Abstract
Disorders of the urinary tract represent a major cause of morbidity and impaired quality of life. To better understand the morphological events responsible for normal urinary tract development, we performed 3-D reconstructive analysis of developing mouse bladders in control, mgb−/−, and Fgfr2Mes−/− mice. Detrusor smooth muscle differentiation initiated in the bladder dome and progressed caudally with the leading edge extending down the right posterior surface of the bladder. Gender-specific differences in detrusor smooth muscle development were observed during early embryonic development. Bladder trigone morphology transitioned from an isosceles to equilateral triangle during development due to the preferential lengthening of the urethra to ureter distance. The primary defect observed in mgb−/− bladders was a significant reduction in detrusor smooth muscle differentiation throughout development. Deviations from normal trigone morphology correlated best with VUR development in Fgfr2Mes−/− mice, while alterations in intravesicular tunnel length did not. In conclusion, multivariate morphometric analysis provides a powerful tool to quantify and assess urinary tract development.
Keywords: bladder, development, detrusor smooth muscle, vesicoureteral reflux
INTRODUCTION
Organogenesis is the complex process by which all organs obtain their mature morphology. The precise organization of tissues within a given organ is critical for normal function, and deviations from this program may result in both structural and functional abnormalities that have significant effects on the subsequent health and survival of an organism. At present, little is known regarding the complex processes controlling bladder organogenesis, including many of the morphometric features associated with its development. The mammalian urinary bladder develops from a partitioning of the cloaca by the urorectal septum into a ventrally placed primitive urogenital sinus and dorsal anorectal canal. The superior region of the developing urogenital sinus gives rise to the definitive bladder while the constricted intermediate region develops into the prostatic and membranous urethra in males and membranous urethra in females. The lower expansion, known as the definitive urogenital sinus, gives rise to the penile urethra in males and vestibule of the vagina in females. Three distinct excretory units developing sequentially from intermediate mesoderm variably contribute to the formation of the remaining urinary tract, with the third metanephric system resulting in the formation of the definitive kidney found in higher vertebrates. Ureteric buds emerging from developing metanephric ducts grow into the metanephric blastema inducing formation of definitive nephrons and a collecting system that empties into the bladder via the ureters.
The proper temporal and spatial development of the bladder is critical to normal urinary tract function with defects in this process having a wide range of effects (Executive Summary, 1999; Foreman and Chang, 1988). Diseases of the urinary bladder affect an estimated 35 million people in the United States often resulting in the development of end-stage renal disease (ESRD). ESRD is extremely important in terms of health care costs, with recent estimates suggesting that as many as 70% of the children presenting with urinary tract abnormalities will progress to ESRD despite clinical intervention (Executive Summary, 1999; Foreman and Chang, 1988; Roth et al., 2001). Clearly, additional studies are needed to clarify the important issues associated with urogenital dysmorphogenesis and its resulting dysfunction.
In this current study, we performed 3-D reconstruction and morphometric analysis of normal murine bladder development at embryonic days (E)13, 14, 15, 16 and 17 and postnatal day (P)1. These results were then compared to two mutant mouse models with known lower urinary tract defects: 1) the mgb−/− mouse that contains a primary defect in detrusor smooth muscle development (Singh et al., 2007), and 2) the Fgfr2Mes−/− mouse that develops primary vesicoureteral reflux (VUR) postnatally (Hains et al., 2009). This analysis demonstrated that detrusor smooth muscle development initiated in the bladder dome and progressed down the posterior bladder wall. Gender-specific differences in the quantity of detrusor smooth muscle were present at E13. Postnatal remodeling of detrusor smooth muscle appeared important in establishing the distinct smooth muscle layers found in the mature bladder. Detrusor smooth muscle development initiated in a normal manner in mgb−/− mice but failed to progress during development. The structural organization of the internal bladder trigone appeared to be the most effective predictor of reflux in Fgfr2Mes−/− mice. In summary, 3-D morphometric analysis of the developing bladder permitted easy quantification of individual morphological features, allowing a more rigorous, objective and statistical comparison to be made between normal and abnormal urinary tract development.
RESULTS
Normal Bladder Development
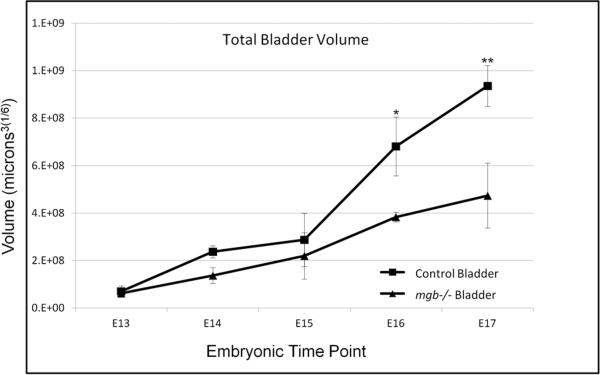
3-D reconstruction permitted the morphometric analysis of murine bladder development including quantification of individual tissue layers as well as the precise geometry of the developing urinary tract in both control and mutant mice (Figure 1; Supplemental Movies 1 & 2). Normal developing bladders showed a 13.3-fold increase in total volume from E13 to E17 (Figure 2; Supplemental Table 1). E13 bladders were composed of 5% urothelium, 62% lamina propria and 33% smooth muscle (Table 1). The percentage of bladder urothelium remained relatively constant throughout development, while the percentage of lamina propria decreased from 62% to 28% mirrored by a corresponding increase in the percentage of bladder smooth muscle from 33% to 67%. At E13, female bladders showed a higher percentage of smooth muscle (37% versus 28%) and correspondingly lower percentage of lamina propria (57% versus 68%) than the male. No additional gender differences in normal bladder development were observed for the remaining time points examined.
Fig. 1.
