained a single open reading frame, encoding a predicted RNA-dependent RNA polymerase of 2,157 amino acids, with a 35- and 79-nucleotide 5'- and 3'- NCR, respectively. Analysis of the JJUV L protein revealed the five conserved motifs (A, B, C, D and E), previously identified for hantavirus RNA polymerases (data not shown) (Kang et al., 2009c; Yadav et al., 2007). The overall high sequence similarity of the L segment between JJUV and rodent- and soricid-borne hantaviruses was consistent with the functional constraints on the RNA-dependent RNA polymerase. Partial L-segment sequences, available from four additional JJUV strains, exhibited 2.0–3.4% nucleotide variation.
Phylogenetic analyses
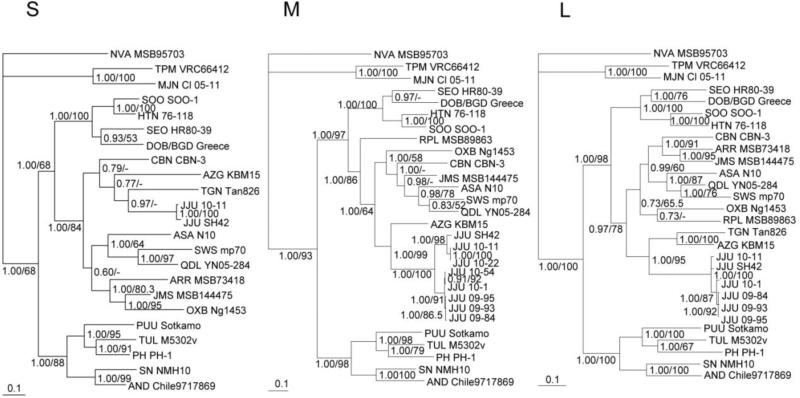
Phylogenetic analyses based on nucleotide sequences of the entire S-, M- and L-genomic segments, generated by the maximum-likelihood method, indicated that JJUV was distinct from MJNV and other hantaviruses with high bootstrap values and high posterior node probabilities based on 150,000 trees (Fig. 2). The average standard deviations of split frequencies of S, M and L segments Markov Chain Monte Carlo generations were 0.000538, 0.000647 and 0.004236, respectively. Phylogenetic trees were derived, using the Bayesian Metropolis–Hastings Markov Chain Monte Carlo tree-sampling method. Since full genomes were unavailable for several viruses, such as TGNV and AZGV, phylogenetic tree based on each genomic segment consisted of different taxa or combinations of taxa (Fig. 2). Despite this, the most strikingly consistent topological feature was the phylogenetic position of JJUV with soricine shrew-borne hantaviruses. Also, JJUV shared a common ancestry with TGNV and AZGV, two crocidurine shrew-borne hantaviruses from Africa, rather than forming a monophyletic group with MJNV and TPMV, both from Asian crocidurine shrews. That is, two distinct, phylogenetically divergent lineages were found.
Fig. 2.
