Abstract
Purpose
We evaluated a novel therapy for primary central nervous system (CNS) lymphoma (PCNSL) using induction immunochemotherapy with high-dose methotrexate, temozolomide and rituximab (MT-R) followed by intensive consolidation with infusional etoposide and high-dose cytarabine (EA). In addition, we evaluated the prognostic value of the minimum apparent diffusion coefficient (ADCmin) derived from diffusion-weighted magnetic resonance imaging (DW-MRI) in patients treated with this regimen.
Experimental Design
Thirty-one patients (median age, 61; median KPS, 60) received induction with methotrexate every 14 days for 8 planned cycles. Rituximab was administered the first 6 cycles and temozolomide administered on odd-numbered cycles. Patients with responsive or stable CNS disease received EA consolidation. Pretreatment DW-MRI was used to calculate the ADCmin of contrast-enhancing lesions.
Results
The complete response rate for MT-R induction was 52%. At a median follow-up of 79 months, the 2-year progression-free and overall survival were 45% and 58%, respectively. For patients receiving EA consolidation, the 2-year progression-free and overall survival were 78% and 93%, respectively. EA consolidation was also effective in an additional 3 patients who presented with synchronous CNS and systemic lymphoma. Tumor ADCmin <384 × 10−6 mm2/s was significantly associated with shorter progression-free and overall survival.
Conclusions
MT-R induction was effective and well-tolerated. MT-R followed by EA consolidation yielded progression-free and overall survival outcomes comparable to regimens using chemotherapy followed by whole-brain radiotherapy consolidation but without evidence of neurotoxicity. Tumor ADCmin derived from DW-MRI provided better prognostic information for PCNSL patients treated with the MTR-EA regimen than established clinical risk scores.
Keywords: Brain Tumors, Non-Hodgkin’s Lymphoma, Immunotherapy, MRI, Biomarkers
INTRODUCTION
Novel therapeutic approaches that improve efficacy but avoid the deleterious neurocognitive effects of treatment, in particular those of standard-dose whole brain radiotherapy (WBRT), are needed in primary CNS lymphoma (PCNSL). The problem of radiation-induced delayed neurotoxicity is particularly significant for the approximate one-half of PCNSL patients older than 60 years (1). While a preliminary report provided evidence that reduced dose whole brain irradiation (23.4 Gy) in conjunction with chemotherapy caused less neurotoxicity than standard dose WBRT (45 Gy) (2), additional follow-up and validation of these results are needed and there remains a general concern that radiation-induced encephalopathy is a particularly undesirable and irreversible, treatment-associated morbidity.
High-dose methotrexate now represents the cornerstone of therapy in PCNSL (3). In contrast to WBRT, treatment with high-dose methotrexate alone does not appear to frequently cause clinically severe neurocognitive impairment (4). However, high-dose methotrexate monotherapy is rarely curative with at least 70% of patients exhibiting disease progression within 2 years (5, 6).
Our goal has been to develop a dose-intensive chemotherapeutic regimen that is tolerated by the majority of PCNSL patients, particularly during the first weeks after diagnosis when neurologic function and performance status are most compromised. For the past 10 years at the University of California, San Francisco (UCSF), newly diagnosed PCNSL patients have been treated with a novel, two-step immunochemotherapy program involving 4 months of induction therapy using intravenous high-dose methotrexate with oral temozolomide and intravenous rituximab (MT-R) followed by high-dose consolidation chemotherapy, without WBRT.
In the regimen, high-dose methotrexate with leucovorin rescue is given every 14 days for a planned 8 treatments. Standard dose intravenous rituximab is administered during the first 2 months of therapy, a window in which the blood-brain barrier is most often significantly compromised (7) and we hypothesized would permit the delivery of rituximab to the tumor. Temozolomide has high relative lipophilicity with reliable CNS penetrance and a superior toxicity and health-related quality of life profile in brain tumor patients compared to procarbazine (8, 9). Temozolomide is active at relapse in PCNSL, both as monotherapy and in combination with rituximab (10-12).
To attempt to potentiate long-term, progression-free survival after MT-R, PCNSL patients with at least stable disease received intensive consolidation chemotherapy with non-cross-resistant agents: 96-hour infusional etoposide plus high-dose, twice-daily cytarabine (EA)(13-15). A similar combination of etoposide plus high-dose cytarabine was shown by Soussain et al. to be active as salvage therapy in recurrent/refractory primary and secondary CNS lymphoma, with 12 of 14 patients exhibiting responses, eight of which were complete responses (16). Moreover 96-hour infusional etoposide has been incorporated within the EPOCH regimen, which is highly active in large cell lymphoma (17, 18), the most common histology to affect the CNS. A variety of reports have demonstrated the activity of etoposide in treating brain tumors, including lymphoid leukemia involving the CNS (19). The use of etoposide has also been associated with a significant reduction in the risk of secondary CNS lymphoma, when given in combination with CHOP in patients with aggressive lymphoma (20). The importance of high-dose cytarabine in PCNSL was also recently underscored in a randomized phase II study (21).
Diffusion-weighted imaging (DWI) is a noninvasive MR imaging technique that produces in vivo images of brain based on differential rate of water diffusion or Brownian motion within the extracellular space. DWI is an essential tool to diagnose acute infarct in the brain due to its ability to detect early changes in altered water diffusion due to cellular damage. DWI has also been widely used in neuro-oncology to assess tumor biology. Specifically, the apparent diffusion coefficient (ADC) values derived from DWI have been shown to correlate with glioma grade (22, 23), tumor cellularity (24), and treatment response (25-31). A recent study also suggests that ADC values may be helpful in predicting clinical outcome in immunocompetent patients with primary CNS lymphoma (32).
In this analysis, we describe the toxicity and long-term outcome of the first PCNSL patients to be treated with combination MT-R followed by EA at UCSF Medical Center, between 2001 and 2006. This study represents the first analysis of the survival of newly-diagnosed PCNSL patients to receive a dose-intensive consolidation chemotherapy regimen that involves neither autologous stem cell transplantation nor WBRT. This study also represents the first analysis of the role of etoposide as a component of consolidation in newly diagnosed patients with CNS lymphoma. Finally, we evaluated diffusion-weighted magnetic resonance imaging (DW-MRI) as a non-invasive tool to determine tumor minimal apparent diffusion coefficient (ADCmin) at diagnosis as a biomarker predictive of prognosis for PCNSL patients who receive MT-R induction therapy.