3-dimensional reconstruction (A, C, E, G & I) and representative H&E stained sections (B, D, F, H, & J) of embryonic (E) day 13, 14, 15, 16 and 17 control (C) and mutant (M) female (F) and male (M) bladders. Outlined structures include bladder lumen (green), urothelium (purple), smooth muscle (red), ureter (blue), and umbilical artery (gold).
Fig. 2.
Comparison of total bladder volume for control and mgb−/− mice from embryonic (E) day 13 through E17. *P=0.0007, **P=0.0005. The transformation of data to meet statistical assumptions resulted in interval units being reported in (microns3)1/6.
Table 1.
Average Percentage of 3-D Bladder Layer Volumes
| E13 | E14 | E15 | E16 | E17 | |
|---|---|---|---|---|---|
| Control Urothelium | 5.35 ± 0.93 | 5.30 ± 0.25 | 5.27 ± 0.30 | 3.99 ± 0.35 | 5.57 ± 0.01 |
| mgb−/− Urothelium | 5.81 ± 1.08 | 5.32 ± 1.09 | 5.14 ± 1.55 | 10.32 ± 2.16** | 11.82 ± 0.04 |
| Control Lamina Propria | 62.30 ± 6.29 | 42.02 ± 2.05 | 41.81 ± 2.27 | 31.84 ± 2.08 | 27.63 ± 0.02 |
| mgb−/− Lamina Propria | 88.17 ± 2.38* | 83.16 ± 2.04* | 66.22 ± 5.15* | 65.29 ± 2.76* | 70.23 ± 0.03* |
| Control Detrusor Muscle | 32.35 ± 5.96 | 52.69 ± 1.91 | 52.93 ± 2.46 | 64.18 ± 1.98 | 66.81 ± 0.01 |
| mgb−/− Detrusor Muscle | 6.03 ± 2.55* | 11.53 ± 1.79* | 28.65 ± 4.15* | 23.89 ± 2.43* | 17.95 ± 0.05* |
P<0.0001
P=0.0005 comparing control versus mgb−/− in each layer at each developmental time point with a significant MANOVA p-value of <0.0033. Volume reported in microns3.
Detrusor smooth muscle differentiation in E13 controls initiated in the upper bladder dome with a finger-like projection extending down the right posterior surface of the developing bladder (Figure 3). Consistent with the morphometric data above, female bladders showed more robust smooth muscle differentiation at E13, with male smooth muscle possessing a more fenestrated appearance than their female counterparts (Figure 3, E13-M vs. E13-F). By E14, control bladders showed uniform detrusor smooth muscle development that encompassed the upper half of the bladder wall, with preferential growth of developing detrusor smooth muscle still evident along the right posterior surface of the bladder. Detrusor smooth muscle differentiation expanded caudally on E15 with the overall thickness of the muscle layer increasing through E17 even though easily discernable smooth muscle layers were not evident by this stage of development (data not shown). A relatively well-defined muscularis mucosa was observed by E16 in control bladders (data not shown).
Fig. 3.
Anterior, right side and caudal views of 3-D reconstruction of the smooth muscle layer in control bladders at embryonic (E) days 13, 14, 15, 16 and 17. E13 male (M) and female (F) bladders are shown for comparison.
During postnatal development, the functioning bladder showed significant growth and remodeling that resulted in a narrowing of the broad embryonic lamina propria and reorganization of the detrusor muscle into well-defined fascicles that consisted of at least two distinct smooth muscle layers presumed to be the inner longitudinal and middle circular layers (Supplemental Figure 1). Even so, these layers were highly intermingled in the adult bladder with individual fascicles readily merging between layers.
Bladder Development in mgb−/− Mice
Initial total bladder volume appeared similar between E13 mgb−/− and control mice, but remained well below control levels at each subsequent developmental time point (Figure 2). E13 mgb−/− bladders were composed of 6% urothelium, 88% lamina propria and 6% smooth muscle (Table 1). The percentage of urothelium remained relatively constant in developing mgb−/− bladders through E15 after which it significantly increased in response to the development of in utero megabladder. The percentage of lamina propria present in mgb−/− bladders was significantly higher at each developmental time point than controls and showed a smaller decrease from E13 to E17 (18%) than controls (34%). The initial percentage of smooth muscle in the E13 mgb−/− bladder was 5.5-fold less than that observed in controls and remained significantly less than controls throughout development. E17 female mgb−/− bladders showed less muscle than male mgb−/− bladders, 13% versus 21%, respectively. No additional gender differences were observed.
Although detrusor smooth muscle differentiation in E13 mgb−/− bladders appeared to initiate in a manner similar to controls, total smooth muscle volume was significantly reduced at each developmental time point examined (Figure 4). As observed in controls, E13 mgb−/− bladder smooth muscle development appeared more robust in female than male mice (Figure 4, E13-M vs. E13-F). E14 and E15 mgb−/− bladders showed modest increases in detrusor smooth muscle development, but remained highly fenestrated and did not extend caudally to the degree observed in control bladders. The preferential extension of smooth muscle differentiation along the right posterior surface of the developing bladder was observed in E13 and E14 mgb−/− mice. In contrast to control bladders, this leading edge of smooth muscle differentiation remained present at E15 in mgb−/− bladders. In addition, the cranial-most regions of differentiated smooth muscle observed in E15 mgb−/− bladders appeared more fenestrated than at E14. Development of in utero megabladder in E16 and E17 mgb−/− mice prevented relative comparison of detrusor smooth muscle and muscularis mucosa development at these time points versus control.
Fig. 4.
Anterior, right side and caudal views of 3-D reconstruction of the smooth muscle layer in mgb−/− bladders at embryonic (E) days 13, 14 and 15. E13 male (M) and female (F) bladders are shown for comparison. E16 and E17 mgb−/− bladders were not reconstructed because they develop in utero megabladder.