Phylogenetic trees generated by the maximum-likelihood method, using the GTR+I+Γ model of evolution as estimated from the data, based on the alignment of the coding regions of the entire (S) 1,287-nucleotide S segment, (M) 3,432-nucleotide M segment and (L) 6,474-nucleotide L genomic segment of JJUV strain 10–11 (S: HQ834695, M: HQ834696, L: HQ834697). The phylogenetic positions of JJUV strain 10–11 are shown in relationship to representative Murinae rodent-borne hantaviruses, including Hantaan virus (HTNV 76–118, S: NC_005218, M: NC_005219, L: NC_005222), Soochong virus (SOOV SOO-1, S: AY675349, M: AY675353, L: DQ056292), Dobrava-Belgrade virus (DOB/BGDV Greece, S: NC_005233, M: NC_005234, L: NC_005235) and Seoul virus (SEOV HR80-39, S: NC_005236, M: NC_005237, L: NC_005238); Arvicolinae rodent-borne hantaviruses, including Tula virus (TULV M5302v, S: NC_005227, M: NC_005228, L: NC_005226), Puumala virus (PUUV Sotkamo, S: NC_005224, M: NC_005223, L: NC_005225) and Prospect Hill virus (PHV PH-1, S: Z49098, M: X55129, L: EF646763); and Neotominae rodent-borne hantaviruses, Sin Nombre virus (SNV NMH10, S: NC_005216, M: NC_005215, L: NC_005217) and Andes virus (ANDV Chile9717869, S: AF291702, M: AF291703, L: AF291704). Also shown are Thottapalayam virus (TPMV VRC66412, S: AY526097, M: EU001329, L: EU001330), Imjin virus (MJNV Cl05-11, S: EF641804, M: EF641798, L: EF641806), Cao Bang virus (CBNV CBN-3, S: EF543524, M: EF543526, L: EF543525), Ash River virus (ARRV MSB 73418, S: EF650086, L: EF619961), Jemez Springs virus (JMSV MSB144475, S: FJ593499, M: FJ593500, L: FJ593501), Seewis virus (SWSV mp70, S: EF636024, M: EF636025, L: EF636026), Qiandao Lake virus (QDLV YN05-284, S: GU566023, M: GU566022, L: GU566021), Oxbow virus (OXBV Ng1453, S: FJ5339166, M: FJ539167, L: FJ593497), Tanganya virus (TGNV Tan826, S: EF050455, L: EF050454), Nova virus (NVAV MSB95703, S: FJ539168, M: HQ840957, L: FJ593498), Asama virus (ASAV N10, S: EU929072, M: EU929075, L: EU929078), Azagny virus (AZGV KBM15, S: JF276226, M: JF276227, L: JF276228) and JJUV strain SH42 (S: HQ663933, M: HQ663934, L: HQ663935). The numbers at each node are posterior node probabilities based on 150,000 trees (left) and bootstrap values of 100 ML replicates executed on the RAxML BlackBox web server (right), respectively. The scale bars indicate nucleotide substitutions per site.
In applying a predictive paradigm based on phylogenetic relationships of known reservoir hosts, we have previously discovered novel hantaviruses (Baek et al., 2006; K. J. Song et al., 2007; Song et al., 2009). The recent isolation of MJNV and the incongruent phylogenies of MJNV and TGNV, two genetically distinct hantaviruses found in crocidurine shrew species in Korea and Guinea, respectively, prompted us to target the discovery of a hantavirus in the Asian lesser white-toothed shrew. Instead, analyses of JJUV strains detected in eight Asian lesser white-toothed shrews showed consistently higher genetic and phylogenetic similarity with TGNV, rather than MJNV or TPMV.
Despite the unexpected phylogenetic positions of JJUV and MJNV, JJUV strains from three capture sites on Jeju Island exhibited phylogenetic segregation according to the geographic origins of the reservoir host species. Thus, JJUV displayed geographic-specific clustering akin to that reported for rodent-borne hantaviruses and recently demonstrated for SWSV in S. araneus (Kang et al., 2009a; Yashina et al., 2010), MJNV in C. lasiura (Gu et al., 2011) and TPMV in S. murinus (Kang et al., 2011c).
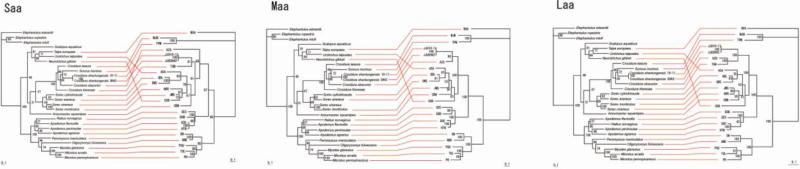
Preferential host switching with local host-specific adaptation, instead of virus-host co-divergence, has been proposed recently to account for the congruent phylogenies of hantaviruses and their reservoir hosts (Ramsden et al., 2009). However, phylogenetic trees reconstructed for co-phylogeny mapping, generated by TreeMap 2.0β, using consensus ML topologies based on the amino acid sequences of the nucleocapsid protein, Gn and Gc glycoproteins and RNA-dependent RNA-polymerase, exhibited segregation of hantaviruses according to the Subfamily of their soricomorph reservoir hosts, with no evidence of host switching for JJUV and MJNV (Fig. 3). The only exceptions were ASAV and OXBV, two hantaviruses harbored by moles (Arai et al., 2008b; Kang et al., 2009b).
