Abstract
Allostasis, originally conceptualized to explain persistent morbidity of arousal and autonomic function, is defined as the process of achieving stability through physiological or behavioral change. Two types of biological processes have been proposed to describe the mechanisms underlying allostasis in drug addiction, a within-system adaptation and a between-system adaptation. In the within-system process, the drug elicits an opposing, neutralizing reaction within the same system in which the drug elicits its primary and unconditioned reinforcing actions, while in the between-system process, different neurobiological systems that the one initially activated by the drug are recruited. In this review, we will focus our interest on alterations in the dopaminergic and corticotropin releasing factor systems as within-system and between-system neuroadaptations respectively, that underlie the opponent process to drugs of abuse. We hypothesize that repeated compromised activity in the dopaminergic system and sustained activation of the CRF-CRF1R system with withdrawal episodes may lead to an allostatic load contributing significantly to the transition to drug addiction.
Keywords: Dopamine, CRF, stress, CeA, extended amygdala, VTA, drugs, dependence, motivation, craving
Allostasis
Allostasis, originally conceptualized to explain persistent morbidity of arousal and autonomic function, is defined as the process of achieving stability through physiological or behavioral change (Sterling and Eyer, 1981, 1988). Allostasis involves a feed-forward mechanism rather than the negative feedback mechanisms of homeostasis, with continuous re-evaluation of need and continuous readjustment of all parameters toward new set points. Thus, the very physiological mechanism that allows rapid responses to environmental challenges becomes the engine of pathology if adequate time or resources are not available to shut off the response (Koob and Le Moal, 2001). The concept of allostasis has served as a framework for a large body of research on integrative health psychology, epidemiology, aging, physiology, and neuroscience. Allostasis is based on the hypothesis that there is a cumulative physiological risk associated with exposure to physiological or psychosocial stressors during an individual’s life. A body of evidence indicates that many psychosocial stressors appear to have small to modest associations with multiple different biological risk factors, reflecting links to most of the known major regulatory systems (e.g., cardiovascular, immune, and central nervous system). For example, disruption of social status impacts cortisol levels, and social support is predictive of cortisol levels (Abbot et al., 2003; Schulkin, 2011). Greater cumulative dysregulation is associated with significantly greater risks for subsequent disease, declines in physical and cognitive functioning, and overall mortality (Seeman et al., 1997, 2001; Karlamangla et al., 2002; Geronimus et al., 2006). Thus, the ability to mobilize resources and use feed-forward mechanisms progressively leads to an allostatic state and ultimately to allostatic load. An allostatic state reflects a new equilibrium, a state of chronic deviation of the regulatory system from its normal (homeostatic) operating level to a pathological (allostatic) operating level (Koob and Le Moal, 1997). Allostatic load can be defined as the “long-term cost of allostasis that accumulates over time and reflects the accumulation of damage that can lead to pathological states” (McEwen, 1998). Cumulative indices of allostatic load have also been positively related to measures of psychosocial stress in young adolescents (Evans et al., 2007) as well as symptoms of posttraumatic stress disorder (PTSD) in the mothers of pediatric cancer survivors (Glover et al., 2006) and adverse perinatal outcomes (Shannon et al., 2007). The concepts of allostasis and allostatic overload provide a conceptual framework to understand the neurobiological mechanisms that underlie vulnerability to disease in general and drug addiction in particular by taking into account the anticipatory neuroadaptations that occur to maintain apparent reward function stability through changes in brain reward mechanisms (Koob and Le Moal, 2001).
Drug Addiction
Drug addiction is a chronically relapsing disorder characterized by compulsion to seek and take the drug, loss of control in limiting drug intake, and emergence of a negative emotional state, reflecting a motivational withdrawal syndrome, when access to the drug is prevented (defined here as dependence; Koob and Le Moal, 1997). Clinically, the occasional but limited use of a drug with the potential for abuse or dependence is distinct from escalated drug intake and the emergence of a chronic drug-dependent state.
Drug addiction has been conceptualized as a disorder that involves elements of both impulsivity and compulsivity (Koob and Le Moal, 2008). The elements of impulsivity and compulsivity yield a composite addiction cycle that comprises three stages—preoccupation/anticipation, binge/intoxication, and withdrawal/negative affect—in which impulsivity often dominates at the early stages and compulsivity dominates at the terminal stages. As an individual moves from impulsivity to compulsivity, a shift occurs from positive reinforcement driving the motivated behavior to negative reinforcement driving the motivated behavior (Koob, 2004). These three stages are conceptualized as interacting with each other, becoming more intense, and ultimately leading to the pathological state known as addiction (Koob and Le Moal, 1997).