Bladder Trigone Development
The generation of a bladder trigone schematic using 3-D reconstructed bladder sections permitted the morphometric analysis and quantification of this developing structure (Figure 5). For morphometric analysis, the bladder trigone was divided into: 1) the internal bladder trigone, which was defined as the region outlined by a triangle connecting the left and right internal ureteral orifices and the internal urethral orifice (ABC), 2) the external bladder trigone, which was defined as the region outlined by a triangle connecting the left and right external ureteral-serosal junction and the internal urethral orifice (CDE), and 3) the intravesicular trapezoid, which was generated by connecting the external ureteral-serosal junctions and internal ureteral orifices (ABED).
Fig. 5.
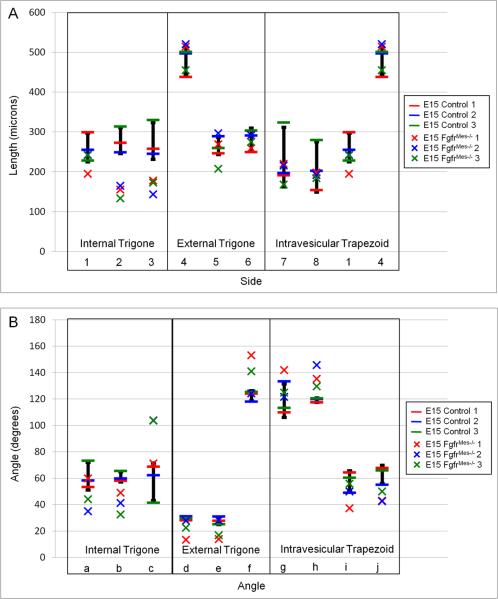
Bladder trigone schematic showing internal bladder trigone (ABC), external bladder trigone (CDE) and intravesicular trapezoid (ABED). The individual lines and angles generated by this schematic are labeled 1–8 and a–j respectively.
We first analyzed the development of the classically defined bladder trigone in control mice (Table 2). At E13, the internal trigone approximated an isosceles triangle with two sides (lines 2 & 3) and angles (angles a & b) of similar dimension between the ureters and urethra and a longer base between the ureters (line 1) and a wider opposing angle (c) at the urethra (Figure 6A). As development progressed, the base of the internal trigone remained relatively constant (line 1), while the two sides of the internal trigone increased in length (lines 2 & 3). This differential growth pattern resulted in the gradual widening of angles “a” and “b” and narrowing of angle “c” altering the geometry of the internal trigone to approximate an equilateral triangle by E16. Between E16 and E17, sides 2 and 3 showed an additional 2.2-fold increase in length, while the base (line 1) only increased 1.5-fold resulting in an isosceles triangle that differed from E13 by possessing a short base (line 1) with long sides (lines 2 & 3). Both the internal trigone perimeter and area showed biphasic linear increases during development resulting in a 3.7-fold and 27.2 fold total increase by E17, respectively (Figure 6B).
Table 2.
Average Trigone Measurements During Normal Bladder Development
| E13 | E14 | E15 | E16 | E17 | |
|---|---|---|---|---|---|
| Line 1 | 167.58 ± 5.93 | 193.36 ± 22.19 | 215.17 ± 25.37 | 198.43 ± 54.25 | 295.33 ± 63.50* |
| Line 2 | 98.79 ± 8.53 | 125.59 ± 22.39 | 163.76 ± 25.81 | 222.29 ± 39.02 | 488.40 ± 22.45** |
| Line 3 | 79.82 ± 0.56 | 140.14 ± 26.55 | 178.53 ± 2.96 | 234.65 ± 38.12 | 504.55 ± 16.56** |
| Angle a | 18.05 ± 0.40 | 44.54 ± 9.07 | 55.03 ± 8.26 | 68.33 ± 6.64 | 75.88 ± 7.68** |
| Angle b | 22.59 ± 2.72 | 39.58 ± 10.28 | 47.71 ± 3.82 | 60.98 ± 1.82 | 69.77 ± 7.38** |
| Angle c | 139.37 ± 3.12 | 95.89 ± 17.38 | 77.26 ± 4.60 | 50.69 ± 6.94 | 34.35 ± 6.96** |
P=0.0010
P<0.0001
P=0.0036 comparing E13 versus E17 for each parameter with a significant MANOVA p-value of <0.0083. Angles reported in degrees and lines in microns.
Fig. 6.
A. Bladder trigone geometry during development. Line numbers represent lengths in microns × 100 (Table 2). B. Comparison of total bladder perimeter and area in control mice. Note biphasic response with break point occurring at E16. *P=0.0009; **P=0.0013; ***P=0.0036; #P=0.0032; #P=0.0002; #P=0.0001 comparing each developmental time point to E13. Both perimeter and area have significant MANOVA p-values of <0.0125. The transformation of data to meet statistical assumptions resulted in interval units being reported in (area)1/6.
Trigone Development in Fgfr2Mes−/− Mice
In an attempt to identify if any of the morphometric parameters analyzed for the developing bladder trigone correlated with the development of VUR, we examined the internal bladder trigone, external bladder trigone and intravesicular trapezoid in E15 and postnatal day 1 (P1) Fgfr2Mes−/− mice and compared them to controls (Figure 7; Supplemental Tables 2 & 3). Modified box plot analysis revealed that E15 Fgfr2Mes−/− bladders possessed an average of 59% ± 2.9% of the morphometric parameters falling outside the normal control range (Figure 8). The E15 Fgfr2Mes−/− internal bladder trigone had 78% of the measured parameters falling outside the normal control range, while the external bladder trigone and intravesicular trapezoid showed 56% and 42%, respectively (Table 3). Interestingly, 100% of the ureter to urethral distances as well as the ureteral angles were reduced in the internal bladder trigone of E15 mutant animals. In contrast, the external bladder trigone and intravesicular trapezoid showed fewer changes in line length and angle when compared to controls.
Fig. 7.
3-D reconstruction of normal (A) and FgfrMes−/− (B) bladders showing orientation of the left (L) and right (R) ureters and urethra. Note low positioning of the right refluxing ureter in the FgfrMes−/− bladder compared to control. Outlined structures include detrusor smooth muscle (red), bladder lamina propria (white) urethral lumen (blue) and ureteral smooth muscle (red).