PATIENTS AND METHODS
Patient Characteristics
The primary study population included 31 immunocompetent patients with newly-diagnosed, histologically or cytologically-proven PCNSL treated at the UCSF Comprehensive Cancer Center beginning in 2001 and ending in 2006. There was no restriction with respect to age or performance status. Ten subjects were enrolled on UCSF protocol 03301, a prospective phase I trial with stopping rules for two safety endpoints: hematologic toxicity (prolonged leucopenia during induction MT-R) and neurotoxicity (grade 3 or 4 neurotoxicity during EA consolidation). The outcome of an additional 3 immunocompetent patients who presented with brain parenchymal involvement of CNS lymphoma of aggressive histology with systemic involvement at pretreatment staging and who were treated with EA consolidation, is presented as well. The retrospective analysis of treatment, toxicities and outcomes for all patients was approved by the UCSF institutional review board (H9414-23160). Pretreatment diagnosis and staging, including complete ophthalmologic examinations, as well as restaging after initiation of treatment, was performed in accordance with guidelines established by the International Primary CNS Lymphoma Collaborative Group (IPCG) (33).
Mandatory baseline lab values required absolute neutrophil count (ANC) >1,500/mcl, AST and ALT ≤2 times the upper limit of normal (ULN), total bilirubin ≤2 times the ULN, and measured creatinine clearance ≥50 ml/minute. Prior to the first dose of methotrexate, creatinine clearance was determined by 24-hour urine collection. In subsequent treatment cycles, the Cockcroft-Gault equation was used to estimate creatinine clearance.
Remission Induction Immunochemotherapy
Treatment cycles were 14 days in length (Table 1). Sulfonamide drugs, trimethoprim, salicylates, non-steroidal anti-inflammatory drugs, penicillins, vitamin C, ciprofloxacin, and proton pump inhibitors were held at least 48 hours prior to methotrexate administration. Hydration and urine alkalinization was achieved by administration of NaHCO3 100-150 mEq/L at 150 ml/hour intravenously until urine output of ≥100 ml/hour and urine pH >7 for 4 hours prior to methotrexate and continued until completion of leucovorin rescue. Intravenous methotrexate 8 grams/m2 was given over 4 hours on day 1 of each 14 day cycle followed 24 hours later by leucovorin 100 mg/m2 IV every 6 hours, as described (34). Serum methotrexate levels were measured every 12 hours after the start of methotrexate. Intravenous leucovorin was continued until serum methotrexate ≤0.5 μM at which time oral leucovorin 10 mg/m2 every 6 hours was administered until methotrexate level <0.05 μM. Methotrexate dose was reduced for decreased creatinine clearance, as described (5). Intravenous rituximab 375 mg/m2 was given on day 3 of each cycle for a total of 6 doses. Diphenhydramine 25-50 mg and acetaminophen 650 mg were administered prior to rituximab. The patient with T-cell lymphoma did not receive rituximab. Oral temozolomide 150 mg/m2 was given daily on days 7-11 during the odd numbered cycles for a total of 4 cycles. No intrathecal therapy was administered. Patients were evaluated for response after six cycles of MT-R. If a complete response (CR) was obtained, the patient was treated with two additional cycles of methotrexate and temozolomide before high dose consolidation chemotherapy. If a partial response (PR) was observed, the patient was treated with between 3-5 additional cycles of induction methotrexate and temozolomide before consolidation.
Table 1.
Treatment schema
| Remission induction therapy, MT-R (14 day cycle, 8 cycles) | |
| Day 1 | Methotrexate 8 grams/m2 IV over 4 hours |
| Day 2 | Leucovorin 100 mg/m2 every 6 hours IV until serum methotrexate <0.05 μM |
| Day 3 | Rituximab 375 mg/m2 IV cycle 1 through 6* |
| Day 7-11 (odd cycles only) |
Temozolomide 150 mg/m2 oral daily |
| Consolidation therapy, EA (one cycle) | |
| Day 1 - 4 | Etoposide 5 mg/kg continuous IV over 12 hours every 12 hours × 8 doses |
| Day 1 - 4 | Cytarabine 2 gm/m2 IV over 2 hours every 12 hours × 8 doses |
Rituximab omitted forT-cell lymphoma
Patients with synchronous brain parenchymal and systemic lymphoma received both high-dose methotrexate at 8 grams/m2 with leucovorin rescue every two weeks, for a total of eight cycles and standard dose R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) every three weeks for six cycles. When R-CHOP and high-dose methotrexate were given during the same week, high-dose methotrexate was administered day 1 and R-CHOP was administered starting on day three.
Consolidation Chemotherapy
Patients with PCNSL as well as the three CNS lymphoma patients with synchronous brain parenchymal and systemic lymphoma, were offered inpatient high-dose therapy with etoposide and cytarabine (EA) if they achieved stable disease or better after eight cycles of methotrexate-based induction. Three patients elected to receive up to three additional cycles of methotrexate before proceeding to intensive consolidation. The median number of methotrexate cycles for all patients who received EA consolidation was eight. Etoposide 5 mg/kg was given by continuous IV infusion every 12 hours for 8 doses (40 mg/kg total dose) with cytarabine 2 grams/m2 IV over 2 hours every 12 hours for 8 doses (total dose 16 gm/m2). Corticosteroid eye drops, 2 drops per eye, were given 4 times per day, days 1-6 to prevent cytarabine keratoconjunctivitis. Patients showered twice daily during cytarabine treatment days. G-CSF 5 mcg/kg/day was given subcutaneously starting day 14 of therapy and continued until ANC ≥500/mcl for 2 days or ≥1,500/mcl for one day. Bacterial prophylaxis with fluoroquinolone (moxifloxacin or levofloxacin) antibiotics was initiated at ANC <500/mcl and continued until ANC ≥500/mcl. Fungal prophylaxis consisted of fluconazole or voriconazole starting day 6 of therapy and continuing until ANC ≥500/mcl. Herpes simplex virus and Varicella zoster virus prophylaxis consisted of acyclovir or valacyclovir. Pneumocystis pneumonia prophylaxis was provided with trimethoprim/sulfamethoxazole or dapsone. Packed red blood cell or platelet transfusions were given for Hct <26% or platelets <10,000/mcl, respectively. All blood products were leukoreduced in-line during transfusion.
Evaluation of toxicity and response
Toxicity was graded by the NCI Common Toxicity Criteria for Adverse Events, version 4.0. Tumor responses were evaluated by gadolinium-enhanced MRI of the brain after every second or third cycle, and in all cases after cycle 8 of HD-MTX and after EA consolidation therapy (33). For patients with initial positive CSF cytology, repeat lumbar punctures were performed to assess response. Response assessment was per guidelines of the International Primary CNS Lymphoma Collaborative Group (IPCG) guidelines (33).