Motivation, Opponent Process, and Addiction
Motivation is a state that can be defined as a “tendency of the whole animal to produce organized activity” (Hebb, 1972). The concept of motivation was linked inextricably with hedonic, affective, or emotional states in addiction in the context of temporal dynamics by Solomon’s opponent process theory of motivation. Solomon and Corbit (1974) postulated that hedonic, affective, or emotional states, once initiated, are automatically modulated by the central nervous system with mechanisms that reduce the intensity of hedonic feelings. This theory postulates that any motivational stimulus activates two opposing motivational processes. The a-process consists of either positive or negative hedonic responses, has a fast onset and offset, correlates with the intensity, quality, and duration of the stimulus, and shows tolerance. The b-process appears after the a-process has terminated, is opposite in direction, sluggish in onset, and slow to build up and decay and gets larger with repeated exposure. The initial acute effect of a drug of abuse (the a-process or positive hedonic response) was hypothesized to be opposed or counteracted by the b-process as homeostatic changes in brain systems. With repeated exposure to drugs, the b-process sensitizes, appears earlier after the unconditioned stimulus, lasts longer, and masks the a-process, leading to apparent tolerance (Laulin et al., 1999). Two types of biological processes have been proposed to describe the mechanisms that underlie allostasis in drug addiction: within-system adaptation and between-system adaptation (Koob and Bloom, 1988). In the within-system process, the drug elicits an opposing, neutralizing reaction within the same system in which the drug elicits its primary and unconditioned reinforcing actions, whereas in the between-system process, neurobiological systems that are different from the ones initially activated by the drug are recruited. This review focuses on alterations in the dopaminergic and corticotropin-releasing factor systems as the within-system and between-system neuroadaptations, respectively, that underlie the opponent process of drug abuse.
Role of the Dopaminergic System in the Motivational Response that Underlies the Opponent Process of Drug Abuse
The mesolimbic dopaminergic system is formed by the dopaminergic cell bodies in the ventral tegmental area (VTA) and their projections to the ventral striatum. The VTA also possesses a population of γ-aminobutyric acid (GABA) neurons that provide inhibitory inputs to dopamine cells and influence other structures, such as the pedunculopontine tegmental nucleus and glutamatergic neurons (Dobi et al., 2010). The VTA receives its main excitatory glutamatergic and cholinergic inputs from the ventromedial prefrontal cortex (ventral prelimbic, infralimbic, and dorsal peduncular cortices), ventral subiculum, subthalamic nucleus, parabrachial nucleus, pedunculopontine tegmental nucleus, and laterodorsal tegmental nucleus (Kalivas, 1993). The VTA also receives prominent inputs from the nucleus accumbens shell and ventromedial ventral pallidum (Oades and Halliday, 1987). The dopamine and GABA neurons in the VTA have been shown to be critical for the rewarding properties of psychostimulants. With the possible exception of opioids, all drugs of abuse when self-administered acutely stimulate the dopaminergic system and increase dopamine release in the nucleus accumbens (Volkow et al., 2007). The pattern of firing of dopaminergic neurons in the VTA in response to drugs of abuse has been hypothesized to encode drug reward, attribution of incentive salience, and establishment of response habits (Wise, 1980, 1987, 2002). The attribution of incentive salience refers to a process that transforms sensory information about reward into attractive incentives (Robinson and Berridge, 1993).
Intracranial self-stimulation has a long history as a measure of activity of the brain reward system and acute reinforcing effects of drugs of abuse. Brain stimulation reward involves widespread neurocircuitry in the brain, but the most sensitive sites defined by the lowest thresholds involve the trajectory of the medial forebrain bundle connecting the ventral tegmental area (VTA) with the basal forebrain (Olds and Milner, 1954). All drugs of abuse, when administered acutely, lower brain stimulation reward thresholds (Kornetsky and Esposito, 1979). The acute reinforcing effects of drugs of abuse are mediated by the activation of dopamine, serotonin, opioid peptides, and GABA systems either by actions in the nucleus accumbens and central nucleus of the amygdala (CeA) or by indirect actions in the VTA (Koob and Le Moal, 2001; Nestler, 2005; Koob 2006).
Converging lines of evidence suggest that dopamine is a key neurotransmitter mediating hedonic allostasis in drug addiction. For instance, cocaine self-administration reduces brain reward threshold acutely (minutes after the injection), but is associated with a compensatory increase in brain reward threshold hours and days after the injection that progressively return to baseline (Kenny et al., 2003, Ahmed et al., 2002). However, with repeated prolonged self-administration sessions and increases in cocaine intake this compensatory increase fail to return to baseline between sessions creating a residual hysteresis leading to increased allostatic load that ultimately will lead to the pathological state of addiction.
Drug withdrawal in humans is associated with fatigue, decreased mood, and psychomotor retardation; in animals it is associated with decreased motivation to work for natural rewards (Barr and Phillips, 1999), elevations in reward thresholds (Koob and Le Moal, 2006), and decreased locomotor activity (Pulvirenti and Koob, 1993), behavioral effects that may involve decreased dopaminergic function. Animals during amphetamine withdrawal show decreased responding on a progressive-ratio schedule for a sweet solution, and this decreased responding was reversed by the dopamine partial agonist terguride (Orsini et al., 2001), suggesting that low dopamine tone contributes to the motivational deficits associated with psychostimulant withdrawal.
Measures of brain reward function using intracranial self-stimulation have revealed elevations in brain reward thresholds during acute abstinence from all major drugs with dependence potential (Markou and Koob, 1991; Schulteis et al., 1994, 1995; Epping-Jordan et al., 1998; Gardner and Vorel, 1998; Paterson et al., 2000).