Fig. 8.
Modified box plot comparing line lengths (A) and angles (B) of the internal bladder trigone, external bladder trigone, and intravesicular trapezoid in embryonic day 15 (E15) control (−) and FgfrMes−/− (x) bladders. The black bar for each parameter represents the normal control range ± standard deviation.
Table 3.
Percentage of Altered Trigone Parameters in E15 and P1 Fgfr2Mes−/− Mice Versus Control
| Internal Trigone | External Trigone | Intravesicular Trapezoid | |
|---|---|---|---|
| E15 | 14/18 (78%) | 10/18 (56%) | 10/24 (42%) |
| P1 | 13/18 (72%) | 14/18 (78%) | 15/24 (63%) |
| P1 NR | 1/6 (17%) | 0/6 (0%) | 3/8 (38%) |
Parameters altered/total parameters examined; (percentage of altered parameters); NR – non-refluxing Fgfr2Mes−/− mice.
Modified box plot analysis of P1 Fgfr2Mes−/− mice revealed that all three refluxing mutants had an average of 69% ± 8.6% of the morphometric parameters falling outside the normal control range, while the one P1 non-refluxing mutant showed alterations in only 17% of those same parameters (Figure 9). 78% of the features measured for the external bladder trigone fell outside the normal control range, while the internal bladder trigone and intravesicular trapezoid showed alterations in 72% and 63%, respectively (Table 3).
Fig. 9.
Modified box plot comparing line lengths (A) and angles (B) of the internal bladder trigone, external bladder trigone, and intravesicular trapezoid in postnatal day 1 (P1) control (−) and FgfrMes−/− (x) bladders. The black bar for each parameter represents the normal control range ± standard deviation.
Since all the Fgfr2Mes−/− mice examined in this study developed right-sided VUR, we closely examined the various morphometric parameters associated with the right, refluxing ureter. The distances between the right ureter and urethra of the internal bladder trigone were decreased in two of three mutant bladders, while the left ureter and both ureteral angles were not consistently different from controls. By comparison, distances between the right ureter and urethra of the external bladder trigone were decreased in all three mutant bladders, but two of the three mutants also showed increases in ureter to urethral lengths in the left ureter. The right ureteral angle of the external trigone showed a consistent increase in all three mutants, while the left ureteral angles all decreased in these same animals. The ureter to urethral distance in the non-refluxing mutant appeared normal for both the external and internal bladder trigones.
A variety of studies have suggested that the intravesicular tunnel traversed by the ureter is critical in preventing the development of VUR (Murawski and Gupta, 2008; Murawski et al., 2011; Oswald et al., 2003; Schwentner et al., 2006; Yu et al., 2004). We initially measured the intramural, submucosal and total intravesicular tunnel lengths for both the right and left ureters of control mice during bladder development (Figure 10; Supplemental Table 4). This analysis revealed that the intravesicular tunnel length of both ureters increased from E13 to E17. In contrast, the length of the submucosal tunnel remained relatively constant during development, while the intramural tunnels lengths showed modest increases that only approached significance in the right ureter.
Fig. 10.
Schematic diagram of the developing bladder showing division of the intravesicular tunnel (1–3) into submucosal tunnel (1–2) and intramural tunnel (2–3). Histological sections stained with α-smooth muscle isoactin show corresponding ureteral landmarks (1, 2, & 3) used to identify the various segments of the intravesicular tunnel.
To determine if the intramural, submucosal and/or intravesicular tunnel lengths were altered in Fgfr2Mes−/− mice, we compared these parameters in E15 and P1 bladders of Fgfr2Mes−/− mice versus controls (Supplemental Table 5). Modified box plot analysis revealed that the E15 Fgfr2Mes−/− intravesicular tunnel length fell outside the range of controls for 100% of the right, refluxing ureters and 50% of the left, non-refluxing ureters (Figure 11). Similar patterns were observed when the intravesicular tunnel length was subdivided into the submucosal and intramural tunnel lengths. Modified box plot analysis of P1 Fgfr2Mes−/− mice indicated that the intravesicular tunnel length of the right, refluxing ureter appeared outside the normal range in all three refluxing mutants, while the non-refluxing mutant appeared “normal”. Similar analysis of the left, non-refluxing ureter indicated that the intravesicular tunnel length fell outside the range of controls for all four Fgfr2Mes−/− mice including the non-refluxing mutant. Although submucosal tunnel length correctly identified the left, non-refluxing ureter in all four P1 Fgfr2Mes−/− mice as normal, this same parameter indicated that the right, refluxing ureter appeared within the control range for two mutant animals and outside the control range for the non-refluxing mutant. Intramural tunnel length appeared to correlate with reflux the least of all three parameters examined, identifying the right, refluxing ureter as normal in two mutant animals and abnormal in the non-refluxing mutant, and the left, non-refluxing ureter as abnormal in three Fgfr2Mes−/− mice including the non-refluxing mutant.
Fig. 11.
Modified box plot comparing the length of the intravesicular tunnel, submucosal tunnel and intramuscular tunnel in embryonic day 15 (E15; A) and postnatal day 1 (P1; B) in control (−) and FgfrMes−/− (x) bladders. The black bar for each parameter represents the normal control range ± standard deviation. Yellow circle in panel B represents a P1 FgfrMes−/− non-refluxing mutant.
DISCUSSION
The results of this study indicate that as total bladder volume increases during development, the percentage of urothelium remains constant, while the percentage of lamina propria decreases in response to the progressive differentiation of detrusor smooth muscle from the peripheral mesenchyme. Detrusor smooth muscle development initiates in the bladder dome and progresses toward the bladder neck, with the leading edge of differentiation extending down the right posterior surface of the bladder. Although the significance of this observation remains to be determined, it is intriguing that it occurs juxtaposed to the right umbilical artery. Interestingly, morphometric analysis also indicated that E13 female bladders possess more smooth muscle than their male counterparts suggesting that gender-based differences in early bladder development may exist. To our knowledge, this represents the first report of these observations and provides evidence that murine bladder development progresses in a distinct cranial to caudal pattern with potential gender-specific differences in smooth muscle development.