MR Imaging and Determination of ADCmin
We previously reported that the minimum apparent diffusion coefficient (ADCmin) of contrast enhancing lesions correlated with outcome in PCNSL patients treated with high-dose methotrexate-based therapies (32). We therefore applied this method to those PCNSL patients treated at our institution who were treated with combination MT-R induction between 2001 and 2006 to assess the relationship between pretreatment intra-tumoral water diffusion and response, progression-free survival, and overall survival in patients treated with this regimen. Eight of these patients were included in our previous report. Prior to therapy, patients underwent brain MR imaging without and with intravenous contrast.
All patients were imaged using a 1.5 Tesla clinical MR scanner (Signa Horizon, GE Healthcare, Milwaukee, Wisconsin). MR imaging examinations included conventional contrast-enhanced T1-weighted imaging and DWI sequences obtained according to a standardized protocol: 3-plane localizer (TR/TE, 8.5/1.6 ms), sagittal T1-weighted spin-echo (TR/TE, 600/17 ms), axial 3D T2-weighted fast spin-echo (TR/TE, 3000/102 ms), axial fluid-attenuated inversion recovery (FLAIR) (TR/TE/TI, 10,000/148/2200 ms), axial DWI echo-planar imaging (TR/TE, 10,000/99 ms; section thickness/intersection gap, 5/0 mm; matrix size, 256 × 256 × 24; FOV, 24 cm; 3-directions, b-value, 0 and 1000 s/mm2) acquired in the transverse plane throughout the infratentorial and supratentorial brain, and contrast-enhanced 3D spoiled gradient-recalled acquisition in the steady state (SPGR) T1-weighted imaging (TR/TE, 34/8 ms; section thickness/intersection gap, 1.5/0 mm).
Gadopentetate dimeglumine (Magnevist, Bayer Healthcare, Wayne, NJ) was the intravenous contrast agent for the MR imaging study and the dose used was 0.1 mmol/kg body weight.
From the raw DWI data set, the apparent diffusion coefficient (ADC) map was reconstructed based on pixel-by-pixel display of diffusion coefficient in 3 different directions and fitting diffusion signal intensities to the Stejskal-Tanner equation, S(b) = S(0) exp(-b ADC), using a least-squares approach. The ADC map was then co-registered with the axial contrast-enhanced T1-weighted images at regions of interest (ROIs). For each patient, the ROIs were drawn on the post-contrast T1-weighted images outlining all contrast enhancing tumor by one investigator. All ROIs were checked and approved by the attending neuroradiologist involved in the study (S.C). In 10 patients, 2 different investigators drew ROIs independently and were verified by the neuroradiologist to be repeatable and reproducible. The minimum ADC coefficient (ADCmin) was determined within the ROIs of the contrast enhancing tumor region as described (32). The neuroradiologist determining the ADCmin was blinded to patient outcomes at the time of ADCmin determination. All ADC values are reported as 100 × 10−6 mm2/s.
Data Analysis
Progression-free (PFS) and overall survival (OS) were determined by Kaplan-Meier analysis. Survival curves were compared with the Log-rank test. Response rates between Memorial Sloan Kettering Cancer Center (MSKCC) risk groups, International Extranodal Lymphoma Study Group (IELSG) risk groups, and ADCmin category were compared with Fisher’s exact test.
RESULTS
Patient Characteristics
Baseline clinical characteristics of the 31 PCNSL patients are summarized in Table 2. Median age was 61 years (range 40-84) with median Karnofsky performance score of 60 (range 50-100). Diffuse large B-cell lymphoma accounted for 25 of the 31 cases (81%) with lymphoblastic lymphoma (n = 2, 6.5%), Burkitt-like lymphoma (n = 2, 6.5%), aggressive B-cell lymphoma, unspecified (n = 1, 3%), and T-cell lymphoma (n = 1, 3%) accounting for the remainder of cases. Twenty-one patients (68%) had deep brain lesions and two patients (6.5%) had isolated leptomeningeal disease. CSF cytology was positive for lymphoma in 6 of 26 cases evaluated (23%). Ocular involvement was evident in 1 patient (3%) at diagnosis.
Table 2.
Baseline patient characteristics
| Number of patients |
% | |
|---|---|---|
| Age | ||
| Median | 61 | |
| Range | 40-84 | |
| KPS | ||
| Median | 60 | |
| Range | 50-100 | |
| Sex | ||
| Female | 17 | 55 |
| Male | 14 | 45 |
| Lymphoma Type | ||
| Diffuse large B-cell | 25 | 81 |
| Lymphoblastic | 2 | 6.5 |
| Burkitt-like | 2 | 6.5 |
| Aggressive B-cell, unspecified | 1 | 3 |
| T-cell | 1 | 3 |
| Deep lesions | 21 | 68 |
| Elevated LDH (n = 29) | 9 | 31 |
| CSF cytology positive (n = 26) | 6 | 23 |
| Elevated CSF protein (n = 24) | 20 | 83 |
| Ocular involvement | 1 | 3 |
| IELSG risk group (n = 24) | ||
| 0-1 | 3 | 12 |
| 2-3 | 11 | 46 |
| 4-5 | 10 | 42 |
| MSKCC risk group (RPA class) | ||
| 1 | 4 | 13 |
| 2 | 9 | 29 |
| 3 | 18 | 58 |
Abbreviations: KPS, Karnofsky performance score; LDH, lactate dehydrogenase; CSF, cerebrospinal fluid; IELSG, International Extranodal Lymphoma Study Group; MSKCC, Memorial Sloan Kettering Cancer Center; RPA, recursive partitioning analysis.
The 3 patients with synchronous brain parenchymal CNS and systemic lymphoma ranged in age from 45-55 years and all had large B-cell lymphoma. Biopsy proven extra-CNS sites of disease for these patients were bone marrow, adrenal gland, and occipital bone with associated musculature.
Toxicity
Toxicity of methotrexate, temozolomide and rituximab (MT-R) induction
MT-R was well tolerated. Grade 3 or 4 adverse events occurring in more than one patient included reversible transaminitis in seven patients and neutropenia in three patients with no episodes of febrile neutropenia. No patient developed grade 2 or greater CNS toxicity. One treatment-related death occurred in an 81 year-old patient from concurrent Pneumocystis jiroveci and Cytomegalovirus pneumonia in the setting of tumor progression. Among the three patients receiving high-dose methotrexate in combination with R-CHOP for systemic lymphoma with CNS involvement, grade 3-4 adverse events included neutropenia (100%), anemia (33%), thrombocytopenia (33%), transaminitis (33%) and neutropenic fever (33%).