Decreases in activity of the mesolimbic dopamine system and decreases in serotonergic neurotransmission in the nucleus accumbens occur during drug withdrawal in animal studies (Weiss et al., 1992, 1996). Decreases in the number of dopamine D2 receptors in human subjects with cocaine dependence and primate cocaine self-administration (Nader et al., 2006), coupled with a decrease in dopaminergic activity in rodent studies, have led to the hypothesis of overall decreased sensitivity of reward circuits to stimulation by natural reinforcers and other drugs in drug-addicted individuals (Martin-Soelch et al., 2001; Volkow and Fowler, 2000). Indeed, cocaine abusers and alcoholics exhibit reduced dopamine release in response to a pharmacological challenge with a stimulant drug (Volkow et al., 1997; Martinez et al. 2007), and increased sensitivity of drug taking in response to dopamine receptor antagonist administration (leftward shift in the dose-response curve) has been observed in rats with extended access compared with rats with limited access to cocaine and methamphetamine (Ahmed et al., 2004; Wee et al., 2007), suggesting the reduced number or function of dopamine receptors in drug-dependent subjects. These findings demonstrate that decreases in the function of the dopaminergic system are implicated in both the acute reinforcing effect of drugs of abuse and the motivational response to drug withdrawal. Moreover, initial activation of the VTA by opiates is required to trigger the subsequent decreased opiate receptor function in the VTA that mediates the withdrawal-induced increases in anxiety-like behavior (Radke et al., 2011), suggesting that the emergence of anxiety during withdrawal from acute opiate exposure begins with activation of VTA mesolimbic dopamine circuitry, providing an early response mechanism for the opponent process view of withdrawal. Further evidence of the dopaminergic system in the development of hedonic allostasis has been obtained in rats with a history of escalation of alcohol drinking (Barak et al., 2011). Withdrawal from chronic alcohol is associated with decreased dopaminergic neurons activity in the VTA and decreased DA release in the nucleus accumbens (Diana et al., 1993; Bailey et al., 2001; Shen 2003; Shen et al., 2007; Darden and Hunt, 1977; Rossetti et al., 1992; Diana et al., 1993; Weiss et al., 1996; Smith et al., 2008). The decreased DA levels in the nucleus accumbens during acute withdrawal from alcohol is associated with increased craving for alcohol, and the emergence of a negative emotional state Ahmed and Koob, 1998; Koob and Le Moal, 2001; Koob, 2003). Moreover, normalizing withdrawal-induced decreases in dopamine levels in the nucleus accumbens with intra-VTA infusion of glial cell-derived neurotrophic factor reduces alcohol intake (Barak et al., 2011), suggesting that decreased dopamine levels in the nucleus accumbens play a key role in allostatic mechanisms and in the reduction of hedonic set point during withdrawal.
The decrease in dopaminergic function associated with drug withdrawal suggests that complete blockade of the dopaminergic system may exacerbate the motivational response to drug withdrawal, but studies in rats and mice show that conditioned place aversion to nicotine withdrawal is blocked by the dopamine receptor antagonist α-flupenthixol and in dopamine D2 receptor knockout mice (Grieder et al., 2010). This result demonstrates that although the decrease in dopaminergic function contributes to the emergence of withdrawal symptoms and is key for the reduction in hedonic set point, dopaminergic function is still required to mediate the motivational response associated with the opponent process of chronic nicotine exposure and suggests that a change in the specific pattern of dopaminergic signaling and not simply decreased signaling may mediate the craving for drugs and motivational response to drug withdrawal.
Role of the Corticotropin-Releasing Factor System in the Motivational Response that Underlies the Opponent Process of Drug Abuse
Within the domain of changes in reward function, the primary deficit is hypothesized to be a neuroadaptational shift in how rewards are processed. More specifically, a loss of positive reinforcement and a recruitment of negative reinforcement are hypothesized to occur within a specific basal forebrain area termed the extended amygdala. The extended amygdala has been identified by neuroanatomical studies (Alheid and Heimer, 1988; Koob et al., 1998) as a separate entity within the basal forebrain and has been hypothesized to be a common neural circuit for the reinforcing actions of drugs (Alheid and Heimer, 1988). The extended amygdala is composed of three major structures: CeA and medial amygdala (MeA), bed nucleus of the stria terminalis (BNST), and a transition zone in the posterior and medial portions of the nucleus accumbens (Alheid et al, 1995). Further examination of this anatomical system reveals two major divisions: central division and medial division. The central division of the extended amygdala includes the CeA, central sublenticular extended amygdala, lateral BNST, and a transition area in the medial and caudal portions of the nucleus accumbens. These structures are within the central division and are largely defined by their network of intrinsic connections and extensive connections to the lateral hypothalamus (Alheid et al., 1995). The medial division of the extended amygdala includes the medial BNST, MeA, and medial sublenticular extended amygdala. These structures, in turn, have been defined as the medial division by their network of intrinsic associative connections and extensive relations to the medial hypothalamus (Alheid et al., 1995). The lateral BNST, which forms a key element of the central division of the extended amygdala, has high amounts of dopamine and norepinephrine terminals, CRF terminals, and CRF cell bodies and receives afferents from the prefrontal cortex, insular cortex, and amygdalopiriform area. The medial BNST, in contrast, contains high amounts of vasopressin, is sexually dimorphic, and receives afferents from structures such as infralimbic cortex, entorhinal cortex, and subiculum (Dong et al., 2001; McDonald et al., 1999; Kozicz, 2001; Gray and Magnuson, 1992; Phelix and Paull, 1990; Allen et al., 1984). Evidence suggests that the central division may be involved in receiving cortical information and regulating the hypothalamic-pituitary-adrenal axis (Gray et al., 1993), whereas the medial division may be more involved in sympathetic and physiological responses and receive olfactory information (Pompei et al., 1991; Lesur et al., 1989; Nijsen et al., 2001).