Detrusor smooth muscle completely invested the developing bladder around E15, after which it thickens creating a distinct octahedral morphology by late embryogenesis. Differentiating smooth muscle cells within the embryonic bladder appear to develop as independent muscle bundles that readily intermingled as they progressively differentiate down the bladder wall. During these early stages of development, the classically defined inner longitudinal, middle circular, and outer longitudinal smooth muscle layers attributed to the mature bladder are not readily apparent. This observation is in stark contrast to gastrointestinal smooth muscle development, where the highly orchestrated and sequential differentiation of distinct smooth muscle layers is evident during embryogenesis (McHugh, 1995; McHugh, 1996).
The initial appearance of distinct smooth muscle layers during postnatal bladder development suggests that significant remodeling of detrusor smooth muscle differentiation occurs following birth. Our studies easily identified an inner longitudinal and middle circular smooth muscle layer in postnatal and adult animals, although a distinct outer longitudinal layer was not readily evident. This observation is consistent with prior studies, which indicate that the outer longitudinal smooth muscle layer is incomplete and primarily found along the anterior and posterior walls of the bladder (Hutch, 1972). The highly intermingled and overlapping detrusor smooth muscle morphology observed in our study is identical to that described by Hutch (1972), and seems well suited to produce the coordinated radial contractions necessary for micturition.
Classic histological descriptions of the developing bladder often describe detrusor smooth muscle development as a direct continuation of ureteral smooth muscle differentiation into the urogenital sinus (Copenhaver et al., 1978). Our studies clearly demonstrate that bladder smooth muscle differentiation independently initiates in the dome of the bladder around E13, well before the appearance of smooth muscle cells within the distal ureter on E15. This observation is consistent with recent molecular studies that clearly demonstrate ureteral and bladder smooth muscle differentiation are independent developmental events modulated by distinct molecular pathways (Airik et al., 2006; Airik and Kispert, 2007, Airik et al., 2010).
Mgb−/− mice possess a transgene-induced mutation that results in a defect in detrusor smooth muscle development (Singh et al., 2007). Morphometric analysis confirmed that the primary defect observed in mgb−/− bladders was a dramatic reduction in smooth muscle differentiation that occurs throughout development. Interestingly, both the temporal and spatial patterning of mgb−/− detrusor smooth muscle development appeared relatively normal, even in the face of dramatically reduced smooth muscle volume. The observed increase and/or maintenance in the percentage of lamina propria present in mgb−/− bladders suggest that the necessary mesenchymal precursors are present but unable to properly initiate and support smooth muscle differentiation. Prior studies support this hypothesis and indicate that E15 mgb−/− bladders possess high levels of apoptosis within the mesenchymal compartment, a fact that may account for the progressive loss of smooth muscle cells and total bladder volume in these animals (Singh et al., 2007). These results are consistent with prior data indicating that short axis patterning is normal in mgb−/− bladders (Singh et al., 2007; Singh et al., 2008).
Development of a mature and functional urinary tract requires the precise temporal and spatial reordering of the bladder, ureters and kidney (Batourina et al., 2005; Mendelsohn, 2009; Viana et al., 2007). A key step in the interconnection of the upper and lower urinary tracts occurs during transposition of the developing ureters from the Wolffian duct to the posterior surface of the urogenital sinus. This process initiates around E11 in the mouse and results in the eventual formation of the bladder trigone, a triangular region of bladder wall that lies between the paired internal ureteral orifices and internal urethral orifice. Morphometric analysis indicated that total bladder trigone area and perimeter increased at distinct linear rates during development. Both parameters showed a biphasic response during development with the point of inflection occurring on E16, when urine production and storage initiates in mice. This point of inflection also correlated with the changes in internal trigone geometry observed during development, suggesting that bladder function plays a key role in the maturation of the internal trigone. These observations are consistent with recent studies and suggest that the temporal and spatial development of the bladder trigone can be modulated by functional (ie:bladder distension and voiding) as well as anatomic parameters (Batourina et al., 2005; Hains et al, 2008; Mackie et al., 1975; Mackie and Stephens, 1975; Mendelsohn, 2009; Oswald et al., 2004; Poladia et al., 2006; Radmayr et al., 2009; Schwentner et al., 2005; Viana et al., 2007).