Toxicity of high-dose etoposide and cytarabine (EA) consolidation
Seventeen patients received EA consolidation chemotherapy. The median length of hospital stay was 20 days (range 19-28). As expected, all patients developed grade 4 neutropenia and thrombocytopenia. Patients had a median of 10 days of severe neutropenia (ANC <500/mcL, range 8-12 days) with 14 patients experiencing fever (temperature ≥ 38.3°C). Grade 4 febrile neutropenia occurred in one patient. Infectious organisms identified in 5 patients were Clostridium difficile (stool, 2 cases), Staphylococcus epidermidis (blood, 2 cases), Enterococcus faecalis (blood, 1 case), Enterococcus faecium (blood, 1 case), Citrobacter freundi (blood, 1 case), Escherichia coli (blood, 1 case; urine, 1 case) and Klebsiella pneumoniae (urine, 1 case). Patients spent a median of 2 days with platelets less than 20,000/mcL (range 1-8 days) and a median of 9 days of platelets less than 50,000/mcl (range 7-20). The median number of platelet transfusions was 2 (range 1-5) and the median number of packed RBC transfusions was 2 (range 0-4). Common grade 1-3 non-hematologic toxicities in >30% of patients included nausea (82%), diarrhea (82%), rash (65%), vomiting (41%), reversible transaminitis (41%), hyperbilirubinemia (35%), and mucositis (35%). The only grade 4 non-hematologic adverse event was hyponatremia associated with confusion in one patient (nadir Na 118 mmol/L). One patient required intensive care monitoring for two days for febrile neutropenia with transient hypotension. There was no grade 3 or 4 neurotoxicity and there were no treatment-related deaths during intensive consolidation (Table 3).
Table 3.
Adverse events with EA consolidation (n=17)
| All grades (%) |
Grade 3-4 (%) |
|
|---|---|---|
| Neutropenia | 100 | 100 |
| Neutropenic fever | 82 | 82 |
| Thrombocytopenia | 100 | 100 |
| Anemia | 100 | 100 |
| Nausea | 82 | 12 |
| Diarrhea | 82 | 6 |
| Rash | 65 | 12 |
| Mucositis | 35 | 6 |
| Hyponatremia | 6 | 6 |
| Vomiting | 41 | - |
| AST elevated | 41 | - |
| ALT elevated | 29 | - |
| Hyperbilirubinemia | 35 | - |
| Alkaline phosphatase elevated | 29 | - |
| Creatinine elevated | 24 | - |
| Hypomagnesemia | 53 | - |
| Hypokalemia | 41 | - |
| Hypophosphatemia | 35 | - |
| Confusion | 6 | - |
Response to Induction Immunochemotherapy
All 31 patients received at least one full cycle of MT-R. Eighteen patients responded in total (ORR=58%) with sixteen patients (52%) achieving a CR, 15 within eight cycles of methotrexate and one after nine cycles (Tables 4 and 5). One of the two partial responders had concomitant primary intraocular and brain parenchymal lymphoma and achieved complete resolution of all intracranial lesions but only partial resolution of intraocular lymphoma after induction MT-R. One patient maintained stable disease throughout MT-R and went on to EA consolidation. Twelve patients exhibited radiographic progression before completion of planned induction MT-R. Each of the 3 patients with synchronous brain parenchymal CNS and systemic lymphoma treated with high-dose methotrexate plus R-CHOP achieved a CR within all sites of disease after four cycles of methotrexate and R-CHOP.
Table 4.
Individual patient outcomes
| Patient | Age (years) |
Histology | Response to MTR | EA consolidation | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|
| 1 | 60 | DLBCL | CR | Yes | 107+ | 107+ |
| 2 | 40 | DLBCL | CR | Yes | 123+ | 123+ |
| 3 | 54 | Lymphoblastic lymphoma | PD | No | 1.1 | 9.8 |
| 4 | 47 | DLBCL | PR | No | 12 | 14 |
| 5 | 71 | DLBCL | PD | No | 3 | 8 |
| 6 | 52 | DLBCL | CR | No | 48+ | 48+ |
| 7 | 43 | DLBCL | CR | Yes | 8.7 | 11 |
| 8 | 68 | DLBCL | CR | No | 104+ | 104+ |
| 9 | 45 | Burkitt-like lymphoma | PD | No | 2 | 22 |
| 10 | 62 | DLBCL | PD | No | 1 | 17 |
| 11 | 68 | DLBCL | PD | No | 8 | 8.5 |
| 12 | 79 | DLBCL | PD | No | 4 | 6.5 |
| 13 | 65 | DLBCL | CR | Yes | 95+ | 95+ |
| 14 | 59 | Aggressive B-cell NHL | CR | Yes | 24 | 66 |
| 15 | 61 | DLBCL | CR | Yes | 91+ | 91+ |
| 16 | 50 | DLBCL | PD | No | 1.2 | 6.4 |
| 17 | 81 | Lymphoblastic lymphoma | CR | No | 77 | 83 |
| 18 | 53 | DLBCL | CR | Yes | 90+ | 90+ |
| 19 | 79 | DLBCL | PD | No | 6 | 9 |
| 20 | 67 | DLBCL | CR | No | 51+ | 51+ |
| 21 | 84 | DLBCL | PD | No | 1 | 3 |
| 22 | 67 | DLBCL | PD | No | 3 | 6 |
| 23 | 49 | DLBCL | PD | No | 3 | 42.3 |
| 24 | 62 | DLBCL | SD | Yes | 80+ | 80+ |
| 25 | 63 | DLBCL | CR | Yes | 79+ | 79+ |
| 26 | 66 | DLBCL | CR | Yes | 54+ | 54+ |
| 27 | 52 | DLBCL | CR | Yes | 71+ | 71+ |
| 28 | 54 | Burkitt-like lymphoma | CR | Yes | 62+ | 62+ |
| 29 | 54 | DLBCL | PR | Yes | 6 | 54+ |
| 30 | 64 | T-cell NHL | PD | No | 3.8 | 5.7 |
| 31 | 54 | DLBCL | CR | Yes | 56+ | 56+ |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; NHL, non-Hodgkin lymphoma; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PFS, progression-free survival; OS, overall survival
Table 5.