Fig. 3.
Tanglegrams, generated by TreeMap 2.0β, using consensus ML topologies based on the amino acid sequences of the nucleocapsid protein (labeled Saa), Gn and Gc glycoproteins (labeled Maa) and viral RNA-dependent RNA-polymerase (labeled Laa) of JJUV 10–11 and JJUV SH42, and representative rodent-, shrew- and mole-borne hantaviruses and cytochrome b mtDNA sequences of the respective reservoir host species. Node support was derived from 100 ML bootstrap replicates executed on the RAxML BlackBox web server. Virus and host names are provided in the legend to Fig. 2.
mtDNA analysis and biogeographic inferences
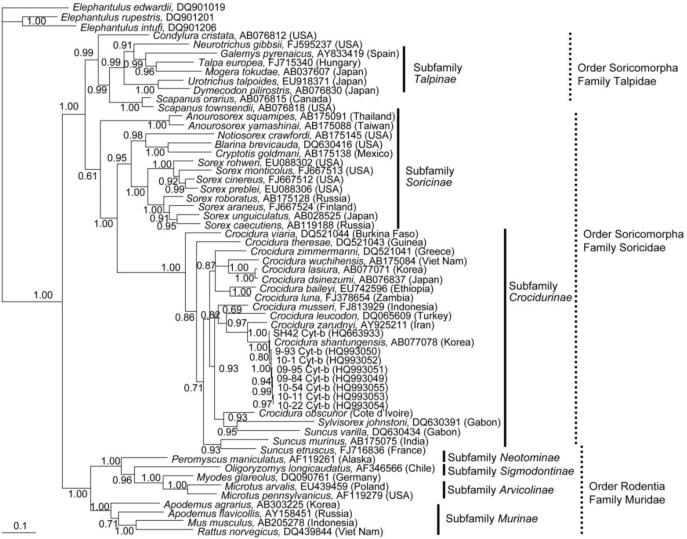
Taxonomic classification of the hantavirus-infected Asian lesser white-toothed shrews was achieved by amplification and sequencing of the 1,140-nucleotide mtDNA cytochrome b gene (SH42: HQ663932; 10–11: HQ834698). Phylogenetic analysis showed distinct grouping of JJUV-infected C. shantungensis from this study with C. shantungensis sequences available in GenBank (Fig. 4). Moreover, rodents and soricomorphs formed distinct lineages, well supported by bootstrap analysis. Also, as previously reported (Dubey et al., 2008), C. shantungensis and C. lasiura were distinct differed species from C. theresae and C. obscurior.
Fig. 4.
Bayesian phylogenetic tree, based on the 1,140-nucleotide cytochrome b region of mtDNA of small mammals within the Order Soricomorpha, Family Talpidae and Soricidae and the Order Rodentia, Family Muridae and Cricetidae. The tree was rooted using Elephantulus (Order Macroscelidea, GenBank accession numbers DQ901019, DQ901206 and DQ901201) as the outgroup. Numbers at nodes indicate posterior probability values.
Distributed widely across Europe, Africa and Asia, members of the genus Crocidura are among the most speciose of mammals, but their biogeographic origin and radiation are unclear (Dubey et al., 2008). As determined by in-depth analysis of karyologic, paleontologic and genetic data, a Palaearctic-Oriental origin has been proposed for Crocidura dating back to the Upper Miocene (6.8 million years before present) (Dubey et al., 2008). The molecular phylogeny of shrews belonging to the genus Crocidura is generally consistent with the biogeographic origins of species into an Afrotropical clade, an Asian clade and an Old World clade, which includes Afrotropical, East Palaearctic-Oriental and West Palaearctic species (Dubey et al., 2008). The geographic and genetic mismatching of the Old World clade is presumed to have resulted from migration and colonization of each species over hundreds of thousands of years or more (Dubey et al., 2008). However, C. shantungensis and C. lasiura group unambiguously into the Asian clade, while C. theresae groups in the Afrotropical clade.