The development of VUR has been postulated to occur by several mechanisms including mal-positioned ureters, alterations in intravesicular tunnel length and/or dysfunction of the non-physiological ureterovesical sphincter (Hains et al, 2008; Mackie et al., 1975; Mackie and Stephens, 1975; Mendelsohn, 2009; Murawski and Gupta, 2008; Murawski et al., 2011; Oswald et al., 2003; Oswald et al., 2004; Poladia et al., 2006; Radmayr et al., 2009; Schwentner et al., 2005; Schwentner et al., 2006; Viana et al., 2007; Yu et al., 2004 19–27). Studies suggest that there is a genetic basis for the development of VUR and several recent animal models have been reported (Atiyeh et al., 1992; Cohen and Kreiborg, 1993; Grieshammer et al., 2004; Guarino et al., 2005; Hains et al., 2009; Jiang et al., 2004; Lu et al., 2007; Mendelsohn, 2009; Murawski and Gupta, 2008; Murawski et al., 2011; Nishimura et al., 1999; Passo-Bueno et al., 1999; Seyedzadeh et al., 2008; Yu et al., 2004). Fgfr2Mes−/− mice were generated by the conditional deletion of Fgfr2 from the metanephric mesenchyme of the developing kidney and possess increased common nephric duct lengths with cranially displaced ureteral buds resulting in the development of postnatal VUR (Hains et al., 2008; Hains et al., 2009; Poladia et al., 2006). Morphometric analysis indicated that the right, refluxing ureter of Fgfr2Mes−/− mice was positioned lower than controls, providing a direct structural basis for our observation that deviation from normal internal bladder trigone geometry the most consistent parameter in identifying Fgfr2Mes−/− bladders at E15 and the presence of reflux in P1 Fgfr2Mes−/− mice. Similar ureteral bud formation defects have been observed in several other mouse models of VUR including Pax2 1Neu± and Hoxb7/Ret± mice (Yu et al., 2004; Murawski and Gupta, 2008; Murawski et al., 2011). However, these animals also possessed shortened intravesicular tunnel lengths associated with the refluxing ureter, an observation that was not noted in our analysis of Fgfr2Mes−/− mice. Although this difference may be a result of the various methodological approaches used to measure intravesicular tunnel length, it most likely represents the variable and complex phenotypes associated with VUR development in these distinct animal models. When these observations are coupled with the fact that ureteral insertion and internal trigone geometry appeared normal in the single non-refluxing Fgfr2Mes−/− mouse, they strongly suggest that the primary defect responsible for the development of VUR in Fgfr2Mes−/− mice is improper ureteral insertion into the developing bladder resulting in altered internal trigone geometry and subsequent function. This conclusion is highly consistent with the original Mackie and Stephens hypothesis of VUR development, which postulated that altered positioning of the ureteral bud during development results in abnormal ureteral insertion sites on the bladder contributing to the subsequent development of VUR (Mackie et al., 1975; Mackie and Stephens, 1975).
In summary, 3-D morphometric analysis of bladder development provided a wealth of novel information regarding normal and abnormal urinary tract development, permitting a variety of downstream permutations to be performed without data re-acquisition. This approach demonstrated that normal detrusor smooth muscle development initiates in the bladder dome and sequentially progresses down the posterior bladder wall, with distinct smooth muscle layers becoming evident during postnatal development. 3-D morphometric analysis easily quantified and confirmed that the primary defect associated with mgb−/− mice is a decrease in the differentiation of detrusor smooth muscle throughout development. In addition, multivariate analysis indicated that significant deviations in the geometry of the internal bladder trigone correlated best with the development of VUR in Fgfr2Mes−/− mice. In conclusion, this study validates the use of a 3-D morphometric approach to quantify the morphological features associated with bladder development, and establishes a valuable research tool for future studies designed to characterize the complex events associated with both normal and abnormal urogenital development.
EXPERIMENTAL PROCEDURES
Animals
Animals were housed and maintained in the vivarium at The Research Institute at Nationwide Children's Hospital according to the NIH Guide for the Care and Use of Laboratory Animals. Timed-pregnant control, mgb−/− (Singh et al., 2007) and Fgfr2Mes−/− (Hains et al., 2009) mice were sacrificed and embryos harvested at E13, E14, E15, E16, and E17. P1 control and Fgfr2Mes−/− mice were identified and sacrificed for analysis. PCR analysis of embryonic DNA tail samples was performed to identify gender and genotype as previously described (Singh et al., 2007).
Immunohistochemistry
All embryos were formalin fixed for 48 hours and processed in a Leica TP 1050 Automatic Tissue Processor (Leica Microsystems, Wetzler, Germany) in the Morphology Core at The Research Institute at Nationwide Children's Hospital using standard procedures. Embryos were embedded tail down in paraffin blocks, closely trimmed and sectioned at 10 microns.to maximize the number of sections/slide. Sequential tissue ribbons were place in a 62°C water bath and collected onto charged slides. Slides were deparaffinized and protein blocked using Mouse-to-Mouse Staining System (Scytek, Logan, UT). Primary antibody used was α-smooth muscle actin (Dako, Carpinteria, CA) at a dilution of 1:100. Color was precipitated using UltraVision Plus Detection System Anti-Polyvalent, HRP/AEC Kit according to manufacturer's protocol (LabVision Corp., Fremont, CA). Sections were counterstained with H & E, covered with Crystal Mount (Biomeda Corp., Foster City, CA) and mounted with Permaslip (Alban Scientific Inc., St. Louis, MO).
3-D Reconstruction of the Developing Urogenital System
All parameters examined by 3-D reconstruction were analyzed in a minimum of three animals/structures per time point. 3-D reconstruction was performed using an Olympus BX51 microscope equipped with a motorized stage, CX9000 camera, StereoInvestigator, Neurolucida Explorer, and Neurolucida software (MBF Bioscience, Williston, VT). Structures of interest including the developing bladder serosa, smooth muscle, lamina propria, urothelium, lumen, ureters, ureteric muscle, ureteric lumen and umbilical arteries were identified, assigned a specific color-coded contour and traced using a pen input display (Wacom, Cintiq 21UX). Bladder images were obtained by tracing a total of 25–50 ten-micron sections for each developmental time point examined, with the total number of sections needed increasing as development progressed. Using serial section manager, section intervals were set to a desired z-depth and the outer limits of each structure were traced at optimal magnification. The objective was switched to map the intricacies of the tissue as needed. Each section was aligned and stacked without disturbing the individual contours contained within the section. Each contour was connected using 3-D solids view in StereoInvestigator. The 3-D model was then subjected to smoothing, transparency, zoom, and rotation to view all aspects of the developing urogenital system. The results of the 3-D rendering were analyzed using the Neurolucida Explorer software to yield an enclosed volume measurement for each contour identified above.
The ureteral path was skeletonized using the Neurolucida program as outlined by supplier. At the central most point of the lumen of each ureter in each section, a specific marker was placed. Markers were started at the vesicoureteral junction and then placed stepwise through the length of the ureter to the bladder serosa. Total ureteral length and pathway was analyzed via segmental summation as outlined by supplier.