Response rates and survival after MT-R, MT-R followed by EA, and by pre-treatment ADCmin values
| Total | Complete response |
Partial response |
Overall response |
Median OS |
Median PFS |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | No. | % | No. | % | No. | % | Months | Months | |
| MT-R | 31 | 16 | 52 | 2 | 6 | 18 | 58 | 66 | 24 |
| MT-R → EA | 14 | NR | NR | ||||||
| ADCmin (× 10−6mm2/s) | |||||||||
| ≥384 | 15 | 10 | 67 | 0 | 0 | 10 | 67 | NR | NR |
| <384 | 8 | 2 | 25 | 1 | 13 | 3 | 38 | 12 | 2 |
Abbreviations: OS, overall survival; PFS, progression-free survival; MT-R, all patients receiving rituximab, methotrexate and temozolomide; MT-R → EA, patients receiving MT-R followed by high-dose etoposide and cytarabine consolidation; NR, not reached; ADCmin, minimum apparent diffusion coefficient
High-dose etoposide and cytarabine consolidation
A total of 14 PCNSL patients received EA consolidation, 12 of whom were in CR after MT-R induction (1 was in PR and 1 with SD). The one patient with stable disease to MT-R had a partial response with EA treatment with concomitant sustained neurologic improvement and is alive without progression 80 months after the start of therapy. One patient had progressive intraocular lymphoma after EA consolidation of an initial PR to induction MT-R and ultimately required external beam radiotherapy after the EA consolidation to eliminate disease in the intraocular compartment. Since completion of EA and ocular radiotherapy the patient has been disease-free for 54 months. Two patients died of progressive disease after receiving EA consolidation.
Of the four PCNSL patients in CR after induction who decided not to pursue EA consolidation, one died from progressive CNS lymphoma six years after completion of MT-R and three are alive at last follow-up, without evidence of disease. One patient in PR after eight cycles of methotrexate-based induction pursued autologous stem cell transplant but succumbed to disease progression three months later.
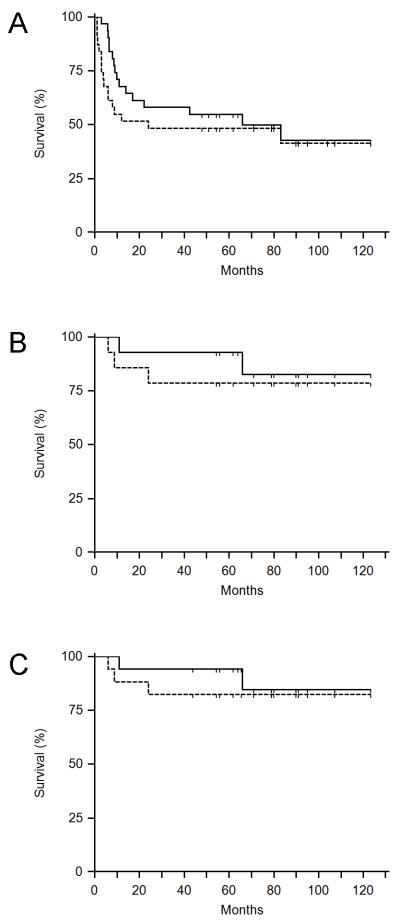
With a median follow-up of 79 months (range 48 -123 months) for all PCNSL patients who received MT-R induction, with or without EA consolidation, the two-year PFS was 45% (95% CI: 30%-58%; Figure 1A). For the subgroup of MT-R patients who also received consolidation with high-dose EA, the two-year PFS was 79% (95% CI: 43%-90%; Figure 1B). None of the 3 patients with synchronous brain parenchymal CNS and systemic lymphoma treated with EA consolidation have relapsed with a median follow-up of 66 months (range, 44 - 79 months; Figure 1C).
Figure 1.
Kaplan-Meier survival curves. (A) Progression-free (PFS) and overall survival (OS) of the entire cohort of PCNSL patients treated with MT-R ± EA (n=31). (B) Survival of the PCNSL patients (n=14) who completed both MTR and EA consolidation. (C) Survival of all CNS lymphoma patients treated with EA consolidation (n=17) including the PCNSL patients (n=14) treated with MTR-EA and the secondary CNS lymphoma patients (n=3) treated with M-R-CHOP-EA.
Prognostic Assessment using Diffusion-Weighted Magnetic Resonance Imaging and by Clinical Parameters
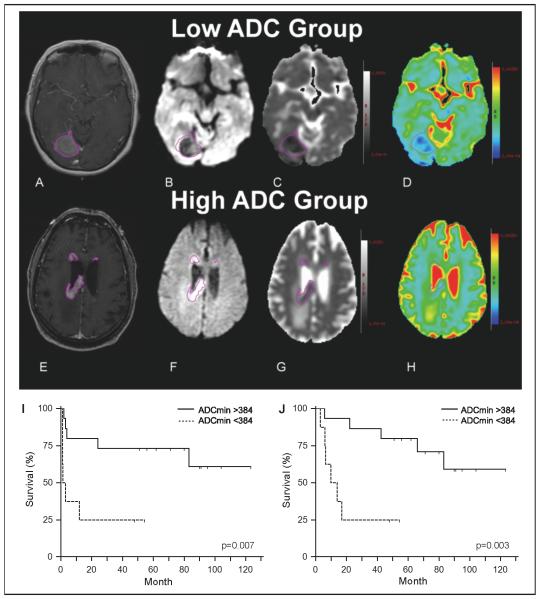
We were able to determine ADCmin by DW-MRI of contrast enhancing lesions at diagnosis for 23 of the 31 patients (74%) in this cohort. Using ADCmin <384 × 10−6 mm2/s as a cut-off, as defined in our earlier report (32), we observed that patients whose tumors exhibited severely reduced water diffusion, (ADCmin <384 × 10−6 mm2/s) had significantly worse outcome compared to patients whose tumors demonstrated an ADCmin >384 × 10−6 mm2/s (Table 5; Figure 2). Patients in the low ADCmin group who received MT-R had a median PFS of only 2 months whereas median PFS was not reached for patients in the high ADCmin group (p=0.007). Overall survival was also shorter in patients with CNS lymphomas that displayed markedly reduced water diffusion when treated with MT-R (p=0.003).
Figure 2.