A role for the extended amygdala in the aversive effects of drug withdrawal includes changes in opioidergic, GABAergic, and CRF neurotransmission during acute withdrawal. The CRF system in the CeA is activated during acute cocaine, alcohol, opioid, Δ9-tetrahydrocannabinol, and nicotine withdrawal as measured by in vivo microdialysis and neuropharmacological probes (Richter and Weiss, 1999; Heinrichs et al., 1995; Merlo-Pich et al., 1995; George et al., 2007). Similar effects have been observed with alcohol in the lateral BNST (Olive et al., 2002). Rats undergoing cocaine withdrawal also show increases in anxiety-like responses that are reversed by administration of a competitive CRF antagonist (Sarynai et al., 1995; Basso et al., 1999). Similar results have been observed with nicotine (George et al., 2007), alcohol (Rassnick et al., 1993, Koob et al., 1994), and opiates (Schulteis et al., 1994, Heinrichs et al., 1995; Basso et al., 1999). Moreover, CRF1 but not CRF2 receptor activation mediates nicotine withdrawal-induced deficits in brain reward function (Bruijnzeel et al., 2009), an effect localized to the CeA and nucleus accumbens shell, but not BNST (Marcinkiewcz et al., 2009). The ability of CRF antagonists to block the anxiogenic-like and aversive-like motivational effects of drug withdrawal would predict the motivational effects of CRF antagonists in animal models of extended access to drugs. Indeed, CRF1 antagonists blocked the increased self-administration of cocaine (Goeders and Guerin, 2000), nicotine (George et al., 2007), heroin (Greenwell et al., 2009) in animals showing compulsive drug seeking after extended access to these drugs. Similar effects have been observed in animal models of compulsive alcohol seeking associated with dependence (Koob, Neuron 2007). Taken together, the increase in CRF levels during drug withdrawal coinciding with the emergence of a negative emotional state that opposes the acute positive hedonic effect of drugs of abuse suggest that activation of the CRF-CRF1R system may represent one of the opponent process mechanisms leading to an allostatic state. Moreover, repeated episodes of withdrawal potentiate the effect of abstinence on the CRF-CRF1R system and may lead to a more intense negative emotional state (George et al., 2007; Zorrilla et al., 2001; Holter et al., 1998; Brown et al., 1998; Breese et al., 2005, 2011). This hyperactivation of the CRF-CRF1R system after repeated withdrawal either by a sensitization mechanism or a failure to return to baseline level after renewed access to the drug may represent the allostatic load responsible for the transition to drug dependence.
Interaction Between the Dopamine and Corticotropin-Releasing Factor Systems
The mesolimbic dopaminergic and extended amygdala CRF systems have long been studied independently and often viewed as mutually exclusive in the drug addiction field. However, recent work has demonstrated that these two systems can powerfully interact with each other, suggesting that dysregulation of this interaction may be lead to the development of drug dependence and relapse. Very few studies have investigated the role of dopamine in CRF release in the extended amygdala, despite the fact that the VTA sends heavy dopamine projections to the extended amygdala (Fallon and Moore, 1978) and PVN (Liposits and Paull, 1989) and directly innervates CRF-containing neurons in the CeA (Eliava et al., 2003) and BNST (Meloni et al., 2006). Early studies with 6-hydroxydopamine lesions of the mesolimbic dopamine system showed a decreased number and staining intensity of CRF neurons in the CeA associated with an increase in CRF mRNA levels (Smialowska et al., 1999). In contrast, stimulation of D1 or D2 receptors stimulates CRF mRNA expression in PVN neurons but not in the CeA (Eaton et al., 1996; Smialowska et al., 2001), suggesting that dopamine does not directly control CRF release in the extended amygdala. However, recent electrophysiological studies suggest a key role for dopamine in CRF neurotransmission. Dopamine enhances glutamatergic transmission in the BNST through activation of D1/2 receptors and CRF1 receptors, suggesting that dopamine stimulates the local release of CRF in the extended amygdala (Kash et al., 2008). Cocaine potentiates the dopamine receptor- and CRF1 receptor-dependent short-term potentiation of N-methyl-D-aspartate (NMDA) receptor function in the BNST (Kash et al., 2008). On the other hand, cocaine withdrawal, a condition known to be associated with decreased dopamine levels is associated with an enhancement of CRF1 receptor-dependent long-term potentiation in the CeA (Fu et al., 2007; Pollandt et al., 2006-75-76), and with an impairment of D1 receptor-dependent long-term potentiation of the intrinsic excitability in the BNST, a deficit that can be mimicked by chronic treatment with CRF (Francesconi et al., 2010). Similarly blockade of D1 receptors prevented the CRF-enhanced startle response, a behavioral assay believed to reflect stress or anxiety-like states (Meloni et al., 2006).