3-D reconstruction of the vesicoureteral junction was performed by placing markers at the bladder neck/urethral junction, the point were each ureter entered the bladder serosa, and the point where each ureter emptied into the bladder lumen. These points were designated as follows: A – left internal ureteral orifice, B – right internal ureteral orifice, C – internal urethral orifice, D – left external ureteral orifice (point where the left ureter pierces the bladder serosa), and E – right external ureteral orifice (point where the right ureter pierces the bladder serosa). Each identified morphological point was then connected by a straight-line permitting calculation of line lengths and angles.
Statistical Analysis
All statistical analyses were performed using SAS software. Analysis of the proportion of urothelium, lamina, and muscle in control and mutant bladders was calculated using Bonferroni adjusted 95% simultaneous confidence intervals based on Student's t-test. MANOVA was initially performed to test all three outcomes jointly, which allowed for covariance between the outcome variables. Confidence intervals were then calculated with a Bonferroni adjustment to provide overall confidence of 95%. Each outcome met the normality assumption required for the use of the t-test. Satterthwaite's approximation was used to adjust for non-constant variance.
Statistical analysis of tunnel lengths and bladder volume was performed using a two-way complete ANOVA model for each tunnel type, with independent variables side, time and genotype. Multiple comparisons using simultaneous 95% Bonferroni adjusted confidence intervals based on Student's t test. Volume measurements were transformed to meet the assumptions of the test resulting in interval units of (volume)1/6.
Statistical analysis of trigone geometry was calculated using a one-way ANOVA model for each line and angle, with the independent variable time. Simultaneous 95% Bonferroni adjusted confidence intervals based on Student's t-test were performed as described above. The assumptions of the test were met for each of the lines and angles. Simultaneous 95% Bonferroni adjusted confidence intervals for perimeter and area were also calculated. Both normality and constant variance assumptions were met. In the case of trigone geometry, it was necessary to transform area to meet the assumptions generating interval units of (area)1/6. Satterthwaite's approximation was also used to adjust for non-constant variance.
Supplementary Material
Acknowledgments
Grant Sponsor: This work was supported by NIH R01-DK70907, R01-DK085242, R01-DK081128 and R01-EY12995.
REFERENCES
- Airik R, Bussen M, Singh MK, Petry M, Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest. 2006;116:663–374. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airik R, Kispert A. Down the tube of obstructive nephropathies: the importance of tissue interactions during ureter development. Kidney Int. 2007;72:1459–1467. doi: 10.1038/sj.ki.5002589. [DOI] [PubMed] [Google Scholar]
- Airik R, Trowe MO, Foik A, Farin HF, Petry M, Schuster-Gossler K, Schweizer M, Scherer G, Kist R, Kispert A. Hydroureteronephrosis due to loss of Sox9-regulated smooth muscle cell differentiation of the ureteric mesenchyme. Hum Mol Genet. 2010;19:4918–4929. doi: 10.1093/hmg/ddq426. [DOI] [PubMed] [Google Scholar]
- Atiyeh B, Husmann D, Baum M. Contralateral renal abnormalities in multicystic-dyspplastic kidney disease. J Pediatr. 1992;121:65–67. doi: 10.1016/s0022-3476(05)82543-0. [DOI] [PubMed] [Google Scholar]
- Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon A, Carrol T, Mendelsohn C. Apoptosis induced by vitamin A signaling is crucial for connecting ureters to the bladder. Nat Genet. 2005;37:1082–1089. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- Cohen M, Kreiborg S. Visceral anomalies in the Apert syndrome. Am J Med Genet. 1993;45:758–760. doi: 10.1002/ajmg.1320450618. [DOI] [PubMed] [Google Scholar]
- Copenhaver W, Kelly D, Wood R. Bailey's Textbook of Histology. Seventh Edition Williams and Wilkins; Baltimore, MD: 1978. pp. 604–609. [Google Scholar]
- Executive summary: United States renal data system Annual data report. Am J Kidney Dis. 1999;34:S9–S19. doi: 10.1053/AJKD034s00009. [DOI] [PubMed] [Google Scholar]
- Foreman J, Chan J. Chronic renal failure in infants and children. J Pediatr. 1988;113:793–800. doi: 10.1016/s0022-3476(88)80003-9. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Le M, Plump AS, Wang F, Tessler-Lvigne M, Martin G. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- Guarino N, Casamassima MG, Tadini B, Marras E, Lace R, Bianchi M. Natural history of vesicoureteral reflux associated with kidney anomalies. Urology. 2005;66:1208–1211. doi: 10.1016/j.urology.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Hains D, Sims-Lucas S, Kish K, Saha M, McHugh KM, Bates CM. Role of fibroblastic growth factor receptor 2 in kidney mesenchyme. Pediatr Res. 2008;64:592–598. doi: 10.1203/PDR.0b013e318187cc12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains D, Sims-Lucas S, Carpenter A, Saha M, Murawsik I, Kish K, Gupta I, McHugh K, Bates C. High incidence of vesicoureteral reflux in mice with deletion of fibroblast growth factor receptor 2 in kidney mesenchyme. J Urol. 2009;148:2077–2084. doi: 10.1016/j.juro.2009.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutch J. Anatomy and physiology of the bladder, trigone and urethra. Butterworths Appleton-Century-Crofts; London, New York: 1972. [Google Scholar]