Representative diffusion-weighted images: low and high ADC groups. (A and E): Contrast-enhanced T1-weighted images with regions of interest (purple) surrounding enhancing lesions obtained pretreatment in patients diagnosed with PCNSL. (B and F): Diffusion-weighted imaging with regions of interest. (C and G): Black and white ADC map with regions of interest. (D and H): Color ADC map with regions of interest. (I and J): Kaplan-Meier plots demonstrating the relationship between low intratumoral ADCmin (< 384 × 10−6 mm2/s) at time of diagnosis and shorter progression-free (I) and overall survival (J) in patients treated with MT-R induction.
We also evaluated clinical prognostic variables proposed in the IELSG scoring system for 24 evaluable patients and by the MSKCC model for all PCNSL patients in this study (Supplemental Table 1; 35, 36). The MSKCC prognostic classification system did not identify significant survival differences in this study. However, IELSG prognostic group 2-3 patients exhibited superior PFS and OS compared to IELSG group 4-5 (2-year PFS 70% vs. 30%, p=0.04; 2-year OS 70% vs. 40%, p=0.05).
Discussion
In this study we analyze the toxicity, responses, and long-term survival of newly diagnosed PCNSL patients treated with a novel two-step intensive immunochemotherapy strategy not involving autologous stem cell transplantation or WBRT. The rate of complete response to MT-R was 52%, comparable to but not substantially higher than the 30-52% rate of CR to high-dose methotrexate alone in PCNSL described in earlier studies (5, 6). Although only one PCNSL patient had an improved response with EA consolidation (SD to CR), our patients as a whole demonstrated excellent PFS and OS and did so without the cognitive morbidity typically associated with WBRT. However, given that the majority of patients who achieved a complete response with MT-R subsequently received a novel intensive consolidation, it is impossible to define the potential contribution of rituximab and temozolomide to long-term progression-free and overall survival within this regimen. Nevertheless, our results with the MT-R regimen demonstrate that it is possible to combine an alkylating agent with high-dose methotrexate without additive toxicity. Moreover, the 2-year PFS of PCNSL patients treated with MT-R followed by intensive consolidation with EA in this series is markedly longer than the rates of 2-year PFS described in previous series using intravenous-chemotherapy alone without brain irradiation (5, 37-39).
Despite a relatively high CR rate, one-third of our patients had clinical and radiographic progression within the first 4 cycles of MT-R therapy, and none of these were long-term survivors. This illustrates the significant clinical problem of primary drug resistance in PCNSL. High rates of primary induction failure are reported in virtually all clinical series in PCNSL (2, 5, 21, 40), yet the issue of early refractory disease has not been emphasized. We suggest that future treatment programs for PCNSL evaluate risk-adapted strategies that selectively implement novel approaches for patients at high risk of early tumor progression, such as those with low ADCmin values at initial diagnosis.
Based upon these data, diffusion-weighted magnetic resonance imaging of contrast-enhancing tumor may be a potentially valuable method for risk-stratification in PCNSL patients at diagnosis. Of note, one of the two patients in the low ADC group who did well with MTR-EA therapy had an intratumoral ADCmin of 365 × 10−6mm2/s, the highest value in the low ADCmin cohort and near the cut-off point of 384 × 10−6 mm2/s, suggesting that further studies are needed to refine ADC as a biomarker of high-risk subpopulations of PCNSL patients. In addition, while increased lymphoma cell density within contrast-enhancing lesions is likely a contributing factor to reduced water diffusion within CNS lymphoma tumors (27, 41) there is a need for further investigation into the genetic and microenvironmental pathophysiology that is the basis for differential ADCmin in CNS lymphoma tumors. Nevertheless, when applied to the MTR-EA regimen in PCNSL, diffusion-weighted imaging provided better prognostic information than established indices based on clinical variables that were developed in the setting of WBRT (35, 36). A limitation of this study is that the IELSG prognostic system could only be applied to 24 of 31 patients, limiting its power, and thus additional studies are required to prospectively validate these preliminary conclusions and to potentially refine the diffusion methodology and cut-point ADCmin value.
On long-term follow-up, our findings suggest that combination high-dose infusional etoposide plus cytarabine (EA) is highly effective as consolidation after MT-R induction in newly diagnosed patients with PCNSL and after R-CHOP plus high-dose methotrexate treatment for patients with stage IV DLBCL with CNS involvement. Of the 14 PCNSL patients who received MT-R followed by EA consolidation, 12 remain in remission with a median follow-up of 79 months and there has not been disease progression outside of the two-year window post-induction. Similarly, none of the 3 patients with synchronous brain parenchymal CNS and systemic lymphoma who were treated with EA have progressed, with a median follow-up of 66 months; results which are markedly superior to non-standardized approaches used in the treatment of synchronous CNS and systemic lymphoma described in a recent series in which the median PFS was only 7 months. (42)
In addition, we have detected no significant acute or delayed neurologic toxicity related to this treatment in long-term evaluation as 15 of the 17 patients treated with EA have regained their pre-CNS lymphoma performance status. Five have maintained their professions at the same level as before MTR-EA and the median mini-mental status examination score evaluated in 12 out of 18 surviving CNS lymphoma patients was 29 (range 25-30) a median of five years after treatment. However it is likely that more detailed neurocognitive testing would identify subtle but persistent disease-associated and potentially treatment-associated deficits. Based upon our data, we propose that EA consolidation be considered as an alternative to WBRT in patients with either primary and secondary CNS lymphoma. These promising results are the basis for a multicenter study through CALGB that is evaluating the response rate, toxicity, and long-term efficacy of the MTR-EA regimen in patients with PCNSL (43). The regimen is also now in development for evaluation in a successor, intergroup randomized phase II study.
In our series, EA was also active as a first-line salvage regimen in patients who progressed on MT-R, in that all four patients with primary refractory disease who received EA exhibited complete responses. However, each of these patients ultimately experienced tumor progression before planned myeloablative therapy and autologous stem cell rescue. Five patients with primary refractory disease received immediate salvage WBRT that was associated with a median survival of only ten months (range 6-42 months). These results highlight a need for the introduction of new biological agents to augment the efficacy of currently available chemotherapeutic and immunotherapeutic approaches, particularly during the induction phase for PCNSL.
Supplementary Material
Statement of Translational Relevance.