In addition, CRF neurons in the BNST, CeA, and PVN project to the VTA (Rodaros et al., 2007; Swanson et al., 1983), and the VTA expresses CRF1 and CRF2 receptors (Sauvage and Steckler, 2001; Ungless et al., 2003). Neuroanatomical studies show that CRF is colocalized in glutamatergic and GABAergic afferents and synapses with dopaminergic as well as non-dopaminergic neurons in the VTA (Tagliaferro and Morales, 2008). Moreover, a subpopulation of GABAergic and dopaminergic neurons in the VTA also express CRF binding protein (CRF-BP), a protein that participates in the regulation of CRF signaling at the synapse (Wang and Morales, 2008). Consistent with these anatomical data, CRF has been found to increase dopamine neuron firing (Kalivas, 1987, Wanat et al., 2008), dopamine release (Bagozi et al., 2006; Muramatsu et al., 2006; Lavicky et al., 1993) and footshock stress induces a long-lasting release in CRF in the VTA (Wang et al., 2005), while CRF1 receptor knock down in the VTA reduce dopamine release in the prefrontal cortex (Refojo et al., 2011). The effect of CRF on the activation of the dopaminergic system may be explained by different mechanisms. CRF has been shown to decrease somatodendritic dopamine release in the VTA through inhibition of voltage-operated Ca2+ gated channels (Kim et al., 2009), a mechanism that may increase dopamine release in the limbic system by decreasing autoreceptor activation. Also, CRF activates CRF1 receptors presynaptically on glutamatergic terminals to stimulate glutamate release in the VTA and activate dopaminergic cells, but only in cocaine-experienced animals (Ungless et al., 2003). CRF receptor antagonists in the VTA have been shown to block glutamate and dopamine release in the VTA and footshock stress-induced reinstatement of cocaine seeking (Wang et al., 2005). More recent studies suggest a direct postsynaptic action on dopaminergic neurons (Hahn et al., 2009), and although CRF can control glutamate release in the VTA through activation of CRF2 receptors in naive animals, it appears that recruitment of CRF1 receptors only occurs in cocaine-experienced subjects (Hahn et al., 2009). These results suggest that exposure to drugs of abuse may sensitize the dopaminergic system to the effect of CRF to facilitate relapse in drug-dependent subjects. However, unanswered questions are whether withdrawal-induced increases in rats that self-administer drugs to the point of dependence also lead to activation of glutamatergic afferents to the VTA and subsequent dopamine release as observed after footshock stress, and whether the glutamatergic afferents contributes to the decreased basal level of dopamine in the nucleus accumbens observed during drug withdrawal through activation of VTA GABAergic neurons (Tagliaferro and Morales, 2008).
Another mechanism of interaction between the CRF and dopaminergic systems is through the dynorphin/κ opioid system. Indeed, CRF stimulates dynorphin release (Nikolarakis et al., 1986; Song and Takemori, 1992; for review, see Bruchas et al., 2010), which can in turn decrease dopamine release in the PFC and striatum through activation of κ receptors on dopaminergic terminals (for review, see Bruijnzeel, 2009).
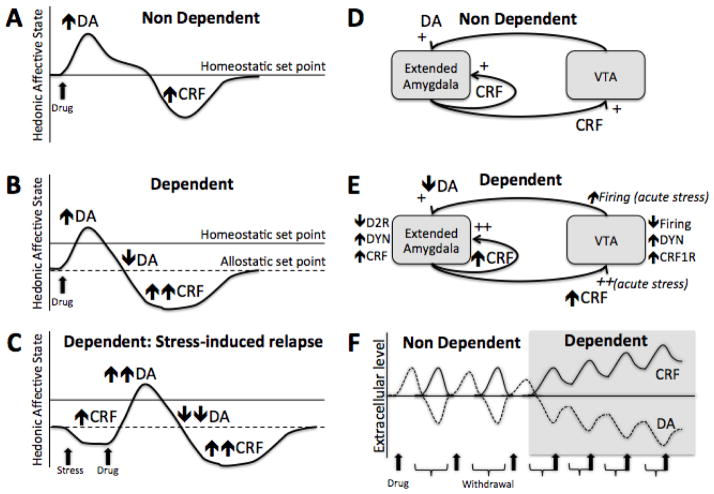
Taken together, these results demonstrate that the dopamine and CRF systems can interact with each other in the VTA and the extended amygdala at every stage of the addiction process. Activation of the dopaminergic system by drugs of abuse may transiently sensitize the CRF system during the initial exposure to the drug, while drug withdrawal will lead to a sustained activation of the CRF system in the extended amygdala. This increase in the CRF-CRF1R system during withdrawal will not only represent the emergence of the b-process but may contribute to the habituation of the a-process through a decrease dopamine release in the limbic system by the recruitment of the dynorphin system. Finally, acute stress after abstinence in dependent subjects may exacerbate CRF release in the VTA and provoke relapse through activation of dopamine neurons (see Figure 1).
Figure 1. Hypothetical interactions between the dopamine and CRF systems as a mechanism underlying the allostatic load in the transition to drug addiction.