The three scenarios, proposed for the molecular phylogeny of crocidurine shrews, presume a colonization of Africa during the Middle Miocene and two independent origins of the Crocidura genus lineages (Dubey et al., 2008). Even less well understood are the evolutionary histories of hantaviruses harbored by crocidurine shrews. The shared common ancestry between JJUV and both TGNV and AZGV would be consistent with replacement of an ancestral Crocidura lineage in Asia with a divergent lineage emerging from Africa.
Future efforts to analyze JJUV sequences from C. shantungensis captured in Taiwan, Buryatia and/or Mongolia (Bannikova et al., 2009), and efforts to detect JJUV-related hantaviruses in the C. dsinezumi, which has been recently introduced into Jeju Island from western Japan (Ohdachi et al., 2004), might yield additional important insights into the biogeography and evolutionary history of crocidurine shrew-borne hantaviruses.
Materials and Methods
Trapping and specimen processing
Asian lesser white-toothed shrews were captured, using Sherman traps (H.B. Sherman, Tallahassee, FL), at several sites on Jeju Island (33°24' N, 126°32' E) during 2007, 2009 and 2010 and on Ulleung Island (37°30' N, 130°50' E), a small, sparsely populated island located in the East Sea, approximately 600 kilometers from Jeju Island (Table 1, Fig. 1A). In addition, 28 Asian lesser white-toothed shrews, from the mainland (15 from Haenam, Jeonllanam province and 13 from Sesan, Chungnam province), were captured. Shrews were sacrificed by cervical dislocation. Lung and liver tissues were dissected using separate instruments, and were stored at −80°C until processed for RT-PCR and mtDNA analysis. All trapping and experimental procedures on animals were approved by the Korea University Institutional Animal Care and Use Committee and the Korean Ministry of Environment.
RNA extraction, cDNA synthesis and RT-PCR amplification
Total RNA was extracted from 20–50 mg of each tissue, using the PureLink Micro-to-Midi total RNA purification kit (Invitrogen, San Diego, CA). cDNA, synthesized using the SuperScript III First-Strand Synthesis Systems (Invitrogen), were analyzed for hantavirus RNA by RT-PCR, using primers designed from highly conserved regions of hantavirus genomes (Arai et al., 2007, 2008a, 2008b; Kang et al., 2009b, 2009c, 2010; Song et al., 2007a, 2007b, 2009).
First- and second-round PCR were performed in 20-μL reaction mixtures, containing 250 μM dNTP, 2.5 mM MgCl2, 1 U of Takara LA Taq polymerase (Takara, Shiga, Japan) and 0.25 μM of each primer. Initial denaturation at 94°C for 2 min was followed by two cycles each of denaturation at 94°C for 30 sec, two-degree step-down annealing from 46°C to 38°C for 40 sec, and elongation at 72°C for 1 min, then 30 cycles of denaturation at 94°C for 30 sec, annealing at 42°C for 40 sec, and elongation at 72°C for 1 min, in a GeneAmp PCR 9700 thermal cycler (Perkin-Elmer, Waltham, MA) (Arai et al., 2008b). PCR products were separated, using MobiSpin S-400 spin columns (MoBiTec, Goettingen, Germany), and amplicons were sequenced directly using an ABI Prism 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Genetic and phylogenetic analyses
Sequences were processed using the Genetyx version 9 software (Genetyx Corporation, Tokyo, Japan) and aligned using Clustal W and Clustal W2 (Thompson et al., 1994) with publicly available hantavirus sequences. For phylogenetic analysis, maximum-likelihood consensus trees were generated by the Bayesian Metropolis-Hastings Markov Chain Monte Carlo (MCMC) tree-sampling methods as implemented by MrBayes 3.1.2 (Ronquist and Huelsenbeck, 2003) using the GTR+I+Γ model of evolution, as selected by hierarchical likelihood-ratio test in MrModeltest v2.3 (Posada and Crandall, 1998) and jModelTest version 0.1 (Posada, 2008), partitioned by codon position, as well as a RAxML Blackbox web server for maximum-likelihood method (Stamatakis et al., 2008). The two replicate MCMC runs each consisted of six chains of 10 million generations sampled every 100 generations with a burn-in of 25,000 (25%), resulting in 150,000 trees. We performed host-parasite phylogenetic comparisons to detect co-divergence or host-switching events using TreeMap2.0β(Charleston, 1998).