Here we report on the long-term follow-up of the first cohort of newly-diagnosed PCNSL patients treated with a novel induction and consolidation regimen, without brain radiotherapy: methotrexate-temozolomide-rituximab (MT-R) followed by 96-hour infusional etoposide plus high-dose cytarabine (EA). This study, which includes the outcomes of a pilot phase I trial that evaluated this regimen, is the first to evaluate a dose-intensive consolidation chemotherapy regimen that includes high-dose etoposide but involves neither autologous stem cell transplantation nor whole brain radiotherapy (WBRT) in newly-diagnosed PCNSL patients. In addition, we evaluated the prognostic utility of diffusion-weighted MR imaging (DW-MRI) in a uniformly-treated cohort of PCNSL patients treated with MTR-EA. Our results demonstrate that MTR-EA therapy resulted in progression-free and overall survival similar to that achieved with high-dose methotrexate-based regimens utilizing consolidation with standard or reduced-dose brain irradiation. In addition, our results, with follow-up of 79 months, suggest that DW-MRI is a potentially important, non-invasive tool to assess prognosis at diagnosis and that this analysis should be prospectively evaluated in future studies using high-dose methotrexate-based induction, in particular trials which evaluate the MT-R regimen. Furthermore, our results suggest that noninvasive DW-MRI characteristics could be used in risk stratification in future clinical trials that evaluate novel biological therapies for patients with PCNSL who are at risk of early tumor progression.
Acknowledgments
This research was supported by the Leukemia & Lymphoma Society and by NIH R01CA139-83-01A1 (JLR)
Footnotes
A portion of this work was presented in abstract form at the 2006 Annual Meeting of the American Society of Hematology, at the 2006 Annual Meeting of the American Society of Clinical Oncology, and at the 11th International Conference on Malignant Lymphoma, Lugano Switzerland, June 2011
Literature Cited
- 1.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–63. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 2.Shah GD, Yahalom J, Correa DD, Lai RK, Raizer JJ, Schiff D, LaRocca R, Grant B, DeAngelis LM, Abrey LE. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730–5. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 3.Blay JY, Conroy T, Chevreau C, Thyss A, Quesnel N, Eghbali H, Bouabdallah R, Coiffier B, Wagner JP, Le Mevel A, Dramais-Marcel D, Baumelou E, Chauvin F, Biron P. High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. J Clin Oncol. 1998;16:864–71. doi: 10.1200/JCO.1998.16.3.864. [DOI] [PubMed] [Google Scholar]
- 4.Fliessbach K, Helmstaedter C, Urbach H, Althaus A, Pels H, Linnebank M, Juergens A, Glasmacher A, Schmidt-Wolf IG, Klockgether T, Schlegel U. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology. 2005;64:1184–8. doi: 10.1212/01.WNL.0000156350.49336.E2. [DOI] [PubMed] [Google Scholar]
- 5.Batchelor T, Carson K, O’Neill A, Grossman SA, Alavi J, New P, Hochberg F, Priet R. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21:1044–9. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Herrlinger U, Schabet M, Brugger W, Kortmann RD, Kuker W, Deckert M, Engel C, Schmeck-Lindenau HJ, Mergenthaler HG, Krauseneck P, Benohr C, Meisner C, Wiestler OD, Dichgans J, Kanz L, Bamberg M, Weller M. German Cancer Society Neuro-Oncology Working Group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma. Ann Neurol. 2002;51:247–52. doi: 10.1002/ana.10102. [DOI] [PubMed] [Google Scholar]
- 7.Ott RJ, Brada M, Flower MA, Babich JW, Cherry SR, Deehan BJ. Measurements of blood-brain barrier permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma. Eur J Cancer. 1991;27:1356–61. doi: 10.1016/0277-5379(91)90009-3. [DOI] [PubMed] [Google Scholar]
- 8.Osoba D, Brada M, Yung WK, Prados MD. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide. Eur J Cancer. 2000;36:1788–95. doi: 10.1016/s0959-8049(00)00165-9. [DOI] [PubMed] [Google Scholar]
- 9.Osoba D, Brada M, Yung WK, Prados M. Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme. J Clin Oncol. 2000;18:1481–91. doi: 10.1200/JCO.2000.18.7.1481. [DOI] [PubMed] [Google Scholar]
- 10.Reni M, Ferreri AJ, Landoni C, Villa E. Salvage therapy with temozolomide in an immunocompetent patient with primary brain lymphoma. J Natl Cancer Inst. 2000;92:575–6. doi: 10.1093/jnci/92.7.575. [DOI] [PubMed] [Google Scholar]
- 11.Reni M, Zaja F, Mason W, Perry J, Mazza E, Spina M, Bordonaro R, Ilariucci F, Faedi M, Corazzelli G, Manno P, Franceschi E, Pace A, Candela M, Abbadessa A, Stelitano C, Latte G, Ferreri AJ. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96:864–7. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong ET, Tishler R, Barron L, Wu JK. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer. 2004;101:139–45. doi: 10.1002/cncr.20339. [DOI] [PubMed] [Google Scholar]
- 13.Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, Lacasce AS, Blum KA, Byrd JC, Kelly M, Stock W, Linker CA, Canellos GP. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–8. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damon L, Damon LE, Gaensler K, Kaplan L, Martin T, 3rd, Rubenstein J, Linker C. Impact of intensive PBSC mobilization therapy on outcomes following auto-SCT for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2008;42:649–57. doi: 10.1038/bmt.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linker CA, Owzar K, Powell B, Hurd D, Damon LE, Archer LE, Larson RA. Auto-SCT for AML in second remission: CALGB study 9620. Bone Marrow Transplant. 2009;44:353–9. doi: 10.1038/bmt.2009.36. [DOI] [PubMed] [Google Scholar]
- 16.Soussain C, Suzan F, Hoang-Xuan K, Cassoux N, Levy V, Azar N, Belanger C, Achour E, Ribrag V, Gerber S, Delattre JY, Leblond V. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19:742–9. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]
- 17.Wilson WH, Bryant G, Bates S, Fojo A, Wittes RE, Steinberg SM, Kohler DR, Jaffe ES, Herdt J, Cheson BD, et al. EPOCH chemotherapy: toxicity and efficacy in relapsed and refractory non-Hodgkin’s lymphoma. J Clin Oncol. 1993;11:1573–82. doi: 10.1200/JCO.1993.11.8.1573. [DOI] [PubMed] [Google Scholar]
- 18.Wilson WH, Dunleavy K, Pittaluga S, Hegde U, Grant N, Steinberg SM, Raffeld M, Gutierrez M, Chabner BA, Staudt L, Jaffe ES, Janik JE. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717–24. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Relling MV, Mahmoud HH, Pui CH, Sandlund JT, Rivera GK, Ribeiro RC, Crist WM, Evans WE. Etoposide achieves potentially cytotoxic concentrations in CSF of children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:399–404. doi: 10.1200/JCO.1996.14.2.399. [DOI] [PubMed] [Google Scholar]