A) The hedonic response to an acute drug administration in a drug-naive individual with activity in the dopaminergic system involved in reward predominating with a minor anti-reward opponent process-like response of the CRF system. B) The hedonic response to an acute drug administration in a drug-dependent individual while taking drug regularly. Initial activity in the dopaminergic system involved in reward is followed by a decrease in function of the dopaminergic system involved in reward, and a major anti-reward opponent process-like response of the CRF system. Note the change in hedonic set point, reflecting an allostatic state produced by chronic dysregulation of the dopamine and CRF systems. C) The hedonic response to an acute stressor followed by a drug administration in a drug-dependent individual during withdrawal. Acute stress produces a recruitment of the CRF system and a further decrease in hedonic response, below the allostatic set point. This major dysphoria triggers drug intake accompanied by an intense activity of the dopaminergic system and is followed by a compensatory decrease in the dopaminergic system and increase in the CRF system to reestablish the allostatic set point. Note that depending on the intensity and frequency of withdrawal episodes and stress-induced relapse, an allostatic load can develop so that the allostatic set point is further shifted away from the homeostatic set point (see C and F). D) Interaction between the dopaminergic system originating from the VTA and the CRF system from the extended amygdala in a drug naïve subject. E) Interaction between the dopaminergic and CRF system in a drug dependent subjects. Note that neuroadaptations in the dopamine, dynorphin and CRF systems represent changes during withdrawal, except for italicized neuroadaptations representing changes during acute stress (see also C). F) Dysregulation of the dopamine and CRF system during chronic access to the drug. When the drug is consumed occasionally (non dependent), changes in the dopamine and CRF systems are maintained within the homeostatic range and have time to return to baseline level. As the frequency of use increases, the initial increase in the dopamine system is blunted and is followed by a greater increase in the CRF system. The failure to self-regulate these systems and the impossibility to return to baseline lead to an allostatic load that will contribute significantly to the transition to drug dependence.
Conclusions
Acute withdrawal from drugs of abuse produces opponent process-like changes in reward neurotransmitters in specific elements of reward circuitry associated with the mesolimbic dopaminergic system and recruitment of the extended amygdala and CRF stress systems that motivationally oppose the acute hedonic effects of drugs of abuse. Such changes in the dopamine and CRF these brain systems associated with the development of motivational aspects of withdrawal are hypothesized to be a major source of neuroadaptive changes that drive and maintain addiction. Decreased dopaminergic function in the nucleus accumbens and extended amygdala may participate in the habituation of the a-process, i.e., or the acute reinforcing efficacy of natural rewards and drugs of abuse, whereas recruitment of the CRF-CRF1 systems and possibly dynorphin/κ opioid system in the CeA, BNST, and VTA during withdrawal may participate in the emergence of the b-process, i.e, or negative emotional state that drives the motivation to seek drugs. Although some tantalizing evidence suggests that the dopaminergic and CRF systems may closely interact with each other, research in this domain is scarce. Unknown is whether the initial activation of the dopaminergic system in the VTA (a-process) is required for the increase in CRF release in the extended amygdala and VTA (b-process) in drug-dependent and withdrawn subjects that leads to compulsive drug seeking and increased craving for the drug. As such, repeated withdrawal episodes and sustained activation of the CRF-CRF1R system may lead to an allostatic load contributing significantly to the transition to drug addiction.
Highlights.
The opponent process theory postulates that drugs trigger two opposing motivational process.
The a-process has fast onset and offset and the b-process is opposite, slower to start and last longer.
Decreased dopaminergic function in the NAC and CeA mediate the habituation of the a-process.
Activation of the CRF systems in the CeA and VTA mediates the increase in b-process in dependent subjects.
Interaction between the dopamine and CRF systems may represent the opponent-process mechanisms of drug withdrawal.
Acknowledgments
This is publication number 21324 from The Scripps Research Institute. This work was supported by National Institutes of Health grant DA04398, DA10072, DA04343, and DA023597 from the National Institute on Drug Abuse, AA08459, and AA06420 from the National Institute on Alcohol Abuse and Alcoholism, and the Pearson Center for Alcoholism and Addiction Research. The authors would like to thank Michael Arends for his help with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003 Jan;43(1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine antagonist in rats with escalating cocaine intake. Psychopharmacology. 2004;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- Alheid GF, De Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. 2. Academic Press; San Diego: 1995. pp. 495–578. [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Allen YS, Roberts GW, Bloom SR, Crow TJ, Polak JM. Neuropeptide Y in the stria terminalis: evidence for an amygdalofugal projection. Brain Research. 1984;321:357–362. doi: 10.1016/0006-8993(84)90193-8. [DOI] [PubMed] [Google Scholar]
- Bagosi Z, Jászberényi M, Bujdosó E, Telegdy G. The effects of corticoptropin-releasing factor and the urocortins on striatal dopamine release induced by electrical stimulation-an in vitro superfusion study. Neurochem Res. 2006 Feb;31(2):209–13. doi: 10.1007/s11064-005-9010-x. Epub 2006 Mar 2. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology. 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology. 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology (Berl) 2005 Apr;178(4):367–80. doi: 10.1007/s00213-004-2016-2. Epub 2004 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011 Feb;129(2):149–71. doi: 10.1016/j.pharmthera.2010.09.007. Epub 2010 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010 Feb 16;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. κ-Opioid receptor signaling and brain reward function. Brain Research Reviews. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biological Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. Journal of Neuroscience. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research Reviews. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Cheung S, Moore KE, Lookingland KJ. Dopamine receptor-mediated regulation of corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 1996 Oct 28;738(1):60–6. doi: 10.1016/0006-8993(96)00765-2. [DOI] [PubMed] [Google Scholar]