Secondary structures
To predict the secondary structures of the JJUV nucleocapsid (N) protein and envelope glycoproteins, sequences were submitted to the NPS@ server (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_server.html) and the CBS prediction server (http://www.cbs.dtu.dk/services/). For the N protein, the DSC method (King and Sternberg, 1996) was used.
mtDNA analysis
To confirm the taxonomic classification of the host species, total DNA was extracted from liver, lung or spleen tissues using the QIAamp Tissue Kit (QIAGEN). The entire 1,140-nucleotide cytochrome b gene of mtDNA was amplified by PCR, using previously described universal primers (Irwin et al., 1991). PCR was performed in 50-μL reaction mixtures, containing 250 μM dNTP and 1 U of Expand Long Template polymerase (Roche Applied Science, Basel, Switzerland). Cycling conditions consisted of an initial denaturation at 95°C for 4 min followed by 35 cycles with denaturation at 94°C for 40 sec, annealing at 57°C for 1 min, and elongation at 72°C for 2 min in a GeneAmp PCR9700 thermal cycler.
Acknowledgements
We thank Satoshi D. Ohdachi for helpful suggestions. This research was supported in part by a Grant-in-Aid Research on Emerging and Re-emerging Infectious Diseases, Health Labour Sciences Research Grant in Japan (H22-Shinko-Ippan-006), by the Korean Ministry of Environment as “The Eco-technopia 21 project”, and by Institute of Biomedical Science & Food Safety, Korea University. Also, support was provided by U.S. Public Health Service grants R01AI075057 from the National Institute of Allergy and Infectious Diseases and P20RR018727 (Centers of Biomedical Research Excellence) and G12RR003061 (Research Centers in Minority Institutions) from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai S, Song J-W, Sumibcay L, Bennett SN, Nerurkar VR, Parmenter C, Cook JA, Yates TL, Yanagihara R. Hantavirus in northern short-tailed shrew, United States. Emerg. Infect. Dis. 2007;13(9):1420–1423. doi: 10.3201/eid1309.070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Bennett SN, Sumibcay L, Cook JA, Song J-W, Hope A, Parmenter C, Nerurkar VR, Yates TL, Yanagihara R. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 2008a;78(2):348–351. [PMC free article] [PubMed] [Google Scholar]
- Arai S, Ohdachi SD, Asakawa M, Kang HJ, Mocz G, Arikawa J, Okabe N, Yanagihara R. Molecular phylogeny of a newfound hantavirus in the Japanese shrew mole (Urotrichus talpoides) Proc. Natl. Acad. Sci. U.S.A. 2008b;105(42):16296–16301. doi: 10.1073/pnas.0808942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek LJ, Kariwa H, Lokugamage K, Yoshimatsu K, Arikawa J, Takashima I, Kang JI, Moon SS, Chung SY, Kim EJ, Kang HJ, Song KJ, Klein TA, Yanagihara R, Song J-W. Soochong virus: an antigenically and genetically distinct hantavirus isolated from Apodemus peninsulae in Korea. J. Med. Virol. 2006;78(2):290–297. doi: 10.1002/jmv.20538. [DOI] [PubMed] [Google Scholar]
- Bannikova AA, Sheftel BI, Lebedev VS, Aleksandrov DY, Muehlenberg M. Crocidura shantungensis, a new species for Mongolia and Buryatia. Dokl. Biol. Sci. 2009;424(1):68–71. doi: 10.1134/s0012496609010207. [DOI] [PubMed] [Google Scholar]
- Carey DE, Reuben R, Panicker KN, Shope RE, Myers RM. Thottapalayam virus: a presumptive arbovirus isolated from a shrew in India. Indian. J. Med. Res. 1971;59(11):1758–1760. [PubMed] [Google Scholar]