- Jiang S, Gitlin J, Deng FM, Liang F-X, Lee A, Atala A, Bauer S, Ehrlich G, Feather S, Goldberg J, Goodship J, Goodship T, Hermanns M, Hu F, Jones K, Malcolm S, Mendelsohn C, Preston R, Retik A, Schneck F, Wright V, Ye X, Woolf A, Wu X-R, Ostrer H, Shapiro E, Yu J, Sun T-T. Lack of major involvement of human uroplakin genes in vesicoureteral reflux: implications for disease heterogeneity. Kidney Int. 2004;66:10–19. doi: 10.1111/j.1523-1755.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- Lu W, van Eerde AM, Fan X, Quintero-Rivera F, Kulkami S, Ferguson H, Kim H, Fan Y, Xi Q, Li Q, Saniaville D, Andrews W, Sundaresan V, Bi W, Yan J, Giltay J, Wijmenga C, de Jong T, Feather S, Woolf A, Rao Y, Lupski J, Eccles M, Quade B, Gusella J, Morton C, Maas R. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet. 2007;80:616–632. doi: 10.1086/512735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie GG, Awang H, Stephens FD. The ureteric orifice: the embryologic key to radiologic status of duplex kidneys. J Pediatr Surg. 1975;10:473–481. doi: 10.1016/0022-3468(75)90187-6. [DOI] [PubMed] [Google Scholar]
- Mackie GG, Stephens FD. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol. 1975;114:274–280. doi: 10.1016/s0022-5347(17)67007-1. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. Using mouse models to understand normal and abnormal urogenital tract development. Organogen. 2009;5:306–314. doi: 10.4161/org.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh KM. Molecular Analysis of Smooth Muscle Development in the Mouse. Developmental Dynamics. 1995;204:278–290. doi: 10.1002/aja.1002040306. [DOI] [PubMed] [Google Scholar]
- McHugh KM. Molecular Analysis of Gastrointestinal Smooth Muscle Development: Invited Review. Journal Pediatric Gastroenterology and Nutrition. 1996;23:379–394. doi: 10.1097/00005176-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Murawski IJ, Gupta IR. Gene discovery and vesicoureteral reflux. Pediatr Nephrol. 2008;23:1021–1027. doi: 10.1007/s00467-007-0704-y. [DOI] [PubMed] [Google Scholar]
- Murawski IJ, Watt CL, Gupta IR. Vesicoureteric reflux: using mouse models to understand a common congenital urinary tract defect. Pediatr Nephrol. 2011;26:1513–1522. doi: 10.1007/s00467-011-1821-1. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley T, Yoshida H, Ichiki T, Threadgill D, Phillips J, Hogan B, Fogo A, Brock J, Inagami T, Ichikawa I. Role of the angiotensin type 2 receptor in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- Oswald J, Brenner E, Schwenter C, Deibl M, Bartsch G, Fritsch H, Radmayr C. The intravesicular ureter in children with vesicoureteral reflux: a morphological and immunohistochemical characterization. J Urol. 2003;170:2423–2427. doi: 10.1097/01.ju.0000097146.26432.9a. [DOI] [PubMed] [Google Scholar]
- Oswald J, Schwentner C, Brenner E, Deibl M, Fritsch H, Bartsch G, Radmayr C. Extracellular matric degredation and reduced nerve supply in refluxing ureteral endings. J Urol. 2004;172:1099–1102. doi: 10.1097/01.ju.0000135673.28496.70. [DOI] [PubMed] [Google Scholar]
- Passos-Bueno M, Wilcox W, Jabs E, Sertie A, Alonso L, Kitoh H. Clinical spectrum of fibroblastic growth factor receptor mutations. Hum Mutat. 1999;14:115–125. doi: 10.1002/(SICI)1098-1004(1999)14:2<115::AID-HUMU3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM. Role of fibroblastic growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol. 2006;291:325–339. doi: 10.1016/j.ydbio.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Radmayr C, Schwentner C, Lunacek A, Karatzas A, Oswald J. Embryology and anatomy of the vesicoureteric junction with special reference to the etiology of vesicoureteral reflux. Ther Adv Urol. 2009;1:243–250. doi: 10.1177/1756287209348985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth K, Carter WH, Jr, Chan J. Obstructive nephropathy in children: long-term progression after relief of posterior urethral valve. Pediatr. 2001;107:1004–1010. doi: 10.1542/peds.107.5.1004. [DOI] [PubMed] [Google Scholar]
- Schwentner C, Oswald J, Lunacek A, Fritsch H, Deibl M, Bartsch G, Radmayr C. Loss of interstitial cells of Cajal and gap junction protein connexin 43 at the vesicoureteral junction in children with vesicoureteral reflux. J Urol. 2005;174:1981–1986. doi: 10.1097/01.ju.0000176818.71501.93. [DOI] [PubMed] [Google Scholar]
- Schwentner C, Oswald J, Lunacek A, Schlenck B, Berger AP, Deibl M, Fritsch H, Bartsch G, Radmayr C. Structural changes of the intravesicular ureter in children with vesicoureteral reflux-does eschemia have a role? J Urol. 2006;176:2212–2218. doi: 10.1016/j.juro.2006.07.062. [DOI] [PubMed] [Google Scholar]
- Seyedzadeh A, Kompani F, Esmailie E, Samadzadeh S, Farshchi B. High-grade vesicoureteral reflux in Pfeiffer syndrome. Urol J. 2008;5:200–202. [PubMed] [Google Scholar]
- Singh S, Robinson M, Nahi F, Coley B, Robinson M, Bates C, Kornacher K, McHugh KM. Identification of a unique transgenic mouse line that develops megabladder, obstructive uropathy, and renal dysfunction. JASN. 2007;18:461–471. doi: 10.1681/ASN.2006040405. [DOI] [PubMed] [Google Scholar]
- Singh S, Robinson M, Ismail I, Saha M, Auer H, Kornacher K, Robinson M, Bates C, McHugh KM. Transcriptional profiling of the megabladder mouse: a unique model of bladder dysmorphogenesis. Dev Dyn. 2008;237:170–186. doi: 10.1002/dvdy.21391. [DOI] [PubMed] [Google Scholar]
- Viana R, Batourina E, Huang H, Dressler G, Kobayashi A, Behringer R, Shapiro E, Hensle T, Lambert S, Mendelsohn C. The development of the bladder trigone, the center of the anti-reflux mechanism. Develop. 2007;134:3763–3769. doi: 10.1242/dev.011270. [DOI] [PubMed] [Google Scholar]
- Yu OH, Murawski IJ, Myburgh D, Gupta IR. Overexpression of RET leads to vesicoureteral reflux in mice. Am J Physiol Renal Physiol. 2004;287:F1123–1130. doi: 10.1152/ajprenal.00444.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.