- 20.Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M, Schmitz N. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) Ann Oncol. 2007;18:149–57. doi: 10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]
- 21.Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci F, Rossi G, Soffietti R, Stelitano C, Vallisa D, Zaja F, Zoppegno L, Aondio GM, Avvisati G, Balzarotti M, Brandes AA, Fajardo J, Gomez H, Guarini A, Pinotti G, Rigacci L, Uhlmann C, Picozzi P, Vezzulli P, Ponzoni M, Zucca E, Caligaris-Cappio F, Cavalli F. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374:1512–20. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 22.Lee EJ, Lee SK, Agid R, Bae JM, Keller A, Terbrugge K. Preoperative grading of presumptive low-grade astrocytomas on MR imaging: diagnostic value of minimum apparent diffusion coefficient. AJNR Am J Neuroradiol. 2008;29:1872–7. doi: 10.3174/ajnr.A1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamasaki F, Kurisu K, Satoh K, Arita K, Sugiyama K, Ohtaki M, Takaba J, Tominaga A, Hanaya R, Yoshioka H, Hama S, Ito Y, Kajiwara Y, Yahara K, Saito T, Thohar MA. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–91. doi: 10.1148/radiol.2353031338. [DOI] [PubMed] [Google Scholar]
- 24.Ellingson BM, Malkin MG, Rand SD, Connelly JM, Quinsey C, LaViolette PS, Bedekar DP, Schmainda KM. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31:538–48. doi: 10.1002/jmri.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chenevert TL, Stegman LD, Taylor JM, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–36. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 26.Kitis O, Altay H, Calli C, Yunten N, Akalin T, Yurtseven T. Minimum apparent diffusion coefficients in the evaluation of brain tumors. Eur J Radiol. 2005;55:393–400. doi: 10.1016/j.ejrad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224:177–83. doi: 10.1148/radiol.2241010637. [DOI] [PubMed] [Google Scholar]
- 28.Politi LS, Forghani R, Godi C, Resti AG, Ponzoni M, Bianchi S, Iadanza A, Ambrosi A, Falini A, Ferreri AJ, Curtin HD, Scotti G. Ocular adnexal lymphoma: diffusion-weighted mr imaging for differential diagnosis and therapeutic monitoring. Radiology. 2010;256:565–74. doi: 10.1148/radiol.10100086. [DOI] [PubMed] [Google Scholar]
- 29.Ross BD, Moffat BA, Lawrence TS, Mukherji SK, Gebarski SS, Quint DJ, Johnson TD, Junck L, Robertson PL, Muraszko KM, Dong Q, Meyer CR, Bland PH, McConville P, Geng H, Rehemtulla A, Chenevert TL. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther. 2003;2:581–7. [PubMed] [Google Scholar]
- 30.Moffat BA, Chenevert TL, Meyer CR, McKeever PE, Hall DE, Hoff BA, Johnson TD, Rehemtulla A, Ross BD. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8:259–67. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee KC, Moffat BA, Schott AF, Layman R, Ellingworth S, Juliar R, Khan AP, Helvie M, Meyer CR, Chenevert TL, Rehemtulla A, Ross BD. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res. 2007;13:443–50. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- 32.Barajas RF, Jr., Rubenstein JL, Chang JS, Hwang J, Cha S. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol. 2010;31:60–6. doi: 10.3174/ajnr.A1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A, Soussain C, DeAngelis LM, Neuwelt EA, O’Neill BP, Thiel E, Shenkier T, Graus F, van den Bent M, Seymour JF, Poortmans P, Armitage JO, Cavalli F. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–43. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 34.Cher L, Glass J, Harsh GR, Hochberg FH. Therapy of primary CNS lymphoma with methotrexate-based chemotherapy and deferred radiotherapy: preliminary results. Neurology. 1996;46:1757–9. doi: 10.1212/wnl.46.6.1757. [DOI] [PubMed] [Google Scholar]
- 35.Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A, Devizzi L, Berger F, Ponzoni M, Borisch B, Tinguely M, Cerati M, Milani M, Orvieto E, Sanchez J, Chevreau C, Dell’Oro S, Zucca E, Cavalli F. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–72. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 36.Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta M, DeAngelis LM. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 37.Sandor V, Stark-Vancs V, Pearson D, Nussenblat R, Whitcup SM, Brouwers P, Patronas N, Heiss J, Jaffe E, deSmet M, Kohler D, Simon R, Wittes R. Phase II trial of chemotherapy alone for primary CNS and intraocular lymphoma. J Clin Oncol. 1998;16:3000–6. doi: 10.1200/JCO.1998.16.9.3000. [DOI] [PubMed] [Google Scholar]
- 38.Pels H, Schmidt-Wolf IG, Glasmacher A, Schulz H, Engert A, Diehl V, Zellner A, Schackert G, Reichmann H, Kroschinsky F, Vogt-Schaden M, Egerer G, Bode U, Schaller C, Deckert M, Fimmers R, Helmstaedter C, Atasoy A, Klockgether T, Schlegel U. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–95. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 39.Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, Roth A, Hertenstein B, von Toll T, Hundsberger T, Mergenthaler HG, Leithauser M, Birnbaum T, Fischer L, Jahnke K, Herrlinger U, Plasswilm L, Nagele T, Pietsch T, Bamberg M, Weller M. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–47. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 40.Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P, Paleologos N, Correa DD, Anderson ND, Caron D, Zelenetz A, Nimer SD, DeAngelis LM. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21:4151–6. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Rubenstein JL, Shen A, Batchelor TT, Kadoch C, Treseler P, Shuman MA. Differential gene expression in central nervous system lymphoma. Blood. 2009;113:266–7. doi: 10.1182/blood-2008-04-152835. author reply 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bazzoli E, Iwamoto FM, Zelenetz AD, Deangelis LM, Abrey LE. Synchronous presentation of systemic and brain non-Hodgkin lymphoma. Leuk Lymphoma. 2008;49:2370–3. doi: 10.1080/10428190802404055. [DOI] [PubMed] [Google Scholar]
- 43.Rubenstein JL, Johnson J, Jung SH, Cheson BD, Kaplan LD. Intensive Chemotherapy and Immunotherapy, Without Brain Irradiation, In Newly Diagnosed Patients with Primary CNS Lymphoma: Results of CALGB 50202. Proc. Am. Soc. of Hematol. 2010 doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.