- Eliava M, Yilmazer-Hanke D, Asan E. Interrelations between monoaminergic afferents and corticotropin-releasing factor-immunoreactive neurons in the rat central amygdaloid nucleus: ultrastructural evidence for dopaminergic control of amygdaloid stress systems. Histochemistry and Cell Biology. 2003;120:183–197. doi: 10.1007/s00418-003-0557-9. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43:341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain: IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Koob GF, Sanna PP. Intrinsic neuronal plasticity in the juxtacapsular nucleus of the bed nuclei of the stria terminalis (jcBNST) Prog Neuropsychopharmacol Biol Psychiatry. 2009 Nov 13;33(8):1347–55. doi: 10.1016/j.pnpbp.2009.08.003. Epub 2009 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiology of Disease. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DA, Stuber M, Poland RE. Allostatic load in women with and without PTSD symptoms. Psychiatry. 2006;69:191–203. doi: 10.1521/psyc.2006.69.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000;23:577–586. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways: role in autonomic, neuroendocrine, and behavioral responses to stress. In: Tache Y, Rivier C, editors. Corticotropin-Releasing Factor and Cytokines: Role in the Stress Response (series title: Annals of the New York Academy of Sciences, vol 697) New York Academy of Sciences; New York: 1993. pp. 53–60. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009 Apr;14(2):130–43. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder TE, Sellings LH, Vargas-Perez H, Ting-A-Kee R, Siu EC, Tyndale RF, van der Kooy D. Dopaminergic signaling mediates the motivational response underlying the opponent process to chronic but not acute nicotine. Neuropsychopharmacology. 2010;35:943–954. doi: 10.1038/npp.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. Journal of Neuroscience. 2009;29:6535–6244. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. Textbook of Psychology. 3. W.B. Saunders; Philadelphia: 1972. [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behavioural Pharmacology. 1995;6:74–80. [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Research Reviews. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987 Sep;242(3):757–63. [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. Journal of Clinical Epidemiology. 2002;55:696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- Kash TL, Matthews RT, Winder DG. Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2008;33:1379–1390. doi: 10.1038/sj.npp.1301504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Park MK, Chung S. Regulation of somatodendritic dopamine release by corticotropin-releasing factor via the inhibition of voltage-operated Ca2+ channels. Neurosci Lett. 2009 Nov 6;465(1):31–5. doi: 10.1016/j.neulet.2009.08.066. Epub 2009 Sep 1. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine and stress. Biological Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Allostatic view of motivation: implications for psychopathology. In: Bevins RA, Bardo MT, editors. Motivational Factors in the Etiology of Drug Abuse (series title: Nebraska Symposium on Motivation. Vol. 50. University of Nebraska Press; Lincoln NE: 2004. pp. 1–18. [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Menzaghi F, Pich EM, Britton KT. Corticotropin releasing factor, stress and behavior. Seminars in the Neurosciences. 1994;6:221–229. [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Animal Models of Drug Addiction, Neurobiology of Addiction. Academic Press; London: 2006. [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Federation Proceedings. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kozicz T. Axon terminals containing tyrosine hydroxylase- and dopamine-®-hydroxylase immunoreactivity form synapses with galanin immunoreactive neurons in the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Research. 2001;914:23–33. doi: 10.1016/s0006-8993(01)02770-6. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, Gallagher JP, Shinnick-Gallagher P. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. European Journal of Neuroscience. 2010;31:1027–1042. doi: 10.1111/j.1460-9568.2010.07148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulin JP, Celerier E, Larcher A, Le Moal M, Simonnet G. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–636. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- Lavicky J, Dunn AJ. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J Neurochem. 1993 Feb;60(2):602–12. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Lesur A, Gaspar P, Alvarez C, Berger B. Chemoanatomic compartments in the human bed nucleus of the stria terminalis. Neuroscience. 1989;32:181–194. doi: 10.1016/0306-4522(89)90117-6. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Paull WK. Association of dopaminergic fibers with corticotropin releasing hormone (CRH)-synthesizing neurons in the paraventricular nucleus of the rat hypothalamus. Histochemistry. 1989;93:119–127. doi: 10.1007/BF00315964. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–1752. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Post-cocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Martin-Soelch C, Chevalley AF, Kunig G, Missimer J, Magyar S, Mino A, Schultz W, Leenders KL. Changes in reward-induced brain activation in opiate addicts. European Journal of Neuroscience. 2001;14:1360–1368. doi: 10.1046/j.0953-816x.2001.01753.