- Charleston MA. Jungles: a new solution to the host/parasite phylogeny reconciliation problem. Math. Biosci. 1998;149(2):191–223. doi: 10.1016/s0025-5564(97)10012-8. [DOI] [PubMed] [Google Scholar]
- Dubey S, Salamin N, Ruedi M, Barriere P, Colyn M, Vogel P. Biogeographic origin and radiation of the Old World crocidurine shrews (Mammalia: Soricidae) inferred from mitochondrial and nuclear genes. Mol. Phylogenet. Evol. 2008;48(3):953–963. doi: 10.1016/j.ympev.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. Eighth Report of the International Committee on the Taxonomy of Viruses. Elsevier Academic Press; London: 2005. Virus Taxonomy. Classification and Nomenclature of Viruses; pp. 704–707. [Google Scholar]
- Gu SH, Kang HJ, Baek LJ, Noh JY, Kim HC, Klein TA, Yanagihara R, Song JW. Genetic diversity of Imjin virus in the Ussuri white-toothed shrew (Crocidura lasiura) in the Republic of Korea, 2004-2010. Virol. J. 2011;8(1):56. doi: 10.1186/1743-422X-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DM, Kocher TD, Wilson AC. Evolution of the cytochrome b gene of mammals. J. Mol. Biol. 1991;32(2):128–144. doi: 10.1007/BF02515385. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Arai S, Hope AG, Song J-W, Cook JA, Yanagihara R. Genetic diversity and phylogeography of Seewis virus in the Eurasian common shrew in Finland and Hungary. Virol. J. 2009a;6(1):208. doi: 10.1186/1743-422X-6-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Bennett SN, Dizney L, Sumibcay L, Arai S, Ruedas LA, Song J-W, Yanagihara R. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii) Virology. 2009b;388(1):8–14. doi: 10.1016/j.virol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Bennett SN, Sumibcay L, Arai S, Hope AG, Mocz G, Song J-W, Cook JA, Yanagihara R. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea) PLoS One. 2009c;4(7):e6149. doi: 10.1371/journal.pone.0006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Arai S, Hope AG, Cook JA, Yanagihara R. Novel hantavirus in the flat-skulled shrew (Sorex roboratus) Vector-Borne Zoonotic Dis. 2010;10(6):593–597. doi: 10.1089/vbz.2009.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Bennett SN, Hope AG, Cook JA, Yanagihara R. Shared ancestry between a newfound mole-borne hantavirus and hantaviruses harbored by cricetid rodents. J. Virol. 2011a;85(15):7496–7503. doi: 10.1128/JVI.02450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kadjo B, Dubey S, Jacquet F, Yanagihara R. Molecular evolution of Azagny virus, a newfound hantavirus in the West African pygmy shrew (Crocidura obscurior) in Côte d'Ivoire. Virol. J. 2011b;8(1):373. doi: 10.1186/1743-422X-8-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kosoy MY, Shrestha SK, Shrestha MP, Pavlin JA, Gibbons RV, Yanagihara R. Genetic diversity of Thottopalayam virus, a hantavirus harbored by the Asian house shrew (Suncus murinus) in Nepal. Am. J. Trop. Med. Hyg. 2011c;85(3):540–545. doi: 10.4269/ajtmh.2011.11-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RD, Sternberg MJ. Identification and application of the concepts important for accurate and reliable protein secondary structure prediction. Protein Sci. 1996;5(11):2298–2310. doi: 10.1002/pro.5560051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempa B, Fichet-Calvet E, Lecompte E, Auste B, Aniskin V, Meisel H, Barriere P, Koivogui L, ter Meulen J, Krüger DH. Novel hantavirus sequences in shrew, Guinea. Emerg. Infect. Dis. 2007;13(3):520–522. doi: 10.3201/eid1303.061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 1978;137(3):298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- Lee HW, French GR, Lee PW, Baek LJ, Tsuchiya K, Foulke RS. Observations on natural and laboratory infection of rodents with the etiologic agent of Korean hemorrhagic fever. Am. J. Trop. Med. Hyg. 1981;30(2):477–482. doi: 10.4269/ajtmh.1981.30.477. [DOI] [PubMed] [Google Scholar]
- Lee HW, Baek LJ, Johnson KM. Isolation of Hantaan virus, the etiologic agent of Korean hemorrhagic fever, from wild urban rats. J. Infect. Dis. 1982;146(5):638–644. doi: 10.1093/infdis/146.5.638. [DOI] [PubMed] [Google Scholar]
- Lin T-T, You E-M, Lin YK. Social and genetic mating systems of the Asian lesser white-toothed shrew, Crocidura shantungensis, in Taiwan. J. Mammal. 2009;90(6):1370–1380. [Google Scholar]
- Maes P, Klempa B, Clement J, Matthijnssens J, Gajdusek DC, Krüger DH, Van Ranst M. A proposal for new criteria for the classification of hantaviruses, based on S and M segment protein sequences. Infect. Genet. Evol. 2009;9(5):813–820. doi: 10.1016/j.meegid.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Ohdachi SD, Iwasa MA, Nesterenko VA, Abe H, Masuda R, Haberl W. Molecular phylogenetics of Crocidura shrews (Insectivora) in east and central Asia. J. Mammal. 2004;85(3):396–403. [Google Scholar]
- Ohshima K. The history of straits around the Japanese Islands in the late-quaternary Quaternary Res. 1990;29(3):193–208. [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Ramsden C, Holmes EC, Charleston MA. Hantavirus evolution in relation to its rodent and insectivore hosts: no evidence for codivergence. Mol. Biol. Evol. 2009;26(1):143–153. doi: 10.1093/molbev/msn234. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Song J-W, Gu SH, Bennett SN, Arai S, Puorger M, Hilbe M, Yanagihara R. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus) Virol. J. 2007a;4:114. doi: 10.1186/1743-422X-4-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W, Kang HJ, Song KJ, Truong TT, Bennett SN, Arai S, Truong NU, Yanagihara R. Newfound hantavirus in Chinese mole shrew, Vietnam. Emerg. Infect. Dis. 2007b;13(11):1784–1787. doi: 10.3201/eid1311.070492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W, Kang HJ, Gu SH, Moon SS, Bennett SN, Song KJ, Baek LJ, Kim HC, O'Guinn ML, Chong ST, Klein TA, Yanagihara R. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura) J. Virol. 2009;83(12):6184–6191. doi: 10.1128/JVI.00371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KJ, Baek LJ, Moon S, Ha SJ, Kim SH, Park KS, Klein TA, Sames W, Kim HC, Lee JS, Yanagihara R, Song J-W. Muju virus, a novel hantavirus harboured by the arvicolid rodent Myodes regulus in Korea. J. Gen. Virol. 2007;88(Pt 11):3121–3129. doi: 10.1099/vir.0.83139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav PD, Vincent MJ, Nichol ST. Thottapalayam virus is genetically distant to the rodent-borne hantaviruses, consistent with its isolation from the Asian house shrew (Suncus murinus. Virol. J. 2007;4(1):80. doi: 10.1186/1743-422X-4-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashina LN, Abramov SA, Gutorov VV, Dupal TA, Krivopalov AV, Panov VV, Danchinova GA, Vinogradov VV, Luchnikova EM, Hay J, Kang HJ, Yanagihara R. Seewis virus: phylogeography of a shrew-borne hantavirus in Siberia, Russia. Vector-Borne Zoonotic Dis. 2010;10(6):585–591. doi: 10.1089/vbz.2009.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]