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. American Journal of Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. In: McGinty JF, editor. Advancing from the Ventral Striatum to the Extended Amygdala: Implications for Neuropsychiatry and Drug Abuse. series title: Annals of the New York Academy of Sciences. Vol. 877. New York Academy of Sciences; New York: 1999. pp. 309–338. [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. In: McCann SM, Lipton JM, Sternberg EM, Chrousos GP, Gold PW, Smith CC, editors. Neuroimmunomodulation: Molecular Aspects, Integrative Systems, and Clinical Advances. series title: Annals of the New York Academy of Sciences. Vol. 840. New York Academy of Sciences; New York: 1998. pp. 33–44. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006 Apr 5;26(14):3855–63. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. Journal of Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T, Inoue K, Iwasaki S, Yamauchi T, Hayashi T, Kiriike N. Corticotropin-releasing factor receptor type 1, but not type 2, in the ventromedial hypothalamus modulates dopamine release in female rats. Pharmacol Biochem Behav. 2006 Oct;85(2):435–40. doi: 10.1016/j.pbb.2006.09.013. Epub 2006 Nov 15. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature Neuroscience. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nijsen MJ, Croiset G, Diamant M, De Wied D, Wiegant VM. CRH signalling in the bed nucleus of the stria terminalis is involved in stress-induced cardiac vagal activation in conscious rats. Neuropsychopharmacology. 2001;24:1–10. doi: 10.1016/S0893-133X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic β-endorphin and dynorphin release by corticotropin-releasing factor (in vitro) Brain Research. 1986;399:152–155. doi: 10.1016/0006-8993(86)90610-4. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Research. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacology Biochemistry and Behavior. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini C, Koob GF, Pulvirenti L. Dopamine partial agonist reverses amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:789–792. doi: 10.1016/S0893-133X(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology. 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Paull WK. Demonstration of distinct corticotropin releasing factor-containing neuron populations in the bed nucleus of the stria terminalis: a light and electron microscopic immunocytochemical study in the rat. Histochemistry. 1990;94:345–364. doi: 10.1007/BF00266441. [DOI] [PubMed] [Google Scholar]
- Pompei P, Tayebaty SJ, De Caro G, Schulkin J, Massi M. Bed nucleus of the stria terminalis: site for the antinatriorexic action of tachykinins in the rat. Pharmacology Biochemistry and Behavior. 1991;40:977–981. doi: 10.1016/0091-3057(91)90114-h. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Lisuride reduces psychomotor retardation during withdrawal from chronic intravenous amphetamine self-administration in rats. Neuropsychopharmacology. 1993;8:213–218. doi: 10.1038/npp.1993.23. [DOI] [PubMed] [Google Scholar]
- Radke AK, Rothwell PE, Gewirtz JC. An anatomical basis for opponent process mechanisms of opiate withdrawal. Journal of Neuroscience. 2011;31:7533–7539. doi: 10.1523/JNEUROSCI.0172-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Research. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schütz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011 Sep 30;333(6051):1903–7. doi: 10.1126/science.1202107. Epub 2011 Sep 1. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates “anxiety-like” behavior induced by cocaine withdrawal in rats. Brain Research. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei: potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Schulkin J. Social allostasis: anticipatory regulation of the internal milieu. Frontiers in Evolutionary Neuroscience. 2011;2:111. doi: 10.3389/fnevo.2010.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob G. Decreased brain reward produced by ethanol withdrawal. Proceedings of the National Academy of Sciences USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. Journal of Pharmacology and Experimental Therapeutics. 1994;271:1391–1398. [PubMed] [Google Scholar]
- Seeman TE, Singer B, Rowe J, McEwen B. Exploring a new concept of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences USA. 2011;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation: allostatic load and its health consequences. MacArthur studies of successful aging Archives of Internal Medicine. 1997;157:2259–2268. [erratum: 159: 1176] [PubMed] [Google Scholar]
- Shannon M, King TL, Kennedy HP. Allostasis: a theoretical framework for understanding and evaluating perinatal health outcomes. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2007;36:125–134. doi: 10.1111/j.1552-6909.2007.00126.x. [DOI] [PubMed] [Google Scholar]
- Smialowska M, Bajkowska M, Prezewłocka B, Maj M, Turchan J, Przewłocki R. Effect of 6-hydroxydopamine on neuropeptide Y and corticotropin-releasing factor expression in rat amygdala. Neuroscience. 1999;94:1125–1132. doi: 10.1016/s0306-4522(99)00393-0. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: 1. Temporal dynamics of affect Psychological Reviews. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Song ZH, Takemori AE. Stimulation by corticotropin-releasing factor of the release of immunoreactive dynorphin A from mouse spinal cords in vitro. European Journal of Pharmacology. 1992;222:27–32. doi: 10.1016/0014-2999(92)90458-g. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. John Wiley; Chichester: 1988. pp. 629–649. [Google Scholar]
- Sterling P, Eyer J. Biological basis of stress-related mortality. Soc Sci Med E. 1981 Feb;15(1):3–42. doi: 10.1016/0271-5384(81)90061-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale W. The organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. Journal of Comparative Neurology. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007 Nov;64(11):1575–9. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008 Apr 15;586(8):2157–70. doi: 10.1113/jphysiol.2007.150078. Epub 2008 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. Journal of Comparative Neurology. 2008;509:302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005 Jun 1;25(22):5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged access. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Research. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. Journal of Neuroscience. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Action of drugs of abuse on brain reward systems. Pharmacol Biochem Behav. 1980;13 (Suppl 1):213–23. doi: 10.1016/s0091-3057(80)80033-5. [DOI] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacology and Therapeutics. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]